Abstract
Both toxic exposure to cadmium and cancer therapy with cisplatin (CDDP) can induce anemia in patients owing to the insufficient production of erythropoietin (EPO). Therefore, the effects of cadmium chloride (Cd) and CDDP in the Hep3B human hepatoma cell line, which up-regulates EPO expression in response to hypoxia and cobalt (Co), were investigated. The induction of binding activity of the HIF-1 transcription factor and EPO mRNA expression and protein production were suppressed by Cd and CDDP in a dose-dependent manner with no apparent cell damage. Mercuric chloride also suppressed hypoxia- and Co-induced EPO production, mRNA expression, and HIF-1 binding in a manner similar to Cd and CDDP, whereas zinc chloride suppressed Co-induced EPO production, mRNA expression, and HIF-1 binding but did not affect hypoxia induction or that observed after simultaneous exposure to hypoxia and Co. In contrast, lead and tin salts had no effect on HIF-1 activation or EPO expression. These results indicate that Cd and CDDP have a strong and specific inhibitory effect on hypoxia- and Co-induced signaling and EPO induction in hepatic cells. It is likely that these agents cause anemia by directly impacting EPO production in the kidney.
Introduction
The red blood cell mass is normally regulated by the erythroid-specific cytokine erythropoietin (EPO).1This glycoprotein hormone, which is produced primarily in the kidneys and the liver, is markedly up-regulated by anemia and by other types of hypoxia. Inadequate renal production of EPO is the primary cause of the often severe anemia encountered in patients with chronic renal failure, irrespective of etiology.
Cadmium (Cd), a metal used in a number of industrial applications, is one of the hazardous pollutants that causes renal tubular dysfunction after long-term exposure.2 The most severe and representative manifestation of chronic Cd intoxication is called Itai-itai disease, which was endemic among the inhabitants of a polluted area in Toyama prefecture, Japan.3 Itai-itai disease is a syndrome that includes renal tubular dysfunction, osteomalacia, generalized pain due to multiple bone fractures, and anemia. The Japanese word itai means “ouch.” A recent clinical report4 demonstrates that the anemia observed in patients with Itai-itai disease is caused primarily by the impaired production of EPO. In addition, long-term exposure of rats to Cd resulted in the marked suppression of EPO mRNA expression in the kidneys and the development of severe anemia.5
Cisplatin [cis-diaminedichloroplatinum (II); CDDP], a metal-based anticancer drug widely used for the treatment of various kinds of malignant tumors, is also known to induce renal tubular dysfunction.6 Anemia, which is often observed during CDDP therapy, appears to be due primarily to EPO deficiency resulting from renal tubular damage.7-9
These clinical observations suggest that metals can selectively suppress renal production of EPO. To better understand this phenomenon, we used the Hep3B human hepatoma cell line, which produces low levels of EPO constitutively with markedly increased amounts after exposure to hypoxia or cobalt chloride (Co).10 We investigated the effect of Cd, CDDP, and other metals on hypoxia- and Co-induced EPO protein production and EPO mRNA expression in Hep3B cells. We also tested the effects of 4 additional metals (mercury, zinc, tin, and lead) on HIF-1 activation and EPO production in Hep3B cells. Mercury induces renal tubular damage similar to Cd in animals and humans,11 though no association with EPO deficiency has been reported. We tested the effect of zinc because this metal has an inhibitory effect on EPO induction in HepG2 cells.12
Materials and methods
Cell culture Hep3B cells were obtained from ATCC (Rockville, MD) and from the Cell Resource Center for Biomedical Research, Institute of Development, Aging, and Cancer, Tohoku University, Sendai, Japan). The Hep3B cells were maintained in α-modified Eagle medium (Gibco BRL, Grand Island, NY) supplemented with 10% fetal bovine serum (Gibco BRL) at 37°C. The final concentration of iron used in our experiments was approximately 18 μg/dL because the medium lacks iron and fetal bovine serum contains approximately 180 μg/dL iron. In all the experiments, cells were resuspended in the medium at a concentration of 2.5 × 105/mL and were preincubated for 24 hours. Aliquots of cells were then exposed to hypoxia or 100 μM CoCl2 with or without increasing concentrations of divalent metal ions, such as cadmium (CdCl2), mercury (HgCl2), zinc (ZnCl2), tin (SnCl2), lead (Pb[acetate]2), and platinum (cisplatin or CDDP), or antioxidants such as 1,1,3,3-tetramethyl-2-thiourea (TMTU) and vitamin E succinate. Cells were usually cultured under normoxic conditions (95% air and 5% CO2), but, when exposed to hypoxia, cells were incubated in an Espec (Grand Rapids, MI) or BIO-LABO (Juji Field, Tokyo, Japan) multi-gas incubator in an atmosphere of 1% O2, 94% N2, and 5% CO2.
Radioimmunoassay for EPO
After exposing the Hep3B cells to hypoxia or Co with or without metals for 24 hours, the culture media were removed and stored at −80°C before analysis. EPO concentrations in the medium were determined by radioimmunoassay using a commercially available kit.13
Cell viability assays
We used 2 kinds of assays to evaluate cell viability, measurement of LDH (lactate dehydrogenase) released from dead cells into medium and an MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide) assay that reflects the activity of mitochondrial dehydrogenase of living cells.14
Ribonuclease protection assay
We used the construct pGRm, which contains a 203-bp fragment of the human EPO gene, including a 151-bp portion of exon 5 and 52 bp of its upstream flanking intron. A fragment comprising nucleotides 2565-2767 (GenBank accession number M11319), was inserted into pGEM3Zf(+).) The EPO gene in this construct is “marked” by site-directed mutagenesis at 3 positions in the coding region.15 As a result, after hybridization of this marked riboprobe to endogenous EPO mRNA, RNase digestion yields 2 major fragments of 75 and 61 bp. After exposing the Hep3B cells to hypoxia or Co with or without the addition of metals for 6 hours, total RNA was purified from the cells by using TRIzol reagent (Gibco BRL). Concentration of total RNA was calculated from absorbances at 260 and 280 nm. The quality of total RNA was assessed by visualization on a UV transilluminator after running small aliquots on a 1% agarose gel. Forty micrograms of each sample of total RNA and an excess of EPO riboprobe, labeled by α32P-CTP (New England Nuclear, Boston MA), were mixed and hybridized at 55°C overnight. After completing hybridization, the single-stranded RNAs were digested by RNase A and RNase T1 and electrophoresed on 10% polyacrylamide gel. The dried gel was analyzed with a PhosphorImager (Molecular Dynamics) or BAS 2000 (Fuji).
Electrophoretic mobility shift assay
After exposing Hep3B cells to hypoxia or Co with or without additional metals for 6 hours, the cells were lysed in 20 mmol/L HEPES, 420 mmol/L NaCl, 1.5 mmol/L MgCl2, 0.5 mmol/L dithiothreitol, and 25% glycerol.16 Concentrations of protein in the whole cell lysates were determined by using Bio-Rad Protein Assay. For the DNA binding reaction, 30μg lysate protein was mixed with double-strand oligonucleotide from the EPO 3′ enhancer (W18),17 labeled with γ32P-adenosine triphosphate (NEN). The samples were electrophoresed on 5% polyacrylamide gel, and the dried gel was analyzed with a PhosphorImager (Molecular Dynamics) or BAS 2000 (Fuji).
Results
After the exposure of EPO-producing cells to either low-oxygen tension or Co, there was rapid activation of the hypoxia-inducible transcription factor HIF-1, which bound to a response element in a critical enhancer, located downstream of the 3′ exon, thereby up-regulating the transcription of EPO mRNA. In all experiments, we assayed these early responses (HIF-1 activation and EPO mRNA expression) 6 hours after induction by hypoxia or cobalt. Because additional time is required for translation, secretion, and accumulation in the medium, EPO protein levels were measured 24 hours after induction.
Cadmium
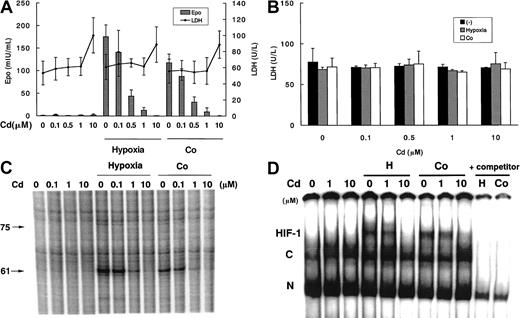
We first investigated the effect of Cd on EPO protein production in Hep3B cells that were exposed to hypoxia or Co for 24 hours (Figure1A). Although Cd itself did not affect EPO production at 21% O2, it showed a dose-dependent (0.1 → 1 μM) inhibition of hypoxia- and Co-induced EPO protein production, which was almost completely suppressed at 1 μM Cd. Cd is one of the heavy metals that in high doses can cause acute cell necrosis.18 Therefore, we checked the viability of Hep3B cells treated with Cd by measuring LDH activity released into medium to ascertain whether global Cd toxicity affected EPO production. Exposure of Hep3B cells to 0.1 to 1 μM Cd for 24 hours had no effect on cell viability. After 6 hours of exposure, Cd did not have any toxic effects on Hep3B cells, even at 10 μM (Figure 1B), suggesting that there is a time lag between EPO suppression and cell toxicity. Using an MTT assay to confirm the evaluation of cell viability, we did not detect any significant difference between the 2 methods (data not shown). These results demonstrate that the inhibitory effect of Cd on EPO protein production was not due to nonspecific toxicity.
Effects of Cd at the indicated doses in Hep3B cells with or without exposure to hypoxia (1% O2) or Co (100 μM).
(A) EPO protein production and cell viability at 24 hours. (B) Cell viability at 6 hours. (C) EPO mRNA expression at 6 hours. (D) DNA binding activity of hypoxia inducible factor-1 (HIF-1) at 6 hours. In C, RNase protection revealed 2 bands of protected EPO mRNA (75 bp and 61 bp). In D, the specificity of HIF-1 bands (H) and constitutive bands (C) was confirmed by the addition of excess unlabeled W18 oligonucleotide as a competitor for HIF-1 binding. N indicates nonspecific band. Data in A and B are expressed as mean ± SD of 3 independent experiments. Results in C and D are representative of 3 independent experiments.
Effects of Cd at the indicated doses in Hep3B cells with or without exposure to hypoxia (1% O2) or Co (100 μM).
(A) EPO protein production and cell viability at 24 hours. (B) Cell viability at 6 hours. (C) EPO mRNA expression at 6 hours. (D) DNA binding activity of hypoxia inducible factor-1 (HIF-1) at 6 hours. In C, RNase protection revealed 2 bands of protected EPO mRNA (75 bp and 61 bp). In D, the specificity of HIF-1 bands (H) and constitutive bands (C) was confirmed by the addition of excess unlabeled W18 oligonucleotide as a competitor for HIF-1 binding. N indicates nonspecific band. Data in A and B are expressed as mean ± SD of 3 independent experiments. Results in C and D are representative of 3 independent experiments.
In addition to EPO protein production, we investigated the effect of Cd on EPO mRNA expression. As shown in Figure 1, panel C, Cd, at doses of 0.1 to 10 μM, inhibited both hypoxia- and Co-induced EPO mRNA induction over a 6-hour period in a dose-dependent manner, similar to that observed for EPO protein production (Figure 1A). Note that in this gel, as in other RPA data (Figures 2C, 3B, and 4B), the 61-bp protected fragment is more pronounced than the larger 75-bp fragment. This difference, which has been consistently observed in our earlier studies using this riboprobe,15 16 is unexplained, but it does not have any effect on the quantitation of EPO mRNA.
Effects of CDDP at the indicated doses in Hep3B cells with or without exposure to hypoxia or Co.
(A) EPO protein production and cell viability at 24 hours. (B) Cell viability at 6 hours. (C) EPO mRNA expression at 6 hours. (D) DNA binding activity of hypoxia inducible factor-1 (HIF-1) at 6 hours. Experimental conditions were as described in the legend to Figure 1.
Effects of CDDP at the indicated doses in Hep3B cells with or without exposure to hypoxia or Co.
(A) EPO protein production and cell viability at 24 hours. (B) Cell viability at 6 hours. (C) EPO mRNA expression at 6 hours. (D) DNA binding activity of hypoxia inducible factor-1 (HIF-1) at 6 hours. Experimental conditions were as described in the legend to Figure 1.
To assess the mechanism responsible for the inhibition of EPO expression by Cd, we examined hypoxia- and Co-induced activation of HIF-1 DNA binding. HIF-1 binding was almost completely suppressed by Cd at 10 μM (Figure 1D). However, 1μM Cd did not completely inhibit HIF-1 binding, whereas EPO protein production and mRNA expression were strongly suppressed. These results indicate that HIF-1 DNA binding may be a less sensitive index of metal-induced inhibition than the measurement of target gene expression.
Because oxygen radicals may mediate the biochemical manifestations of Cd toxicity,19-22 we investigated the effects of 2 kinds of oxygen radical scavengers, TMTU (1 mmol/L) and vitamin E (10 μM),19 22-24 on the inhibition of EPO production in Hep3B cells treated with Cd. Neither radical scavenger affected the inhibition of EPO protein production (data not shown). The concentrations of TMTU and vitamin E used in these experiments, and those described below, were maximum doses that had no effect on Hep3B cell viability.
Platinum (CDDP)
We next investigated the effect of cisplatin (CDDP), another metal-based chemical substance that, like Cd, can induce renal tubular injury and impair the renal production of EPO9; 10 μM CDDP suppressed hypoxia- and Co-induced EPO production more than 60% to 70% without any cell damage (Figure 2A). EPO production was almost completely suppressed by CDDP at 50 to 100 μM, but this additional inhibition was likely due in part to decreased cell viability. At 6 hours' incubation, even 100 μM CDDP had no effect on cell viability (Figure 2B).
Compared with the strong inhibition of 10 μM CDDP on EPO protein levels at 24 hours, the same dose caused only partial inhibition of EPO mRNA expression at 6 hours (Figure 2C). Almost complete suppression of EPO mRNA expression was observed at 50 to 100 μM CDDP with no decrease in cell viability (Figure 2B), again suggesting that there is a time lag between EPO suppression and cell damage. These results indicate that CDDP, in a similar manner to Cd, suppresses EPO induction in the absence of cell toxicity.
CDDP at doses of 10 μM and 100 μM showed almost progressive inhibition of HIF-1 binding activation (Figure 2D), consistent with that of EPO mRNA. These results indicate that CDDP inhibits EPO production mainly by suppressing HIF-1 binding activity, but it may also affect EPO mRNA stability and rate of translation.
CDDP is also known to generate oxygen radicals. Neither TMTU nor vitamin E affected the ability of CDDP to inhibit the induction of EPO production (data not shown).
Mercury
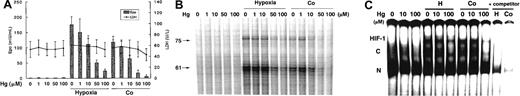
Because mercury (Hg) is another metal that can induce renal tubular injury in vivo, we investigated the effects of HgCl2 on EPO protein production at 24 hours and EPO mRNA expression at 6 hours. We observed a dose-dependent suppression of hypoxia- and Co-induced EPO protein production (Figure 3A) and mRNA expression (Figure 3B) without any decrease in cell viability. In addition, 10 to 100 μM Hg also suppressed HIF-1 binding (Figure 3C). The addition of either TMTU or vitamin E had no effect on the inhibition by Hg of EPO induction (data not shown). These results indicate that Hg has a suppressive effect on EPO induction through the inhibition of HIF-1 DNA binding, though the effective doses are relatively high compared with those of Cd and CDDP.
Effects of Hg at the indicated doses in Hep3B cells with or without exposure to hypoxia or Co.
(A) EPO protein production and cell viability at 24 hours. B) EPO mRNA expression at 6 hours.(C) DNA binding activity of hypoxia inducible factor-1 (HIF-1) at 6 hours. Experimental conditions were as described in the legend to Figure 1.
Effects of Hg at the indicated doses in Hep3B cells with or without exposure to hypoxia or Co.
(A) EPO protein production and cell viability at 24 hours. B) EPO mRNA expression at 6 hours.(C) DNA binding activity of hypoxia inducible factor-1 (HIF-1) at 6 hours. Experimental conditions were as described in the legend to Figure 1.
Other metals
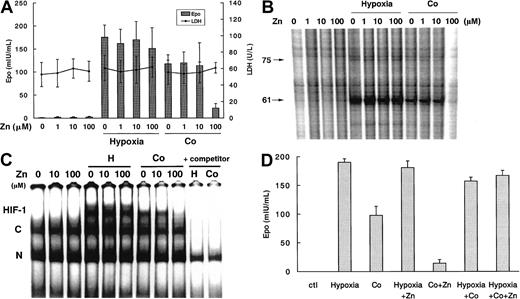
To assess the specificity of Cd, CDDP, and Hg, we also investigated the effects of zinc (Zn), lead (Pb), and tin (Sn), on EPO induction in Hep3B cells. Pb and Sn at doses as high as 100 μM had no effect on EPO induction (data not shown). On the other hand, Zn at 100 μM suppressed Co-induced EPO protein production at 24 hours without any apparent cell damage but had no effect on hypoxia induction (Figure4A). In addition, comparable patterns of suppression of hypoxically induced EPO mRNA expression and HIF-1 binding were observed at 6 hours (Figure 4B-C). Oxygen radical scavengers again did not affect the inhibition of Co-induced EPO induction by Zn (data not shown).
Effects of Zn at the indicated doses in Hep3B cells with or without exposure to hypoxia or Co.
(A) EPO protein production and cell viability at 24 hours. (B) EPO mRNA expression at 6 hours. (C) DNA binding activity of hypoxia inducible factor-1 (HIF-1) at 6 hours. (D) Effect of zinc (Zn) (100 μM) on either hypoxia (1% O2), cobalt (Co) (100 μM), or both stimulants on induced EPO protein production in Hep3B cells for 24 hours. Data are expressed as mean ± SD of 3 independent experiments. Experimental conditions were as described in the legend to Figure 1.
Effects of Zn at the indicated doses in Hep3B cells with or without exposure to hypoxia or Co.
(A) EPO protein production and cell viability at 24 hours. (B) EPO mRNA expression at 6 hours. (C) DNA binding activity of hypoxia inducible factor-1 (HIF-1) at 6 hours. (D) Effect of zinc (Zn) (100 μM) on either hypoxia (1% O2), cobalt (Co) (100 μM), or both stimulants on induced EPO protein production in Hep3B cells for 24 hours. Data are expressed as mean ± SD of 3 independent experiments. Experimental conditions were as described in the legend to Figure 1.
To further assess zinc's differential effect on EPO induction by hypoxia versus Co, we investigated the effect of simultaneous exposure to hypoxia and Co on EPO protein production in the presence and absence of Zn (Figure 4D). The level of EPO produced from Hep3B cells exposed to both hypoxia and Co simultaneously for 24 hours was not higher than the total value of EPO produced from exposure to hypoxia and to Co separately, indicating that, as observed,25 there was no additive effect of hypoxia and Co on EPO production. Furthermore, Zn did not affect EPO levels after simultaneous exposure to both hypoxia and Co.
These results indicate that Cd, CDDP, and Hg—metals that can induce renal tubular injury in vivo—suppress EPO induction in Hep3B cells.
Discussion
In patients with kidney disease, anemia results primarily from the insufficient production of EPO. However, there is only a crude inverse correlation between levels of EPO in the plasma and global measurements of renal function such as creatinine clearance. This marked variability in EPO responsiveness depends in part on the etiology of the kidney disease. For example, patients with uremia and polycystic kidney disease have less severe anemia than patients with more common causes of renal failure.
EPO is produced in a subset of interstitial cells located at the boundary between cortex and medulla, adjacent to proximal tubules.26-29 In addition, EPO may also be produced in proximal tubule cells.30,31 Accordingly, drugs and toxins that are filtered through the glomeruli and reabsorbed in the proximal tubule may directly and selectively impair renal production of EPO. It is noteworthy that patients with chronic Cd intoxication (Itai itai disease)3,4 and those exposed to cisplatin chemotherapy9 have suppression of plasma EPO levels that correlate better with measurements of renal tubular dysfunction than with tests of overall nephron function, such as creatinine clearance. In this study we demonstrated that, in comparison to other metals tested, Cd, CDDP, and Hg suppressed hypoxia- and Co-induced EPO induction in Hep3B cells in a dose-dependent manner. Our results strongly support the contention that exposure to Cd in Itai-itai disease, or to platinum in patients with cancer who receive CDDP therapy, can perturb sites of EPO production in the kidney, resulting in anemia. This metal specificity is unlikely to be caused by differences in cellular uptake. The divalent metal cation transport protein DMT1 is expressed in a wide variety of tissues, including liver parenchyma and proximal tubule of the kidney.32 Among the metals tested in our study, Co2+, Cd2+, Zn2+, and Pb2+ have been shown to be substrates for DMT.
Hg has toxic effects similar to those of Cd and CDDP, including renal tubular dysfunction in vivo and, as shown here, inhibition of EPO induction in vitro. However, patients with chronic mercury intoxication have not been reported to be anemic and therefore are unlikely to have impaired EPO production. The reason for this discrepancy is unclear, but one explanation may be difference in doses. Our results show that in Hep3B cells, EPO induction is inhibited by 1 μM Cd, whereas 50 to 100 μM Hg is required for the same effect. In general, chronic exposure is necessary to induce anemia secondary to impaired EPO production, but high doses of Hg are likely to cause other toxic manifestations before the development of anemia.
Although Pb induces anemia along with renal tubular dysfunction, like Cd or CDDP, the anemia is probably caused by the inhibition of heme synthesis or hemolysis rather than by EPO deficiency.33Lead-induced renal disease includes the formation of intranuclear inclusion bodies in proximal tubular cells but is subtle compared with what is observed with Cd, CDDP, or Hg.34 The clinical features of Pb poisoning are consistent with the failure to inhibit EPO induction in Hep3B cells. The close correlation between clinical manifestations of metal toxicity and effects on EPO induction in vitro further support the conclusion that metals that have specific effects on renal tubular cells cause anemia by impairing EPO production in the kidney.
Dittmer and Bauer12 previously reported that Zn inhibited both hypoxia-induced and Co-induced EPO protein production in HepG2 cells. Inspection of their data reveals that the latter effect was stronger than the former. They also reported that up to 2 μM Cd failed to affect hypoxic induction of EPO protein and only slightly reduced Co induction. The reason for these minor discrepancies is unclear. Our measurements include EPO protein levels, EPO mRNA, HIF-1 activity, and an assay of cell viability, all of which are internally consistent. Using different cell lines may be relevant. The hypoxic and Co induction of EPO and HIF-1 in Hep3B cells is more robust and reliable than that in HepG2 cells.10
Our studies on the effects of metals on the induction of EPO in cell culture may provide fresh insight into the mechanism by which oxygen is sensed and transduces a signal that regulates HIF-1. We had proposed that Co and certain other transition metals (nickel and manganese) simulated hypoxia by substituting for iron in the heme of the oxygen sensor.25 However, we have recently reported35 experiments using inhibitors of heme biosynthesis that challenge this hypothesis. It seems more likely that these metals act by competing with intracellular iron and inhibiting iron's ability to catalyze the generation of reactive oxygen intermediates (ROI) through Fenton chemistry.36,37Generation of ROI may underlie the ability of other metals to inhibit HIF-1 activation and EPO induction. Indeed, Cd has been shown to generate intracellular ROI.19-22 Moreover, the efficacy of CDDP as an antineoplastic agent is predicated on its ability to generate DNA and protein cross-links through the formation of ROI.38 We found that the effects of Cd and CDDP could not be reversed by the free radical scavengers TMTU and vitamin E. In contrast, vitamin E has been shown to protect both rat hepatocytes in suspension19 and rat liver in vivo22 from Cd toxicity. This apparent inconsistency may be reconciled if the signaling process responsible for HIF-1 activation in EPOgene expression were compartmentalized in a subcellular site to which the scavengers cannot gain access.
In conclusion, we have shown that Cd and platinum, metals that induce renal tubular dysfunction and EPO deficiency anemia in vivo, suppressed EPO induction in Hep3B cells in vitro. Our experiments may contribute to a better understanding of both the basic mechanisms underlyingEPO gene induction and the pathobiology of EPO production in renal disease.
Supported by National Institutes of Health grant DK 4-1234 (H.F.B.), a Grant-in-Aid for the Encouragement of Young Scientists (A) (no. 09770237) from the Ministry of Education, Science, and Culture of Japan (H.H.), the Uehara Memorial Foundation for Medical Research in Japan (H.H.), and CREST (F.K.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
H. Franklin Bunn, Hematology Division, Brigham and Women's Hospital, Harvard Medical School, Rm 223 LMRC, 221 Longwood Ave, Boston MA 02155; e-mail: bunn@calvin.bwh.harvard.edu.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal