Abstract
Platelets are known to contain platelet factor 4 and β-thromboglobulin, α-chemokines containing the CXC motif, but recent studies extended the range to the β-family characterized by the CC motif, including RANTES and Gro-α. There is also evidence for expression of chemokine receptors CCR4 and CXCR4 in platelets. This study shows that platelets have functional CCR1, CCR3, CCR4, and CXCR4 chemokine receptors. Polymerase chain reaction detected chemokine receptor messenger RNA in platelet RNA. CCR1, CCR3, and especially CCR4 gave strong signals; CXCR1 and CXCR4 were weakly positive. Flow cytometry with specific antibodies showed the presence of a clear signal for CXCR4 and weak signals for CCR1 and CCR3, whereas CXCR1, CXCR2, CXCR3, and CCR5 were all negative. Immunoprecipitation and Western blotting with polyclonal antibodies to cytoplasmic peptides clearly showed the presence of CCR1 and CCR4 in platelets in amounts comparable to monocytes and CCR4 transfected cells, respectively. Chemokines specific for these receptors, including monocyte chemotactic protein 1, macrophage inflammatory peptide 1α, eotaxin, RANTES, TARC, macrophage-derived chemokine, and stromal cell–derived factor 1, activate platelets to give Ca++ signals, aggregation, and release of granule contents. Platelet aggregation was dependent on release of adenosine diphosphate (ADP) and its interaction with platelet ADP receptors. Part, but not all, of the Ca++ signal was due to ADP release feeding back to its receptors. Platelet activation also involved heparan or chondroitin sulfate associated with the platelet surface and was inhibited by cleavage of these glycosaminoglycans or by heparin or low molecular weight heparin. These platelet receptors may be involved in inflammatory or allergic responses or in platelet activation in human immunodeficiency virus infection.
Introduction
Chemokines (chemotactic cytokines) are small proteins that induce chemotaxis of cells by interacting with specific receptors. Chemokines are subdivided into 2 major classes based on the sequence of the cysteine pair domain near to the NH2terminus.1 The α-chemokines are characterized by the CXC motif and include platelet factor 4 (PF4), platelet basic protein-derived peptides, interleukin-8 (IL-8), interferon γ inducible protein 10 (γIP-10), and stromal cell-derived factor 1 (SDF-1). The β-chemokines are characterized by the CC motif and include monocyte chemotactic protein 1 (MCP-1), macrophage inflammatory peptide 1 (MIP-1) α and β, RANTES, TARC,2and macrophage-derived chemokine (MDC).3 In addition, there are related molecules such as lymphotactin with only one disulfide bond4 and a chemokine-like molecule with a CX3C spacing.4 An ever-increasing number of receptors for CXC and CC chemokines have been characterized and both specific (ie, one chemokine, one receptor) as well as nonspecific (ie, several chemokines, several receptors) interactions have been demonstrated. CXCR45 has only been shown to interact with SDF-1 as ligand so far, whereas CCR46 recognizes several CC chemokines.
The major physiologic function of platelets is hemostasis and maintenance of the vessel wall but, in addition, they are thought to have roles in inflammation and host defense.7 Platelets were the source of the first-described molecules of the chemokine CXC class with PF4 and β-thromboglobulin synthesized in megakaryocytes and stored in major amounts in α-granules that release their contents on platelet activation.8 Although they were used as markers of platelet activation, the biologic function of these molecules remained obscure for a long time. Studies over the past few years support a role for PF4 in controlling megakaryopoiesis9 and showed that β-thromboglobulin is one cleavage product of the precursor molecule, platelet basic protein.10 Platelet basic protein is also cleaved by physiological proteases to yield peptides such as neutrophil-activating peptide 2,11 which is chemotactic for neutrophils and acts via the CXCR1b and CXCR2 receptors.12 PF4 forms complexes with heparin in blood, as well as possibly with other glycosaminoglycans on the platelet surface, to form the major antigen implicated in heparin-induced thrombocytopenia13 and was recently shown to activate neutrophils via specific binding to a chondroitin sulfate proteoglycan.14
More recently platelets have also been shown to contain other members of the chemokine family, both of the CXC class, such as IL-8 and epithelial neutrophil-activating protein 78 (ENA-78),15and of the C-C class, such as RANTES,16MIP-1α,17 and monocyte chemotactic protein-3 (MCP-3).15 Evidence for the expression of chemokine receptors in platelets was also obtained, first by polymerase chain reaction (PCR) of a platelet-derived complementary DNA (cDNA) library15 but also by flow cytometry with specific antibodies.18 These studies point to the expression of at least CXCR1, CXCR4, and CCR4 on platelets in major amounts. In addition, there was evidence for messenger RNA (mRNA) for CCR3.15 Nevertheless, the functionality of these receptors as well as the role of the chemokines stored in the α-granules has remained obscure or even controversial.
Recent studies have shown that platelets are activated in patients infected with human immunodeficiency virus (HIV) and that plasma levels of RANTES are raised.19,20 It was also shown by using platelet inhibitors that the plasma levels could be brought back to normal levels, strongly supporting the idea that the platelets were the main source of the plasma RANTES in patients infected with HIV.19 Because chemokine receptors are known to play a major role in infection of T cells and macrophages by the virus21 and it appeared possible that in HIV-infected patients the virus may induce platelet activation, we investigated the expression and function of chemokine receptors on platelets in more detail. These studies indicate that the CCR1, CCR3, CCR4, and CXCR4 receptors function on platelets, signaling to the platelet interior as well as activating the fibrinogen receptor, αIIbβ3, to cause aggregation, and, as a consequence, they may have important roles in physiology and pathology.
Materials and methods
Chemicals
The ADP receptor inhibitor AR-C66096 (2-propylthio-D-β,γ-difluromethylene adenosine triphosphate trisodium salt) was a kind gift from Mr R.G. Humphries, Astra Charnwood (Loughborough, United Kingdom). Apyrase (grade III), chondroitinase ABC (EC 4.2.2.4), heparinase I (EC 4.2.2.7), heparinase III (EC 4.2.2.8), heparin (sodium salt, from porcine intestinal mucosa), goat antirabbit immunoglobulin G (IgG) peroxidase-coupled antibody, and rabbit antimouse IgG peroxidase-coupled antibody were from Sigma Chemical (St Louis, MO). Low-molecular-weight heparin (Fragmin, KabiVitrum AB) was a kind gift from Globopharm AG (Küsnacht, Switzerland). Thrombin receptor–activating peptide (TRAP, SFLLRN) was from Bachem AG (Bubendorf, Switzerland). Bovine thrombin was from Hoffmann-LaRoche (Basel, Switzerland). Chemokines IL-8, MIP-1α, MCP-1, MCP-3, eotaxin, RANTES, MDC, TARC, and SDF-1 were from Peprotech (Rocky Hill, NJ). All other chemicals were reagent grade or better. Methylated type I calf skin collagen was a kind gift from Dr Beate Kehrel, Münster, Germany. PolyScreen polyvinylidene difluoride (PVDF) membrane was from NEN Research Products (Boston, MA). The chemiluminescence detection system was SuperSignal (Pierce, Rockford, IL). Fuji RX autoradiographic films were from Fujifilm (Dielsdorf, AG, Switzerland).
Antibodies
Polyclonal antibodies to CCR1, CCR4, and phospholipase Cγ2 (PLCγ2), and p72SYK were from Santa Cruz Biotechnology (Santa Cruz, CA). Monoclonal antibodies to CCR3, CCR5, CXC3, CXCR4 were from R & D Systems (Minneapolis, MN). Antiphosphotyrosine antibody 4G10 (isotype IgG2b) was from Upstate Biochemicals (Lake Placid, NY). Antiphosphotyrosine antibody PY-7E1 (isotype IgG1) was from Zymed Laboratories (South San Francisco, CA). Fab fragments of anti-FcγRII (IV.3) were from Medarex (Annandale, NJ). The polyclonal rabbit antibody against the Fc receptor was a kind gift from Dr J. P. Kinet (National Institutes of Health, Rockville, MD).
Platelet preparation
Human blood platelets were isolated from buffy coats, within 12 hours after blood collection, obtained from the Central Laboratory of the Blood Transfusion Service of the Swiss Red Cross in Berne. The buffy coats were transferred into one third of their volume of 100 mmol/L sodium citrate, pH 6.5. The platelets were collected by centrifugation at 1500g for 7 minutes and washed twice in 40 mL Tyrode solution pH 6.5 (24.4 mmol/L NaH2PO4, 4.3 mmol/L Na2HPO4, 4.3 mmol/L K2HPO4, 113 mmol/L NaCl, 5.5 mmol/L glucose). The platelets were suspended in 20 mmol/L Hepes, 136 mmol/L NaCl, 4.8 mmol/L KCl, pH 7.4, and stirred at 1000 rpm. Activation studies were performed in the presence of 2 mmol/L CaCl2.
Polymerase chain reaction
Poly A+ mRNA was isolated from human platelets as described previously.15 Reverse transcriptase reactions were performed on 1 μg poly A+ RNA using an oligo dT primer with the Superscript preamplification system (Gibco-BRL-Life Technologies, Basel, Switzerland) and Amplitaq (PerkinElmer-Cetus, Rotkreuz, Switzerland). One twentieth of the reverse transcriptase reaction mixture was then subjected to 35 cycles of PCR (2 minutes, 94°C; 2 minutes, 55°C; and 2 minutes, 72°C) in a reaction mixture containing 50 pmoles sense and antisense primer pairs for each of the following chemokine receptors: CCR1-9, CXCR1-5, CX3CR1, XCR1, DARC, and GAPDH as a control for the quality of the cDNA used in each PCR reaction, in an MJ Research DNA engine (Waltham, MA). Primers were designed to amplify the full coding sequence (approximately 1.1 kb), based on the receptor sequences obtained from the GenBank database. The predicted size of the GAPDH product was 1 kb. In addition, control PCR reactions were performed with each primer pair on RNA samples that had been incubated in the absence of reverse transcriptase (results not shown). The identity of PCR products migrating at the predicted size was verified following gel purification using a Wizard PCR preps kit (Promega, Wallisellen, Switzerland), by direct sequencing using the same primers as for the PCR reaction, in an ABI 377 DNA sequencer (PerkinElmer, Hünenberg, Switzerland).
Intracellular Ca++ measurements
Washed platelets (108/mL) were loaded with 2 μm fura-2/AM (Fluka, Buchs, Switzerland) for 20 minutes, washed once with Hepes buffer, pH 7.4, and resuspended in Hepes buffer, pH 7.4, containing 2 mmol/L Ca++. Fluorescence of fura-2/Ca++ complex in platelets was measured in a multichannel fluorimeter. Ca++ measurements were standardized using ionomycin (1 μmol/L) and Mn++ (5 mmol/L) to give maximum and minimum levels, respectively. Platelet activation by ADP (10 μmol/L) and thrombin (0.05 U/mL) was used to control platelet viability.
Immunoblotting
Aliquots (5 μL or 20 μL, 5 × 108platelets/mL) of control, resting platelets as well as activated platelets were solubilized in sodium dodecyl sulfate (SDS) electrophoresis buffer containing 1 mmol/L PMSF, 5 mmol/L EDTA, 2 mmol/L N-ethylmaleimide, 2 mmol/L benzamidine, and 2 mmol/L sodium orthovanadate and separated by polyacrylamide gel electrophoresis (PAGE) on 7% to 20% acrylamide gels using the Laemmli method. Proteins were blotted to PVDF membranes using a semidry technique. Proteins were then detected by staining the membranes using specific antibodies, polyclonal or monoclonal, followed by chemiluminescence using peroxidase-coupled second antibody, luminol-enhancer substrate, and autoradiography or using a phosphatase-coupled second antibody, then 5-bromo-4-chloro-3-indolyl phosphate plus nitroblue tetrazolium.
Immunoprecipitation
Aliquots (700 μL, 5 × 108/μL) of control, resting as well as activated platelets were solubilized in phosphate-buffered saline containing either 1% Triton X-100, 0.5% sodium deoxycholate, and 0.1% SDS (RIPA) or 1% Triton X-100 with 1 mmol/L PMSF, 5 mmol/L EDTA, 2 mmol/L N-ethylmaleimide, 2 mmol/L benzamidine, and 2 mmol/L sodium orthovanadate. After platelet solubilization, the lysates were left for 30 minutes on ice, centrifuged for 30 minutes at 10 000g, in most cases cleared with 10 μL Protein A–Sepharose and then agitated for 2 hours with specific antibodies before adding 20 μL Protein A–Sepharose followed by a 6- to 8- hour incubation. The Protein A–Sepharose pellets were washed 4 times in the platelet solubilizing buffer, boiled in Laemmli buffer, and the supernatants collected. The immunoprecipitated or affinity-precipitated proteins were separated by gel electrophoresis, transferred to PVDF membranes, and incubated with specific antibodies. They were detected by chemiluminescence. Before reprobing with another antibody the membranes were stripped for 30 minutes at 60°C in 62.5 mmol/L Tris-HCl, pH 7.0, 2% SDS, and 100 mmol/L β-mercaptoethanol.
Results
Detection of platelet chemokine receptors
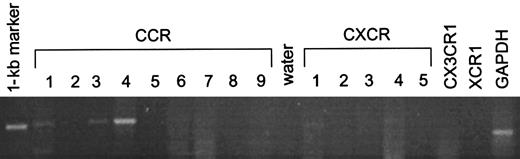
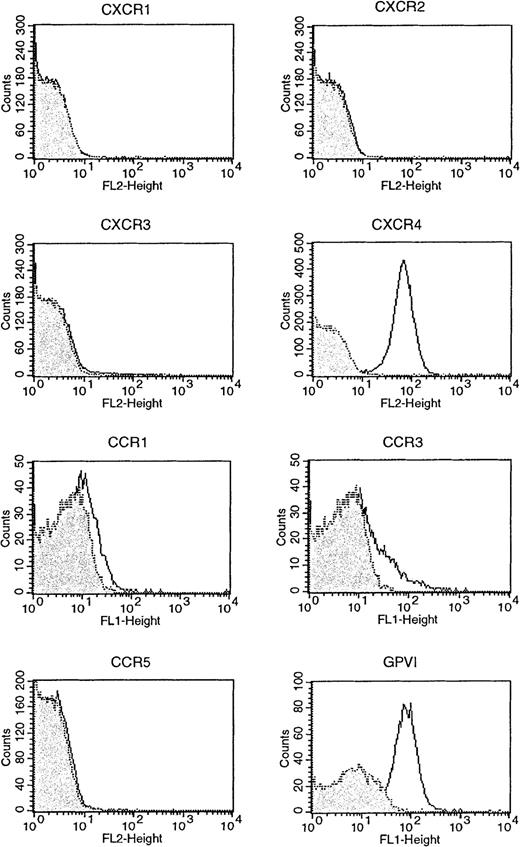
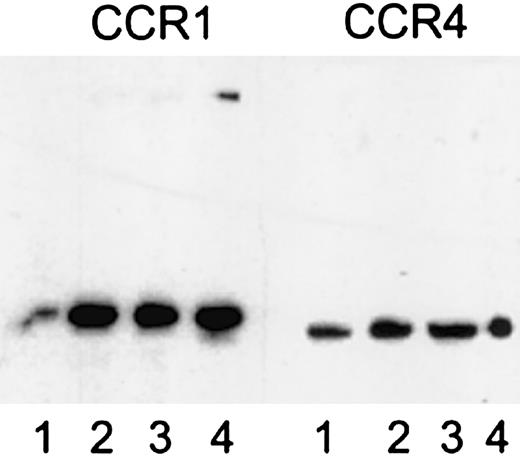
Evidence for the presence of platelet chemokine receptors was obtained by several methods. PCR of platelet mRNA with specific primers for CCR1-9 and CXCR1-5 showed the presence of mRNA for CCR1, CCR3, CCR4, CXCR1, and, weakly, CXCR4 (Figure1). Flow cytometry with specific antibodies showed the presence of a clear signal for CXCR4 and weaker signals for CCR1 and CCR3, whereas CXCR1, CXCR2, CXCR3, and CCR5 were all negative (Figure 2). Immunoprecipitation and Western blotting with polyclonal antibodies to cytoplasmic peptides clearly showed the presence of CCR1 and CCR4 in platelets in comparable amounts to monocytes and CCR4 transfected cells, respectively (Figure 3).
Expression of chemokine receptor mRNA in platelets.
PCR analysis was performed using primers against the chemokine receptors CCR1-9, CXCR1-5, CX3CR1, and XCR1 with GAPDH as a housekeeping probe.
Expression of chemokine receptor mRNA in platelets.
PCR analysis was performed using primers against the chemokine receptors CCR1-9, CXCR1-5, CX3CR1, and XCR1 with GAPDH as a housekeeping probe.
Surface expression of CCR1, CCR3, CCR5, CXCR1, CXCR3, and CXCR4 on platelets assessed by fluorescence-activated cell sorter analysis.
Surface expression of GPVI, a collagen receptor, is shown for comparison. Platelets were labeled with 10 μg/mL antireceptor antibody (black line) or an isotype control antibody (or preimmune polyclonal) (gray filled), then labeled with an fluorescein isothiocyanate (FITC) conjugate. Washed platelets were analyzed by flow cytometry and accumulated events were gated against the isotype control. The experiment shown is representative of 3 similar experiments.
Surface expression of CCR1, CCR3, CCR5, CXCR1, CXCR3, and CXCR4 on platelets assessed by fluorescence-activated cell sorter analysis.
Surface expression of GPVI, a collagen receptor, is shown for comparison. Platelets were labeled with 10 μg/mL antireceptor antibody (black line) or an isotype control antibody (or preimmune polyclonal) (gray filled), then labeled with an fluorescein isothiocyanate (FITC) conjugate. Washed platelets were analyzed by flow cytometry and accumulated events were gated against the isotype control. The experiment shown is representative of 3 similar experiments.
Expression of CCR1 and CCR4 in platelets assessed by immunoprecipitation and immunoblotting using antipeptide polyclonal antibodies.
Left panel, detection with anti-CCR1 polyclonal antibodies; 1, platelet lysate; 2, immunoprecipitate from platelets of donor A with anti-CCR1 polyclonal antibodies; 3, immunoprecipitate from platelets of donor B with anti-CCR1 polyclonal antibodies; 4, immunoprecipitate from monocytes with anti-CCR1 polyclonal antibodies. Right panel, detection with anti-CCR4 polyclonal antibodies; 1, platelet lysate; 2, immunoprecipitate from platelets of donor A with anti-CCR4 polyclonal antibodies; 3, immunoprecipitate from platelets of donor B with anti-CCR4 polyclonal antibodies; 4, immunoprecipitate from CCR4 transfected cells with anti-CCR4 polyclonal antibodies.
Expression of CCR1 and CCR4 in platelets assessed by immunoprecipitation and immunoblotting using antipeptide polyclonal antibodies.
Left panel, detection with anti-CCR1 polyclonal antibodies; 1, platelet lysate; 2, immunoprecipitate from platelets of donor A with anti-CCR1 polyclonal antibodies; 3, immunoprecipitate from platelets of donor B with anti-CCR1 polyclonal antibodies; 4, immunoprecipitate from monocytes with anti-CCR1 polyclonal antibodies. Right panel, detection with anti-CCR4 polyclonal antibodies; 1, platelet lysate; 2, immunoprecipitate from platelets of donor A with anti-CCR4 polyclonal antibodies; 3, immunoprecipitate from platelets of donor B with anti-CCR4 polyclonal antibodies; 4, immunoprecipitate from CCR4 transfected cells with anti-CCR4 polyclonal antibodies.
Chemokines induce a Ca++ response in platelets
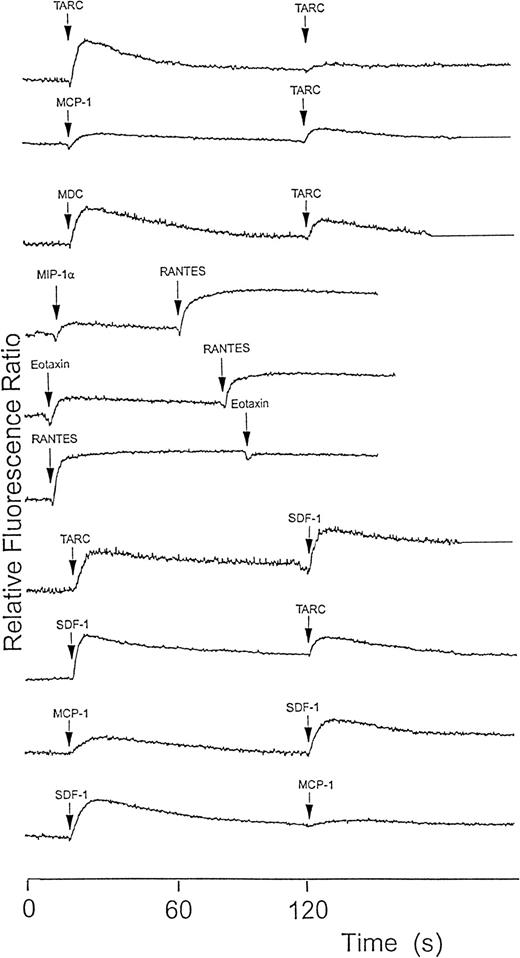
To determine whether chemokine receptors on platelets are functional, we tested several chemokines on platelets to assess their ability to induce Ca++ responses. Of those tested, IL-8 and MCP-3 had essentially no effect (a very marginal effect was seen with 100 nmol/L IL-8). MCP-1, MIP-1α, eotaxin, RANTES, TARC, MDC, and SDF-1 all induced a rapid, dose-dependent (10-100 nmol/L) rise in [Ca++]i. Figure4 shows results with 100 nmol/L amounts. Figure 5 shows a dose-dependent response with lower amounts of ligands typical for 3 receptors. To clarify which receptors respond and the ligand specificity, cross-desensitization was carried out between these chemokines. The known CCR4 ligands showed cross-desensitization; however, SDF-1, which is a CXCR4-specific ligand, also showed cross-desensitization with MCP-1, MIP-1α, RANTES, TARC, and MDC. All of these chemokines also showed a desensitized response to a second dose of the same chemokine.
Platelet cytoplasmic Ca++ response to various chemokines measured using fura-2 fluorescence.
Washed platelets (108/mL) were loaded with fura-2 and then activated with 100 nmol/L chemokine. When the signal had returned to baseline or stabilized (generally at 2 minutes) either a second dose of the same chemokine or of another one was added. Partial mutual desensitization of the Ca++ signal response by chemokines using different receptors was observed but was almost complete when the same chemokine was used twice or when chemokines with the same receptor were used. The results shown are typical of at least 3 similar experiments.
Platelet cytoplasmic Ca++ response to various chemokines measured using fura-2 fluorescence.
Washed platelets (108/mL) were loaded with fura-2 and then activated with 100 nmol/L chemokine. When the signal had returned to baseline or stabilized (generally at 2 minutes) either a second dose of the same chemokine or of another one was added. Partial mutual desensitization of the Ca++ signal response by chemokines using different receptors was observed but was almost complete when the same chemokine was used twice or when chemokines with the same receptor were used. The results shown are typical of at least 3 similar experiments.
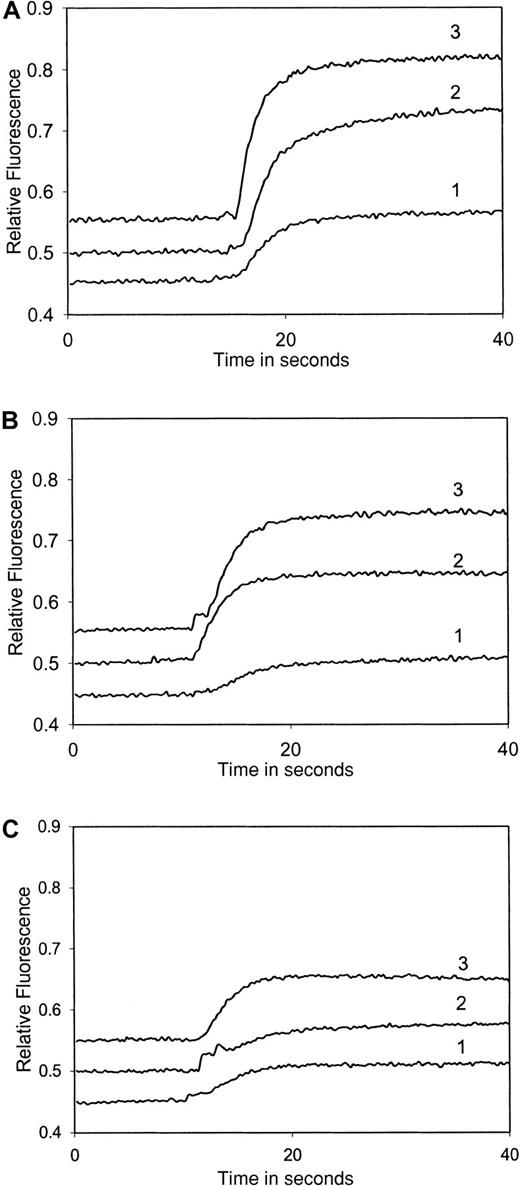
Titration of platelet cytoplasmic Ca++response to increasing amounts of chemokines measured using fura-2 fluorescence.
Washed platelets (108/mL) loaded with fura-2 were activated with various concentrations of chemokines. (A) TARC: plot 1, 10 nmol/L; plot 2, 20 nmol/L; and plot 3, 50 nmol/L. (B) Eotaxin: plot 1, 10 nmol/L; plot 2, 20 nmol/L; and plot 3, 50 nmol/L. (C) MIP-1α: plot 1, 10 nmol/L; plot 2, 20 nmol/L; and plot 3, 30 nmol/L. The results shown are typical of at least 3 similar experiments.
Titration of platelet cytoplasmic Ca++response to increasing amounts of chemokines measured using fura-2 fluorescence.
Washed platelets (108/mL) loaded with fura-2 were activated with various concentrations of chemokines. (A) TARC: plot 1, 10 nmol/L; plot 2, 20 nmol/L; and plot 3, 50 nmol/L. (B) Eotaxin: plot 1, 10 nmol/L; plot 2, 20 nmol/L; and plot 3, 50 nmol/L. (C) MIP-1α: plot 1, 10 nmol/L; plot 2, 20 nmol/L; and plot 3, 30 nmol/L. The results shown are typical of at least 3 similar experiments.
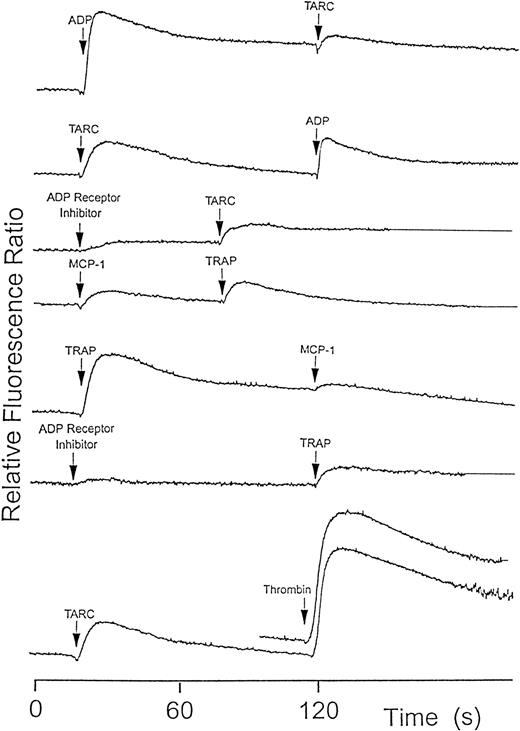
ADP and ADP receptor inhibitor induced effects on chemokine signals
Unlike most cells on which chemokines have been tested, platelets show rapid positive feedback responses to many agonists, via release of storage granule contents or by thromboxane generation. ADP is a major component of dense granules and also causes a rapid rise in [Ca++]i when used as agonist. To check if ADP release in response to chemokines might be responsible for some desensitization, we tested both ADP as a desensitization agent as well as the ADP receptor inhibitor (AR-C66096). ADP as first agonist gave a signal and partially desensitized the response to the chemokines. ADP receptor inhibitor or, alternatively, apyrase to destroy released ADP both reduced, but did not eliminate, the Ca++ signal in response to the chemokines (Figure 6).
Platelet cytoplasmic Ca++ response to various agonists measured using fura-2 fluorescence.
Several agonists such as ADP (10 μmol/L), TRAP (1 μmol/L), and thrombin (0.05 U/mL) caused a partial reduction in a second chemokine-induced signal or vice-versa. The ADP receptor inhibitor, AR-C66096 (100 nmol/L), also caused a partial but not complete inhibition of Ca++ signaling by chemokines and the other agonists. The signals were lower but not cross-desensitized in the presence of apyrase (5 U/mL, not shown). The results shown are typical of at least 3 similar experiments.
Platelet cytoplasmic Ca++ response to various agonists measured using fura-2 fluorescence.
Several agonists such as ADP (10 μmol/L), TRAP (1 μmol/L), and thrombin (0.05 U/mL) caused a partial reduction in a second chemokine-induced signal or vice-versa. The ADP receptor inhibitor, AR-C66096 (100 nmol/L), also caused a partial but not complete inhibition of Ca++ signaling by chemokines and the other agonists. The signals were lower but not cross-desensitized in the presence of apyrase (5 U/mL, not shown). The results shown are typical of at least 3 similar experiments.
Thrombin receptor peptide causes desensitization of chemokine responses
Platelets gave a strong Ca++ elevation in response to SFLLRN (TRAP) but a following challenge by MCP-1, MIP-1α, RANTES, TARC, MDC, and SDF-1 was partially desensitized (Figure 6). In reverse order, following a challenge by these chemokines, the [Ca++]i signal induced by TRAP was also reduced compared to when it was given as first agonist. Similarly, a first challenge by a chemokine reduced somewhat the Ca++response to thrombin (Figure 6).
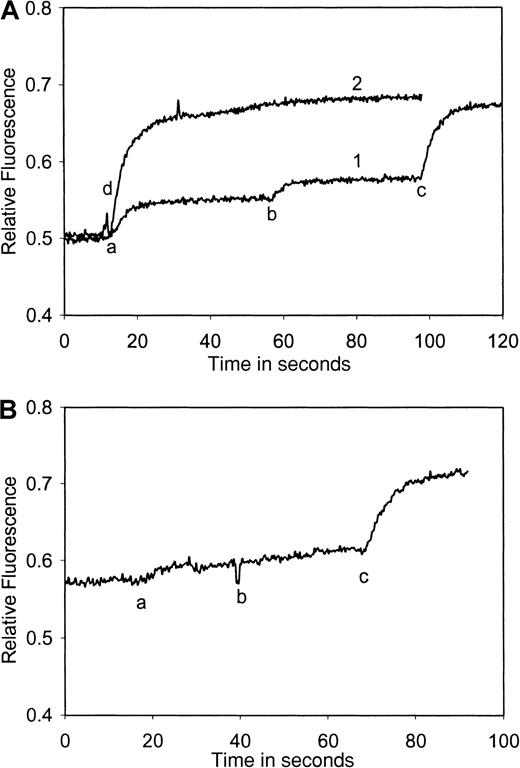
Chemokines signal synergistically via Ca++ and signals are inhibited by specific receptor antibodies
When platelets were treated with a mixture of low amounts of 2 chemokines acting on different receptors, the calcium signal obtained was much higher than when they were treated with the same amounts of each chemokine subsequently (eg, eotaxin and TARC, Figure7A) or with double the amount of each chemokine (compare with Figure 5). The platelets nevertheless still responded well to thrombin. A synergistic aggregation response to mixed chemokines was also detected (see below).
Platelet cytoplasmic Ca++ response to eotaxin and TARC alone and in the presence of a blocking antibody directed against CCR3, measured using fura-2 fluorescence.
(A) Plot 1: washed platelets (108/mL) loaded with fura-2 were activated with eotaxin (10 nmol/L) (a), followed by TARC (10 nmol/L) (b) and thrombin (0.1U/mL) (c). Plot 2: eotaxin (10 nmol/L) (d) and TARC (10 nmol/L) were added together. The Ca++ signal is equivalent to that of 0.1 U/mL thrombin in plot 1. (B) Washed platelets (108/mL) loaded with fura-2 were incubated with Fab fragments of anti-CD32 antibodies and then treated with anti-CCR3 antibodies (a) that cause the inhibition of Ca++ signaling by eotaxin (b) but not TARC (c). The results shown are typical of at least 3 similar experiments.
Platelet cytoplasmic Ca++ response to eotaxin and TARC alone and in the presence of a blocking antibody directed against CCR3, measured using fura-2 fluorescence.
(A) Plot 1: washed platelets (108/mL) loaded with fura-2 were activated with eotaxin (10 nmol/L) (a), followed by TARC (10 nmol/L) (b) and thrombin (0.1U/mL) (c). Plot 2: eotaxin (10 nmol/L) (d) and TARC (10 nmol/L) were added together. The Ca++ signal is equivalent to that of 0.1 U/mL thrombin in plot 1. (B) Washed platelets (108/mL) loaded with fura-2 were incubated with Fab fragments of anti-CD32 antibodies and then treated with anti-CCR3 antibodies (a) that cause the inhibition of Ca++ signaling by eotaxin (b) but not TARC (c). The results shown are typical of at least 3 similar experiments.
To demonstrate that signals were specific to a given ligand/receptor a blocking antibody against CCR3 (MAB155) was used (Figure 7B). This antibody caused a calcium signal by itself, which could be eliminated by pretreating the platelets with Fab fragments of the anti-FcγRII antibody, IV.3.
Chemokines induce platelet aggregation
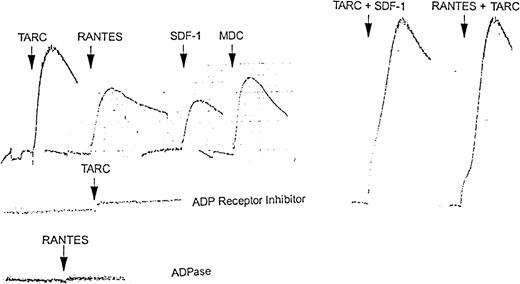
Platelet suspensions (500 μL, 5 × 108platelets/mL) were treated with chemokines in an aggregometer. TARC, RANTES, and MDC gave rapid aggregation responses that were slowly and partly reversible. SDF-1 gave a lower but still clear response (Figure8), as did MCP-1 and MIP-1α (data not shown). Following an aggregation response to TARC or SDF-1, platelets were still capable of responding to RANTES (data not shown). Combinations of TARC and SDF-1 or RANTES and TARC gave an additive aggregation response when given simultaneously (Figure 8) but not when added successively (not shown).
Aggregation response of platelets to chemokines alone and in presence of ADP receptor inhibitor AR-C66096 or ADPase.
In the upper left series, washed platelets (5 × 108/mL) were treated with 100 nmol/L TARC, RANTES, SDF-1, or MDC, respectively. In the upper right series, washed platelets (5 × 108/mL) were treated with a mixture of 100 nmol/L each TARC plus SDF-1 or RANTES plus TARC, respectively. No signal addition was seen when the same doses were given successively (Figure4). In the lower 2 aggregation profiles, washed platelets were treated with 100 nmol/L TARC in the presence of 100 nmol/L ADP receptor inhibitor (AR-C66096) or (bottom profile) with 100 nmol/L RANTES in the presence of 5 μmol/L apyrase. The results shown here are representative of at least 3 similar experiments.
Aggregation response of platelets to chemokines alone and in presence of ADP receptor inhibitor AR-C66096 or ADPase.
In the upper left series, washed platelets (5 × 108/mL) were treated with 100 nmol/L TARC, RANTES, SDF-1, or MDC, respectively. In the upper right series, washed platelets (5 × 108/mL) were treated with a mixture of 100 nmol/L each TARC plus SDF-1 or RANTES plus TARC, respectively. No signal addition was seen when the same doses were given successively (Figure4). In the lower 2 aggregation profiles, washed platelets were treated with 100 nmol/L TARC in the presence of 100 nmol/L ADP receptor inhibitor (AR-C66096) or (bottom profile) with 100 nmol/L RANTES in the presence of 5 μmol/L apyrase. The results shown here are representative of at least 3 similar experiments.
Chemokine-induced platelet aggregation is dependent on ADP release and ADP receptor engagement
Platelet suspensions (500 μL, 5 × 108platelets/mL) were pretreated with either AR-C66096 ADP receptor inhibitor or apyrase in an aggregometer. After 2 minutes, RANTES, TARC, MDC, or SDF-1 was added. Aggregation was inhibited in a dose-dependent way with complete inhibition at above 100 nmol/L ADP receptor inhibitor or 2 U/mL apyrase. Figure 8 shows the results obtained with TARC and with RANTES in the presence of apyrase.
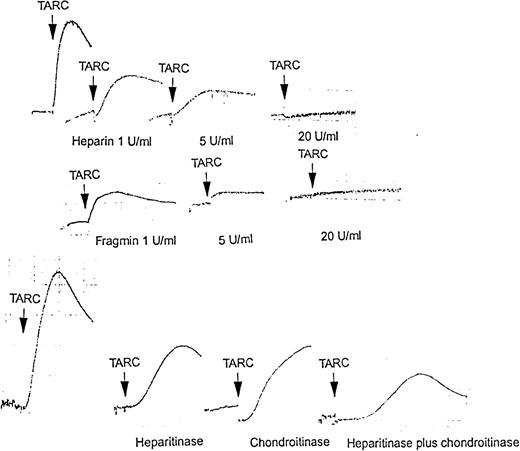
Heparin and low-molecular-weight heparin inhibit the platelet response to chemokines in a dose-dependent way
Addition of 1 U/mL heparin to a suspension of washed platelets reduced the aggregation response to TARC by 50% (Figure9). Amounts greater than 7 U/mL essentially prevented platelet aggregation in response to TARC. Similar results were obtained with the other chemokines. Use of low-molecular-weight heparin (Fragmin) in place of unfractionated heparin gave similar results (Figure 9).
Aggregation response of platelets to chemokines alone and in combination as well as in the presence of heparin, low-molecular-weight heparin (Fragmin), heparinase, or chondroitinases.
Upper series of aggregation profiles shows washed platelets activated with 100 nmol/L TARC, from left, control, in the presence of 1 U/mL, 5 U/mL, and 20 U/mL heparin, respectively. Middle series shows washed platelets activated with 100 nmol/L TARC, from left, in the presence of 1 U/mL, 5 U/mL, and 20 U/mL Fragmin, respectively. Lower series shows washed platelets activated with 100 nmol/L TARC, from left, control, after incubation for 30 minutes at 37°C with 5 U/mL heparinases I and III, 5 U/mL chondoitinases ABC, and 5 U/mL heparitinase I and III plus 5 U/mL chondoitinase ABC, respectively. These results are typical of at least 3 similar experiments.
Aggregation response of platelets to chemokines alone and in combination as well as in the presence of heparin, low-molecular-weight heparin (Fragmin), heparinase, or chondroitinases.
Upper series of aggregation profiles shows washed platelets activated with 100 nmol/L TARC, from left, control, in the presence of 1 U/mL, 5 U/mL, and 20 U/mL heparin, respectively. Middle series shows washed platelets activated with 100 nmol/L TARC, from left, in the presence of 1 U/mL, 5 U/mL, and 20 U/mL Fragmin, respectively. Lower series shows washed platelets activated with 100 nmol/L TARC, from left, control, after incubation for 30 minutes at 37°C with 5 U/mL heparinases I and III, 5 U/mL chondoitinases ABC, and 5 U/mL heparitinase I and III plus 5 U/mL chondoitinase ABC, respectively. These results are typical of at least 3 similar experiments.
Heparinases and chondroitinases reduce the platelet aggregation response to chemokines
A suspension of washed platelets, which was incubated at 37°C for 30 minutes with heparinases I and III (5 U/mL) or chondroitinases ABC (5 U/mL), showed a reduced aggregation response to TARC (Figure 9), SDF-1, and RANTES (not shown) chemokines. Incubation with a mixture of heparinases and chondroitinases (each 5 U/mL) was more effective than either alone but still did not completely prevent platelet aggregation.
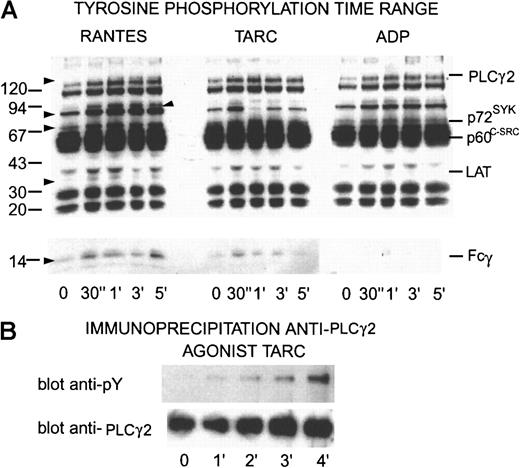
Chemokines induce tyrosine kinase signaling in platelets
Platelets (700 μL, 5 × 108/mL) were treated with chemokines as for aggregation; aliquots were taken at 0, 30, 60, 90, 120, and 300 seconds and dissolved in 1% SDS, 1 mmol/L N-ethylmaleimide, 1 mmol/L EDTA, and 2 mmol/L sodium orthovanadate. After separation by SDS-PAGE (7%-20% acrylamide gradient) and transfer to PVDF membranes, the proteins were incubated with the antiphosphotyrosine antibody 4G10 before detection by a peroxidase-linked second antibody and chemiluminescence (Figure10). Clear dose-related responses to chemokines were detected, which showed some distinct differences from signals induced by ADP. The results shown are those obtained with different doses of 2 different agonists, in one case 100 nmol/L RANTES and, in the other, 40 nmol/L TARC. However, similar results were also obtained with different doses of the same agonist. The lower dose of agonist caused a rapid, transient tyrosine phosphorylation of several typical components, including p72SYK and Fcγ. The higher dose also caused a rapid increase in tyrosine phosphorylation of these components but they remained phosphorylated and were only slowly dephosphorylated. Comparison with the pattern of tyrosine phosphorylation obtained under the same conditions using ADP or thrombin as agonists showed some similarities but also differences.
Time dependence of tyrosine phosphorylation in proteins from platelets activated by RANTES, TARC, or ADP and immunoprecipitation of PLCγ2.
(A) Washed platelets (5 × 108) were activated with 100 nmol/L RANTES, TARC, or 10 μmol/L ADP, respectively. Aliquots were removed at times indicated and dissolved in SDS containing inhibitors. After separation by SDS-PAGE and transfer to PDVF membranes the proteins were incubated with the antiphosphotyrosine antibody 4G10 before detection by chemiluminescence. Proteins with increased tyrosine phosphorylation are indicated by arrows on the left and identified phosphoproteins are indicated on the right. The results shown here are typical of at least 3 experiments performed with these doses and chemokines but analogous experiments with other chemokines interacting with platelets also gave similar results. (B) Aliquots of 108 platelets from points on a time range activated with TARC (50 nmol/L) solubilized in RIPA buffer were immunoprecipitated with antibodies against PLCγ2. After SDS-PAGE and Western blotting, the proteins were detected with 4G10 antiphosphotyrosine antibody. The membranes were stripped and treated with anti-PLCγ2antibodies.
Time dependence of tyrosine phosphorylation in proteins from platelets activated by RANTES, TARC, or ADP and immunoprecipitation of PLCγ2.
(A) Washed platelets (5 × 108) were activated with 100 nmol/L RANTES, TARC, or 10 μmol/L ADP, respectively. Aliquots were removed at times indicated and dissolved in SDS containing inhibitors. After separation by SDS-PAGE and transfer to PDVF membranes the proteins were incubated with the antiphosphotyrosine antibody 4G10 before detection by chemiluminescence. Proteins with increased tyrosine phosphorylation are indicated by arrows on the left and identified phosphoproteins are indicated on the right. The results shown here are typical of at least 3 experiments performed with these doses and chemokines but analogous experiments with other chemokines interacting with platelets also gave similar results. (B) Aliquots of 108 platelets from points on a time range activated with TARC (50 nmol/L) solubilized in RIPA buffer were immunoprecipitated with antibodies against PLCγ2. After SDS-PAGE and Western blotting, the proteins were detected with 4G10 antiphosphotyrosine antibody. The membranes were stripped and treated with anti-PLCγ2antibodies.
Chemokines induce tyrosine phosphorylation of Fcγ as well as p72SYK and phospholipase Cγ2 via platelet receptors
As shown in Figure 10, both TARC and RANTES induced tyrosine phosphorylation of p72SYK but also of Fcγ although to a lesser degree. Although the ADP-activated platelets showed a similar dose-dependent increase in phosphorylation of p72SYK, the Fcγ was not detectably phosphorylated. In addition, a band at 36 to 38 kd corresponding to Linker for Activation ofT cells (LAT) was also phosphorylated in a dose-dependent way that was not detectable in the ADP-activated platelets. Immunoprecipitation showed a time-dependent increase in tyrosine phosphorylation of PLCγ2 (Figure 10B), a major downstream enzyme target of these pathways responsible for calcium release and protein kinase C (PKC) activation. Thus, chemokine-activated platelets showed an increase in tyrosine phosphorylation of some components independent of the effects of ADP alone.
Discussion
Chemokine family proteins were first detected stored in platelet α-granules and released on activation. Detection of mRNA for several additional chemokines and chemokine receptors in platelets together with direct evidence for release of some of these chemokines from storage granules on platelet activation suggested that chemokines may have a more direct role in platelet function. Various chemokines that are ligands for different receptors were tested for effects on Ca++ levels in platelets. These were IL-8, MCP-3, MCP-1, MIP-1α, eotaxin, RANTES, TARC, MDC, and SDF-1. Of those tested, ligands for the CCR1, CCR3, CCR4, and CXCR4 receptors gave clear signals, although IL-8 also gave a barely measurable signal. Signals obtained with RANTES were somewhat weaker than those with the CCR4 ligands, TARC, and MDC. PCR studies with RNA or cDNA libraries derived from platelets have shown that mRNA for both these receptors is present in platelets15,18 and reports have indicated that CXCR4 can be readily detected on platelets by flow cytometry.18 The results presented here provide clear evidence for the presence of functional CCR1, CCR3, CCR4, and CXCR4 receptors based on the physical presence of these receptors as proteins as well as an appropriate cellular response to chemokines specific for the receptors present. Thus, responses to MIP1-α and RANTES but not to MCP-3 in the absence of CCR2 and CCR5 implicate CCR1. Responses to eotaxin and RANTES implicate CCR3, responses to TARC and MDC implicate CCR4, and responses to SDF-1 implicate CXCR4.
Desensitization of the signal from a second stimulus was also observed, whether the same chemokine was used twice or followed by another, which was active as a first stimulus. Normally, this is a classic method for testing for G-protein–linked receptor specificity because only ligands that interact with the same receptor mutually desensitize the response. It was therefore surprising to observe desensitization occurring between chemokines such as TARC, specific for CCR4, and SDF-1, specific for CXCR4. However, platelets are different from many other cells where chemokine receptors have been studied because of the many rapid feedback mechanisms that operate. Further experiments showed that, following platelet activation by ADP or TRAP, derived from the protease activated receptor 1 (PAR-1) thrombin receptor, the platelet Ca++ response to chemokines was also partially down-regulated. Chemokines as first stimulus also influenced the platelet response to ADP as well as that to TRAP (which causes ADP release). This suggested that the desensitization effect could be due to ADP release from platelet-dense granules caused by the first agonist feeding back to ADP receptors and desensitizing these to ADP released by the second agonist. To test this hypothesis we used either the ADP receptor inhibitor, AR-C66096, to prevent feedback to the ADP receptor involved in activating the fibrinogen receptor αIIbβ3, or alternatively, apyrase, to destroy released ADP and prevent activation of the ADP receptor. In both cases the strength of the signals caused by chemokines as first agonist was reduced, but that of the second agonist was also affected. Apyrase had a stronger effect than the ADP receptor inhibitor, which is thought to inhibit only the P2T receptor and which affected the latter part of the signal only. Cross-desensitization experiments were therefore not made easier. Our interpretation is that part of the Ca++ signal, probably the slower part, is due to ADP release feeding back to its receptors. ADP or TRAP also desensitized platelets to activation by chemokines, presumably again because of desensitization of the ADP receptor, in the first case directly and in the second case due to ADP released by the activated platelets. Previous difficulties in detecting platelet responses to chemokines18 could possibly have been due to exposure to ADP during the washing procedure.
When small amounts of 2 chemokines were mixed before adding to platelets they gave a much stronger effect, whether Ca++signal or aggregation response, than when the same reagents were added sequentially (Figures 7 and 10). This suggests a synergistic effect due to simultaneous activation of Gαq- and Gαi-linked receptors where down-regulation of ADP receptors was not possible.
In addition to a Ca++ signal chemokines also induce platelet aggregation but weakly compared to normal amounts of classic reagents such as thrombin and collagen, although within the range found with ADP. TARC and RANTES gave the strongest signals among the CCR ligands and SDF-1 gave a slightly weaker signal as a CXCR4 agonist. Prevention of ADP receptor activation by either the ADP receptor inhibitor or by apyrase resulted in a complete inhibition of platelet aggregation demonstrating that the aggregation response is completely dependent on feedback of released ADP to the ADP receptor(s). Thus, activation of platelets by chemokines causes release of ADP from dense granules and ADP feedback is a prerequisite for aggregation in common with plasma from patients with heparin-induced thrombocytopenia22 and collagen as agonists, which suggests common mechanisms for all these agonists. A possible explanation may lie in studies that showed that simultaneous activation via Gαi- and Gαq-coupled receptors was necessary for induction of platelet activation.23,24 Indeed, this is also thought to be true for ADP alone acting via several receptors.25 26 Thus, the signal from a single class of chemokine receptor needs to be supplemented by that from ADP acting via the complementary G-protein–coupled receptor to give an aggregation response.
Both TARC and RANTES induce tyrosine phosphorylation of signaling proteins, including p72SYK, Fcγ, LAT, and PLCγ2. All of these showed clear differences compared to an ADP-activated control (Figure 10). Both LAT27 and PLCγ228 have been shown to be phosphorylated by, and to lie downstream of, p72SYK. Recently, MIP-1β stimulation of CCR5 was shown to activate p72SYK by a related adhesion focal tyrosine kinase (RAFTK) pathway.29 Signaling pathways involving p72SYK may therefore be common to some β-chemokine receptors. In platelets p72SYK is important for glycoprotein (GP) IIb-IIIa activation as well as in outside-in signaling via GPIIb-IIIa after aggregation.30
It was shown earlier that cell surface proteoglycans play a role in the activation of endothelial cells by chemokines, either by enhancing binding to the cell surface or by multiplying interactions with receptors or both.31 Similarly, the interactions between SDF-1 and cell-bound glycosaminoglycans were suggested to enhance SDF-1 interactions with CXCR4 on epithelial cells, possibly by presenting the chemokine to its receptor in a more concentrated form.32It has also been shown previously that platelet surface proteoglycans are involved in interactions between certain platelet agonists and the platelet surface. These include human group II PLA2, which uses a glycophosphatidylinositol-anchored heparan sulfate proteoglycan to interact with the platelet surface,33 as well as the PF4-heparin antibody complex involved in heparin-induced thrombocytopenia, which requires surface proteoglycans for effective platelet activation and also acts via released ADP.22Because of these observations various heparinases and chondroitinases were tested to see if they affected platelet activation by chemokines. All of those tested reduced platelet aggregation by typical chemokines by 20% to 30%. A mixture of glycosaminoglycanases reduced platelet aggregation response by 60% to 70%, as had been noted earlier for endothelial cells, suggesting that the glycosaminoglycans involved belong to different classes that are not all cleaved off when single-specificity enzymes were used. This interpretation was strengthened by the effect of heparin and low-molecular-weight heparin (Fragmin) on platelet activation by chemokines. In both cases a dose-dependent inhibition was observed, and at the higher concentrations platelet aggregation was completely inhibited.
It has been suggested that those chemokine receptors present on platelets are simply carried over from megakaryocytes and have no direct function in platelet physiology. CXCR4 receptors expressed on mature polyploid megakaryocytes responded to SDF-1 and caused chemotaxis, proplatelet formation, and transmigration through bone marrow endothelial cells.34 The proplatelets and platelet-like particles that were formed expressed CXCR4. The results described here show clearly that platelets express functional CCR4 and CXCR4 receptors that are capable of signaling, inducing release of storage granules, and causing aggregation, albeit at a low level. The demonstration of the presence of functional CCR4 suggests that chemokines that are ligands for this receptor might also function in megakaryocyte maturation and platelet formation. Platelets themselves contain some of the chemokines that are ligands for CCR1 and CCR3, such as RANTES, so that one role may be in feeding back to receptors on the same or other platelets to amplify response in an aggregatory situation.
Recently, vascular endothelial cells35 as well as smooth muscle cells36 have been shown to express several chemokine receptors. In addition several cells associated with atherosclerotic plaque were found to express RANTES, which was absent in normal vascular tissue. Raised levels of RANTES as well as other CC chemokines were found in patients with congestive heart failure,37 and a role for both platelets and monocytes was suggested.38Recent studies found elevated levels of RANTES in plasma from patients with HIV-1 infections. This was demonstrated to come predominantly from activation of platelets because platelet activation inhibitors reduced plasma levels considerably.19,20 In patients on antiretroviral therapy higher maintained levels of RANTES were associated with nonprogressors, suggesting that RANTES had a beneficial effect.39 Patients with asymptomatic HIV infections have thrombocytopenia in about 10% of cases, which has not yet been satisfactorily explained, although several hypotheses have been proposed.40 Shortened platelet lifetime is certainly one reason. Activation of platelets by HIV envelope glycoprotein 120 (gp120) or by herpesvirus proteins41 as well as feedback through RANTES release from their granules or from neighboring platelets could be contributory factors. Following activation, platelets aggregate via plasma proteins such as fibrinogen and the aggregates are removed by the spleen leading to thrombocytopenia. Thrombotic microangiopathy is also increased in patients with acquired immunodeficiency syndrome and HIV infections.42 Platelets were recently shown to be able to take up and transport HIV, and the platelets containing virus were activated.43
Several studies have been made of signal transduction via chemokine receptors.44 HIV gp120 induced signal transduction in cells expressing CXCR4 or CCR5 in addition to CD4.45 An important question is what activates the platelets in patients infected with HIV-1. Because platelets do not express CD4 but do express CXCR4 and CCR4, which are or can be coreceptors, a possibility exists that another molecule can replace CD4. Recently, it was shown that gp120 from HIV can activate human neuronal tera (hNT) neuronal cell lines directly via CXCR4 without a requirement for CD446 so that a direct mechanism may also exist for platelets. Taken together, these results suggest that RANTES release from platelets could be a defense mechanism in HIV infections by blocking or down-regulating CCR3 or CCR5 receptors on immune cells with RANTES and that chemokine receptors on platelets may be involved in feedback mechanisms.
Because of the inhibitory effects of both ADP receptor inhibitors as well as heparin and low-molecular-weight heparin on the platelet response, it should be borne in mind that antithrombotic treatment of patients with these classes of inhibitors could affect the development of the disease in HIV-infected patients and that other types of treatment such as GPIIb-IIIa or thrombin inhibitors should also be considered. It was recently shown that soluble complexes of RANTES and glycosaminoglycans could suppress HIV-1 infection of monocytes but did not induce calcium signaling,47 suggesting that, as in platelets, binding to glycosaminoglycan-carrying macromolecules on the cell surface may supply a critical component of the signal response.
It was recently shown that the platelet CXC chemokine PF4 promotes monocyte survival and induces monocyte differentiation into macrophages.48 Activated platelets cause monocytes to release chemokines49 so that mutual activation by specific chemokines could be an important mechanism for interaction between these cells under a variety of physiologically relevant conditions. After this manuscript was submitted Abi-Younes and coworkers50 demonstrated that SDF-1 induces platelet aggregation and calcium signaling and that human atheroma is rich in SDF-1. It is likely that atheroma is also enriched in other platelet-active chemokines, supporting a role for platelet-chemokine interactions in atherosclerosis.
Release of RANTES from platelets with a role in accumulation of eosinophils has been implicated in asthma.51 Here again platelets may have a role in amplifying this response. Platelets do not contain either CCR4 (MDC, TARC) or CXCR4 (SDF-1) agonists. Therefore, the role of these receptors may be to involve platelets in situations where these agonists are provided by other cells. In general, the part that platelets play in many inflammatory and allergic responses has not yet been carefully examined. The availability of clinically approved, efficient inhibitors of platelet aggregation as well as those that affect platelet release should allow the role of platelet activation to be tested in a variety of diseases other than those directly related to thrombosis.
Acknowledgment
We thank the Central Laboratory of the Swiss Red Cross Blood Transfusion Service for the supply of buffy coats.
Work at the Theodor Kocher Institute was supported by Swiss National Science Foundation grant 31-52396.97 to K.J.C. and 31-055996.98 to M.B.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Kenneth J. Clemetson, Theodor Kocher Institute, University of Berne, Freiestrasse 1 CH-3012, Berne, Switzerland; e-mail: clemetson@tki.unibe.ch.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal