Basophilic granulocytes (usually referred to as basophils) are a small population of peripheral blood leukocytes containing cytoplasmatic granules that stain with basophilic dyes. They were first described in 1879 by Paul Ehrlich,1 who 1 year earlier had found a morphologically similar cell type present in tissues that he termed Mastzellen.2 Based on their similarity to mast cells, basophils have often been considered (and neglected) as minor and possibly redundant “circulating mast cells.” Like mast cells, basophils possess high-affinity immunoglobulin (Ig) E receptors (FcεRI) that are cross-linked upon engagement of receptor-bound IgE with corresponding antigens (“allergens”), resulting in the release of a number of mediators that are in part common for both cell types. Until recently, it has been very difficult for most laboratories to obtain basophils without major contaminating cell populations, because the percentage of basophils in peripheral blood is low (< 1%) and they share physicochemical properties with other blood cells. This lack of satisfactory purification protocols has considerably hampered basophil research and negatively affected the interest in this cell type. Nevertheless, recent findings have provided new insights into the possible role of basophils in allergic disease and immunity to pathogens. Most notably, the discovery that basophils rapidly produce large amounts of the regulatory cytokines interleukin (IL)-4 3,4 and IL-13,5-7 together with the constitutive expression of CD40L 8,9 and CCR310 on their surface, has fueled speculations that extend beyond their recognized role as effector cells in IgE-mediated reactions. As discussed in this review, the increasing knowledge about the chemokine network has provided a robust framework for understanding the mechanisms underlying basophil recruitment to the tissues in allergic diseases. This development has been instrumental in identifying very promising new targets for the treatment of asthma and other allergic diseases. We also review recent aspects regarding basophil growth and development and their role in allergic late-phase reactions (LPRs). Furthermore, we have included a section on basophil signal transduction, which expands our understanding of the mechanisms operating downstream of the high-affinity IgE receptor and are also expected to yield new and better targets for pharmacologic intervention. Finally, we discuss the possible roles of basophils in innate immunity and finish with a section on the currently available tools for basophil research.
Growth and development of basophils
Although the phenotype of mature basophils has been extensively studied,11,12 the early stages of basophil maturation and their relationship to other cell lineages are not well understood. Basophils, as well as mast cells, monocytes, eosinophils, and neutrophils, are thought to arise from CD34+ progenitors found in cord blood, peripheral blood, and the bone marrow. Basophils, in particular, have been suggested to evolve from CD34+/IL-3Rα+/IL-5+eosinophil/basophil progenitors,13 as supported by the occurrence of granulocytes with a hybrid eosinophil/basophil phenotype in patients with chronic or acute myelogenous leukemia or in cell culture.14-16
Although mast cell progenitors have been documented as a separate lineage, eg, characterized as CD34+/CD38+ 17 or CD34+/c-kit+/CD13+,18most recently a new monoclonal antibody (97A6) has been described as specific for mature mast cells and basophils and their progenitors.19 Monoclonal antibody 97A6 did not react with any other hematopoietic or nonhematopoietic cell type. The epitope recognized by 97A6 may therefore be associated with a commitment of the CD34+ precursor to a mast cell or basophil lineage distinct from other lineages. The possibility of a common mast cell or basophil lineage also arises from the surprising observation that basophils with phenotypic features characteristic of mast cells (presence of tryptase, chymase, c-kit, carboxypeptidase A) can be found in patients with asthma, allergy, or allergic-drug reactions.20Therefore, the currently predominant concept that mast cells and basophils originate from separate lineages17,21 22 may have to be revised.
Various protocols using different combinations of cytokines and growth factors have been described for the in vitro cultivation of basophils from blood or bone marrow progenitors,23-25 with the presence of exogenous IL-3 being a common prerequisite for the successful development and maturation of basophils. Other growth factors for basophils are IL-5,26 granulocyte-macrophage colony-stimulating factor (GM-CSF),27 transforming growth factor-β (TGF-β), and nerve growth factor (NGF).28There is a general consensus, however, that the main growth and differentiation factor for basophils is IL-3,24,29 whereas the growth and development of mast cells requires the presence of stem cell factor (SCF).30 In conformity with their relative cytokine requirements, mature basophils can be distinguished phenotypically from mast cells by their differential surface expression of IL-3 α chain (CD123, found on basophils and other cells but not mast cells) and SCF receptor (c-kit/CD117, strongly expressed on mast cells but not, or only weakly, on mature basophils).
Role of basophils in allergic late-phase reactions
It is well established that mast cells, which are found in the tissues in the proximity of small blood vessels and postcapillary venules, play a key role in the early phase of IgE-mediated allergic reactions.31 Unlike mast cells, which differentiate and mature in the tissues, mature basophils are found in the circulatory system, where they constitute less than 1% of circulating leukocytes. Basophils, eosinophils, and Th2 lymphocytes are recruited to the site of inflammation during LPRs. Because until recently specific markers for immunohistochemical detection of basophils were not available (see “Tools for basophil research”), the participation of basophils during LPRs has been documented mainly by indirect means,32-34 ie, by determining the pattern of mast cell– or basophil-specific mediators like histamine (derived from both), prostaglandin D2 (from mast cells), or leukotriene C4 (LTC4; from basophils). It is now well established that basophils are rapidly recruited to the skin,35 lung,36 or nose37 after allergen challenge.
More recently, a better understanding of the processes leading to inflammatory cell recruitment in LPRs has sprung up from the study of chemokines and their receptor counterparts, identifying potentially important new targets for the treatment of asthma and other allergic diseases.38-40 These studies complement previous knowledge regarding the molecules involved in basophil adhesion to the endothelium,41-43 a critical step in the process of extravasation. The aim of the following section is to highlight the potential roles of basophils in LPRs. We use the new nomenclature for chemokines proposed by the Keystone Chemokine Conference (Keystone, CO, January 18-23, 1999).40
Several reports have pinpointed eotaxin (CCL11) and the eotaxin receptor (CCR3) as key molecules in the selective recruitment of eosinophils to the lung during allergic airway inflammation,44 both in animal models45-49 and asthmatic patients.50-53 Sensitized mice with a disrupted eotaxin gene showed a 70% reduction in the number of eosinophils recruited by antigen in comparison with the identically treated wild-type mice.54 Enhanced eotaxin expression in allergic inflammation may also account for the influx of basophils, which strongly express CCR3 on their surface and respond to eotaxin in vitro.10,55 In addition, human basophils also respond to other chemokines such as the CC chemokines eotaxin-2 (CCL24), RANTES, MCP-3 (CCL7), MCP-1, and MIP-1α,56,57 which also play a role in antigen-induced tissue recruitment of eosinophils.58-60 Moreover, basophils may be responsive to eotaxin-3 (CCL26),61,62 the most recent addition to a growing list of CCR3 agonists. Although it remains to be proven, it is likely that the presence of CCR3 and other CC chemokine receptors shared between eosinophils and basophils account for the previously demonstrated strong temporal association of their influx into tissues such as the nose37,63 or the lung36 during LPRs.
A further insight regarding tissue influx of basophils and their subsequent activation is also given by the actions of hematopoietic cytokines such as IL-3, IL-5, and GM-CSF. An increased expression of these Th2 lymphocyte–derived cytokines in allergen-induced cutaneous LPRs in atopics was clearly demonstrated by Kay and colleagues,64 and these cytokines have also been shown to facilitate basophil migration.65-67 In addition, IL-3, IL-5, and GM-CSF also enhance mediator release following either IgE-dependent stimulation or activation due to platelet-activating factor, C5a, and C3a. Similar properties have additionally been described for the neurotrophic cytokine NGF.68 Moreover, increased levels of NGF have recently been reported in allergic rhinitis69 and asthma.70
IL-3, IL-5, GM-CSF, and NGF have similar efficacies with respect to potentiating IgE-mediated basophil histamine and LTC4releases, and they bind to receptors of the same subfamily but, of these, IL-3 is the most potent71 and its effects on basophils have been appreciated for some time.72,73 In vitro, IL-3 alone causes little mediator release by itself, with the exception of IL-13 production in some donors. However, a short preincubation or coculture of basophils with IL-3 causes significant enhancement of histamine and LTC4 release to a number of immunologic and nonimmunologic stimuli such as anti-IgE, C5a, C3a, the eosinophil product major basic protein, and platelet-activating factor.74-77 Moreover, studies by Ochensberger78 demonstrate that LTC4 synthesis in basophils primed by IL-3 before stimulation with C5a is continuously maintained over many hours. This clearly highlights the ability of basophils to maintain a state of activity over longer periods than previously anticipated. Additionally, these authors showed an elevated production of IL-4 and IL-13 from basophils under the same conditions,78 which may exacerbate LPRs because of the up-regulatory effects of these cytokines on vascular cell adhesion molecule-1 (VCAM-1), thus promoting further influx of inflammatory cells (discussed in detail below).
The ability of basophils to continuously produce LTC4 and cytokines may also be particularly important in maintaining chronic allergic inflammatory diseases, such as asthma. Furthermore, because IL-3 can, in conjunction with C5a, cause basophil mediator release independently of IgE-receptor cross-linking, this suggests a role for basophils in nonallergic inflammatory diseases caused by either bacterial infections or IgG immune complexes. In addition to the combined effects of C5a and IL-3, C5a by itself is a highly potent and efficacious inducer of histamine release from basophils79following binding to the C5a receptor, CD88.80 In contrast, most human mast cell subtypes do not express CD88 and are unresponsive to C5a except skin mast cells.80 Moreover, unlike basophils, mature human mast cells, including those of the skin, also do not express receptors for IL-3 and GM-CSF.81 This clearly suggests that, compared with mast cells, basophils are more sensitive to the effects of immunomodulatory cytokines and proinflammatory factors, which are likely to be in abundance in both LPRs and other (nonallergic) inflammatory conditions.
Unfortunately, the interplay of these various basophil activators, in terms of the net clinical outcome of their combined effects, in allergic disease remains to be elucidated. However, the importance of IL-3 in vivo as both a modulator of basophil growth and activation as well as its ability to cause clinical symptoms of allergic inflammation was shown by van Gils et al.82 Using rhesus monkeys treated with the IL-3, the authors observed a clear increase in both basophil numbers together with an up-regulation of IL-3 receptors without a concomitant rise of IL-3R+ monocytes or mast cells.82 Moreover, the same authors also demonstrated that IL-3 treatment caused severe hypersensitivity reactions including urticaria, vomiting, diarrhea, edema, arthritis, and stimulation of hemopoiesis.83 Given the above evidence, IL-3 and related hematopoietic growth factors, as well as NGF, potentially play a pivotal role in exacerbating LPRs in addition to CC chemokines describedearlier.
Another indirect line of evidence that reveals basophils as potential major regulatory cells in allergic inflammation comes from the study of their cytokine profile. As described earlier, human basophils are an important source of the proallergic cytokines IL-4 and IL-13. Kasaian et al84 have shown that allergen-induced IL-4 from primary cultures of unfractionated peripheral blood leukocytes of atopic patients is mainly basophil-derived. This finding has been confirmed and extended to IL-13 by Devouassoux and coworkers,85 who found that basophils are the main source of both cytokines early after allergen stimulation of peripheral blood cells. IL-4 is of central importance for the recruitment of inflammatory cells to the tissues, because it induces the expression of the adhesion molecule VCAM-1 on endothelial cells in vitro42 and it recruits eosinophils in vivo, eg, when injected in rodents.86,87 Transgenic mice expressing IL-4 in the lung under the control of a lung-specific promoter showed eosinophil infiltrations in the airways.88Recently, Hickey et al89 have used intravital microscopy to demonstrate that the VLA (very late antigen)-4/VCAM-1 interaction is necessary and sufficient for IL-4–induced eosinophil recruitment in mice. On the other hand, it has been shown that IL-4, especially in combination with tumor necrosis factor (TNF)–α, induces the production and release of eotaxin by human dermal fibroblasts;90 moreover, eotaxin-3, but not eotaxin, is up-regulated in vascular endothelial cells by IL-4.62 The induction of eotaxins by IL-4 could represent a strong positive feedback loop operating in eosinophilic skin diseases (eg, atopic dermatitis), with the induced eotaxins recruiting more eosinophils, basophils, and Th2 lymphocytes and the subsequent release of more IL-4 upon activation (Figure 1). Interestingly, eotaxin can potentiate the antigen-dependent IL-4 production by basophils91 without having a direct effect on basophils by itself,78 thus generating a further potential amplification loop in allergic inflammation. The aforementioned proallergic properties of IL-4 therefore make the identification of its cellular source(s) an important prerequisite for the successful therapy of asthma and other allergic diseases.
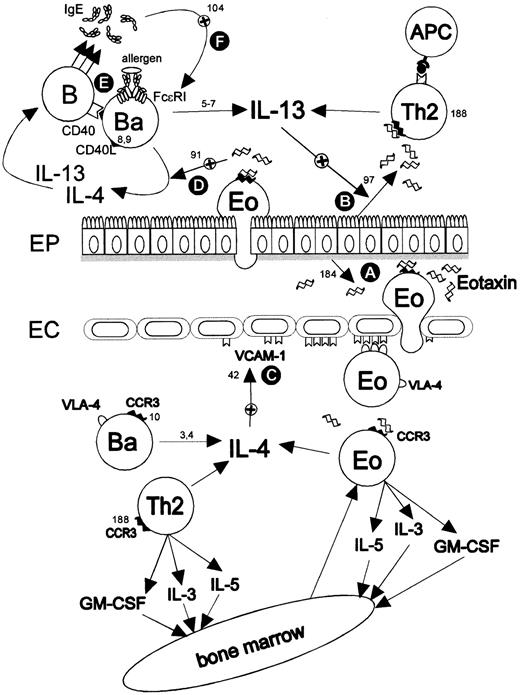
LPR scenario emphasizing the putative regulatory roles of basophils in allergic inflammation.
Eotaxin (CCL11) (A) derived from epithelial cells,184eosinophils,51 or mast cells185 can be detected in the lungs of asthmatic patients50-53 and recruits cells expressing the eotaxin receptor (CCR3), ie, eosinophils,186,187 basophils,10,55 Th2 cells,188,189 and mast cells.190,191Recruited basophils are a major source of the regulatory cytokines IL-43,4 and IL-13.5-7 There are several potential amplification loops, such as the up-regulation of eotaxin in airway epithelial cells97 (B) and VCAM-1 on vascular endothelium (C) by IL-1396 or IL-442 or, also, the up-regulation of eotaxin by IL-4 in fibroblasts.90Eotaxin has also been shown to potentiate the IL-4 production by activated basophils91 (D). Furthermore, basophils can deliver the signals necessary for switching B cells to IgE (E), ie, IL-4, IL-13, and CD40L.8,9 The increased IgE production is thought to up-regulate the expression of FcεRI on basophils and mast cells103,104 (F).
LPR scenario emphasizing the putative regulatory roles of basophils in allergic inflammation.
Eotaxin (CCL11) (A) derived from epithelial cells,184eosinophils,51 or mast cells185 can be detected in the lungs of asthmatic patients50-53 and recruits cells expressing the eotaxin receptor (CCR3), ie, eosinophils,186,187 basophils,10,55 Th2 cells,188,189 and mast cells.190,191Recruited basophils are a major source of the regulatory cytokines IL-43,4 and IL-13.5-7 There are several potential amplification loops, such as the up-regulation of eotaxin in airway epithelial cells97 (B) and VCAM-1 on vascular endothelium (C) by IL-1396 or IL-442 or, also, the up-regulation of eotaxin by IL-4 in fibroblasts.90Eotaxin has also been shown to potentiate the IL-4 production by activated basophils91 (D). Furthermore, basophils can deliver the signals necessary for switching B cells to IgE (E), ie, IL-4, IL-13, and CD40L.8,9 The increased IgE production is thought to up-regulate the expression of FcεRI on basophils and mast cells103,104 (F).
Recent evidence has implicated the pleiotropic cytokine IL-13, which is also produced in large amounts by activated basophils, as a key mediator of allergic asthma. In humans, IL-13 is secreted following local allergen challenge in subjects with mild atopic asthma.92 Work performed by 2 independent laboratories, using an IL-13R–Fc fusion protein that specifically neutralizes IL-13, has indicated that this cytokine is necessary and sufficient for establishment of an asthmatic phenotype in a murine model.93,94 Administration of soluble recombinant IL-13 was effective in inducing airway hyperresponsiveness and in generating a significant recruitment of eosinophils to the lung, as shown by their appearance in bronchoalveolar lavages.93 In vitro, IL-13 induces recruitment and prolongs the survival of eosinophils,95 up-regulates VCAM-1 expression on vascular endothelium,96 and is a very potent inducer of eotaxin in airway epithelial cells.97 The importance of IL-13 in the pathogenesis of asthma is also corroborated by a recent study using transgenic mice that express IL-13 selectively in the lung.98 The authors of this study found that transgenic mice, in the absence of a specific sensitization, developed a phenotype very similar to the asthmatic phenotype, including eosinophil infiltration, up-regulation of eotaxin, mucus hypersecretion by goblet cells, subepithelial airway fibrosis, and airway hyperresponsiveness to methacholine.98
Finally, basophils have also been proposed to play a key role in allergy by directly inducing the switch to the IgE isotype in B cells independently of T cells (Figure 1). Gauchat et al have shown that the basophil cell line KU812, as well as purified peripheral blood basophils, the mast cell line HMC-1, and human lung mast cells, can provide the necessary signals for IgE production in vitro, ie, IL-4, IL-13, and CD40L.9 Basophils generated from human umbilical cord blood mononuclear cells, after cultivation in the presence of appropriate cytokines, expressed detectable levels of CD40L and induced IgG4 and IgE synthesis in B cells when stimulated with allergen.8 The induction of IgE synthesis was completely abrogated by neutralizing IL-4 and IL-13 with monoclonal antibodies or CD40L with soluble CD40.8
Taken together, the data about the involvement of IL-4, IL-13, and eotaxin as key players in the pathogenesis of asthma, together with the proven infiltration and activation of basophils in the tissues during LPRs, makes it reasonable to assume that basophils, as a major source of IL-4 and IL-13 and by constitutively expressing CCR3 and CD40L on their surface, may play an important but underestimated role in the pathology of asthma and other allergic conditions. The knowledge about the relative roles and contribution of basophils, eosinophils, mast cells, and T cells to these processes as well as about their interplay32 is still preliminary and needs further refinement.
Regulation of IgE-dependent signaling and mediator secretion in human basophils
The effector role of basophils in IgE-mediated allergy is well known to depend on cross-linking of FcεRI-bound IgE by allergens. The diversity of basophil mediators that are released following IgE-receptor cross-linking is accomplished by a highly complex and interrelated network of secondary messengers. Their specific roles in controlling mediator release are not nearly as well characterized for human basophils compared with their counterparts from other species or cell lines. Several examples given below show that a mere extrapolation of observations derived from basophil lines or basophils from other species does not clearly reflect the situation in primary human basophils. Although it may be obvious, this point must be stressed because future successful pharmacologic approaches to reducing basophil mediator production hinge crucially on understanding basophil biology taken from studies on primary basophils.
Early IgE-dependent signaling events in basophils
High-affinity IgE-receptor (FcεRI) cross-linking culminates in 3 main results. The first, and most rapid, is fusion of basophil granules to the plasma membrane resulting in histamine release99; the second is an activation of lipid metabolism and subsequent LTC4 production; and the third is synthesis of immunomodulatory cytokines. The initial signaling events following FcεRI cross-linking are currently modeled on studies largely carried out on rodent mast cell lines (such as RBL-2H3 cells), where phosphorylation of tyrosine, serine, and threonine residues contained in the β and γ chains of FcεRI receptor have been observed (Figure 2). In human basophils, as in RBL-2H3 cells, this has been shown to involve Lyn and subsequent recruitment of SH-2 domain–containing proteins such as Syk and other ZAP-70–related protein tyrosine kinases.101 The possibility that Syk may be involved in IgE-dependent basophil signaling was recently also demonstrated by Kepley and coworkers,102 who showed that nonreleaser human basophils, occurring in about 20% of the population, have a deficiency of this protein. In mast cells, most of the known major regulatory proteins known to be involved in FcεRI activation are downstream of Syk and thus crucially hinged to Syk activity. These include PLCγ1 and PLCγ2, Shc, Vav, mitogen-activated protein (MAP) kinases, phosphatidylinositol (PI)-3 kinase, and JNK, among others, although this has yet to be conclusively demonstrated for human basophils.
Schematic diagram of possible IgE-mediated signaling pathways in basophils.
Following high-affinity IgE-receptor cross-linking, various tyrosine kinases are activated, including Lyn and Syk, which consequently lead to the activation of PI 3-kinases, ERK-associated MAP kinases (Vav, Ras, Raf1, MEK), and PLC. PI 3-kinase targets include both p38 MAP kinase (p38) and Rac/Rho GTPases (Rac/Rho), which are involved in cytokine production. Further, Rac/Rho affect the cytoskeletal processes100 during degranulation as well as extracellular signal–related kinase-activating kinase (MEK). IgE-dependent ERK activation in basophils is largely limited to controlling LTC4 generation; PKC, activated by diacylglycerol (DAG) as a result of hydrolysis of PIP2 by PLC, is involved in degranulation. PKC may also affect cytokine transcription, although the mechanism is not yet clear. The release of calcium from intracellular stores by IP3 together with the influx of the ion through calcium channels affects degranulation, PLA2 translocation, and calcineurin activity. Thicker print is used to highlight what is currently known in human basophils. The remaining has been extrapolated from studies with rodent mast cells or cell lines.
Schematic diagram of possible IgE-mediated signaling pathways in basophils.
Following high-affinity IgE-receptor cross-linking, various tyrosine kinases are activated, including Lyn and Syk, which consequently lead to the activation of PI 3-kinases, ERK-associated MAP kinases (Vav, Ras, Raf1, MEK), and PLC. PI 3-kinase targets include both p38 MAP kinase (p38) and Rac/Rho GTPases (Rac/Rho), which are involved in cytokine production. Further, Rac/Rho affect the cytoskeletal processes100 during degranulation as well as extracellular signal–related kinase-activating kinase (MEK). IgE-dependent ERK activation in basophils is largely limited to controlling LTC4 generation; PKC, activated by diacylglycerol (DAG) as a result of hydrolysis of PIP2 by PLC, is involved in degranulation. PKC may also affect cytokine transcription, although the mechanism is not yet clear. The release of calcium from intracellular stores by IP3 together with the influx of the ion through calcium channels affects degranulation, PLA2 translocation, and calcineurin activity. Thicker print is used to highlight what is currently known in human basophils. The remaining has been extrapolated from studies with rodent mast cells or cell lines.
Although several of the early IgE-dependent signaling events in basophils are known, there are currently no pharmacologic approaches to the treatment of allergy that specifically modulate very early signaling events following cross-linking with allergen. However, the molecules known to be involved in early post–cross-linking are also used by other immunoreceptor systems affecting many other immune cell functions, thus making selective pharmacologic intervention difficult. Therefore, an alternative strategy is to inhibit either FcεRI expression itself or the binding of IgE molecules to the receptor. It has long been observed that there is a positive correlation between circulating IgE antibodies and FcεRI expression in basophils,103,104 and recent evidence clearly demonstrates that IgE does directly induce high-affinity IgE-receptor expression by acting through FcεRI itself.104 Thus, basophil (and mast cell) function may significantly be down-regulated by disrupting the binding of IgE to its receptor at the sensitization stage. This has been successfully achieved in atopic patients treated with a monoclonal anti-IgE antibody (rhuMAb-E25), which prevents IgE binding to the FcεRI α chain without cross-linking high-affinity receptor-bound IgE,105 and is a promising new therapeutic advancement in the treatment of allergic disorders.
PI 3-kinase is a universal regulator of basophil mediator release
PI 3-kinase is composed of a p85 regulatory subunit and a 110-kd catalytic domain that catalyses the reaction of membrane PdtIns4,5P2 principally to PdtIns3,4,5P3. Indirect observations using specific PI 3-kinase antagonists suggest that PI 3-kinase plays a crucial role in the release of all major basophil mediator types, suggesting that this enzyme is closely associated with initial events in IgE-receptor signaling.106,107 We have recently shown that the PI 3-kinase inhibitors wortmannin and LY-294003 almost completely abrogate the release of histamine, LTC4, IL-4, and IL-13 in human basophils stimulated with anti-IgE.107Moreover, inhibition of both IL-4 and IL-13 production did not result in increased cellular contents of the cytokines, showing that PI 3-kinase blockade is not restricted to impairing secretory events.
With respect to mast cell and basophil degranulation, there is a universal consensus that PI 3-kinase inhibitors abolish granule-associated release.108-112 However, in regard to LTC4 production, Marquardt et al reported that the PI 3-kinase inhibitor wortmannin had no effect on the production of the eicosanoid in murine mast cells.109 The same authors also failed to detect any effects of this compound on cytokine release, whereas other reports have described inhibition of either leukotriene synthesis or TNF-α production.113 114 These differences show that a marked heterogeneity exists between basophils and various mast cell lineages to PI 3-kinase signaling.
Extracellular signal–related kinases control basophil LTC4 production but not histamine or cytokine release
The extracellular signal–related kinases (ERKs) consist of 2 forms, ERK-1 and ERK-2 (sometimes referred to as p44 and p42 MAP kinases), which are considerably homologous to another in both structure and function.115 ERKs are activated after FcεRI cross-linking via Vav, Ras-Raf1, and MEK1 and activate phospholipase A2 (PLA2) as well as several transcription factors (Figure 2). The role of ERKs in basophils, however, has only recently been partially investigated. Studies from our own laboratory and from Miura et al116 have shown that ERK phosphorylation occurs within 3 to 10 minutes of basophil stimulation, after which there is a decrease to basal levels. The MEK inhibitor PD-098059 abolishes both anti-IgE–induced ERK activation and LTC4 production in basophils but has little effect on histamine release or cytokine synthesis (IL-4 and IL-13).107,116 The above studies highlight that ERK activation does not seem to affect cytokine transcription in basophils although it is an important regulator of cJun, cFos, STAT, and other transcription factors in diverse cell types. Therefore, in basophils ERKs appear to be largely involved only in LTC4 production because of the activation of PLA2. Nevertheless, we have also demonstrated that ERK activation is not by itself sufficient for leukotriene synthesis because IL-3, which strongly activates ERK in the absence of IgE-dependent stimulation,116 is almost ineffective at causing LTC4release.74,107,117,118 Despite this, in combination with anti-IgE, IL-3 leads to both a striking potentiation and prolongation of ERK phosphorylation compared with the effects of IL-3 or anti-IgE alone and leads to twice as much LTC4 production than anti-IgE alone. Although activation of PLA2 is dependent on ERK, translocation of soluble PLA2 from the cytosol to the membrane is dependent on calcium. However, studies by Krieger et al119 and MacGlashan and Hubbard118 have shown that the priming caused by briefly (< 15 minutes) preincubating basophils with IL-3 neither requires extracellular calcium nor induces calcium elevations. Taken together, these findings strongly suggest that, despite there being an important regulatory input from both ERK phosphorylation and intracellular calcium mobilization regarding PLA2 activity, one or more additional signals, possibly from protein kinase C (PKC)–dependent routes,120 are vital for LTC4 synthesis in basophils.
Comparing human basophils with other rodent histamine-producing cells, there are important differences with respect to regulation of cytokine synthesis controlled through ERKs. For example, IgE-dependent TNF-α production in RBL-2H3 cells is inhibited by the MEK inhibitor PD-098059, suggesting ERK involvement. In contrast, the release of IgE-dependent TNF-α from MC/9 murine mast cells is not affected by the antagonist. Although basophils have not been shown to produce TNF-α, the above examples highlight further the variable abilities of ERKs to control the transcription of cytokine factors between human basophils and their counterparts in rodents. Further evidence that the MEK-ERK route does not predominantly influence basophil IL-4 production is provided by Miura et al,116 who report that the bacterial peptide fMLP induces basophil ERK activation without IL-4 release.
Calcium dependence of basophil mediator secretion
It has long been appreciated that basophil degranulation is strikingly reduced in the absence of extracellular calcium.121 Similarly, production of both IL-4 113,122,123 and IL-13 (B.F.G. et al, unpublished data, 1998) is just as dependent on the ion for optimum response to IgE-receptor activation. Calcium responses are in 2 phases.124 The first takes place by the release of intracellular calcium stores from the endoplasmic reticulum because of the effects of inositol 1,4,5-trisphosphate (IP3). The second phase is caused by the opening of calcium channels allowing an influx of the ion from the extracellular milieu and is modulated by cyclic adenosine monophosphate (cAMP)–elevating drugs.125A rise in intracellular free calcium ions can be mimicked by using calcium ionophores such as A23187, which directly transport extracellular calcium into the cells, release internal stores of the ion, and evoke degranulation, eicosanoid generation, and cytokine production.
In terms of IgE-dependent cytokine secretion, both IL-4 and IL-13 are controlled by calcium-calcineurin pathways, which promote the translocation of the transcription factor NFAT to the nucleus.126 This pathway is blocked by macrolides such as FK506 and cyclosporin A, which reduce both IL-4 and IL-13 synthesis.127 Additionally, the release of these cytokines is also effectively blocked by cAMP-elevating drugs such as theophylline or β-2 agonists (salmeterol), further highlighting the importance of calcium influx in basophil activation.128
IL-4 and IL-13 are differentially controlled by IL-3 and PKC
In terms of IgE-dependent stimulation of IL-4 and IL-13, there is no clear evidence of differential control regarding the production of these cytokines. However, the kinetics of their release from basophils is strikingly different, suggesting that factors other than the signaling routes already described above are involved. IL-4 is released rapidly, peaking within 4 hours of stimulation, and is accompanied in some donors by the release of preformed stores of this cytokine.6,129,130 In contrast, IL-13 is detectable in basophil supernatants beginning 2 hours after FcεRI cross-linking, and the amounts of this factor continue to rise for at least 16 hours.6 At present, the reasons for these differing kinetics are unknown, but factors such as autocrine effects are now being investigated.
In terms of the levels of IL-4 and IL-13 produced, there is growing evidence that signals employed by priming factors such as IL-3 favor the production of IL-13 rather than IL-4. IL-3 potentiates the IgE-dependent release of both factors but, in addition to this, Schroeder et al, as well as Redrup et al, have reported that IL-3 stimulation alone is sufficient itself to give rise to IL-13 generation without a marked increase in IL-4 production.126 127 These authors further showed that, in stark contrast to IgE signaling, this IL-3-dependent IL-13 release is insensitive to FK506, suggesting the involvement of an activatory pathway independent of calcineurin and NFAT.
A further tier of differential control of IL-4 and IL-13 release is seen with respect to PKC. Following PMA-induced PKC activation in basophils, Schroeder and coworkers have reported that although messenger RNA expressions for both IL-4 and IL-13 are increased only IL-13 protein is released.126 127 It is not yet clear, however, which isozymes of PKC are involved. The same authors have shown that the selective PKC inhibitor bisindolylmaleimide II not only reverses the effects of PMA activation but potentiates the anti-IgE induced IL-4 release but not that of IL-13. Interestingly, bisindolylmaleimide II, as well as FK506, does not affect IL-3–induced secretion of IL-13, thus showing that IL-3–associated signaling in basophils diverges considerably from both calcium and PKC-sensitive routes compared with IgE-dependent pathways.
Future aspects
The brief summary above regarding the control of IgE signaling in basophils highlights 2 major problems for future consideration. First, functional differences exist between primary basophils and mast cells or basophil cell lines and, second, IgE-dependent signaling is greatly affected by the actions of priming factors such as IL-3. Thus, the net outcome of IgE-triggered basophil activation in vivo is likely to be modulated by varying degrees of the local concentrations of hematopoietic cytokines (such as IL-3, IL-5, and GM-CSF) as well as neurotrophic cytokines (NGF), complement factors, and histamine-releasing factors. Because basophils are geared to the rapid release of Th2-type cytokines, it would be of considerable interest to establish the concentrations of the above factors in the in vivo environment, particularly during an allergic reaction where acute (IgE-mediated) basophil activation occurs.
What is the physiologic role of basophils in the immune system?
Although their existence has been known for more than a century, the physiologic role of basophils in immunity remains a mystery. It is conceivable that basophils, like eosinophils, neutrophils, macrophages, and mast cells, fulfill important roles in innate immunity against pathogenic organisms. Important clues could be derived from the study of natural or experimentally induced gene deficiencies, as happened with W/Wv c-kit–deficient mice,131 which have proven very useful in dissecting the role of mast cells in immunity to intestinal nematodes, or IL-3−/− mice.132Unfortunately, in the absence of a precise knowledge about the unique developmental pathway of basophils, none of the currently available gene-deficient mice are likely to elucidate the exclusive role of basophils in immunity.
Mast cells and basophils have been proposed to play an important role in the innate immune response to a variety of pathogens.133 For mast cells such a role is corroborated by an increasing body of evidence,133,134 whereas the current evidence for a comparable involvement of basophils is, at best, circumstantial. Such a role would require the ability of basophils to be activated in a non–antigen-specific manner. Indeed, several such non–antigen-specific stimuli, derived from various organisms, have been shown to induce mediator or cytokine release from basophils. For example, protein Fv, an endogenous Ig-binding protein released in the intestine of patients affected by viral hepatitis, as well as the HIV-1 glycoprotein gp 120 have recently been shown to induce IL-4 and IL-13 production from basophils and mast cells by binding to the VH3 region of IgE.135-137 Similarly, antigens derived from the egg stage of the parasite Schistosoma mansoni (SEA, or soluble egg antigen) induce the release of IL-4 and other mediators from basophils of nonimmune donors.138This activation was dependent on the presence of IgE because a short incubation at low pH (under conditions that induce dissociation of IgE from its receptor)139 abrogated, whereas resensitization with purified IgE or other IgE-containing materials restored the activating effect of SEA (Falcone et al138 and K. Haisch et al, unpublished data, 2000). Furthermore, proteases secreted by the hookworm Necator americanus induce the de novo synthesis of IL-4 and IL-13 in the basophilic cell line KU812 (C. Phillips et al, unpublished data, 1999). The filarial parasiteBrugia malayi strongly expresses the geneBm-tph-1,140 which is closely related to a human IgE-dependent histamine releasing factor,141 but it is not known whether Bm-TPH-1 can also activate basophils. Finally, the recombinant form of the IgE-dependent human histamine-releasing factor induces histamine and IL-4 release from basophils. This factor, in contrast to original findings,142,143 has recently been shown to act independently of the presence of IgE and is now thought to bind to a receptor present on the surface of basophils.144
Because basophils rapidly release considerable amounts of IL-4 upon stimulation, they may have a decisive impact on the outcome of a primary infection by inducing T-cell differentiation to the Th2 phenotype. To the best of our knowledge, basophils are the only leukocytes containing preformed IL-4, although in relatively small amounts and not detected in all donors. Constitutive IL-4 expression in unstimulated basophils has been demonstrated by intracellular cytokine staining in saponin-permeabilized cells130 as well as by enzyme-linked immunosorbent assay.6 Apart from basophils, cells such as Th2 cells, NK1.1+ cells, γδ+T cells, eosinophils, and mast cells also have been found to produce IL-4 and have been considered as a cellular source of the so-called “early IL-4.”145 The discussion about the in vivo source of early IL-4 has led to much controversy146; the bottom line is that several different cell types may play a not mutually exclusive role in initiating Th2 responses.145 146 It is also possible that part of the controversy is ultimately due to fundamental differences in the relative roles of mast cells and basophils as a source of IL-4 in rodents versus humans.
Another line of evidence suggesting a possible role of basophils as a source of early IL-4 comes from the study of the effects of plant lectins on this cell type. It has long been known that lectins such as concanavalin A can trigger degranulation and histamine release from basophils.147,148 Using a panel of 16 plant lectins with different carbohydrate specificities, we have recently shown that lectins differentially induce the release of IL-4 (and IL-13) from basophils.149 The capacity of the studied lectins to trigger cytokine release correlated with their binding to different myeloma IgE molecules—IgE-PS, IgE-(T+H), IgE-PE, IgE-VL—suggesting non–antigen-specific cross-linking of FcεRI-bound IgE via its carbohydrate moieties as the initial step of signal generation.149 However, when unsensitized RBL-2H3 cells, ie, cells devoid of receptor-bound IgE, were stimulated, most of the cytokine-inducing lectins also triggered the release of β-hexosaminidase (a granule mediator in rodent mast cells). OnlySambucus nigra and Ricinus communis agglutinins clearly required sensitization of the RBL-2H3 cells with rat IgE to elicit a full response (H. Haas et al, unpublished data, 1999). By analogy, a similar IgE-independent mechanism may be expected for IL-4 release induced by most lectins. Although the significance of these in vitro findings needs to be confirmed in vivo, it nevertheless points to a whole category of molecules with the ability to trigger the release of IL-4 from basophils in a non–antigen-specific manner. It is therefore intriguing to note that several parasitic helminths, which are well known to induce a strong Th2 response in their host, have been recently shown to produce or secrete lectins.150 For instance, infective larvae of the nematode parasite Toxocara canis secrete a novel C-type lectin (TES-32) that surprisingly binds both mannose- and galactose-type monosaccharides,151 which are both present on the carbohydrate side chains of IgE.152,153 TES-32 is the most abundant glycoprotein secreted by this parasite in vitro. The hematophagous parasite N americanus produces a major allergen that is a putative homolog of calreticulin,154 a lectin with a carbohydrate affinity155 that also matches the glycosylation pattern of human IgE. Thus, several pathogenic organisms may induce the activation of basophils by the release of superallergens, a term introduced by Patella et al,135 136which in this context would define molecules with the ability to cross-link FcεRI either directly or via receptor-bound IgE in a non–antigen-specific manner.
Taken together, the observation that different classes of molecules derived from pathogens or endogenous sources can directly activate basophils independently of the presence of specific IgE on their surface fulfills the theoretical requirement for a role of this cell type in innate immunity or in bridging innate and specific immunity by skewing the differentiation of naive T cells to the Th2 phenotype. Thus, more research is needed to address the fundamental question concerning the true physiologic role of basophils in the immune system.
Tools for basophil research
Investigation into the role of basophils in health and disease has previously been curtailed by the lack of suitable experimental tools. The following section, therefore, summarizes the currently available tools for basophil research.
Monoclonal antibodies
Because basophils are quickly recruited to the tissues in a variety of allergic diseases, monoclonal antibodies allowing specific detection of basophils by immunohistochemical staining would be a highly desirable tool, eg, for studying the relative contribution of this cell type to pathogenesis. Earlier protocols relied on the presence of FcεRI and the absence of tryptase for immunohistochemical detection.156,157 It has become clear, however, that the expression of FcεRI is not restricted to mast cells and basophils, because it can be up-regulated on other cells such as Langerhans cells158 and other dendritic cells,159eosinophils,160 and monocytes.161Furthermore, the expression of tryptase cannot be used for discrimination of mast cells from basophils, because the gene for tryptase α is transcribed in the latter cell type162 and is present in substantial amounts in peripheral blood basophils of patients with asthma or other allergic diseases.20Therefore, several laboratories have generated antibodies for specific detection of basophils in flow cytometry or immunohistochemistry.
There are currently 3 basophil-specific monoclonal antibodies: BSP-1, 2D7, and BB-1. BSP-1 is an IgM class monoclonal antibody that recognizes an epitope expressed on the surface of human basophils and was originally raised against the human erythroblastic leukemia cell line (HEL).163 It reacts with a 45-kd surface antigen on HEL cells in Western blots.163 Because of its low sensitivity, BSP-1 is not suitable for immunohistochemistry.164 Monoclonal antibody 2D7 is an IgG1κ monoclonal antibody that reacts with a ligand localized to the secretory granules of human basophils.164 Western blots with basophil extracts exhibit 2 major bands with apparent molecular weights of 72 and 76 kd and a minor band of 124 kd.164Immunologic activation of basophils leads to the loss or attenuation of the staining intensity, indicating release (or decay) of the 2D7 antigen.164 Monoclonal antibody 2D7 has successfully been used for immunohistochemical staining of basophils in human skin during the LPR after cutaneous allergen challenge.165
The most recent addition to the pharmacopoeia is BB-1, an IgG2a monoclonal antibody that recognizes a 124- ( ± 11) kd antigen (estimated by the method of Hedrick and Smith)166 localized mainly to the secretory granules with a comparatively small expression on the surface.167 The BB-1 antigen was released upon activation with anti-IgE or the calcium ionophoreA23187.167 Because the BB-1 antigen was not detected in any other cell types, the authors suggest its use as a discriminating marker of basophil activation, particularly in the tissues, where it has been successfully used for immunohistochemical detection of basophils in lung sections fixed in Carnoy's fluid.167
Cell lines
Several studies have used the cell line KU812, originally established from a patient with chronic myelogenous leukemia.168 KU812 is seen as an immature basophil precursor169 and has served as a model for basophil differentiation. Several cytokines can induce its differentiation into basophil-like cells, including TNF-α and IL-6,170,171IL-3,172 and IL-4.173 As shown by Hara et al,173 incubation of KU812 cells with IL-4 induced several changes, including a 10-fold increase of total histamine content, appearance of metachromatic staining with toluidine blue, and up-regulation of functionally active FcεRI on the surface after 7 days, with increased transcription of FcεRI α, β, and γ chains' messenger RNA. Another cell line, termed LAMA-84, was established in 1987 by Seigneurin et al174 and has also been described as basophil-like.175 Kepley and coworkers176 have recently described the in vitro generation of fully functional basophils from cord blood. The protocol consists in pulsing normal cord blood leukocytes with IL-3 for 3 to 4 hours and subsequently incubating them with fetal calf serum. The resulting cells are mostly 2D7+, FcεRI+, express a series of integrins also found on normal peripheral blood basophils (CD11b, CD18, CD29, and CD49d), and display basophil-like morphology by light or electron microscopy. Activation through the high-affinity IgE receptor results in a time- and dose-dependent release of histamine. The use of basophil-like cell lines, however, must be cautiously examined given the fundamental differences in morphology and function compared with primary human basophils.
Purification protocols
A high degree of basophil purity is often required for functional studies with basophils, especially when mediator secretion from contaminating cells masks what is released by basophils. Pure basophil populations are also mandatory when investigating either intracellular signaling events or to exclude the possibility of priming cytokines (eg, IL-3, GM-CSF), derived from cellular contaminants, to influence basophil function. Therefore, several protocols for basophil purification have been published over the last decade (see references in Haisch et al177). When purifying basophils, one is faced with several challenges. First, the average percentage of basophils in the peripheral blood of healthy individuals is low (< 1%), which restricts the possible yield. Furthermore, a large donor variation in the proportion of basophils makes the final recovery of basophils unpredictable, especially when dealing with blood from unknown donors (ie, from buffy coats).178
Most of the currently published purification techniques rely on initial physical separation methods (density gradients or elutriation) that, by themselves, do not reliably result in very high purity but are successful in enriching basophils sufficiently enough for further purification by immunoselection. Some protocols have relied on the expression of the high-affinity receptor FcεRI on the surface of human basophils.179,180 This positive selection technique, although resulting in very high basophil purity, is not desirable for many purposes because of the likelihood of activation during purification. The use of negative selection with magnetic beads176,181,182 consistently resulted in viable, functional (nonpreactivated) basophils but, in our experience, high yields were usually obtained only with hyperbasophilic donors.178 A recently published protocol, however, has overcome this limitation (Figure 3) and uses a commercially available kit,177 therefore making the purification protocol widely accessible to any laboratory with an interest in granulocyte function. A similar protocol combines density gradient centrifugation with negative selection with magnetic beads.183
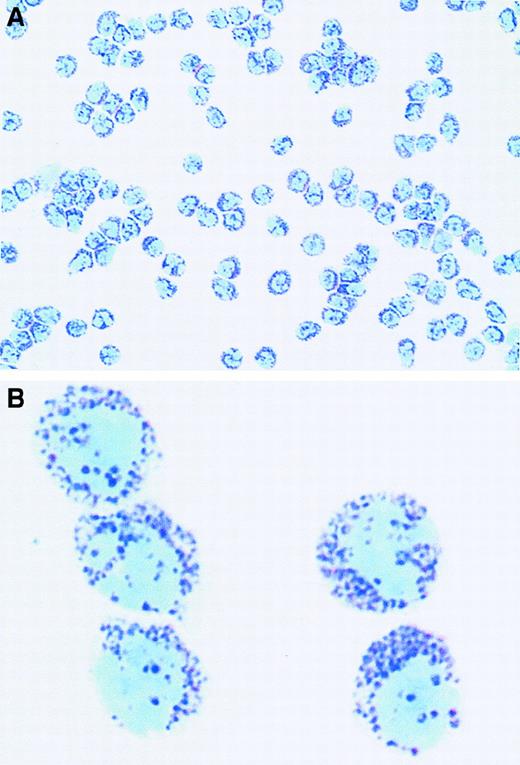
Purified human peripheral blood basophils stained with May-Grünwald-Giemsa obtained with a recently published basophil purification protocol.177
(A) Original magnification × 160. (B) Original magnification × 1000. Courtesy of A. Gronow and K. Haisch, Forschungszentrum Borstel, Germany.
Purified human peripheral blood basophils stained with May-Grünwald-Giemsa obtained with a recently published basophil purification protocol.177
(A) Original magnification × 160. (B) Original magnification × 1000. Courtesy of A. Gronow and K. Haisch, Forschungszentrum Borstel, Germany.
Taken together, the availability of effective protocols for the purification of nearly homogeneous basophils or for the generation of this cell type from peripheral blood precursors as well as the availability of monoclonal antibodies for immunohistochemical detection and of basophil-like cell lines should encourage further study of human basophil function. The development of similar tools for the study of murine basophils would be extremely useful because it would enable the research to be extended to experimental models of disease.
Acknowledgment
The authors thank Dr Martin J. Holland for critical comments and suggestions.
Supported by the Deutsche Forschungsgemeinschaft (Ha 1590/2-1 and Fa 359/1-1).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Bernhard F. Gibbs, Department of Dermatology, Medical University of Lübeck, Ratzeburger Allee 160, D-23538 Lübeck, Germany.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal