Abstract
Megakaryocytopoiesis is a complex multistep process involving cell division, endoreplication, and maturation and resulting in the release of platelets into the blood circulation. Megakaryocytes (MK) progressively express lineage-restricted proteins, some of which play essential roles in platelet physiology. Glycoprotein (GP)Ib-V-IX (CD42) and GPIIb (CD41) are examples of MK-specific proteins having receptor properties essential for platelet adhesion and aggregation. This study defined the progressive expression of the GPIb-V-IX complex during in vitro MK maturation and compared it to that of GPIIb, an early MK marker. Human cord blood CD34+ progenitor cells were cultured in the presence of cytokines inducing megakaryocytic differentiation. GPIb-V-IX expression appeared at day 3 of culture and was strictly dependent on MK cytokine induction, whereas GPIIb was already present in immature CD34+ cells. Analysis by flow cytometry and of the messenger RNA level both showed that GPV appeared 1 day later than GPIb-IX. Microscopy studies confirmed the late appearance of GPV, which was principally localized in the cytoplasm when GPIb-IX was found on the cell surface, suggesting a delayed program of GPV synthesis and trafficking. Cell sorting studies revealed that the CD41+GPV+ population contained 4N and 8N cells at day 7, and was less effective than CD41+GPV− cells in generating burst-forming units of erythrocytes or MK colonies. This study shows that the subunits of the GPIb-V-IX complex represent unique surface markers of MK maturation. The genes coding for GPIb-IX and GPV are useful tools to study megakaryocytopoiesis and for tissue-specific or conditional expression in mature MK and platelets.
Introduction
Megakaryocytopoiesis is the cellular differentiation process that leads to the release of platelets into the circulation. Megakaryocyte (MK) progenitor cells proliferate, polyploidize, increase in size, and develop lineage-specific cell surface and cytoplasmic markers. The regulation of megakaryocytopoiesis occurs in the bone marrow within 2 major compartments. The first one contains different populations of proliferative bipotent erythromegakaryocytic progenitor cells (burst-forming units of erythrocytes and megakaryocytes [BFU-E/MK]).1 In the second compartment, terminal maturation is accompanied by a decrease in proliferation, an increase in cell ploidy, and cytoplasmic maturation.2 The most immature MK progenitors are the burst-forming units of megakaryocytes (BFU-MK), made of primitive cells expressing the CD34 antigen and HLA-DR−. The colony-forming units of megakaryocytes (CFU-MK) are the most mature progenitors, which still express CD34 but are also HLA-DR+.3
Proliferation and maturation of MK precursors are controlled by several pleiotropic cytokines.4,5 Although thrombopoietin (TPO) plays a major role in regulating MK and platelet production,6-8 many studies point to additional contributions from stem cell factor (SCF), interleukin-3 (IL-3), interleukin-6 (IL-6), interleukin-11 (IL-11), granulocyte-macrophage colony-stimulating factor (GM-CSF), and basic fibroblast growth factor (bFGF).4 5
Megakaryocytic differentiation is accompanied by changes in cell morphology, notably an increase in size and the appearance of demarcation membranes, and by the sequential expression of a number of genes coding for specific cell surface markers, cytokines, and cytokine receptors. Early genes, such as mpl coding for the TPO receptor9 and the GPIIb gene10 are first expressed in CD34+ cells, but are still expressed in the late stages leading to platelet production. On the other hand, platelet factor 4 (PF4), β-thromboglobulin, von Willebrand factor (vWF),11,12 and GPIbα,13 one of the 4 subunits of the GPIb-V-IX complex with GPIbβ, GPV and GPIX,14 appear at later, more differentiated stages. During maturation surface expression of CD34 gradually disappears.
GPIIb-IIIa (integrin αIIbβ3), the platelet receptor for fibrinogen and vWF, is a well-studied example of an early MK marker. GPIIb is weakly expressed on bipotent BFU-E/MK1,15 and early expression of GPIIb on multilineage hematopoietic progenitors has been recently demonstrated in the avian hematopoietic system.16 In conditional knockout mice in which the thymidine kinase gene was driven by the GPIIb promoter,17 the administration of gancyclovir led to dramatic reduction in the platelet count. In the bone marrow, erythroid and myeloid progenitors were also affected, which indicated the presence of GPIIb on progenitor cells. In addition, GPIIb expression has been detected on leukemic cell lines displaying erythroid and MK markers such as HEL,18 OCIM-2,19 KU 812,20 LAMA-84,21 M-07e,22Meg-01,23 MKPL-1,24 Dami,25CMK,26 CHRF-288,27 UT-7,28MEG-A2,29 and EST-IU30 cells.
In contrast to GPIIb-IIIa, but also PF4 or vWF, the time course of GPIb-V-IX expression during MK maturation has not been studied in detail. The GPIb-V-IX complex, a member of the leucine-rich glycoprotein family, is a vWF receptor supporting the initial rolling and adhesion of platelets on the subendothelium of damaged blood vessels.14,31,32 Previous studies of megakaryoblasts and MK cultured from bone marrow cells suggested a delayed time course of GPIbα expression as compared to GPIIb-IIIa.13 In addition, among 13 leukemic cell lines expressing GPIIb-IIIa,18-30 only 3 (Dami, CHRF-288, and M-07e) show significant surface expression of GPIb. This proportion is slightly higher for cells differentiated by phorbol ester treatment, confirming that GPIb is a later marker than GPIIb-IIIa.
Another unanswered question concerns the timing of the expression of the 4 subunits forming the GPIb-V-IX complex. These subunits are encoded by separate genes and despite their coexpression at the platelet surface there is no clear indication of coordinated expression during megakaryocytopoiesis. On the contrary, there is indirect evidence in favor of the separate regulation of GPV expression in MK because (1) studies in transfected cells show that the GPIb-IX complex does not require GPV for its efficient expression33; (2) among GPIb+ cells only the Dami cell line expressed GPV34; and (3) GPV knockout mice have platelets displaying normal surface expression of the GPIb-IX complex.35 36
The aims of this study were to use techniques of in vitro MK culture using cord blood CD34+ cells to follow the temporal expression of the 4 subunits of the GPIb-V-IX complex during MK maturation, to compare the results to those for GPIIb-IIIa, and to assess possible differences in the temporal expression of the 4 subunits of GPIb-V-IX.
Materials and methods
Purification and culture of CD34+ cells
The CD34+ cells were purified from human umbilical cord blood samples obtained immediately after delivery in accordance with institutional guidelines. Briefly, cord blood was diluted one third in 0.9% NaCl and centrifuged. The upper phase containing platelets was removed. Low-density mononuclear cells were isolated by Ficoll-HiPaque (1.077 g/mL) density gradient centrifugation, washed in phosphate-buffered saline (PBS), and resuspended in tissue culture flasks for overnight adherence in RPMI 1640 (GIBCO BRL, Gaithersburg, MD) supplemented with 10% fetal calf serum (Boehringer, Mannheim, Germany), 2 mmol/L glutamine, and 100 U/mL penicillin and streptomycin (GIBCO BRL).
The CD34+ progenitor cells were isolated using an immunomagnetic separation system (MACS: Miltenyi Biotec, Bergisch Gladbach, Germany), as previously described by Miltenyi.37Mononuclear cells were labeled with an anti-CD34 monoclonal antibody (QBEND/10) recognizing a denaturation-resistant epitope on the CD34 antigen, washed in PBS, and incubated with microbeads, which bind QBEND/10. The labeled cells were then resuspended in PBS containing 1% bovine serum albumin (BSA) and 5 mmol/L EDTA to prevent platelet contamination and purified on the MACS system according to the manufacturer's instructions. To improve the CD34+ purity, the cells were passed twice over the column, and the percentage of CD34+ cells as determined by flow cytometry generally exceeded 98% to 99%.
These cells were cultured in serum-free STEMαA medium (TEBU, Le Parray en Yvelines, France) supplemented with 2.5 ng/mL IL-3 (TEBU), 10 ng/mL IL-6 (TEBU), 10 ng/mL IL-11 (Genzyme Diagnostics, Paris, France) and 50 ng/mL TPO (Genzyme Diagnostics), at a density of less than 3 × 105 cells/mL. Cultures were maintained at 37°C in a humidified 5% CO2 atmosphere.
Monoclonal antibodies
Mouse monoclonal antibodies (MoAbs) directed against GPIbα (ALMA.12), GPIX (ALMA.16), and GPV (V.1) were produced in our laboratory.38 Fluorescein isothiocyanate–conjugated goat antimouse IgG F(ab′)2 (FITC-GAM) was purchased from Jackson ImmunoResearch Laboratories (West Grove, PA); the FITC-conjugated MoAbs 80H5 against CD15 and T16 against CD38 were obtained from Immunotech (Marseille-Luminy, France). The R-phycoerythrin (PE)–conjugated 5B12 MoAb against CD41 was from DAKO (Glostrup, Denmark) and the FITC- or peridin chlorophyll a–binding protein (PerCP)-conjugated 8G12 MoAb against CD34 from Becton Dickinson (Mountain View, CA). V.1 was directly coupled to the Cy3 fluorochrome with a Cy3 labeling kit (Amersham, Les Ulis, France) and ALMA.12 to Alexa 488 with an Alexa 488 signal-amplification kit (Molecular Probes, Leiden, The Netherlands). The 6 nonspecific control MoAbs were PerCP-conjugated IgG1 (Becton Dickinson), PE- and FITC-conjugated IgG1, FITC-conjugated IgM (Immunotech), Cy3-conjugated IgG1, and Alexa 488–conjugated IgG1 (Molecular Probes).
Flow cytometry
The MoAbs used in flow cytometry were ALMA.12, ALMA.16, V.1, ALMA.12–Alexa 488, V.1-Cy3, FITC-GAM, FITC–anti-CD15, FITC–anti-CD38, PE–anti-CD41, and PerCP–anti-CD34.
Indirect labeling.
Cells (2 × 104) were incubated with 2 μg ALMA.12, ALMA.16, or V.1 in PBS containing 1% BSA (PBS/BSA) for 15 to 20 minutes at 4°C. After 2 washes in PBS/BSA, the samples were resuspended in 100 μL PBS/BSA and further labeled by addition of 2 μL FITC-GAM for 15 to 20 minutes at 4°C in the dark. The cells were then washed twice, incubated with 2 μL each of PerCP–anti-CD34 and PE–anti-CD41 for 15 to 20 minutes at 4°C in the dark and finally washed and resuspended in 200 μL PBS/BSA.
Direct labeling.
Cells (2 × 104) in PBS/BSA were triple labeled by incubation for 15 to 20 minutes at 4°C in the dark with different combinations of PerCP–anti-CD34, PE–anti-CD41, FITC–anti-CD15, FITC–anti-CD38, V.1-Cy3, and ALMA.12–Alexa 488. The cells were then washed and resuspended in 200 μL PBS/BSA.
Samples were analyzed on a FACSCalibur flow cytometer using CellQuest software (Becton Dickinson) and PerCP-IgG1, PE-IgG1, FITC-IgM, and FITC-IgG1 as nonspecific controls.
Cell sorting
The CD34+ cells purified as described above were double labeled with PE–anti-CD41 and ALMA.12–Alexa 488, or with PE–anti-CD41 and an anti-GPV revealed by an Alexa 488–GAM for 30 minutes at 4°C, and washed twice. Cells were sorted using a Coulter Elite flow cytometer (Coulter Electronics, Margency, France). CD41+GPIbα− and CD41+GPIbα+ populations were selected at day 4, and CD41+GPV− and CD41+GPV+ populations were selected at day 7. The purity of the sorted populations was confirmed by analysis on a FACSCalibur cytometer (Becton Dickinson).
Clonogenic cell assay
Methylcellulose assays for burst-forming units-erythrocytes.
Freshly sorted CD41+GPIbα+, CD41+GPIbα−, CD41+GPV+, and CD41+GPV− cells were plated on 35-mm plastic culture dishes at a concentration of 103 cells/mL in semisolid growth media that consisted of 1% methylcellulose in Iscove modified Dulbecco medium (IMDM; GIBCO BRL), 30% heat-inactivated FCS, 1% BSA, 0.1 mmol/L β-mercaptoethanol, 2 U erythropoietin (Epo; TEBU), 2 ng IL-3 (TEBU), 100 U IL-1 (TEBU), 1 ng IL-6 (TEBU), 100 U GM-CSF (TEBU), 100 ng G-CSF (TEBU), and 10 ng SCF (TEBU) (Le Bousse-Kerdiles, oral communication, June 1999). Cultures were incubated for 14 days at 37°C in a humidified 5% CO2atmosphere. The colonies (≥ 30 cells) were counted on 5 individual plates using an inverted microscope. BFU-E–type colonies were evaluated at days 12 through 14. Similarly, BFU-E/MK, colony-forming units of granulocytes (CFU-G), colony-forming units of macrophages (CFU-M), colony-forming units of granulocytes and macrophages (CFU-GM), and colony-forming units of granulocytes, erythrocytes, monocytes, macrophages (CFU-GEMM) colonies were identified morphologically and counted.
Megakaryocytic progenitor assays.
Freshly sorted CD41+GPIbα+, CD41+GPIbα−, CD41+GPV+, and CD41+GPV− cells were plated at a concentration of 2 × 103 cells/mL in collagen medium on 35-mm plastic culture dishes. The medium contained 0.1% collagen in STEMαA medium supplemented with 2.5 ng IL-3 (TEBU), 10 ng IL-6 (TEBU), 10 ng/mL IL-11 (Genzyme Diagnostics), and 50 ng TPO (Genzyme Diagnostics). Cultures were incubated for 8 to 10 days at 37°C in a humidified 5% CO2 atmosphere. Colonies (≥ 3 MK) were counted on 5 plates.
Ploidy values
Cells labeled with anti-GPV and anti-CD41 were fixed overnight in 70% ethanol at 4°C. The fixed cells were washed and resuspended in 300 μL PBS containing propidium iodide (50 μg/mL; Sigma, Saint Quentin Fallavier, France) and RNase A (0.2 μg/mL; Sigma) for 30 minutes at 4°C. Samples were analyzed on a FACSCalibur flow cytometer using CellQuest software. The ploidy distribution was determined by setting markers at the nadirs between peaks.
Immunocytochemistry
Cells in RPMI 1640 were seeded on glass coverslips coated with poly-l-lysine, at a density of 5 × 104/mL, and incubated for 30 to 40 minutes at 37°C. The medium was removed and the cells were fixed in PBS-3.7% paraformaldehyde for 10 minutes at room temperature, rinsed twice in PBS, and quenched by incubation in PBS-0.1 mol/L glycine for 10 minutes at room temperature. After 2 washes in PBS, the cells were permeabilized in PBS buffer containing 0.02% BSA and 0.005% saponin (SBP buffer) for 20 minutes, at room temperature. The cells were subsequently incubated with the primary antibodies (V.1, anti-CD41, or ALMA.12–Alexa 488) diluted in SBP for 30 minutes at room temperature, washed 3 times for 5 minutes in SBP, incubated for 30 minutes with the secondary antibodies (Cy3-GAM, Alexa 488–GAM, or FITC-GAM), washed 3 times for 5 minutes in SBP, and then incubated for 30 minutes with the tertiary antibodies (ALMA.12, ALMA.16, and V.1 directly conjugated with Cy3). Finally, the coverslips were washed twice for 5 minutes in SBP and twice for 5 minutes in PBS and mounted upside down on a slide in Mowiol.
The following dilutions were used: ALMA.16, 1:200; V.1, 1:200; anti-CD41, 1:200; FITC-GAM, 1:150; ALMA.12-Cy3, 1:500; ALMA.16-Cy3, 1:66; V.1-Cy3, 1:500; ALMA.12–Alexa 488, 1:250; Cy3-GAM, 1:800; and Alexa 488–GAM, 1:400. Samples were examined and photographed under a DMR HC fluorescence microscope (Leica, Vienna, Austria) using an oil immersion objective (63 × or 100 ×).
Confocal laser scanning microscopy and image analysis
Confocal microscopy was performed using a Zeiss laser scanning microscope (LSM 410 invert, Göttingen, Germany) equipped with an oil immersion lens (63 ×, numerical aperture = 1.4). Cy2, Alexa 488, and FITC emissions were excited with the argon 488 nm line, Cy3 with the He/Ne 543 nm line, and Cy5 with the He/Ne 633 nm line. The emission signals were filtered with a Zeiss 515-565 nm filter (Cy2, Alexa 488, and FITC), with a long pass 595 nm filter (Cy3) or with a long pass 650 nm filter (Cy5). Nonspecific fluorescence was assessed by incubating the cells with the secondary fluorescent-labeled antibodies and measuring the average emission intensity for each fluorochrome, which was then subtracted from all specific images.
Analysis of transcripts
Total cellular RNA was extracted by the thiocyanate-guanidium method of Chomczynski and Sacchi.39 Briefly, 107 cells were lysed in 1 mL TriPure (Boehringer), extracted with 0.2 mL chloroform and centrifuged at 11 000 rpm for 15 minutes at 4°C. The aqueous phase containing RNA was precipitated with isopropanol and the pellet washed in 70% (v/v) cold ethanol.
Complementary DNA (cDNA) was synthesized from 50 ng total RNA from CD34+ cells using a Ready To Go: T-Primed First-Strand Kit (Pharmacia Biotech, Uppsala, Sweden), in a reaction volume of 33 μL. Polymerase chain reaction (PCR) amplifications were carried out with an Expand High Fidelity PCR System (Boehringer) in a volume of 100 μL, using 200 μmol/L each dNTP, 0.4 μmol/L of the reverse and forward primers, 1.5 mmol/L MgCl2, 2 μL cDNA, and 2.6 U of a mix containing Taq DNA polymerase and Pwo DNA polymerase. Amplifications were performed in a DNA Thermal Cycler (Perkin Elmer Cetus, Norwalk, CT) for 2 minutes at 94°C, followed by 30 cycles of 1 minute at 94°C, 2 minutes at 55°C (for glyceraldehyde-3-phosphate dehydrogenase [GAPDH], GPIbα, and GPV) or 60°C (for GPIIb) and 2 minutes at 72°C, and finally 7 minutes at 72°C. The PCR products were loaded on ethidium bromide–stained agarose gels.
The direct and reverse primers were respectively P1 and P2 for GAPDH (EMBL/Genbank: HSGAPDR-X01677),40 P3 and P4 for GPIIb (EMBL/Genbank: HUMGPIIBA-M34480),41 P5 and P6 for GPV (EMBL/Genbank: HSGPV-Z23091),42 and P7 and P8 for GPIbα (Genbank: HSGPIBAA-M22403).43 P1: 5′-CCA CCC ATG GCA AAT TCC ATG GCA-3′; P2: 5′-TCT AGA CGG CAG GTC AGG TCC ACC-3′; P3: 5′-GTT GGT GAG CGT GGG GAA TC-3′; P4: 5′-TTC ACA GTC CCA GGG CCA TT-3′; P5: 5′-TCA GTT ACT TTG GAG TGC AGA ACC AT-3′; P6: 5′-AAG ATG CGT GAT TTT GTT GCG CGA C-3′; P7: 5′-GAA TTC ATG GCA GAA GGC TGT TTG GAG GAG TCC-3′; P8: 5′-GGC AGC GCT GTC AGA TTC CTC TTG TCA CAG-3′. The GPV and GPIbα primers were located on both sides of the 5′ intron to discriminate between messenger RNA (mRNA) and genomic amplification products.
Results
Maturation of megakaryocytes during the course of the liquid culture
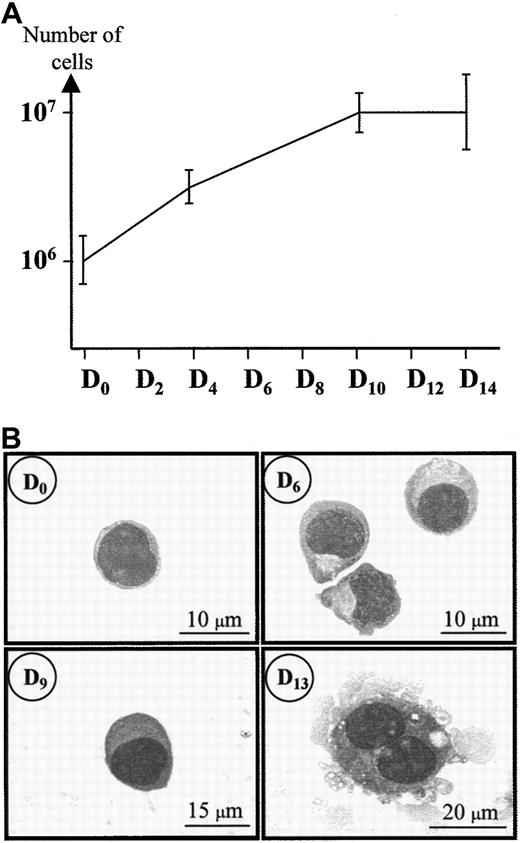
During the first days of culture, the proliferation was maximal: the number of cells was amplified 3 times during the first 4 days (Figure 1A). Then, the proliferation decreased, reaching a plateau between days 10 to 14. Figure 1B shows that during the high proliferative stage of the culture, the cells had the aspect of undifferentiated progenitors. These cells were about 8 μm in diameter with thin chromatin. They grew and differentiated from day 6 to day 9 into 15-μm diameter megakaryoblasts with denser chromatin. At day 12, most of the cells were about 25 to 30 μm in diameter with polylobular nuclei. By day 13, they measured approximately 30 to 40 μm in diameter and cells with the appearance of mature MK were frequent in the culture, with filopodia and polylobular nuclei.
May-Grünwald-Giemsa (MGG) staining of hematopoietic cord blood cells during MK maturation.
Day 0 corresponds to the day of CD34+ cell isolation. These cells were then cultured under conditions optimized for MK growth and maturation (see “Materials and methods”) and analyzed on days 0, 6, 9, and 13 of culture. MGG was performed as described by Löffler.44
May-Grünwald-Giemsa (MGG) staining of hematopoietic cord blood cells during MK maturation.
Day 0 corresponds to the day of CD34+ cell isolation. These cells were then cultured under conditions optimized for MK growth and maturation (see “Materials and methods”) and analyzed on days 0, 6, 9, and 13 of culture. MGG was performed as described by Löffler.44
Early expression of GPIIb-IIIa and delayed expression of GPIb-V-IX during megakaryocyte maturation
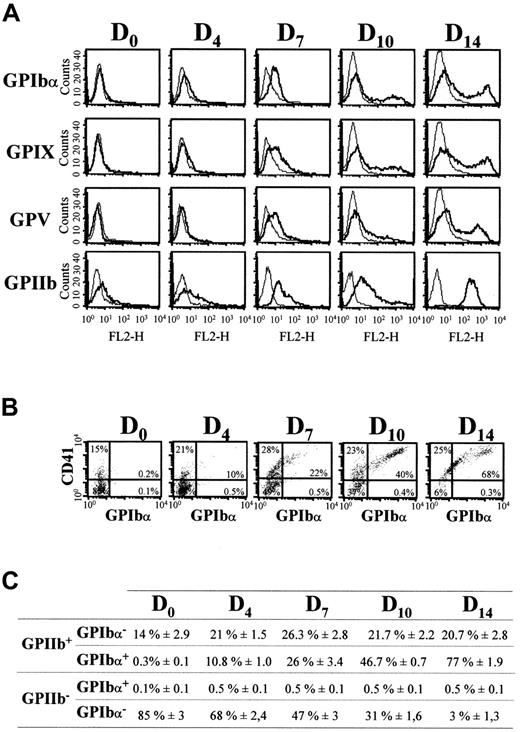
Surface expression of GPIbα, GPIX, and GPV was determined by flow cytofluorimetry and compared to that of GPIIb in double-labeling experiments (Figure 2). Typical expression profiles of these markers are shown on Figure 2A and 2B. Values for GPIIb and GPIbα obtained for one CB sample, representative of 6 individual experiments, are shown in Figure 2C. GPIbα, GPIX, and GPV were not detected at day 0, whereas about 15% of the cells were positive for GPIIb. The GPIIb labeling did not correspond to background generated by residual platelets because EDTA treatment of the cells during labeling did not change the percentage of CD41+cells (data not shown). From days 4 to 14, there was increased expression of the GPIbα, GPV, and GPIX subunits at the cell surface. At day 14, when most cells had differentiated morphologically into MK, GPIbα+ cells represented about 77% of the total population, whereas about 95% of the cells expressed GPIIb. Dot-plot analysis indicated that all GPIbα+ cells were also GPIIb+ (Figure 2B). Similar results were obtained for GPV and GPIX (data not shown). Interestingly, although GPIIb+cells evolved as a single population from mildly to strongly labeled cells from day 7 to day 14, GPIbα+, GPV+, and GPIX+ cells evolved from a single to 2 distinct populations, with mild and strong expression, respectively. This suggests that at different maturation stages, 2 megakaryocytic populations could coexist, both expressing CD41, but displaying different levels of GPIb-V-IX complexes.
Flow cytometric analyses of GPIbα, GPIX, GPV, and GPIIb-IIIa (CD41) surface expression during MK differentiation of CD34+ cells.
(A) Cell surface expression of GPIbα, GPIX, GPV (FL1 channel), and GPIIb-IIIa (FL2 channel) was analyzed on days 0, 4, 7, 10, and 14 of culture. Negative control curves represent labeling with PE- and FITC-conjugated IgG1 (see “Materials and methods”). (B) Dot-plot analysis of cells doubly labeled for GPIIb-IIIa (PE-CD41) and GPIbα (FITC-ALMA.12). The upper right quadrant indicates the percentage of cells doubly positive for GPIIb-IIIa and GPIbα. (C) Percentages of GPIIb+/GPIbα+, GPIIb+/GPIbα−, GPIIb−/GPIbα+, and GPIIb−/GPIbα− cells determined by double labeling as in panel B. Results are the means ± SEM of at least 6 separate cultures (C), or from a single experiment representative of at least 6 cultures (A and B).
Flow cytometric analyses of GPIbα, GPIX, GPV, and GPIIb-IIIa (CD41) surface expression during MK differentiation of CD34+ cells.
(A) Cell surface expression of GPIbα, GPIX, GPV (FL1 channel), and GPIIb-IIIa (FL2 channel) was analyzed on days 0, 4, 7, 10, and 14 of culture. Negative control curves represent labeling with PE- and FITC-conjugated IgG1 (see “Materials and methods”). (B) Dot-plot analysis of cells doubly labeled for GPIIb-IIIa (PE-CD41) and GPIbα (FITC-ALMA.12). The upper right quadrant indicates the percentage of cells doubly positive for GPIIb-IIIa and GPIbα. (C) Percentages of GPIIb+/GPIbα+, GPIIb+/GPIbα−, GPIIb−/GPIbα+, and GPIIb−/GPIbα− cells determined by double labeling as in panel B. Results are the means ± SEM of at least 6 separate cultures (C), or from a single experiment representative of at least 6 cultures (A and B).
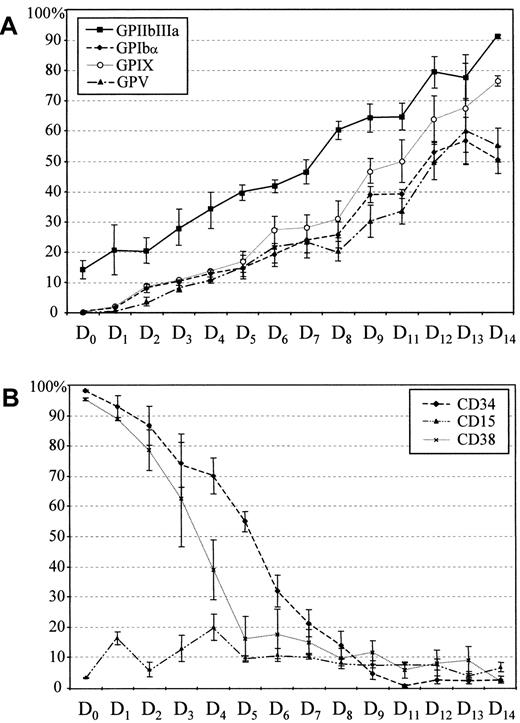
The delay in GPIbα, GPIX, and GPV expression as compared to GPIIb-IIIa is clearly visible in Figure3A. At day 5, the level of the 3 subunits of the GPIb-V-IX complex was comparable to that of GPIIb at day 0. During the first 6 to 8 days of culture, 25% of the cells were positive for the 3 subunits. Approximately 25% of the cells remained GPIbα−, GPV−, and GPIX− at day 15, indicating that even at this stage a significant proportion of the MK remained immature.
Kinetics of cell surface protein expression.
Kinetics of CD34, CD15, and CD38 (A) and GPIbα, GPIX, GPV, and GPIIb-IIIa (B) surface expression during MK differentiation of CD34+ cells. Cells were harvested at different time intervals and the percentages of cells positive for the different markers were determined by flow cytometry after double labeling (Figure2). Values are the means ± SEM of 5 separate experiments.
Kinetics of cell surface protein expression.
Kinetics of CD34, CD15, and CD38 (A) and GPIbα, GPIX, GPV, and GPIIb-IIIa (B) surface expression during MK differentiation of CD34+ cells. Cells were harvested at different time intervals and the percentages of cells positive for the different markers were determined by flow cytometry after double labeling (Figure2). Values are the means ± SEM of 5 separate experiments.
We also tested the expression of CD34, CD15, and CD38 (Figure 3B). CD34 expression decreased progressively during differentiation, as previously described for bone marrow cell cultures11 and was totally absent by days 9 to 10. At day 0, 95% of CD34+cells were also CD38+, but this later marker disappeared more rapidly than CD34 during the MK differentiation. To check for the presence of myeloid precursors, we analyzed the expression of CD15, the Lewis x (Lex) antigen, which is a granulomonocytic marker, absent from the MK surface. This marker remained at low levels (10%-15%) throughout culture, confirming that cells committed to the MK pathway were selectively amplified.
GPV appears later than GPIbα during megakaryocyte differentiation
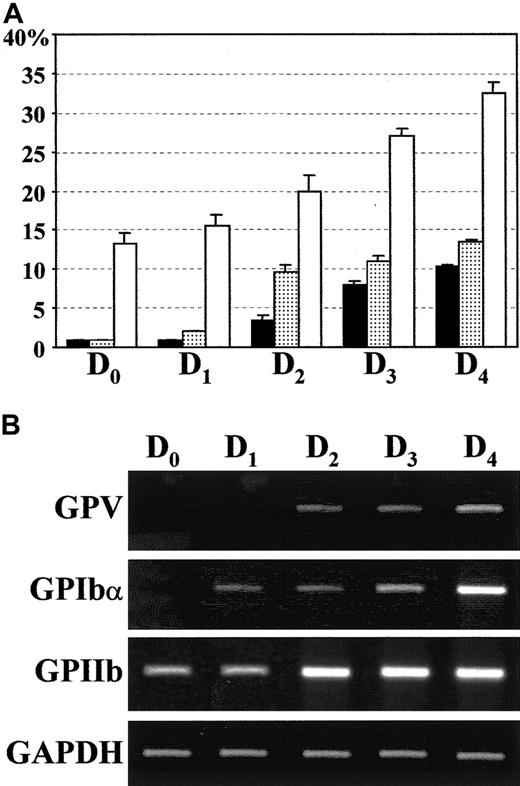
The kinetics of GPV expression appears to be slightly delayed compared to GPIbα and GPIX (Figure 3). To confirm this observation, we performed additional FACS analysis of GPV and GPIbα expression during the first 5 days of culture. To avoid potential bias generated by the secondary antibodies, we used MoAbs directly coupled to fluorophores (anti-GPIbα–Alexa 488 and anti-GPV–Cy3). At day 2, GPV expression was low (approximately 3%) as compared to 10% of GPIbα (Figure 4A). At day 3, the difference between both expressions decreased (8% and 11%, respectively). The delay of GPV expression was also observed at the mRNA level, because GPV transcript was detected, in parallel reverse transcription (RT)–PCR analysis, a day later than GPIbα. Futhermore, FACS analysis and RT-PCR experiments confirmed the presence of GPIIb protein and transcript at day 0 (Figure 4). In conclusion, GPIIb, GPIbα, and GPV appear sequentially during the early stages of megakaryocytopoiesis.
Cell surface protein and mRNA expression of GPV, GPIbα, and GPIIb during the first days of culture.
(A) Cell surface expression of GPV (▪), GPIbα (░), and GPIIb-IIIa (CD41, ■) was followed by flow cytometry (Figure 2) until day 4 of culture. Results are expressed as the percentage of positive cells and are the mean ± SEM of 3 separate experiments. (B) The presence of GAPDH (positive control), GPV, GPIbα, and GPIIb transcripts was assessed by RT-PCR analysis.
Cell surface protein and mRNA expression of GPV, GPIbα, and GPIIb during the first days of culture.
(A) Cell surface expression of GPV (▪), GPIbα (░), and GPIIb-IIIa (CD41, ■) was followed by flow cytometry (Figure 2) until day 4 of culture. Results are expressed as the percentage of positive cells and are the mean ± SEM of 3 separate experiments. (B) The presence of GAPDH (positive control), GPV, GPIbα, and GPIIb transcripts was assessed by RT-PCR analysis.
CD41+GPIb-V-IX− sorted cells produce more colonies than CD41+GPIb-V-IX+ cells
To analyze if CD41+GPIb-V-IX− and CD41+GPIb-V-IX+ cell populations had different clonogenic properties, we sorted CD41+GPIbα−and CD41+GPIbα+ day 4 and CD41+GPV− and CD41+GPV+ cells at day 7. Figure5A shows that at day 4 double-positive sorted cells produce fewer BFU-E than CD41+GPIbα− cells (65% ± 10% BFU-E versus 90% ± 9%), in methylcellulose assay. This observation was confirmed at day 7 for the CD41+GPV+ and the CD41+GPV− populations. Moreover, cells sorted at day 7 produced fewer BFU-E than cells sorted at day 4, indicating that as the cells differentiate, they progressively lose their clonogenic capacities. Using collagen medium, we observed only MK colonies. The number of MK colonies obtained with the double-positive cells at day 4 was significantly lower than that obtained with the CD41+GPIbα− population (233 ± 37 versus 321 ± 42, respectively). The number of MK colonies decreased significantly at day 7 (4- to 5-fold), whereas the ratio between the 2 populations was the same as at day 4 (Figure 5B).
Colony assay for BFU-E and for MKs after cell sorting at day 4 or day 7.
The cells were sorted at day 4 using anti–CD41-PE and anti-GPIbα–Alexa 488, and at day 7 with an anti–CD41-PE and an anti-GPV revealed by an Alexa 488–GAM. ▪, CD41+GPIbα+; ░, CD41+GPIbα+; ▨, CD41+GPV−; ▩, CD41+GPV+. (A) The percentage of positive cells represents the proportion of BFU-E colonies compared to the total number of colonies obtained in methylcellulose medium. (B) Number of megakaryocytic colonies obtained in collagen medium.
Colony assay for BFU-E and for MKs after cell sorting at day 4 or day 7.
The cells were sorted at day 4 using anti–CD41-PE and anti-GPIbα–Alexa 488, and at day 7 with an anti–CD41-PE and an anti-GPV revealed by an Alexa 488–GAM. ▪, CD41+GPIbα+; ░, CD41+GPIbα+; ▨, CD41+GPV−; ▩, CD41+GPV+. (A) The percentage of positive cells represents the proportion of BFU-E colonies compared to the total number of colonies obtained in methylcellulose medium. (B) Number of megakaryocytic colonies obtained in collagen medium.
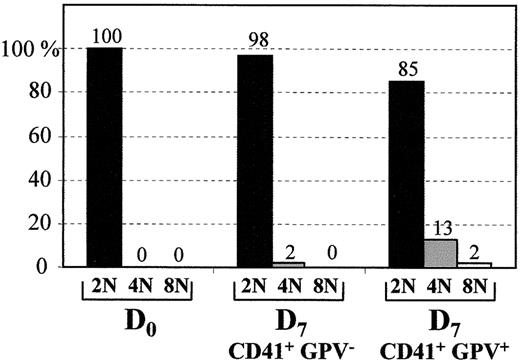
Cell ploidy distribution of the CD41+GPV+cells at day 7 is different from that of CD41+GPV− cells
Cell ploidy distribution was investigated at day 0 and day 7 (Figure 6). At day 0, all the CD34+ cells were 2N. At day 7, cells were sorted according to the presence of CD41 and the presence or absence of GPV. Most of CD41+GPV− cells were 2N, whereas 13% and 2% of the CD41+GPV+ cells were 4N and 8N, respectively.
Cell ploidy distribution.
The percentage of 2N, 4N, or 8N cells was calculated at day 0 for CD34+ population and at day 7 for both CD41+GPV− and CD41+GPV+ sorted populations.
Cell ploidy distribution.
The percentage of 2N, 4N, or 8N cells was calculated at day 0 for CD34+ population and at day 7 for both CD41+GPV− and CD41+GPV+ sorted populations.
Fluorescence microscopy analysis of the GPIbα and GPV subunits on permeabilized cells
Permeabilized cells were analyzed by immunofluorescence microscopy to determine whether the intracellular trafficking of GPIIb, GPIbα, and GPV would account for the differences observed in cell surface expression of these molecules. At day 0, labeling of GPIIb was observed, but labeling of GPIbα or GPV (Figure7) was negative, consistent with the FACS and RT-PCR studies. By day 7 a large number of permeabilized cells contained GPV molecules within their cytoplasm, whereas at this stage, the membrane-bound GPV was only detected on 25% of the cells (Figure3A). Almost all the GPIIb+ cells were also stained intracellularly for GPIbα. All GPIbα+ or GPV+ cells were positive for GPIIb, consistent with the FACS results. Results for GPIX were identical to those obtained for GPIbα (data not shown). These experiments clearly demonstrate the sequential expression of GPIIb, GPIbα, and GPV during MK maturation, with stages including intracellular retention of the subunits of the GPIb-V-IX complex.
Double-labeling fluorescence microscopy of GPIIb/GPV and GPIIb/GPIbα expression on permeabilized cultured CD34+cells.
CD34+ cells were permeabilized on the day of collection (day 0) and double labeled with anti-GPIIb revealed by FITC-GAM and anti-GPV–Cy3 or anti-GPIbα–Cy3. Cells at day 7 were permeabilized and labeled with anti-GPIIb revealed by GAM-Cy3 and anti-GPIbα–Alexa 488 or anti-GPV revealed by an Alexa 488–conjugated secondary antibody. The arrows indicate cells that are CD41+ but GPIbα− or GPV−. Neg represents the fluorescence of cells incubated with FITC-IgG1, Cy3-IgG1, or Alexa 488–IgG1.
Double-labeling fluorescence microscopy of GPIIb/GPV and GPIIb/GPIbα expression on permeabilized cultured CD34+cells.
CD34+ cells were permeabilized on the day of collection (day 0) and double labeled with anti-GPIIb revealed by FITC-GAM and anti-GPV–Cy3 or anti-GPIbα–Cy3. Cells at day 7 were permeabilized and labeled with anti-GPIIb revealed by GAM-Cy3 and anti-GPIbα–Alexa 488 or anti-GPV revealed by an Alexa 488–conjugated secondary antibody. The arrows indicate cells that are CD41+ but GPIbα− or GPV−. Neg represents the fluorescence of cells incubated with FITC-IgG1, Cy3-IgG1, or Alexa 488–IgG1.
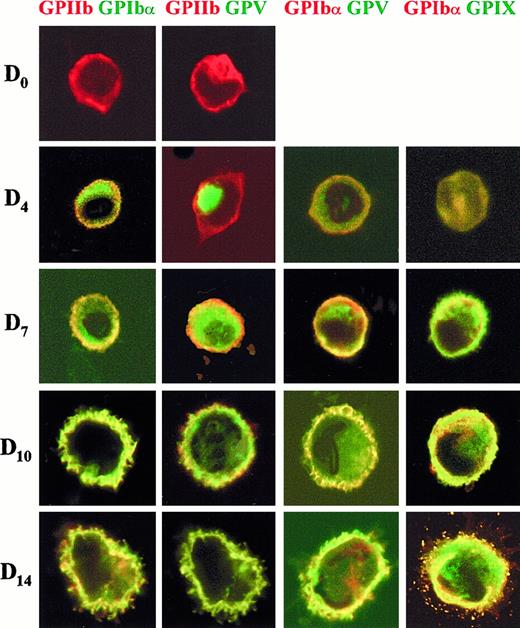
Cellular localization of GPIbα, GPV, GPIX, and GPIIb by confocal microscopy
A more precise determination of the cellular localization of GPIbα, GPIX, and GPV from day 0 to day 14 was obtained by confocal laser scanning microscopy (Figure 8). Different combinations of antibodies against the 3 subunits and against GPIIb were used in double- and triple-labeling experiments. At day 0, GPIIb was present on the cell surface, whereas GPIbα and GPV were undetectable. At day 4, GPIbα was found in the cytoplasm and on the cell surface. At day 7, some GPV and GPIbα surface colocalization (yellow) was observed (GPIbαGPV double labeling). However, most GPIbα was localized at the cell surface, whereas the GPV molecule was principally detected in a cytoplasmic pool (green in GPIIbGPV double labeling and in GPIbαGPV). In contrast, by day 7 GPIX and GPIbα subunits were almost completely colocalized at the cell surface. At day 10, all 3 subunits colocalized on the cell surface, and the cells began to produce filopodia. Finally, by day 14 mature MK were observed with colocalization of all the markers tested on the cell body and on filopodia.
Double-labeling confocal microscopy of the cellular localization of GPIIb, GPIbα, GPV, and GPIX at different stages of MK maturation.
Representative cells are shown for days 0, 4, 7, 10, and 14 of culture. The cells were permeabilized and labeled with anti-GPIIb revealed by a Cy3-GAM, anti-GPIbα–Alexa 488 or –Cy3, anti-GPV revealed by an Alexa 488–GAM and anti-GPIX–Cy2. Colored lettering matches the dye used for the corresponding fluorophore: red for Cy3 and green for Cy2 or Alexa 488. Confocal immunofluorescence images were recorded with a minimum pinhole size in the plane of the nuclei, using excitation and emission filtering as described in “Materials and methods.” Images in the rhodamine (Cy3) and fluorescein (Cy2 or Alexa 488) channels were recorded simultaneously in the same focal plane by a double exposure procedure.
Double-labeling confocal microscopy of the cellular localization of GPIIb, GPIbα, GPV, and GPIX at different stages of MK maturation.
Representative cells are shown for days 0, 4, 7, 10, and 14 of culture. The cells were permeabilized and labeled with anti-GPIIb revealed by a Cy3-GAM, anti-GPIbα–Alexa 488 or –Cy3, anti-GPV revealed by an Alexa 488–GAM and anti-GPIX–Cy2. Colored lettering matches the dye used for the corresponding fluorophore: red for Cy3 and green for Cy2 or Alexa 488. Confocal immunofluorescence images were recorded with a minimum pinhole size in the plane of the nuclei, using excitation and emission filtering as described in “Materials and methods.” Images in the rhodamine (Cy3) and fluorescein (Cy2 or Alexa 488) channels were recorded simultaneously in the same focal plane by a double exposure procedure.
Discussion
This is the first report addressing in detail the relative kinetics of expression of the platelet GPIb-V-IX subunits during MK maturation. Although GPIb-V-IX is considered to be a very specific marker of platelets, unlike GPIIb, it has been little studied during MK differentiation. This complex is essential for the adhesive functions of platelets, but its role in MK maturation is unknown. That GPIb plays an active role in late stage of MK differentiation is suggested by the presence of abnormally large platelets in GPIb-deficient Bernard-Soulier patients as well as abnormal demarcation membranes in their bone marrow MK, which was recently confirmed on GPIb−/− mice.45 Furthermore, a recent study by Takahashi and coworkers46 showed that infusion of a GPIb-specific MoAb into mice led to MK with abnormal morphology, impaired platelet production, and abnormal platelet morphology. It appeared to us therefore of interest to identify at which stage the GPIb-V-IX complex was expressed at the cell surface during MK maturation.
The study of the sequential development of MK and temporal surface expression of the GPIb-V-IX complex was made possible due to improved culture conditions allowing in vitro differentiation of MK. These culture conditions included TPO, IL-6 and IL-11, a mixture of powerful MK differentiation inducing cytokines,47,48 and IL-3, that has been described for acting synergistically with TPO to support the formation of multiples types of hematopoietic colonies including multilineage colonies.5 It has also been reported that IL-3 alone could support CFU-MK colony growth in vitro.4 The combination of IL-3, IL-6, and TPO appears to be additive as measured by colony growth.4Moreover, the combination of IL-11 plus TPO resulted in a synergistic enhancement of the number of CFU-MK colonies. Using this cocktail of cytokines, we observed that the cells underwent typical MK morphologic maturation with development of polylobular nuclei and formation of filopodia and proplatelets. The time course of cell surface markers was typical for MK differentiation with down-regulation of CD34 and up-regulation of GPIb-V-IX and GPIIb-IIIa.
Our present results performed at early stages of MK differentiation revealed that GPIb-V-IX appearance was markedly delayed as compared to that of GPIIb-IIIa. The CD34+ population already contained a significant fraction of GPIIb+ cells (15%), consistent with previous studies using CD34+ cells derived from bone marrow1,11,13,49 or cord blood.50 51 FACS analysis and immunofluorescence studies of permeabilized cells detected no expression of GPIb on the first day of culture, which was confirmed at the mRNA level by RT-PCR studies. We then tested whether the cells that already expressed GPIIb-IIIa, but not yet the GPIb-V-IX complex, were functionally different from those expressing both markers. We observed that the cells only expressing GPIIb-IIIa reproducibly generated more BFU-E than the double-positive cells, which confirms that single-positive GPIIb-IIIa+ cells are more immature cells. The observation that cells sorted for the expression of the 2 distinct megakaryocytic markers GPIIb-IIIa and GPIbα are able to produce a significant number of BFU-E is striking. Two hypotheses might account for this observation: (1) GPIIb-IIIa and GPIb are expressed on a subpopulation of precursor cells still displaying an erythromegakaryocytic potential, and (2) cells expressing both markers are already committed to the megakaryocytic lineage, but they retain some plasticity allowing them to be reprogrammed by inductive culture conditions.
Using megakaryocytic-inducing conditions, CD41+GPIbα− cells generated more MK colonies than the double-positive population at D4. This suggests that the single-positive cells represent a more immature population than the double-positive cells, with a greater proliferative capacity. This capacity decreases at D7.
We then tested whether the ploidy distribution of the cells expressing only GPIIb-IIIa was different from that of GPIIb-IIIa/GPIb-V-IX double-positive cells. We were unable to detect 4N population before day 7. At day 7, 4N and 8N cells were detected in the double-positive population, whereas only 2N cells were detected in the GPIIb-IIIa single-positive population. However, we did not detect any MKs displaying ploidy above 8N, even at late stages of the differentiation. These results are consistent with previous studies,50 51and suggest that MKs from CB could undergo fewer endoreplications than those of adult bone marrow. The physiologic significance of this difference in ploidization is not clear.
The observation that most cell lines expressing GPIIb-IIIa do not express GPIb-V-IX might be explained by the delayed appearance of GPIb-V-IX, because most cell lines display an erythromegakaryocytic phenotype fitting with a blockage at an immature stage. Hence, it is possible that the few cell lines that express GPIb-V-IX such as Dami25 or M-O7e22 have acquired a more mature phenotype, although this issue requires further clarification.
There is to date no clear explanation for the different temporal expression of GPIIb and GPIb-V-IX. Detailed analysis of the GPIIb gene promoter has revealed the importance of Ets and GATA elements for transcription in the megakaryocytic lineage52 and the additional role of a repressor element active in nonmegakaryocytic cell lines.53 Initial functional analyses of the promoters of GPIbα,54 GPIX,55 and GPV34point to a similar role of GATA and Ets in the transcription of these proteins and the presence on the GPV gene of a silencer domain resembling that of GPIIb. Additional studies will be needed to identify the elements specifically involved in the temporal control of genes sequentially activated during MK differentiation.
Our detailed analysis on the early days of culture revealed a lag of a day in GPV expression (at the protein and mRNA levels), possibly due to different transcriptional regulation. The same delay was observed when analyzing the intracellular and membrane compartments by fluorescence or confocal microscopy. At day 0, cells were only positive for surface GPIIb; GPIbα, GPIX, and GPV were undetectable. From day 4 onward, GPIbα was found in the cytoplasm and on the cell surface of many cells. At days 5 to 7, cells that were clearly positive for surface GPIbα contained only low levels of GPV, mainly located in the cytoplasm of the cells. After day 7, cells were positive for surface GPIb-V-IX complex. This can be related to our previous observation of the dissociated expression of GPIb-IX and GPV in leukemic cell lines34 and concurs with transfection and gene inactivation studies, showing that GPIb-IX could be expressed efficiently in the absence of GPV.33
In conclusion, this study confirms that GPIb-V-IX represents a later marker of MK maturation than GPIIb and further demonstrates that GPV has a delayed temporal pattern of expression. This distinct behavior, as compared to GPIbα, GPIX, and presumably GPIbβ, may serve an as yet unknown specialized function of GPV. GPV should prove to be a useful tool to study normal or pathologic megakaryocytopoiesis and to improve our understanding of the final stages of MK maturation. Selection of GPIIb+/GPIb-V-IX− cells will discriminate between early and more committed MK precursors and with the help of new differential screening approaches should allow identification of cytokines and transcription factors that are turned off or on during the final stages of differentiation. In addition, isolation of mature MK could be useful for functional studies or to identify elements of the transduction pathway leading to agonist-induced activation of GPIIb-IIIa56 and platelet aggregation. Finally, GPV promoter could be instrumental to direct the in vivo expression in MK and platelets of therapeutic proteins such as antithrombotics or antitumor agents.
Acknowledgments
The authors wish to thank Sylvette Chasserot-Golaz (INSERM U.338, Strasbourg, France) for confocal laser scanning microscopy, Annie Falkenrodt (EFS-Alsace, Strasbourg, France) for help with morphologic analyses, and Denis Clay (INSERM U.268, Villejuif, France) for helpful discussions and support during this study. We are also grateful to Pierre Charbord (INSERM U.506, Villejuif, France) and Jean-Philippe Rosa (INSERM U.348, Paris, France) for critical reading of the manuscript.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Georges Uzan, Hôpital Paul Brousse, 12-14 avenue Paul Vaillant Couturier, F-94807 Villejuif Cedex, France; e-mail: guzan@infobiogen.fr.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal