Abstract
Several chemokines have been shown to act as angiogenic molecules or to modulate the activity of growth factors such as fibroblast growth factor 2 (FGF-2) and vascular endothelial growth factor (VEGF). The detection of the CC chemokine receptor (CCR) 8 message in human umbilical vein endothelial cells (HUVECs) by reverse transcription– polymerase chain reaction (RT-PCR) and RNase protection assay (RPA), prompted us to investigate the potential role exerted by the CC chemokine I-309, a known ligand of such receptor, in both in vitro and in vivo angiogenesis assays. We show here that I-309 binds to endothelial cells, stimulates chemotaxis and invasion of these cells, and enhances HUVEC differentiation into capillary-like structures in an in vitro Matrigel assay. Furthermore, I-309 is an inducer of angiogenesis in vivo in both the rabbit cornea and the chick chorioallantoic membrane assay (CAM).
Introduction
During angiogenesis, new blood vessels emerge and grow from preexisting ones through an invasive process that requires proteolysis of the extracellular matrix, migration and proliferation of endothelial cells, and recruitment of cell types, such as pericytes and smooth muscle cells, to different segments of the vasculature.1
The mature vascular system undergoes angiogenic remodeling in a number of pathophysiologic states. During embryonic growth, endometrial proliferation, and wound healing, neovascularization occurs and is strictly regulated by a balance between stimulatory and inhibitory factors. Disregulation of these control mechanisms may lead to abnormal growth of newly generated blood vessels, during pathologic states such as tumor growth and diabetic retinopathy.2
Chemokines are a family of structurally related leukocyte chemoattractant cytokines that play a central role during inflammation. The presence and spacing of critical cysteine residues defines the 2 major subgroups of chemokines, namely, CXC and CC, as well as the C and CX3C chemokines that, at present, are constituted by single members. Chemokines interact with 7 transmembrane-spanning receptors, coupled to G proteins (mostly Gi or Gq), and their triggering induces biochemical events that may result in the control of chemotaxis, proliferation, apoptosis and adhesion.3
In addition to their well-characterized functions of leukocyte activation and trafficking, members of the chemokine family have been shown to regulate angiogenesis. In fact, a number of CXC chemokines has been reported to induce endothelial cell migration and/or proliferation in vitro and angiogenesis in vivo4,5 or to act as angiostatic molecules, as they inhibit growth factor–induced activation of endothelial cells and angiogenesis in vivo.4,6,7 Accordingly, few selective chemokine receptors have been shown to be expressed by endothelial cells of different origins,8-12 and chemokine-binding sites identified on endothelium.13
Although most molecules showing angiogenic or angiostatic activity are members of the CXC class of chemokines, an emerging role for CC chemokines in the modulation of angiogenesis has recently been demonstrated.12,14-17 In fact, 6Ckine, an atypical 6 cysteine–containing chemokine, is an angiostatic molecule,12 and monocyte chemotattractant protein 1 (MCP-1) increases collateral artery growth and conductance after femoral artery occlusion14 and is angiogenic in vivo in the rabbit cornea, chick chorioallantoic membrane (CAM) assay, and Matrigel plug assays.17,18 Moreover, MCP-1 and macrophage inflammatory protein 1α (MIP-1α) binding sites on human brain microvessels have been reported.13 Lastly, the viral MIP-I (vMIP-I), MIP-II (vMIP-II), and MIP-III (vMIP-III) chemokines, encoded by Kaposi-associated herpesvirus (KSHV), are potent angiogenic molecules in vivo, and their possible pathogenic role in Kaposi sarcoma has been suggested.15 16
In this study we found CCR8 messenger RNA (mRNA) expression by human umbilical vein endothelial cells (HUVECs) and hypothesized a potential regulation of endothelial cell functions by the CC chemokine I-309, a known ligand for such a receptor. We report here that I-309 binds to HUVECs and induces their chemotaxis, invasion, and differentiation. Furthermore, we show the angiogenic activity of I-309 in vivo in both the rabbit cornea and the CAM assays, indicating that this molecule may be regarded as a novel modulator of angiogenesis.
Materials and methods
Cell culture and reagents
HUVECs were purchased from Mascia Brunelli srl (Milan, Italy) and maintained in complete medium, ie, endothelial cell basal medium (EBM-2) 2% fetal bovine serum (FBS) plus human epidermal growth factor (hEGF), hFGF-2, human vascular endothelial growth factor (hVEGF), and insulin growth factor 1 (R3-IGF-1) (Biowittaker Italia srl, Caravaggio, Italy). Human I-309 was purchased from R&D Systems (Minneapolis, MN), human interferon-inducible protein 10 (IP-10), human B-cell–attracting protein 1 (BCA-1) from PeproTech (London, Great Britain), and pertussis toxin (PTX) from Sigma (St Louis, MO).
Reverse transcriptase–polymerase chain reaction analysis
Total cellular RNA was extracted from 2 × 106HUVECs (passage 4-7) using Trizol reagent and standard procedures (Life Technologies, Grand Island, NY), and RNA was treated with 2 units of RNase-free DNase and 10 units RNasin (Promega, Madison, WI) for 50 minutes at 37°C. Four micrograms of total RNA were used for complementary DNA (cDNA) first-strand synthesis in a 20-μL reaction volume using Superscript II RNase HRT (Life Technologies) and oligo-(dT)12-18 primers. One microliter of the cDNA sample was used for polymerase chain reaction (PCR) amplification using AmpliTaq (Perkin Elmer, Branchburg, NJ). Forward and backward primers used for CC chemokine receptor 8 (CCR8) were CATCACCCTCATGAGTGTGG and CACGTTGAATGGGACCCAGA, respectively. The cDNAs were subjected to 35 cycles of PCR amplification (94°C for 40 seconds, 63°C 40 seconds, and 72°C for 1 minute), products were run on 1.5% agarose gel and DNA fragments visualized by ethidium bromide staining. cDNAs were also subjected to 25 cycles of PCR amplification with glyceraldehyde-3-phosphate dehydrogenase (GAPDH) primers for sample normalization.
RNase protection assay
HUVECs mRNA was prepared and extracted as above and hybridized to CC chemokine receptor RNA probes using a Multiprobe RPA System (Riboquant, Pharmingen, San Diego, CA), following the manufacturer's instructions. Briefly, 10 μg of total RNA from HUVECs were hybridized to a 32P-labeled hCR5 template set (6 × 105cpm per sample) for 16 hours at 56°C, then subjected to RNase A+T1 digestion, proteinase K digestion, phenol-chloroform extraction, and ethanol precipitation. The samples were then run on a 5% acrylamide/bis acrylamide (29:1) urea-containing gel, dried, and autoradiographed using Biomax films (Sigma) at −80°C. RNA from Th2 cells was used as a positive control for CCR8 expression.23
125I-labeled I-309 binding to HUVECs
HUVECs (4 × 104 cells per well) were seeded on 24-well plates 18 to 24 hours before the experiment. Plates were washed twice with cold binding buffer (BB): 125 mmol/L NaCl, 25 mmol/L HEPES, 1 mmol/L CaCl2, 5 mmol/L MgCl2, and 0.5% bovine serum albumin (BSA) pH 7. The binding of [125I]I-309, (7400 × 1010 Bq/mmol [2000 Ci/mmol]) (Amersham Intl, Buckinghamshire, Great Britain) was performed incubating the cells in BB containing 0.2 nmol/L [125I]I-309 in the absence or the presence of unlabeled I-309 (0-400 nmol/L) for 2 hours at 4°C. Cells were then washed twice with cold BB and lysed with 2% sodium dodecyl sulfate (SDS) in phosphate-buffered saline (PBS), and radioactivity counted in a gamma counter. Data were analyzed using the LIGAND program, the graphs derived using the GraphPad Prism program (GraphPad Software, Inc, San Diego, CA) and expressed as the average of triplicates. Three independent experiments allowed calculation of the approximateKd.
Chemotaxis assay
Cell migration was assayed using a 48-well microchamber (Neuroprobe, Cabin John, MD) as described.19 HUVECs (passage 4-7) were incubated for 36 to 48 hours in RPMI 15% FBS. Then, cells were detached and resuspended in a migration medium (MM): RPMI 25 mmol/L HEPES, 0.01% BSA, at 0.5 × 106 cells/mL. Eight micrometers of pore-size polycarbonate filters (Costar, Cambridge, MA), coated with murine collagen type IV (Becton Dickinson), was used. After 4 hours of incubation at 37°C, filters were removed and stained with Diff-Quick (DADE, Dudingen, Switzerland). The number of migrating cells was determined by counting 6 fields at × 400 magnification. The migration index (MI) was calculated by dividing the number of migrated cells in the presence of I-309 or FGF-2 by the cells migrated in MM alone. A range of doses between 0.1 and 50 ng/mL was assayed in chemotaxis to assess the optimal PTX dose. The sensitivity of HUVEC chemotaxis to PTX was evaluated incubating the cells with either 5 ng/mL of PTX or control medium, at 37°C before (20 minutes) and throughout the chemotaxis assay. The data were plotted as the percentage of migration. Viability of cells after PTX treatment was more than 95%, as evaluated by trypan blue exclusion. The invasion assay was performed as the chemotaxis assay, except that filters were coated with Matrigel (Becton Dickinson) (400 μg per filter).
Matrigel assay
After 36 hours of incubation in EBM-2 0.5% FBS, HUVECs were trypsinized, and 8 × 104 cells seeded, in the presence or absence of I-309, in 24-well plates precoated with 250 μL of Matrigel (Becton Dickinson) for 0.5 to 1 hour at 37°C. After 1 to 24 hours of incubation, capillary-like structure formation was examined under an inverted phase photomicroscope. Cells were fixed with PBS containing 0.2% glutaraldehyde, 1% paraformaldehyde, and photographed. The assays were performed in triplicate wells. Tube formation was quantitated by counting the number of intersection points (magnification × 50) of each experiment ± SD.
Rabbit cornea assay
The angiogenic activity of I-309 was assessed in vivo using the rabbit cornea assay as described.20 Corneal assays were performed in male New Zealand albino rabbits (Charles River, Calco, Italy) in accordance with the guidelines of the European Economic Community for animal care and welfare (EEC Law No 86/609). Briefly, after being anesthetized with sodium Pentothal (30 mg/kg), a micropocket (1.5 × 3 mm) was surgically produced in the lower half of the cornea. Slow-release pellets of elvax-40 (DuPont, Wilmington, DE) containing the human I-309 (400 ng) were implanted into the micropocket. Subsequently, daily observation of the implants was made with a slit-lamp stereomicroscope without anesthetic. An angiogenic response was scored positive when the budding of vessels from the limbal plexus occurred within 4 days, and capillary progression persisted over time to reach the implant. The potency of angiogenic activity was evaluated on the basis of the number and growth rate of newly formed capillaries, and an angiogenesis score was calculated as previously reported.21 Corneas were removed at the end of the experiment, as well as at defined intervals after treatment, and fixed in formalin for histologic examination.
Implantation of gelatin sponges onto the CAMs and quantitation of the angiogenic response
Gelatin sponges (Gelfoam, Upjohn, Kalamazoo, MI) adsorbed with 3 μL I-309 (400 ng) in PBS were placed on top of the CAMs at day 8 of incubation as described.22 Sponges containing the vehicle alone or 1 μg FGF-2 (Pharmacia, Milano, Italy) were used as negative and positive controls, respectively. CAMs were examined daily until day 12 and photographed in ovo under a Zeiss stereomicroscope SR equipped with the Camera System MC 63 (Zeiss, Oberkochen, Germany). At day 12, CAMs were processed for light microscopy. Briefly, the embryos and their membranes were fixed in ovo. The sponges and the underlying and immediately adjacent CAM portions were removed and paraffin embedded. Serial sections were cut, stained with 0.5% aqueous solution of toluidine blue (Merck Biochemica, Darmstadt, Germany), and observed under a Leitz-Dialux 20 light microscope (Leitz, Wetzlar, Germany). The angiogenic response was assessed by a planimetric method of “point counting.”22 Briefly, every third section within 30 serial slides was analyzed by a 144-point mesh inserted in the eyepiece of a Leitz-Dialux 20 photomicroscope. Six randomly chosen microscopic fields were evaluated for each section at × 250 magnification. To this purpose, the total number of the intersection points occupied by vessels transversely cut (diameter: 3-10 μm) inside the sponge and at the boundary between the sponge and the surrounding CAM mesenchyme were counted. Mean values ± SD were determined for each analysis. The vascular density was indicated by the final mean number of the occupied intersection points. The statistical significance of the differences between the mean values of the intersection points in the experimental and control CAMs was determined by the Student t test for unpaired data. The same experimental procedure was used to quantify the mononuclear cell infiltrate.
Results
Expression of CCR8 mRNA by HUVECs
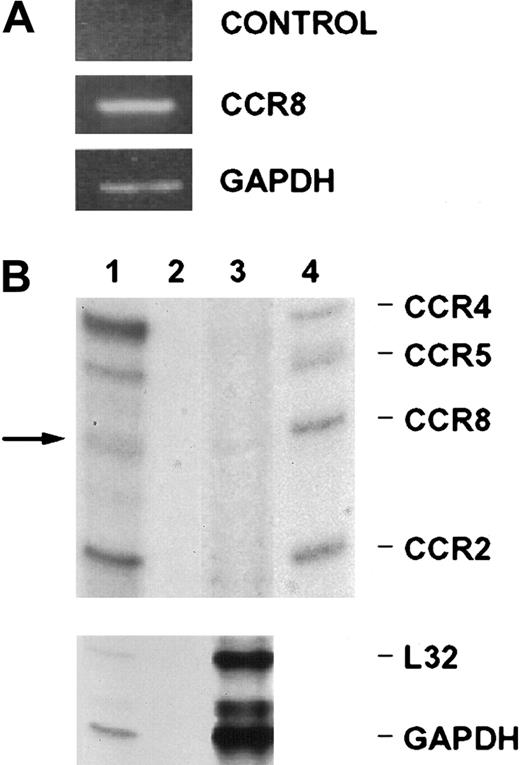
Endothelial cells have been shown to express the Duffy antigen/receptor for chemokine (DARC), CCR1, CCR2, CCR5, CXCR3, and CXCR4.8-13 18 In our effort to characterize the CCR8 receptor in nonlymphoid cell types, we analyzed its expression by endothelial cells. By both reverse transcriptase–PCR (RT-PCR) (Figure1A) analysis and RPA (Figure 1B), we detected CCR8 message in exponentially growing HUVECs.
Analysis of CCR8 mRNA expression in HUVECs.
(A) PCR amplification was performed on RNAs or cDNAs of HUVECs grown in complete medium. Shown is a representative experiment of 4 performed. RT-PCR was performed with CCR8 primers on RNA samples (absence of reverse transcription) (control), and cDNA samples (CCR8). Also shown is a representative reaction with GAPDH primers on the cDNA template (GAPDH). (B) The upper panel shows an RNase protection assay performed using 200 ng Th2 positive control total RNA (lane 1), 2 μg yeast total RNA (lane 2), and 10 μg of total RNA extracted from HUVECs (lane 3) using the hCR5 multiprobe from Pharmingen. Approximately 500 cpm of 32P-labeled multiprobe were loaded on lane 4, and the corresponding RNA species marked on the right-hand side. The arrow shows the CCR8 message visible in lane 1 and lane 3. The RNA species in lane 4 (probe) show, as expected, a slightly higher molecular weight than in lane 1 and 3, due to the presence of flanking regions in the in vitro transcript. The bottom panel shows the L32 and GAPDH RNA species. The dried gel was subjected to autoradiography at −80°C and developed with an automatic Kodak developer. The upper panel was exposed for 16 hours and the lower panel for 20 minutes.
Analysis of CCR8 mRNA expression in HUVECs.
(A) PCR amplification was performed on RNAs or cDNAs of HUVECs grown in complete medium. Shown is a representative experiment of 4 performed. RT-PCR was performed with CCR8 primers on RNA samples (absence of reverse transcription) (control), and cDNA samples (CCR8). Also shown is a representative reaction with GAPDH primers on the cDNA template (GAPDH). (B) The upper panel shows an RNase protection assay performed using 200 ng Th2 positive control total RNA (lane 1), 2 μg yeast total RNA (lane 2), and 10 μg of total RNA extracted from HUVECs (lane 3) using the hCR5 multiprobe from Pharmingen. Approximately 500 cpm of 32P-labeled multiprobe were loaded on lane 4, and the corresponding RNA species marked on the right-hand side. The arrow shows the CCR8 message visible in lane 1 and lane 3. The RNA species in lane 4 (probe) show, as expected, a slightly higher molecular weight than in lane 1 and 3, due to the presence of flanking regions in the in vitro transcript. The bottom panel shows the L32 and GAPDH RNA species. The dried gel was subjected to autoradiography at −80°C and developed with an automatic Kodak developer. The upper panel was exposed for 16 hours and the lower panel for 20 minutes.
Binding of [125I]I-309 to HUVECs
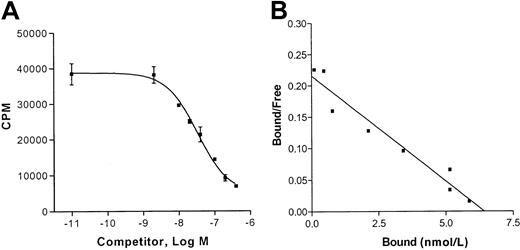
To assess whether the presence of the CCR8 receptor on HUVECs would be relevant to endothelial cell functions, we first tested the ability of I-309, whose only reported receptor is CCR8, to bind to HUVECs. We performed competition binding experiments using [125I]I-309 (0.2 nmol/L) in the presence of increasing amounts of I-309 cold molar excess. Shown in Figure2A are the displacement data that allowed us to detect single affinity binding sites (Figure 2B) on HUVECs and to calculate a Kd of approximately 20 nmol/L and 3 × 105 sites per cell.
Competition binding of 125I-labeled I-309 to HUVECs.
(A) Cells were incubated for 2 hours at 4°C with 0.2 nmol/L125I-labeled I-309 in the presence of increasing amounts of unlabeled recombinant I-309 (2-200 nmol/L). Indicated are the numbers of total counts (cpm) versus increasing concentrations of I-309 (Log M). Shown is a representative experiment of 3 performed in triplicate. (B) Scatchard tranformation of the data shown in panel A.
Competition binding of 125I-labeled I-309 to HUVECs.
(A) Cells were incubated for 2 hours at 4°C with 0.2 nmol/L125I-labeled I-309 in the presence of increasing amounts of unlabeled recombinant I-309 (2-200 nmol/L). Indicated are the numbers of total counts (cpm) versus increasing concentrations of I-309 (Log M). Shown is a representative experiment of 3 performed in triplicate. (B) Scatchard tranformation of the data shown in panel A.
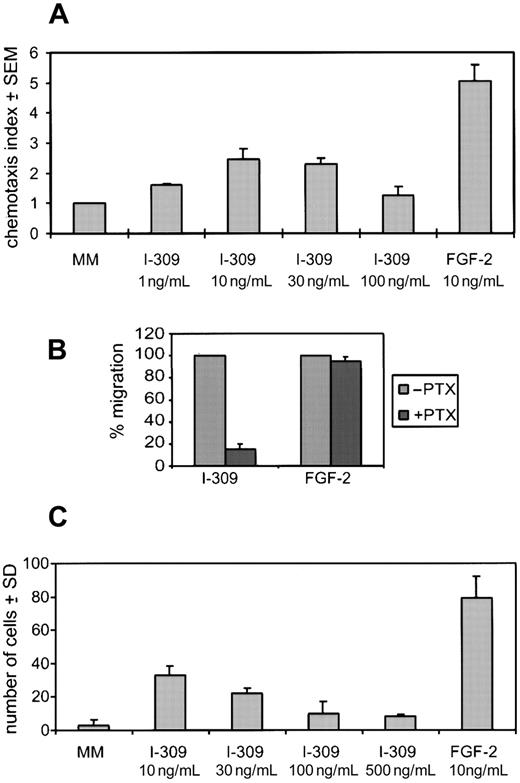
I-309 induces HUVEC chemotaxis and invasion
I-309 ability to affect endothelial cell chemotaxis was assayed by measuring the ability of HUVECs to cross a polycarbonate filter in a 48-well microchamber. I-309 induced chemotaxis of endothelial cells in a dose-dependent manner and with a typical bell-shaped curve (Figure3A). Maximal MI (2-2.5) was observed at doses of I-309 of 10 to 30 ng/mL. Other chemokines tested, ie, IP-10 and BCA-1, had no effect on endothelial cell migration (not shown). FGF-2, a well-known chemoattractant for endothelial cells included as an internal positive control in each chemotaxis experiment, was more active than I-309 by about 2-fold (MI = 5).
Effect of I-309 on HUVEC chemotaxis and invasion.
(A) Chemotaxis was assayed using the 48-well microchamber. Results are expressed as the migration index ± SEM of 8 experiments performed in triplicate using a range of I-309 concentrations. (B) Treatment with PTX (5 ng/mL) selectively inhibited I-309– but not FGF-2–induced chemotaxis (used at 30 ng/mL and 20 ng/mL, respectively). Shown is the percentage migration of untreated versus PTX-treated HUVECs of an average of 3 experiments performed in triplicate. (C) HUVEC invasion was evaluated with the 48-well microchamber. Shown is a representative experiment of 4 performed in triplicate. The results are expressed as the average number of cells per high-power field ± SD of 6 counted.
Effect of I-309 on HUVEC chemotaxis and invasion.
(A) Chemotaxis was assayed using the 48-well microchamber. Results are expressed as the migration index ± SEM of 8 experiments performed in triplicate using a range of I-309 concentrations. (B) Treatment with PTX (5 ng/mL) selectively inhibited I-309– but not FGF-2–induced chemotaxis (used at 30 ng/mL and 20 ng/mL, respectively). Shown is the percentage migration of untreated versus PTX-treated HUVECs of an average of 3 experiments performed in triplicate. (C) HUVEC invasion was evaluated with the 48-well microchamber. Shown is a representative experiment of 4 performed in triplicate. The results are expressed as the average number of cells per high-power field ± SD of 6 counted.
To determine whether I-309–stimulated chemotaxis of HUVECs could be mediated through a G-protein–coupled receptor, we treated cells with PTX. As shown in Figure 3B, HUVECs treated with 5 ng/mL of PTX inhibited I-309–induced chemotaxis by more than 90%, suggesting the involvement of Gαi proteins in I-309–mediated activity, differently than FGF-2–induced chemotaxis.
Once activated, endothelial cells acquire the capacity to degrade and cross an extracellular matrix barrier. As shown in Figure 3C, I-309 induces HUVEC invasion through a Matrigel barrier in a range of doses comparable to those active in chemotaxis. The invasive capacity of HUVECs was increased about 6-fold over the control at the maximal effective dose of 10 ng/mL.
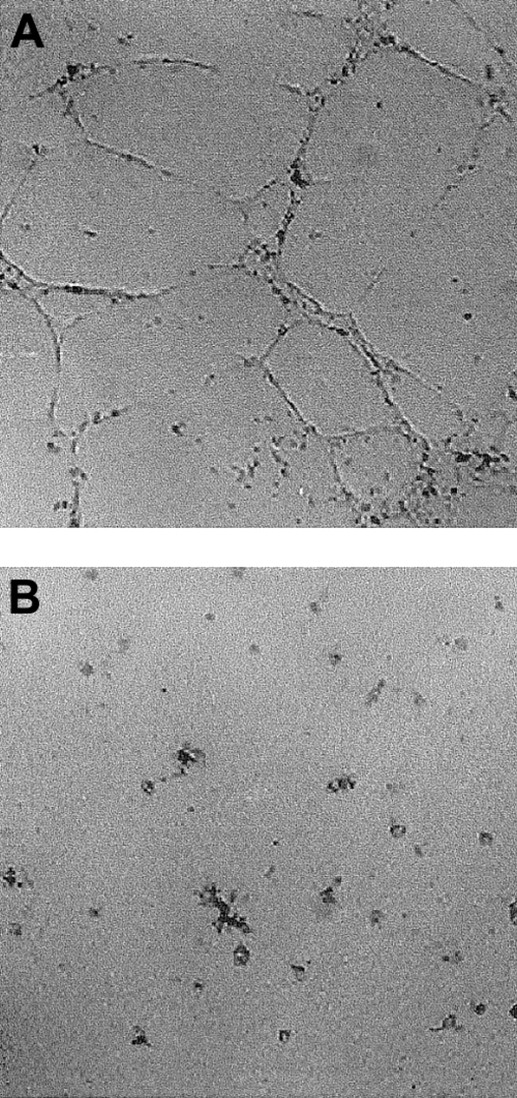
I-309 induces HUVEC differentiation on Matrigel
The ability of endothelial cells to differentiate in response to specific stimuli can be evaluated using the in vitro Matrigel morphogenic assay. As shown in Figure 4A, HUVECs form capillary-like structures in the Matrigel layer after stimulation with I-309 at 300 ng/mL. This effect was already maximal after 4 to 6 hours of incubation at 37°C, whereas untreated cells showed little or no differentiation (Figure 4B). Positive controls, represented by complete medium–treated cells, were included in each experiment (not shown). In 4 independent experiments performed, we detected induction of HUVEC differentiation after I-309 treatment. The number of intersection points, in the untreated and I-309–stimulated cells of the single experiments, were as follows: 0, 35 ± 6; 33 ± 10, 94 ± 12; 0, 43 ± 9; and 19 ± 10, 67 ± 15.
Morphogenic activity of I-309.
HUVECs were seeded on Matrigel and incubated in the presence of 300 ng/mL I-309 (A) or medium alone (B). After 6 hours, cells were fixed and photographed using an inverted phase contrast photomicroscope. Magnification 50 ×. Shown is a representative experiment of 4.
Morphogenic activity of I-309.
HUVECs were seeded on Matrigel and incubated in the presence of 300 ng/mL I-309 (A) or medium alone (B). After 6 hours, cells were fixed and photographed using an inverted phase contrast photomicroscope. Magnification 50 ×. Shown is a representative experiment of 4.
I-309 is angiogenic in the rabbit cornea and CAM in vivo assays
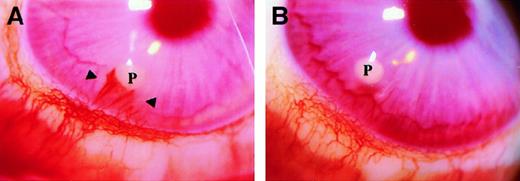
I-309 was tested for its angiogenic activity in the avascular rabbit cornea assay, and its activity was compared with that induced by FGF-2. After 2 weeks, I-309 (400 ng per pellet) had induced an angiogenic response in 60% of the implants (3 of 5 implants) (Figure5A), whereas control pellets were negative (Figure 5B). Although at this time the extent of angiogenesis in the positive implants was comparable to that induced by FGF-2 (not shown), the progression of the neovascular response elicited by I-309 appeared to have a different kinetics. In all samples tested, FGF-2 (200 ng per pellet, n = 4) produced the maximal angiogenic score (7) by day 6, whereas I-309 implants triggered capillary growth 1 week later and with a delayed pattern of progression into the corneal stroma. In fact, the calculated angiogenic scores for I-309 were 2, 4, and 7 at day 8, 13, and 15, respectively. The histologic examination (not shown) of the implanted corneas treated with I-309 showed no relevant inflammatory cell infiltrate during the early stage of neovascularization (day 8). However, few leukocytes could be detected in the proximity of the neovascular growth reaching the pellet implant at day 15, suggesting a contribution of these cell types to capillary progression into the corneal stroma at later stages.
Angiogenic activity of I-309 in the rabbit cornea.
Representative pictures of angiogenesis induced by 400 ng I-309 (A) and vehicle containing pellet (B). Photographs were taken at 15 days after implant through a slit-lamp stereomicroscope (Nikon, Tokyo, Japan). Magnification × 18. P indicates pellets.
Angiogenic activity of I-309 in the rabbit cornea.
Representative pictures of angiogenesis induced by 400 ng I-309 (A) and vehicle containing pellet (B). Photographs were taken at 15 days after implant through a slit-lamp stereomicroscope (Nikon, Tokyo, Japan). Magnification × 18. P indicates pellets.
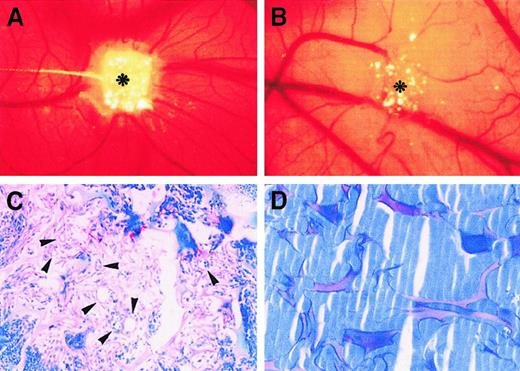
The angiogenic effect of I-309 was also investigated in vivo in the CAM assay. Embryonic chorioallantoic membranes at day 8 of incubation were implanted with sponges containing I-309 (400 ng). Vehicle alone (PBS) and FGF-2 were used as negative and positive controls, respectively. Macroscopic observation of the membrane at day 12 showed that the gelatin sponges adsorbed with I-309 were surrounded by allantoic vessels that developed radially toward the implant in a “spoked-wheel” pattern (Figure 6A), whereas no vascular reaction was detectable around the sponges treated with PBS only (Figure 6B). At microscopic level, a highly vascularized tissue was recognizable among the trabeculae of the I-309–treated sponges 4 days after implantation. The tissue consisted of newly formed blood vessels, mainly capillaries with diameters of 3 to 10 μm growing perpendicularly to the plane of the CAMs, and a dense mononuclear cell infiltrate within an abundant network of collagen fibers (Figure 6C). In contrast, the vessels were absent among trabeculae of implants treated with PBS (Figure 6D). Quantitation of the angiogenic response, performed at day 12 of incubation, demonstrated that the microvessel density in the sponges treated with I-309 was comparable to that of FGF-2 (Table1).
Neovascularization induced by I-309 in the CAM.
(A) CAM of 12-day-old chick embryo incubated for 4 days with a sponge adsorbed with 400 ng I-309. Note the presence of an increased number of blood vessels with a radially arranged “spoked wheel” pattern around the implant (asterisk). (B) CAM of 12-day-old chick embryo incubated for 4 days with a sponge absorbed with vehicle alone (PBS), used as negative control. No vascular response is detectable around the sponge (asterisk). (C) Histologic section of a sponge treated with I-309. Note, among the sponge trabeculae, a collagenous matrix containing numerous capillaries (arrowheads) and a mononuclear cell infiltrate. (D) Histologic section of a sponge treated with PBS. No collagenous matrix and blood vessels are detectable among the sponge trabeculae. Original magnifications: A, B, × 50; C, D, × 250.
Neovascularization induced by I-309 in the CAM.
(A) CAM of 12-day-old chick embryo incubated for 4 days with a sponge adsorbed with 400 ng I-309. Note the presence of an increased number of blood vessels with a radially arranged “spoked wheel” pattern around the implant (asterisk). (B) CAM of 12-day-old chick embryo incubated for 4 days with a sponge absorbed with vehicle alone (PBS), used as negative control. No vascular response is detectable around the sponge (asterisk). (C) Histologic section of a sponge treated with I-309. Note, among the sponge trabeculae, a collagenous matrix containing numerous capillaries (arrowheads) and a mononuclear cell infiltrate. (D) Histologic section of a sponge treated with PBS. No collagenous matrix and blood vessels are detectable among the sponge trabeculae. Original magnifications: A, B, × 50; C, D, × 250.
Quantitation of angiogenic response at day 12 of incubation
| Treatment . | Number of samples . | Number of intersection points (mean ± SD) . | Microvessel density (%) . |
|---|---|---|---|
| PBS | 10 | 0 | 0 |
| FGF-2 | 10 | 29 ± 1 | 23.6 |
| I-309 | 30 | 25 ± 2 | 17.4 |
| Treatment . | Number of samples . | Number of intersection points (mean ± SD) . | Microvessel density (%) . |
|---|---|---|---|
| PBS | 10 | 0 | 0 |
| FGF-2 | 10 | 29 ± 1 | 23.6 |
| I-309 | 30 | 25 ± 2 | 17.4 |
Discussion
In this paper we demonstrated that the CC chemokine I-309 binds to endothelial cells, induces their functional activation and acts as an angiogenic molecule in vivo.
The human CC chemokine I-309 is a product of activated T-lymphocytes, mast cells, and monocytes. I-309 and its murine homologue T-cell-activation–specific gene 3 (TCA3) are monocyte- and Th2-cell chemoattractant23,24 and protect murine thymic lymphoma cell lines against dexamethasone-induced apoptosis. TCA3 also induces activation of neutrophils, microglial, mesangial, and vascular smooth muscle cells.25 I-309/TCA3, differently from most described chemokines, is a selective ligand for the CCR8 receptor.26-28 CCR8 mRNA expression was previously reported to be restricted to lymphoid tissues such as thymus, spleen, and lymph nodes and to Th2 cells and monocytes.19,24 26-28
Similar to what has been described for other chemokines modulating angiogenesis, we report here the expression of a chemokine receptor (CCR8) on endothelial cells and describe the biologic activities of its major ligand on these cells. On the basis of these findings, although we have not directly demonstrated that I-309 is inducing angiogenesis through CCR8, we hypothesize an involvement of this receptor in such modulation. In fact, it is the only known receptor for I-309, and the I-309–induced chemotaxis of HUVECs is PTX sensitive, indicating that at least some of the effects reported may indeed be chemokine receptor mediated, as these receptors are typically Gi coupled. Furthermore, the range of effective doses of I-309 we observed in vitro in both chemotaxis and invasion assays (about 1-3 nmol/L) is compatible with described EC50 and Kd for human and murine CCR8.26-30
Viruses have developed multiple ways to subvert a number of biologic functions, mostly immune responses, to escape control and favor the spread of infection.31 The viral chemokines vMIP-I and vMIP-II are reported to be potent angiogenic molecules.15Although vMIP-II is a shared ligand of chemokine receptors, including CCR8, acting either as an agonist or antagonist,19,30,32vMIP-I selectively binds to CCR829,30 but not to all other known chemokine receptors and this further supports the hypothesis that CCR8-mediated signals may regulate both endothelial cell functions and angiogenesis. The analysis of CCR8 knockout (KO) mice, whose generation is in progress and, in particular, the functional evaluation of endothelial cells derived from both macrovascular and microvascular districts of wild-type and deficient animals as well as the use of the recently described viral antagonist MCC-1/MC148,30 32 a product of molluscum contagiosum poxvirus, may provide further experimental evidence to address such an issue.
Surprisingly, we could only detect low-intermediate affinity I-309–binding sites on HUVECs, likely to be constituted by proteoglycans, whereas no higher affinity binding sites were observed on these cells in our experimental conditions.
Chemokines, in addition to the 7 transmembrane receptors, have been shown to bind heparan sulfate moieties,3 similar to a number of growth factors.33 Chemokine binding to glycosaminoglycans on endothelial cells is believed to promote the generation of an aptotactic gradient and a firmer adhesion of leukocytes to the vessels, an important step preceding transendothelial migration.34,35 Moreover, chemokine binding to proteoglycans may be required for their full functional activity, similar to growth factors such as FGF-2 that utilizes cell surface proteoglycans as part of its receptor complex.33 In fact, heparin and heparin sulfate augment neutrophil responses to interleukin 8 (IL-8)36; furthermore, IP-10 and platelet factor 4 (PF-4) bind to specific heparin sulfate sites, and heparin blocks their inhibitory activity on endothelial cell proliferation.37Moreover, proteoglycans such as syndecans have been shown to act as direct signaling molecules.38 I-309 activities on endothelial cells, like other chemokines, may therefore be mediated by binding to both chemokine receptors and heparin sulfate proteoglycans.
Not many data are yet available on the role of chemokine receptors in the modulation of endothelial cell functions exerted by both angiogenic as well as angiostatic chemokines. Few possible mechanisms of action have been suggested for the angiostatic chemokines such as heterodimer formation between chemokines and growth factors39competition on heparin sulfate moieties for growth factor binding, resulting for PF4 in an impaired down-modulation of p21CIP1/WAF1.40
The only direct evidence of the role of chemokine receptors in promoting angiogenesis is gained by the analysis of CXCR4 KO mice. These animals have impaired vascular development, hematopoiesis, and cardiogenesis,41,42 a phenotype similar to that shown by animals defective in stromal cell–derived factor 1 (SDF-1), a CXCR4 ligand.42 The expression of CXCR4 by endothelial cells and the angiogenic activity reported for SDF-19-11 further support the role for this ligand-receptor pair in the control of angiogenesis.
In summary, our in vitro observations indicate that I-309 may play a role in both the early invasive phase of the angiogenic process by increasing endothelial cell motility and invasive activity, as well as the differentiation phase in which such cells form the lumen of new capillaries. These activities may be triggered by I-309 binding to both chemokine receptors and glycosaminoglycans on the surface of endothelium.
In addition to the in vitro effects, we have reported here the in vivo angiogenic activity of I-309 in both the rabbit cornea and CAM assays. In the cornea assay, I-309 exhibited an angiogenic score similar to that of FGF-2 at day 15, but with a slower progression rate. While in a first stage, capillaries were slowly recruited into the avascular stroma, in a second stage, the stabilized vascular sprouts lodging a few leukocytes expressed a burst of angiogenic activity. It is therefore possible to hypothesize that, although I-309 is able to trigger the angiogenic switch of the endothelium, the complete response is acquired when other potential angiogenic molecules become available into the stroma. Our recent observation that I-309 rapidly induces activation of the p38 MAPK in HUVECs suggests that this molecule may exert a direct effect on endothelium (not shown).
The CAM assay showed a potent angiogenic response of I-309, comparable to that obtained with FGF-2. In this system we detected a dense mononuclear cell infiltrate among the sponge trabeculae, possibly recruited by I-309, and a strong angiogenic response. The mononuclear cell infiltrate may act synergistically with I-309 and enhance its response by secreting factors such as FGF-2, VEGF, IL-8, and tumor necrosis factor-α.43
The angiogenic activity of I-309 in the CAM assay is different from that reported for other cytokines tested in the same assay, such as erythropoietin that was active in the absence of a significant mononuclear cell infiltrate.44 In conclusion, both in vivo assays allowed us to show the angiogenic activity of I-309 and the difference in the ability to induce a mononuclear infiltrate may be related to the angiogenic backgrounds of the rabbit cornea (avascular) and chicken chorioallantoic membrane (strongly vascularized).
Angiogenesis and inflammation can be considered closely linked processes. In fact, in the course of chronic inflammation, ie, in rheumatoid arthritis and psoriatic lesions, there may be the presence of newly generated blood vessels, as several inflammatory mediators may induce angiogenesis, either directly or indirectly. Blood supply may, in turn, sustain chronic inflammation by providing leukocytes and nutrients to tissues.45
Chemokines, key molecules involved in both the regulation of immune responses and angiogenesis may therefore play an important role in several processes such as tumor growth, development, and wound repair, and their overall effect may be the result of the multiplicity of their biologic functions. In fact, the murine homologue of I-309, ie, TCA3, is a growth inhibitor of xenogeneic- and syngeneic-transfected mouse myeloma cells, inducing tumor-specific immunity,46 whereas we demonstrated that I-309 is an angiogenic factor and may therefore promote tumor growth.
In conclusion, we provide the first evidence for the CC chemokine I-309 as an inducer of angiogenesis, potentially relevant in pathophysiologic conditions, and that may represent a novel tool for therapeutic intervention.
Acknowledgments
We thank the members of the Laboratory of Vascular Pathology for their helpful suggestions, Dr Daniele D'Ambrosio for kindly providing Th2 total RNA, and Mrs Marina Vultaggio of Medical Image Service for excellent technical assistance.
Partially supported by the National Research Council Biotechnology Program (M.N. and A.S.), ISS-National AIDS Research Program (M.N.), and Ministry of Health-Wound Healing Project (M.C.C.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Monica Napolitano, Laboratory of Vascular Pathology, Istituto Dermopatico dell'Immacolata-IRCCS, Via Monti di Creta 104, 00167, Rome, Italy; email: m.napolitano@idi.it.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal