Chronic myeloid leukemia (CML) is probably the most extensively studied human malignancy. The discovery of the Philadelphia (Ph) chromosome in 19601 as the first consistent chromosomal abnormality associated with a specific type of leukemia was a breakthrough in cancer biology. It took 13 years before it was appreciated that the Ph chromosome is the result of a t(9;22) reciprocal chromosomal translocation2 and another 10 years before the translocation was shown to involve the ABLproto-oncogene normally on chromosome 93 and a previously unknown gene on chromosome 22, later termed BCR for breakpoint cluster region.4 The deregulated Abl tyrosine kinase activity was then defined as the pathogenetic principle,5 and the first animal models were developed.6 The end of the millennium sees all this knowledge transferred from the bench to the bedside with the arrival of Abl-specific tyrosine kinase inhibitors that selectively inhibit the growth of BCR-ABL–positive cells in vitro7,8and in vivo.9
In this review we will try to summarize what is currently known about the molecular biology of CML. Because several aspects of CML pathogenesis may be attributable to the altered function of the 2 genes involved in the Ph translocation, we will also address the physiological roles of BCR and ABL. We concede that a review of this nature can never be totally comprehensive without losing clarity, and we therefore apologize to any authors whose work we have not cited.
The physiologic function of the translocation partners
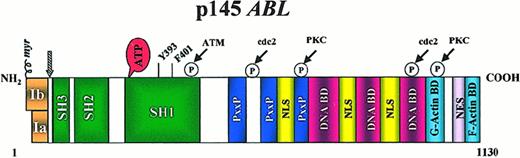
The ABL gene is the human homologue of the v-abl oncogene carried by the Abelson murine leukemia virus (A-MuLV),10 and it encodes a nonreceptor tyrosine kinase.11 Human Abl is a ubiquitously expressed 145-kd protein with 2 isoforms arising from alternative splicing of the first exon.11 Several structural domains can be defined within the protein (Figure 1). Three SRC homology domains (SH1-SH3) are located toward the NH2terminus. The SH1 domain carries the tyrosine kinase function, whereas the SH2 and SH3 domains allow for interaction with other proteins.12 Proline-rich sequences in the center of the molecule can, in turn, interact with SH3 domains of other proteins, such as Crk.13 Toward the 3′ end, nuclear localization signals14 and the DNA-binding15 and actin-binding motifs16 are found.
Structure of the Abl protein.
Type Ia isoform is slightly shorter than type Ib, which contains a myristoylation (myr) site for attachment to the plasma membrane. Note the 3 SRC-homology (SH) domains situated toward the NH2terminus. Y393 is the major site of autophosphorylation within the kinase domain, and phenylalanine 401 (F401) is highly conserved in PTKs containing SH3 domains. The middle of each protein is dominated by proline-rich regions (PxxP) capable of binding to SH3 domains, and it harbors 1 of 3 nuclear localization signals (NLS). The carboxy terminus contains DNA as well as G- and F-actin–binding domains. Phosphorylation sites by Atm, cdc2, and PKC are shown. The arrowhead indicates the position of the breakpoint in the Bcr-Abl fusion protein.
Structure of the Abl protein.
Type Ia isoform is slightly shorter than type Ib, which contains a myristoylation (myr) site for attachment to the plasma membrane. Note the 3 SRC-homology (SH) domains situated toward the NH2terminus. Y393 is the major site of autophosphorylation within the kinase domain, and phenylalanine 401 (F401) is highly conserved in PTKs containing SH3 domains. The middle of each protein is dominated by proline-rich regions (PxxP) capable of binding to SH3 domains, and it harbors 1 of 3 nuclear localization signals (NLS). The carboxy terminus contains DNA as well as G- and F-actin–binding domains. Phosphorylation sites by Atm, cdc2, and PKC are shown. The arrowhead indicates the position of the breakpoint in the Bcr-Abl fusion protein.
Several fairly diverse functions have been attributed to Abl, and the emerging picture is complex. Thus, the normal Abl protein is involved in the regulation of the cell cycle,17,18 in the cellular response to genotoxic stress,19 and in the transmission of information about the cellular environment through integrin signaling.20 (For a comprehensive review of Abl function, see Van Etten21). Overall, it appears that the Abl protein serves a complex role as a cellular module that integrates signals from various extracellular and intracellular sources and that influences decisions in regard to cell cycle and apoptosis. It must be stressed, however, that many of the data are based solely on in vitro studies in fibroblasts, not hematopoietic cells, and are still controversial. Unfortunately, the generation of ABL knockout mice failed to resolve most of the outstanding issues.22 23
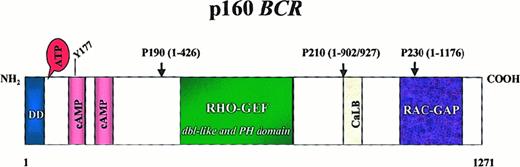
The 160-kd Bcr protein, like Abl, is ubiquitously expressed.11 Several structural motifs can be delineated (Figure 2). The first N-terminal exon encodes a serine–threonine kinase. The only substrates of this kinase identified so far are Bap-1, a member of the 14-3-3 family of proteins,24 and possibly Bcr itself.11 A coiled–coil domain at the N-terminus of Bcr allows dimer formation in vivo.25 The center of the molecule contains a region withdbl-like and pleckstrin-homology (PH) domains that stimulate the exchange of guanidine triphosphate (GTP) for guanidine diphosphate (GDP) on Rho guanidine exchange factors,26 which in turn may activate transcription factors such as NF-κB.27 The C-terminus has GTPase activity for Rac,28 a small GTPase of the Ras superfamily that regulates actin polymerization and the activity of an NADPH oxidase in phagocytic cells.29 In addition, Bcr can be phosphorylated on several tyrosine residues,30 especially tyrosine 177, which binds Grb-2, an important adapter molecule involved in the activation of the Ras pathway.31 Interestingly, Abl has been shown to phosphorylate Bcr in COS1 cells, resulting in a reduction of Bcr kinase activity.31,32 Although these data argue for a role of Bcr in signal transduction, their true biologic relevance remains to be determined. The fact that BCR knockout mice are viable and the fact that an increased oxidative burst in neutrophils is thus far the only recognized defect33 probably reflect the redundancy of signaling pathways. If there is a role for Bcr in the pathogenesis of Ph-positive leukemias, it is not clearly discernible because the incidence and biology of P190BCR-ABL-induced leukemia are the same in BCR−/− mice as they are in wild-type mice.34
Structure of the Bcr protein.
Note the dimerization domain (DD) and the 2 cyclic adenosine monophosphate kinase homologous domains at the N terminus. Y177 is the autophosphorylation site crucial for binding to Grb-2. The center of the molecule contains a region homologous to Rho guanidine nucleotide exchange factors (Rho-GEF) as well as dbl-like and pleckstrin homology (PH) domains. Toward the C-terminus a putative site for calcium-dependent lipid binding (CaLB) and a domain with activating function for Rac-GTPase (Rac-GAP) are found. Arrowheads indicate the position of the breakpoints in the BCR-ABL fusion proteins.
Structure of the Bcr protein.
Note the dimerization domain (DD) and the 2 cyclic adenosine monophosphate kinase homologous domains at the N terminus. Y177 is the autophosphorylation site crucial for binding to Grb-2. The center of the molecule contains a region homologous to Rho guanidine nucleotide exchange factors (Rho-GEF) as well as dbl-like and pleckstrin homology (PH) domains. Toward the C-terminus a putative site for calcium-dependent lipid binding (CaLB) and a domain with activating function for Rac-GTPase (Rac-GAP) are found. Arrowheads indicate the position of the breakpoints in the BCR-ABL fusion proteins.
Molecular anatomy of the BCR-ABLtranslocation
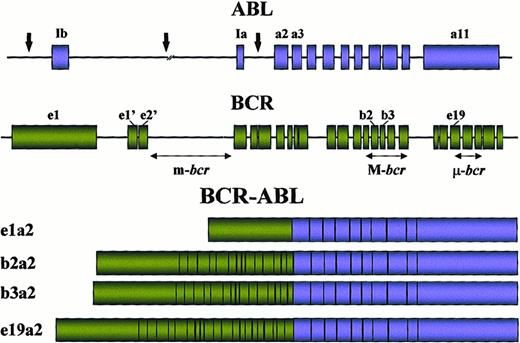
The breakpoints within the ABL gene at 9q34 can occur anywhere over a large (greater than 300 kb) area at its 5′ end, either upstream of the first alternative exon Ib, downstream of the second alternative exon Ia, or, more frequently, between the two35 (Figure 3). Regardless of the exact location of the breakpoint, splicing of the primary hybrid transcript yields an mRNA molecule in which BCR sequences are fused to ABL exon a2. In contrast to ABL, breakpoints within BCR localize to 1 of 3 so-called breakpoint cluster regions (bcr). In most patients with CML and in approximately one third of patients with Ph-positive acute lymphoblastic leukemia (ALL), the break occurs within a 5.8-kb area spanning BCR exons 12-16 (originally referred to as exons b1-b5), defined as the major breakpoint cluster region (M-bcr). Because of alternative splicing, fusion transcripts with either b2a2 or b3a2 junctions can be formed. A 210-kd chimeric protein (P210BCR-ABL) is derived from this mRNA. In the remaining patients with ALL and rarely in patients with CML, characterized clinically by prominent monocytosis,36,37the breakpoints are further upstream in the 54.4-kb region between the alternative BCR exons e2′ and e2, termed the minor breakpoint cluster region (m-bcr). The resultant e1a2 mRNA is translated into a 190-kd protein (P190BCR-ABL). Recently, a third breakpoint cluster region (μ-bcr) was identified downstream of exon 19, giving rise to a 230-kd fusion protein (P230BCR-ABL) associated with the rare Ph-positive chronic neutrophilic leukemia,38 though not in all cases.39 If sensitive techniques such as nested reverse transcription–polymerase chain reaction are used, transcripts with the e1a2 fusion are detectable in many patients with classical P210BCR-ABL CML.40 The low level of expression of these P190-type transcripts compared to P210 indicates that they are most likely the result of alternative splicing of the primary mRNA. Occasional cases with other junctions, such as b2a3, b3a3, e1a3, e6a2,41 or e2a2,42 have been reported in patients with ALL and CML. These “experiments of nature” provide important information as to the function of the various parts of BCR and ABL in the oncogenic fusion protein. Interestingly, ABL exon 1, even if retained in the genomic fusion, is never part of the chimeric mRNA. Thus, it must be spliced out during processing of the primary mRNA; the mechanism underlying this apparent peculiarity is unknown. Based on the observation that the Abl part in the chimeric protein is almost invariably constant while the Bcr portion varies greatly, one may deduce that Abl is likely to carry the transforming principle whereas the different sizes of the Bcr sequence may dictate the phenotype of the disease. In support of this notion, rare cases of ALL express aTEL-ABL fusion gene,43,44 indicating that theBCR moiety can in principle be replaced by other sequences and still cause leukemia. Interestingly, a fusion betweenTEL(ETV6) and the ABL-related gene ARGhas recently been described in a patient with AML.45Although all 3 major Bcr-Abl fusion proteins induce a CML-like disease in mice, they differ in their ability to induce lymphoid leukemia,46 and, in contrast to P190 and P210, transformation to growth factor independence by P230BCR-ABLis incomplete,47 which is consistent with the relatively benign clinical course of P230-positive chronic neutrophilic leukemia.38
Locations of the breakpoints in the ABL andBCR genes and structure of the chimeric mRNAs derived from the various breaks.
Locations of the breakpoints in the ABL andBCR genes and structure of the chimeric mRNAs derived from the various breaks.
One of the most intriguing questions relates to the events responsible for the chromosomal translocation in the first place. From epidemiologic studies it is well known that exposure to ionizing radiation (IR) is a risk factor for CML.48,49 Moreover,BCR-ABL fusion transcripts can be induced in hematopoietic cells by exposure to IR in vitro50; such IR-induced translocations may not be random events but may depend on the cellular background and on the particular genes involved. Two recent reports showed that the physical distance between the BCR and theABL genes in human lymphocytes51 and CD34+ cells52 is shorter than might be expected by chance; such physical proximity could favor translocation events involving the 2 genes. However, the presence of theBCR-ABL translocation in a hematopoietic cell is not in itself sufficient to cause leukemia because BCR-ABL fusion transcripts of M-bcr and m-bcr type are detectable at low frequency in the blood of many healthy individuals.53,54 It is unclear why Ph-positive leukemia develops in a tiny minority of these persons. It may be that the translocation occurs in cells committed to terminal differentiation that are thus eliminated or that an immune response suppresses or eliminates Bcr-Abl–expressing cells. Indirect evidence that such a mechanism may be relevant comes from the observation that certain HLA types protect against CML.55 Another possibility is thatBCR-ABL is not the only genetic lesion required to induce chronic-phase CML. Indeed, a skewed pattern of G-6PD isoenzymes has been detected in Ph-negative Epstein-Barr virus-transformed B-cell lines derived from patients with CML, suggesting that a Ph-negative pathologic state may precede the emergence of the Ph chromosome.56
Mechanisms of BCR-ABL–mediated malignant transformation
Essential features of the Bcr-Abl protein
Mutational analysis identified several features in the chimeric protein that are essential for cellular transformation (Figure4). In Abl they include the SH1, SH2, and actin-binding domains (Figure 1), and in Bcr they include a coiled–coil motif contained in amino acids 1-63,25 the tyrosine at position 177,57 and phosphoserine–threonine-rich sequences between amino acids 192-242 and 298-41358 (Figure 2). It is, however, important to note that essential features depend on the experimental system. For example, SH2 deletion mutants of Bcr-Abl are defective for fibroblast transformation,59 but they retain the capacity to transform cell lines to factor independence and are leukemogenic in animals.60
Signaling pathways activated in
BCR-ABL–positive cells. Note that this is a simplified diagram and that many more associations between Bcr-Abl and signaling proteins have been reported.
Signaling pathways activated in
BCR-ABL–positive cells. Note that this is a simplified diagram and that many more associations between Bcr-Abl and signaling proteins have been reported.
Deregulation of the Abl tyrosine kinase
Abl tyrosine kinase activity is tightly regulated under physiologic conditions. The SH3 domain appears to play a critical role in this inhibitory process because its deletion14 or positional alteration61 activates the kinase; it is replaced by viral gag sequences in v-abl.62 Both cis- and trans-acting mechanisms have been proposed to mediate the repression of the kinase. Several proteins have been identified that bind to the SH3 domain.63-65 Abi-1 and Abi-2 (Abl interactor proteins 1 and 2) activate the inhibitory function of the SH3 domain; even more interesting, activated Abl proteins promote the proteasome-mediated degradation of Abi-166 and Abi-2. Another candidate inhibitor of Abl is Pag/Msp23. On exposure of cells to oxidative stress such as ionizing radiation, this small protein is oxidized and dissociates from Abl, whose kinase is in turn activated.67These results are in line with previous observations that highly purified Abl protein is kinase-active,61 suggesting that its constitutive inhibition derives from a trans-acting mechanism. Alternatively, the SH3 domain may bind internally to the proline-rich region in the center of the Abl protein, causing a conformational change that inhibits interaction with substrates.68Furthermore, a mutation of Phe401 to Val (within the kinase domain) leads to the transformation of rodent fibroblasts. Because this residue is highly conserved in tyrosine kinases with N-terminal SH3 domains, it may bind internally to the SH3 domain.69 It is conceivable that the fusion of Bcr sequences 5′ of the Abl SH3 domain abrogates the physiologic suppression of the kinase. This might be the consequence of homodimer formation; indeed, the N-terminal dimerization domain is an essential feature of the Bcr-Abl protein but can be functionally replaced by other sequences that allow for dimer formation, such as the N-terminus of the TEL(ETV-6) transcription factor in the TEL-ABLfusion associated with the t(9;12).43,70 It is possible that deregulated tyrosine kinase activity is a unifying feature of chronic myeloproliferative disorders. Several other reciprocal translocations have been cloned from patients with chronicBCR-ABL–negative myeloproliferative disorders. Remarkably, most of these turn out to involve tyrosine kinases such as fibroblast growth factor receptor 171 and platelet-derived growth factor β receptor (PDGFβR).72
A host of substrates can be tyrosine phosphorylated byBcr-Abl (Table 1). Most important, because of autophosphorylation, there is a marked increase of phosphotyrosine on Bcr-Abl itself, which creates binding sites for the SH2 domains of other proteins. Generally, substrates of Bcr-Abl can be grouped according to their physiologic role into adapter molecules (such as Crkl and p62DOK), proteins associated with the organization of the cytoskeleton and the cell membrane (such as paxillin and talin), and proteins with catalytic function (such as the nonreceptor tyrosine kinase Fes or the phosphatase Syp). It is important to note that the choice of substrates depends on the cellular context. For example, Crkl is the major tyrosine-phosphorylated protein in CML neutrophils,73 whereas phosphorylated p62DOKis predominantly found in early progenitor cells.74
Substrates of BCR-ABL
| Protein . | Function . | Reference . |
|---|---|---|
| P62DOK | Adapter | 74 |
| Crkl | Adapter | 73 |
| Crk | Adapter | 13 |
| Shc | Adapter | 75 |
| Talin | Cytoskeleton/cell membrane | 76 |
| Paxillin | Cytoskeleton/cell membrane | 77 |
| Fak | Cytoskeleton/cell membrane | 78 |
| Fes | Myeloid differentiation | 79 |
| Ras-GAP | Ras-GTPase | 80 |
| GAP-associated proteins | Ras activation? | 214 |
| PLCγ | Phospholipase | 80 |
| PI3 kinase (p85 subunit) | Serine kinase | 127 |
| Syp | Cytoplasmic phosphatase | 83 |
| Bap-1 | 14-3-3 protein | 24 |
| Cbl | Unknown | 81 |
| Vav | Hematopoietic differentiation | 82 |
| Protein . | Function . | Reference . |
|---|---|---|
| P62DOK | Adapter | 74 |
| Crkl | Adapter | 73 |
| Crk | Adapter | 13 |
| Shc | Adapter | 75 |
| Talin | Cytoskeleton/cell membrane | 76 |
| Paxillin | Cytoskeleton/cell membrane | 77 |
| Fak | Cytoskeleton/cell membrane | 78 |
| Fes | Myeloid differentiation | 79 |
| Ras-GAP | Ras-GTPase | 80 |
| GAP-associated proteins | Ras activation? | 214 |
| PLCγ | Phospholipase | 80 |
| PI3 kinase (p85 subunit) | Serine kinase | 127 |
| Syp | Cytoplasmic phosphatase | 83 |
| Bap-1 | 14-3-3 protein | 24 |
| Cbl | Unknown | 81 |
| Vav | Hematopoietic differentiation | 82 |
Tyrosine phosphatases counterbalance and regulate the effects of tyrosine kinases under physiologic conditions, keeping cellular phosphotyrosine levels low. Two tyrosine phosphatases, Syp83 and PTP1B,84 have been shown to form complexes with Bcr-Abl, and both appear to dephosphorylate Bcr-Abl. Interestingly, PTP1B levels increase in a kinase-dependent manner, suggesting that the cell attempts to limit the impact of Bcr-Abl tyrosine kinase activity. At least in fibroblasts, transformation by Bcr-Abl is impaired by the overexpression of PTP1B.85Interestingly, we recently observed the up-regulation of receptor protein tyrosine phosphatase κ (RPTP-κ) with the inhibition of Bcr-Abl in BV173 cells treated with the tyrosine kinase inhibitor STI571,86 which suggests that the opposite effect may also occur. Thus, though the pivotal role of Bcr-Abl tyrosine kinase activity is clearly established, much remains to be learned about the significance of tyrosine phosphatases in the transformation process.
Activated signaling pathways and biologic properties of BCR-ABL–positive cells
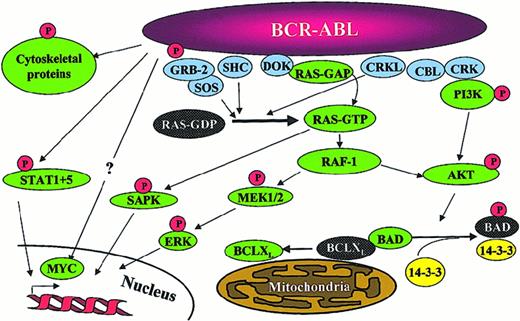
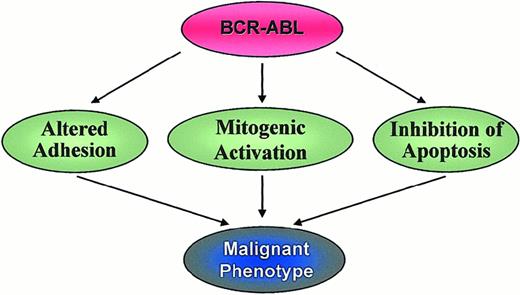
Three major mechanisms have been implicated in the malignant transformation by Bcr-Abl, namely altered adhesion to stroma cells and extracellular matrix,87 constitutively active mitogenic signaling88 and reduced apoptosis89(Figure 5). A fourth possible mechanism is the recently described proteasome-mediated degradation of Abl inhibitory proteins.66
Altered adhesion properties
CML progenitor cells exhibit decreased adhesion to bone marrow stroma cells and extracellular matrix.87,90 In this scenario, adhesion to stroma negatively regulates cell proliferation, and CML cells escape this regulation by virtue of their perturbed adhesion properties. Interferon-α (IFN-α), an active therapeutic agent in CML, appears to reverse the adhesion defect.91Recent data suggest an important role for β-integrins in the interaction between stroma and progenitor cells. CML cells express an adhesion-inhibitory variant of β1 integrin that is not found in normal progenitors.92 On binding to their receptors, integrins are capable of initiating normal signal transduction from outside to inside93; it is thus conceivable that the transfer of signals that normally inhibit proliferation is impaired in CML cells. Because Abl has been implicated in the intracellular transduction of such signals, this process may be further disturbed by the presence of a large pool of Bcr-Abl protein in the cytoplasm. Furthermore, Crkl, one of the most prominent tyrosine-phosphorylated proteins in Bcr-Abl–transformed cells,73 is involved in the regulation of cellular motility94 and in integrin-mediated cell adhesion95 by association with other focal adhesion proteins such as paxillin, the focal adhesion kinase Fak, p130Cas,96 and Hef1.97 We recently demonstrated that Bcr-Abl tyrosine kinase up-regulates the expression of α6 integrin mRNA,86 which points to transcriptional activation as yet another possible mechanism by which Bcr-Abl may have an impact on integrin signaling. Thus, though there is sound evidence that Bcr-Abl influences integrin function, it is more difficult to determine the precise nature of the biologic consequences, and, at least in certain cellular systems, integrin function appears to be enhanced rather than reduced by Bcr-Abl.98
Activation of mitogenic signaling
Ras and the MAP kinase pathways.
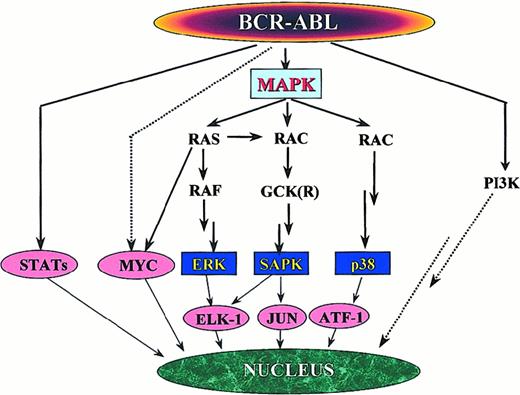
Several links between Bcr-Abl and Ras have been defined. Autophosphorylation of tyrosine 177 provides a docking site for the adapter molecule Grb-2.57 Grb-2, after binding to the Sos protein, stabilizes Ras in its active GTP-bound form. Two other adapter molecules, Shc and Crkl, can also activate Ras. Both are substrates of Bcr-Abl73,99 and bind Bcr-Abl through their SH2 (Shc) or SH3 (Crkl) domains. The relevance of Ras activation by Crkl is, however, questionable because it appears to be restricted to fibroblasts.100 Moreover, direct binding of Crkl to Bcr-Abl is not required for the transformation of myeloid cells.101 Circumstantial evidence that Ras activation is important for the pathogenesis of Ph-positive leukemias comes from the observation that activating mutations are uncommon, even in the blastic phase of the disease,102 unlike in most other tumors. This implies that the Ras pathway is constitutively active, and no further activating mutations are required. There is still dispute as to which mitogen-activated protein (MAP) kinase pathway is downstream of Ras in Ph-positive cells. Stimulation of cytokine receptors such as IL-3 leads to the activation of Ras and the subsequent recruitment of the serine–threonine kinase Raf to the cell membrane.103 Raf initiates a signaling cascade through the serine–threonine kinases Mek1/Mek2 and Erk, which ultimately leads to the activation of gene transcription.104 Although some data indicate that this pathway may be activated only in v-abl– but not in BCR-ABL–transformed cells,105 this view has recently been challenged.106 Moreover, activation of the Jnk/Sapk pathway by Bcr-Abl has been demonstrated and is required for malignant transformation107; thus, signaling from Ras may be relayed through the GTP–GDP exchange factor Rac108 to Gckr (germinal center kinase related)109 and further down to Jnk/Sapk (Figure 6). There is also some evidence that p38, the third pillar of the MAP kinase pathway, is also activated in BCR-ABL–transformed cells, and there are other pathways with mitogenic potential. In any case, the signal is eventually transduced to the transcriptional machinery of the cell.
Signaling pathways with mitogenic potential in
BCR-ABL–transformed cells. The activation of individual paths depends on the cell type, but the MAP kinase system appears to play a central role. Activation of p38 has been demonstrated only in v-abl–transformed cells, whereas data forBCR-ABL–expressing cells are missing.
Signaling pathways with mitogenic potential in
BCR-ABL–transformed cells. The activation of individual paths depends on the cell type, but the MAP kinase system appears to play a central role. Activation of p38 has been demonstrated only in v-abl–transformed cells, whereas data forBCR-ABL–expressing cells are missing.
It is also possible that Bcr-Abl uses growth factor pathways in a more direct way. For example, association with the βc subunit of the IL-3 receptor110 and the Kit receptor111 has been observed. Interestingly, the pattern of tyrosine-phosphorylated proteins seen in normal progenitor cells after stimulation with Kit ligand is similar to the pattern seen in CML progenitor cells.112 Dok-1 (p62DOK), one of the most prominent phosphoproteins in this setting, forms complexes with Crkl, RasGAP, and Bcr-Abl. In fact, there may be a whole family of related proteins with similar functions—for example, the recently described Dok-2 (p56DOK2).113 Somewhat surprisingly, p62DOK is essential for transformation of Rat-1 fibroblasts but not for growth-factor independence of myeloid cells114; thus, its true role remains to be defined.
Jak-Stat pathway.
The first evidence for involvement of the Jak-Stat pathway came from studies in v-abl–transformed B cells.62 Constitutive phosphorylation of Stat transcription factors (Stat1 and Stat5) has since been reported in several BCR-ABL–positive cell lines115 and in primary CML cells,116 and Stat5 activation appears to contribute to malignant transformation.117 Although Stat5 has pleiotropic physiologic functions,118 its effect in BCR-ABL–transformed cells appears to be primarily anti-apoptotic and involves transcriptional activation of Bcl-xL.119,120In contrast to the activation of the Jak-Stat pathway by physiologic stimuli, Bcr-Abl may directly activate Stat1 and Stat5 without prior phosphorylation of Jak proteins. There seems to be specificity for Stat6 activation by P190BCR-ABL proteins as opposed to P210BCR-ABL.115 It is tempting to speculate that the predominantly lymphoblastic phenotype in these leukemias is related to this peculiarity.
The role of the Ras and Jak-Stat pathways in the cellular response to growth factors could explain the observation that BCR-ABLrenders a number of growth factor–dependent cell lines factor independent.105,121 In some experimental systems there is evidence for an autocrine loop dependent on the Bcr-Abl–induced secretion of growth factors,122 and it was recently reported that Bcr-Abl induces an IL-3 and G-CSF autocrine loop in early progenitor cells.123 Interestingly, Bcr-Abl tyrosine kinase activity may induce expression not only of cytokines but also of growth factor receptors such as the oncostatin M β receptor.86 One should bear in mind, however, that during the chronic phase, CML progenitor cells are still dependent on external growth factors for their survival and proliferation,124though less than normal progenitors.125 A recent study sheds fresh light on this issue. FDCPmix cells transduced with a temperature-sensitive mutant of BCR-ABL have a reduced requirement for growth factors at the kinase permissive temperature without differentiation block.126 This situation resembles chronic-phase CML, in which the malignant clone has a subtle growth advantage while retaining almost normal differentiation capacity.
PI3 kinase pathway.
PI3 kinase activity is required for the proliferation ofBCR-ABL–positive cells.127 Bcr-Abl forms multimeric complexes with PI3 kinase, Cbl, and the adapter molecules Crk and Crkl,95 in which PI3 kinase is activated. The next relevant substrate in this cascade appears to be the serine–threonine kinase Akt.128 This kinase had previously been implicated in anti-apoptotic signaling.129 A recent report placed Akt in the downstream cascade of the IL-3 receptor and identified the pro-apoptotic protein Bad as a key substrate of Akt.130Phosphorylated Bad is inactive because it is no longer able to bind anti-apoptotic proteins such as BclXL and it is trapped by cytoplasmic 14-3-3 proteins. Altogether this indicates that Bcr-Abl might be able to mimic the physiologic IL-3 survival signal in a PI3 kinase-dependent manner (see also below). Ship131 and Ship-2,132 2 inositol phosphatases with somewhat different specificities, are activated in response to growth factor signals and by Bcr-Abl. Thus, Bcr-Abl appears to have a profound effect on phosphoinositol metabolism, which might again shift the balance to a pattern similar to physiologic growth factor stimulation.
Myc pathway.
Overexpression of Myc has been demonstrated in many human malignancies. It is thought to act as a transcription factor, though its target genes are largely unknown. Activation of Myc by Bcr-Abl is dependent on the SH2 domain, and the overexpression of Myc partially rescues transformation-defective SH2 deletion mutants whereas the overexpression of a dominant-negative mutant suppresses transformation.133 The pathway linking Myc to the SH2 domain of Bcr-Abl is still unknown. However, results obtained in v-abl–transformed cells suggest that the signal is transduced through Ras/Raf, cyclin-dependent kinases (cdks), and E2F transcription factors that ultimately activate the MYC promoter.134 Similar results were reported for BCR-ABL–transformed murine myeloid cells.135 How these findings relate to human Ph-positive cells is unknown. It seems likely that the effects of Myc in Ph-positive cells are probably not different from those in other tumors. Depending on the cellular context, Myc may constitute a proliferative or an apoptotic signal.136 137 It is therefore likely that the apoptotic arm of its dual function is counterbalanced in CML cells by other mechanisms, such as the PI3 kinase pathway.
Inhibition of apoptosis
Expression of Bcr-Abl in factor-dependent murine138and human122 cell lines prevents apoptosis after growth-factor withdrawal, an effect that is critically dependent on tyrosine kinase activity and that correlates with the activation of Ras.88,139 Moreover, several studies showed thatBCR-ABL–positive cell lines are resistant to apoptosis induced by DNA damage.89,140 The underlying biologic mechanisms are still not well understood. Bcr-Abl may block the release of cytochrome C from the mitochondria and thus the activation of caspases.141,142 This effect upstream of caspase activation might be mediated by the Bcl-2 family of proteins. Bcr-Abl has been shown to up-regulate Bcl-2 in a Ras-143 or a PI3 kinase-dependent128 manner in Baf/3 and 32D cells, respectively. Moreover, as mentioned previously, BclxL is transcriptionally activated by Stat5 in BCR-ABL–positive cells.119 120
Another link between BCR-ABL and the inhibition of apoptosis might be the phosphorylation of the pro-apoptotic protein Bad. In addition to Akt, Raf-1, immediately downstream of Ras, phosphorylates Bad on 2 serine residues.144,145 Two recent studies provided evidence that the survival signal provided by Bcr-Abl is at least partially mediated by Bad and requires targeting of Raf-1 to the mitochondria.146,147 It is also possible that Bcr-Abl inhibits apoptosis by down-regulating interferon consensus sequence binding protein (ICSBP).148,149 These data are interesting because ICSBP knockout mice develop a myeloproliferative syndrome,150 and hematopoietic progenitor cells from ICSBP−/− mice show altered responses to cytokines.151 The connection to interferon α, an active agent in the treatment of CML, is obvious.
It becomes clear that the multiple signals initiated by Bcr-Abl have proliferative and anti-apoptotic qualities that are frequently difficult to separate. Thus, Bcr-Abl may shift the balance toward the inhibition of apoptosis while simultaneously providing a proliferative stimulus. This is in line with the concept that a proliferative signal leads to apoptosis unless it is counterbalanced by an anti-apoptotic signal,152 and Bcr-Abl fulfills both requirements at the same time. There is, however, controversy. One report found 32D cells transfected with BCR-ABL to be more sensitive to IR than the parental cells,153 whereas 2 other studies failed to detect any difference between CML and normal primary progenitor cells with regard to their sensitivity to IR and growth factor withdrawal.124,154 Furthermore, based on results obtained in transfected cell systems, it was suggested that Bcr-Abl inhibits apoptosis mediated by the Fas receptor/Fas ligand system.155 However, though there may be a role for this system in mediating the clinical response to interferon-α,156 there is no indication that Fas-triggered apoptosis is defective in primary CML cells or in “natural” Ph-positive cell lines.157 Moreover, Bcr-Abl accelerates C2 ceramide-induced apoptosis,158 and it does not protect against natural killer cell-induced apoptosis.159 These inconsistencies may reflect genuine differences between cell lines and primary cells. On the other hand, it is debatable whether complete growth-factor withdrawal and IR constitute stimuli that have much physiologic relevance. To allow for a representative comparison, it would be crucial to define the signals that induce apoptosis in vivo.
Degradation of inhibitory proteins.
The recent discovery that Bcr-Abl induces the proteasome-mediated degradation of Abi-1 and Abi-2,66 2 proteins with inhibitory function, may be the first indication of yet another way by which Bcr-Abl induces cellular transformation. Most compelling, the degradation of Abi-1 and Abi-2 is specific for Ph-positive acute leukemias and is not seen in Ph-negative samples of comparable phenotype. The overall significance of this observation remains to be seen, and one must bear in mind that the data refer to acute leukemias and not to chronic phase CML. It is nevertheless tempting to speculate that other proteins, whose level of expression is regulated through the proteasome pathway, may also be degraded. A good candidate would be the cell cycle inhibitor p27, but to our knowledge no data are available yet.
Experimental models of CML
Various experimental systems have been developed to study the pathophysiology of CML. All of them have their advantages and shortcomings, and it is probably fair to say that there is still no ideal in vitro or in vivo model that would cover all aspects of the human disease.
Cell lines
Fibroblasts.
Fibroblast lines have been used extensively in CML research because they are easy to manipulate. Fibroblast transformation—that is, anchorage-independent growth in soft agar—is the standard in vitro test for tumorigenicity.160 However, it became clear that the introduction of BCR-ABL into fibroblasts has diverse effects, depending on the type of fibroblast used. Thus, though P210BCR-ABL transforms Rat-1 fibroblasts,161there is no such effect in NIH3T3.162 Moreover, transformation to serum-independent growth occurs only in few cells (permissive cells163), whereas most undergo growth arrest. These observations show that certain cellular requirements must be met if a cell is to be transformed by BCR-ABL. Interestingly, this is also the case for the various parts of the Bcr-Abl protein. Thus, a BCR-ABL mutant that lacks the SH2 domain retains the capacity to transform hematopoietic 32D cells to growth factor independence60 but is defective for fibroblast transformation.59 In addition, there are differences between hematopoietic cells and fibroblasts in terms of interactions with other proteins such as Crkl. The latter is functional in Ras activation and transformation in fibroblasts100 but not in hematopoietic cells.101 Thus, results obtained from studies in fibroblasts must be interpreted with great caution.
Hematopoietic cell lines.
Until relatively recently, only a few BCR-ABL–positive lines derived from CML were available, but their number has grown considerably in the past few years.164 They include cell lines with myeloid differentiation, such as the well-known K562, and lymphoid phenotype, such as BV173. The main drawback common to all these lines is the fact that they are derived from blast crisis and, thus, contain genetic lesions in addition to BCR-ABL. Consequently, they may reflect blast crisis fairly well but are insufficient models of chronic phase CML. Until now, no cell line from chronic phase CML has been established, just as no cell line could be derived from normal human bone marrow. Even attempts to immortalize Ph-positive B-cells from patients in the chronic phase of disease were not successful because these lines have a limited life span,165 in contrast to their Ph-negative counterparts. One could therefore speculate that the very establishment of a line from a patient with CML would be diagnostic of advanced disease. In this context, it is surprising that most human CML lines remain dependent on Bcr-Abl tyrosine kinase activity for their proliferation and survival, as shown by their susceptibility to the effects of the Abl-specific tyrosine kinase inhibitor STI571.8 However, the phenotype of these cell lines is that of an acute leukemia. Therefore great caution is warranted if experimental results are to be transferred to chronic phase CML. A striking example is the fact that inhibition of apoptosis by Bcr-Abl is easily demonstrable in cell lines139 but not in primary cells.124 It should also be noted that many of the lines used have undergone hundreds, if not thousands, of rounds of replication, and different laboratories frequently house lines that have little in common but their names and BCR-ABL positivity.
Transformation of factor-dependent cell lines to growth-factor independence is an important feature of Bcr-Abl, and, in fact, other oncoproteins that contain an activated tyrosine kinase.43,166 Although it is usually difficult to obtain stable expression of BCR-ABL in previously immortalized cell lines, this is relatively easily achieved in factor-dependent lines, presumably because BCR-ABL expression is an advantage to the latter but useless or even detrimental to the former. Murine cell lines such as Baf/3 and 32D and human cell lines such as MO7 were used to study the effects of BCR-ABL by direct comparison between transduced and parental cells. A particular advantage of the murine lines is the fact that they are derived from normal nonmalignant hematopoietic cells. Unfortunately, this does not rule out the development of additional mutations167 that confer a selective growth advantage. Furthermore, it is not clear how the transformation to complete factor independence relates to clinical CML in which the cells are still factor-dependent, though obviously less so than normal hematopoietic cells.123 The subject of growth factor independence and BCR-ABL transformation has been reviewed recently.168
None of the cell lines mentioned above is capable of multilineage hematopoietic differentiation. Two strategies are promising in overcoming this restraint. A recent report126 shows that murine FDCPmix cells, transduced with a temperature-sensitive mutant ofBCR-ABL, become partially factor-independent at the permissive temperature, in analogy to chronic phase CML. They retain the capacity for terminal differentiation, similar to chronic phase CML cells. Another approach is the study of embryonic stem (ES) cells transduced with BCR-ABL. In one such experimental system, it was possible to reproduce one cardinal feature of the clinical disease in the model, namely the expansion of the myeloid compartment at the expense of the erythroid compartment.169 Interestingly, the increase in total cell numbers in the BCR-ABL-transduced ES cells was found to result from increased proliferation though there was little effect on apoptosis, another finding in line with observations in primary Ph-positive cells.124,154 In this system, a stromal cell layer is used on which the ES cells removed from leukemia-inhibitory factor (LIF) differentiate into hemangioblasts and then into hematopoietic cells. This may explain why these results are not strictly comparable to those of another study, in whichBCR-ABL resulted in the decreased formation of embryonal bodies along with increased output of all kinds of hematopoietic progenitors.170 In yet another study,BCR-ABL–transformed ES cells transplanted into irradiated mice induced a leukemic syndrome with many features of CML.171 If developed further, these models may well be able to retain the major advantage of cell lines—their ease of manipulation—while at the same time moving the in vitro system closer to the clinical disease.
Bearing all these caveats in mind, there is no doubt that the study of cell lines contributed significantly to our understanding of CML. Particularly, many of the proteins that interact with Bcr-Abl were identified in Ph-positive cell lines, where they are more abundantly expressed than in primary cells. Thus, though these lines are invaluable tools for screening, it is important to confirm the results in primary cells.
Primary cells.
The study of patient material and its comparison with normal hematopoietic progenitor cells is certainly the gold standard of CML research, particularly for the chronic phase of the disease. Much of the data refer to operationally defined cellular properties of CML versus normal cells, such as clonogenicity or adherence to bone marrow stroma; to give a comprehensive account of the cellular biology of CML would require a review in its own right. We will therefore focus on some areas in which the study of primary CML cells has been particularly instrumental to the study of the molecular biology of the disease.
One of the main problems when studying primary cells is inherent in the very nature of chronic phase cells—they tend to mature when placed in culture. Thus, the window of time for in vitro studies is narrow, and expansion of very primitive cells, the least prevalent but most interesting population, is difficult and carries the risk for introducing nonphysiologic alterations.172 Furthermore, there is considerable variation between patients that frequently results in an overlap rather than a clear distinction between normal cells and CML cells. Last, results are unreliable unless clearly defined cell populations such as CD34+ cells are studied. To a large extent, these problems can be overcome by the introduction of retroviral BCR-ABL expression vectors to murine or human primary bone marrow cells (see “Animal models” below).
A striking example of how fruitful the comparison of primary cell populations can be is the study of tyrosine-phosphorylated proteins in CD34+ cells.112 This study led to the subsequent identification of p62DOK74;173 and SHIP2132 as mediators of Bcr-Abl–induced transformation. Moreover, it produced the important notion that Bcr-Abl tyrosine kinase activity may have consequences similar to the activation of the KIT receptor.112 Another example is the identification of CRKL as the major tyrosine-phosphorylated protein in CML neutrophils.73
The recent possibility of turning off the Bcr-Abl tyrosine kinase activity in cell lines and primary cells with STI5717 8provided the opportunity to study the effects of the BCR-ABLgene when expressed from its natural BCR promoter at “physiological” levels. This is certainly an advantage over transduced cell systems; the drawback, however, is that effects related to inhibition of the KIT and PDGFRβ kinases, and potentially other unidentified tyrosine kinases, cannot be ruled out. Furthermore, the Bcr-Abl protein, though kinase-inactive, is still present in the cells and may interfere with other proteins.
Animal models
Thus far, no animals other than mice have been used for the study of CML in vivo. Various approaches have been taken.
Engraftment of BCR-ABL–transformed cell lines in syngeneic mice.
Murine factor-dependent cell lines such as 32D transduced withBCR-ABL give rise to an aggressive leukemia when transplanted into syngeneic recipients.60,174 This is an excellent in vivo model to test the efficacy of new drugs, such as the tyrosine kinase inhibitor STI571, in vivo. Furthermore, the impact on leukemogenicity of modifications within the Bcr-Abl protein and modifications to the respective cell lines (such as the introduction of co-stimulatory molecules174) can be tested. The main drawback is that the disease is a form of acute leukemia and is thus far from chronic phase CML.
Engraftment of immunodeficient mice with human BCR-ABL–positive cells.
Cell lines derived from human CML blast crisis are relatively easily propagated in severe combined immunodeficiency (SCID) mice.175 The distribution of the leukemia cells is fairly similar to the human disease, that is, they home to bone marrow and peripheral blood before they metastasize to nonhematopoietic tissues. More recently, it was shown that SCID mice can be engrafted with chronic phase CML cells if the cell inoculum is large enough (in the range of 1 × 108 cells).176 Up to 10% human cells were detectable in the recipient bone marrow and showed multilineage differentiation. Interestingly, most colonies wereBCR-ABL negative and thus were derived from the patient's residual normal hematopoiesis. This is reminiscent of long-term bone marrow cultures of CML177 and shows that host factors modify the disease to a great extent, a problem that will persist, even if higher percentages of engraftment can eventually be achieved. A step into this direction is the use of nonobese diabetes-SCID mice. In addition to the SCID defect in V(D)J recombination, these animals lack functional natural killer cells. Chronic phase CML cells and, even more so, cells from accelerated phase or blast crisis readily engraft in these mice, and there is a significant correlation between engraftment and disease state.178 Interestingly, the cells were exclusively Ph-positive in most cases, in contrast to cells engrafted in SCID mice, as mentioned above. This may be attributed to technical reasons but may also reflect a genuine difference between the different strains of mice. We can anticipate that these murine models will be useful for studying certain aspects of CML, such as the response to novel forms of treatment. Their value in investigating the human disease will be limited because it is difficult to see how disease modification by host factors could ever be ruled out.
Transgenic mouse models.
Attempts to use BCR-ABL transgenic mice as a CML model go back to the late 1980s, when a full-length cDNA of BCR-ABLwas not yet available and an artificial construct of human BCR sequences fused to v-abl was used instead.179Since then, a number of studies have been published that clearly prove the oncogenic potential of BCR-ABL. Several different promoters were used to direct the expression to the desired target tissues. However, some problems were encountered. First, it became clear that Bcr-Abl has a toxic effect on embryogenesis,180 perhaps the consequence of a cytostatic effect in nonhematopoietic tissues.181 Recently, the expression of BCR-ABL from a tetracycline-repressible promoter effectively overcame this problem.182 Most striking, the leukemia in these transgenic mice is completely reversible on re-addition of tetracycline. The second problem with transgenic mice is that the P210 BCR-ABL variant relevant to CML is difficult to study because it is less efficient in inducing leukemia than P190, a finding that was again confirmed in a recent study.47 Third and most important, the types of leukemia that developed in these mice were acute and of either B- or T-lymphoid phenotype, regardless of whether they arose in P190 or P210 transgenic animals. Thus, they resembled BCR-ABL–positive ALL but not chronic-phase CML183,184. In fact, myeloid leukemias developed rarely, if at all. A recent report185 may represent a major advance in this respect. In this study,BCR-ABL was expressed from the Tec promoter, a cytoplasmic tyrosine kinase predominantly expressed in hematopoietic cells. Although the founder mice exhibited excessive proliferation of lymphoblasts, their progeny developed a CML-like disease, albeit after a relatively long latency period of approximately 10 months. Thus, it is likely that the problems of the transgenic models will eventually be resolved if the gene is targeted to the appropriate cell.
Transduction of murine bone marrow cells with BCR-ABL retroviruses.
In 1990, several groups reported that a CML-like myeloproliferative syndrome could be induced when P210BCR-ABL–infected marrow was transplanted into syngeneic recipients.6,186,187Transplantation into secondary recipients frequently produced an identical disease while some mice developed acute leukemias of T- or B-cell phenotype, analogous to the development of lymphoid blast crisis in the clinical disease. Clonality was demonstrated in many cases. Roughly a quarter of the mice showed the myeloproliferative disease, whereas other recipients developed other distinct hematologic malignancies, such as macrophage tumors, B-ALL, T-ALL, and erythroleukemia. Most likely, these different diseases are the consequence of infection of different committed progenitor cells that, after transformation, give rise to the respective progeny. Not surprisingly, the infection conditions have a major impact on the disease phenotype.188 Building on the foundations of this early work, major improvements to the transduction–transplantation system have been made in the past few years. High-titerBCR-ABL retroviral stocks can be produced rapidly by transient transfection of packaging lines; the culture conditions have been refined, and the murine stem cell virus LTR has been introduced that allows for more efficient expression of BCR-ABL in the desired target cell. Combining all 3 improvements, 2 recent studies189 190 reported the induction of a transplantable CML-like disease in 100% of recipients. Pulmonary hemorrhage, a complication not found in human CML, was a frequent cause of death in both studies, demonstrating that these novel models, though a major step forward, may have their own distinct problems. Nevertheless, bone marrow transduction–transplantation most faithfully reproduces human CML, and further improvements are likely in the near future.
Transformation to blast crisis
Clinically, chronic-phase CML does not represent a major management problem because the elevated white blood cell count is readily controlled with cytotoxic agents in most patients, and neutrophil and platelet functions are largely normal. However, the disease progresses inexorably to acceleration and blast crisis, often within 5 years of diagnosis. The mechanisms underlying this evolution remain enigmatic. Deletion or inactivation of p16,191p53,192 and the retinoblastoma gene product193 have been reported but occur relatively rarely and, similar to the overexpression of EVI-1,194are not specific for blast crisis CML. This probably indicates that a wide variety of lesions, possibly multiple “cooperating” lesions, are required to induce the phenotype of blast crisis. Perhaps even more intriguing is why the cells acquire these additional lesions in the first place. A recent report shows that Bcr-Abl enhances the mutation rate in the Na-K-ATPase and in the HPRT genomic loci, both commonly used markers to measure mutation frequency. Along with this goes enhanced expression of DNA polymerase β, the mammalian DNA polymerase with the least fidelity.195P210BCR-ABL, but not P190BCR-ABL, phosphorylates and potentially interacts with xeroderma pigmentosum group B protein (XPB); as a result, the catalytic function of XPB may be reduced, and DNA repair may be impaired.196 In a recent study p210BCR-ABL transgenic mice were cross-mated with p53 heterozygous mice. In the offspring, the remaining p53 was rapidly lost because of somatic mutation, and the mice developed a disease that resembled blast crisis.197 Although this is still not a perfect model of human CML because the blasts are of T lineage, it strongly supports the concept of genomic instability inBCR-ABL–transformed cells. How Bcr-Abl leads to these phenomena is unclear, but they might form the basis of the presumed genomic instability of chronic phase CML. It is also possible that the alleged anti-apoptotic effect of Bcr-Abl favors inaccurate DNA repair where apoptosis would ensue in normal cells. In line with this concept, a prolonged G2 arrest after IR has been observed inBCR-ABL–expressing cell lines exposed to DNA-damaging agents.140 This arrest could allow for DNA (mis)repair, whereas in a normal cell the damage would induce apoptosis. Over time this could lead to the accumulation of mutations inBCR-ABL–positive cells that finally result in blastic transformation. There is no doubt that the excessive proliferation, with its high cell turnover, must be a risk factor per se for additional genetic lesions.
Molecular targets for therapy
Attempts at designing therapeutic tools for CML based on our current knowledge of the molecular and cell biology of the disease have concentrated on 3 main areas—the inhibition of gene expression at the translational level by “antisense” strategies, the stimulation of the immune system's capacity to recognize and destroy leukemic cells, and the modulation of protein function by specific signal transduction inhibitors. The antisense oligonucleotide198,199 and ribozyme200 approaches received much attention in the last decade but have in general failed to fulfill their theoretical promises. New modifications to the system, such as the use ofBCR-ABL junction-specific catalytic subunits of RNase P,201 may revitalize the field. The issue of immunologic stimulation, be it in the form of adoptive immunotherapy by donor lymphocyte infusions202 or of BCR-ABL junction peptide vaccination,203 is another avenue being extensively explored for the treatment of CML.
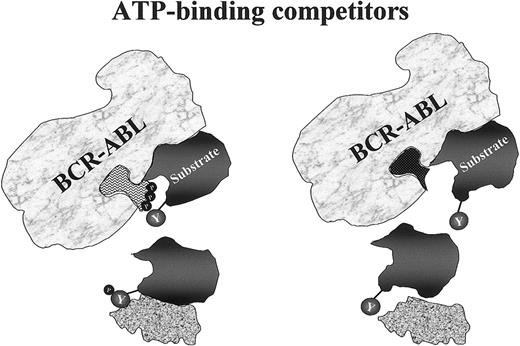
Perhaps the most exciting of the molecularly designed therapeutic approaches was brought about by the advent of signal transduction inhibitors (STI), which block or prevent a protein from exerting its role in the oncogenic pathway. Because the main transforming property of the Bcr-Abl protein is effected through its constitutive tyrosine kinase activity, direct inhibition of such activity seems to be the most logical means of silencing the oncoprotein. To this effect, several tyrosine kinase inhibitors have been evaluated for their potential to modify the phenotype of CML cells. The first to be tested were compounds isolated from natural sources, such as the iso-flavonoid genistein and the antibiotic herbimycin A.204 Later, synthetic compounds were developed through a rational design of chemical structures capable of competing with the adenosine triphosphate (ATP) or the protein substrate for the binding site in the catalytic center of the kinase205 (Figure7).
Mechanism of action of tyrosine kinase inhibitors.
The drug competes with ATP for its specific binding site in the kinase domain. Thus, whereas the physiologic binding of ATP to its pocket allows Bcr-Abl to phosphorylate selected tyrosine residues on its substrates (left diagram), a synthetic ATP mimic such as STI571 fits this pocket equally well but does not provide the essential phosphate group to be transferred to the substrate (right diagram). The downstream chain of reactions is then halted because, with its tyrosines in the unphosphorylated form, this protein does not assume the necessary conformation to ensure association with its effector.
Mechanism of action of tyrosine kinase inhibitors.
The drug competes with ATP for its specific binding site in the kinase domain. Thus, whereas the physiologic binding of ATP to its pocket allows Bcr-Abl to phosphorylate selected tyrosine residues on its substrates (left diagram), a synthetic ATP mimic such as STI571 fits this pocket equally well but does not provide the essential phosphate group to be transferred to the substrate (right diagram). The downstream chain of reactions is then halted because, with its tyrosines in the unphosphorylated form, this protein does not assume the necessary conformation to ensure association with its effector.
The most promising of these compounds is the 2-phenylaminopyrimidine STI571 (formerly CGP57418B; Novartis Pharmaceutics, Basel, Switzerland), which specifically inhibits Abl tyrosine kinase at micromolar concentrations.206 Inhibition of the Bcr-Abl kinase activity by this compound results in the transcriptional modulation of various genes involved in the control of the cell cycle, cell adhesion, and cytoskeleton organization, leading the Ph-positive cell to an apoptotic death.86 Furthermore, STI571 selectively suppresses the growth of CML primary cells and cell lines in vitro7,8 and in mice.7,207 Its remarkable specificity and efficacy led to consideration of the drug for therapeutic use. Thus, in the spring of 1998, a phase 1 clinical trial was initiated in the United States in which patients with CML in chronic phase resistant to IFN-α were treated with STI571 in increasing doses. The drug showed little toxicity but proved to be highly effective. All patients treated with 300 mg/d or more entered a complete hematologic remission. Even more striking, many of the patients had cytogenetic responses.9 This might mean that STI571 changes the natural course of the disease, though it is far too early to arrive at any definite conclusions. Altogether, the results were convincing enough to justify the initiation of phase 2 studies that included patients with acute Ph-positive leukemias (CML in blast crisis and Ph-positive ALL) and, at a later stage, a large cohort of interferon-intolerant or -resistant patients. These studies are ongoing. It turned out that STI571 is effective in many patients with acute Ph-positive leukemia, particularly of lymphoid phenotype. Although in many patients the remissions are not sustained, the advent of an effective oral medication with little toxicity represents a major step forward in this very poor risk group. Clearly, elucidation of the mechanisms underlying the resistance208 will be of critical importance for the development of further treatment strategies, such as a combination of STI571 with conventional cytotoxic drugs or, perhaps, with other STIs (see below). In this context, the most interesting question is whether STI571 will be able to eradicate the malignant clone, at least in some patients with chronic-phase CML. From what we know about the disease, this seems unlikely—colony formation by CML progenitor cells is much reduced but not abrogated in the presence of STI5717 8—which might mean that a subset of these cells proliferates independently of Bcr-Abl tyrosine kinase activity and still relies on external growth factors. There is no doubt, however, that the clinical efficacy and low toxicity of STI571 sets a precedent for the further development of targeted forms of therapy in malignant disease.
An alternative or a supplement to direct inhibition of Bcr-Abl is interference with proteins that are critical for Bcr-Abl–induced transformation (Figure 4). One of these proteins is Grb2, whose SH2 domain binds directly to Bcr-Abl through the phosphorylated tyrosine 177 within the Bcr portion of the chimera57 and is essential for activation of the Ras pathway (Figure 6).209Another good candidate is Ras itself, whose activity depends on its attachment to the cell membrane through a prenyl (usually a farnesyl) group. Thus, farnesyl transferase inhibitors (FTI) have been studied for their effect in inhibiting the proliferation of ALL210and juvenile myelomonocytic leukemia cells,211 and they may be useful for the control of CML cells. Additional targets worth considering are represented by PI-3 and Src kinases, of which the available inhibitors have been shown to suppress colony formation of primary progenitors,127 proliferation ofBCR-ABL–transfected cell lines, or both.212 213 It is envisaged that the progressive unraveling of which pathways are really essential for the development of the disease, coupled to rapid advances in biotechnology, will bring us the ideal combination of rationally designed drugs that can tip the balance toward the re-establishment of normal hematopoiesis in CML.
Conclusion
Though this be madness, yet there is method in it.(Shakespeare W., Hamlet. Act 2, scene 2.)
There are 2 ways to conclude this review after going through the vast amount of data presented. Surely one could argue that despite all these data, there is still no clear picture emerging and each piece of additional information adds only more confusion. Alternatively, what might help us against capitulation in the face of complexity is to try to simplify without oversimplification.
Can we build a model of CML that incorporates all the scientific data available but still retains clarity? In other words, could we explain how Bcr-Abl works in a few sentences to somebody who has never heard of it? Perhaps the most promising approach might be to try to link the biologic behavior of a CML cell to the underlying molecular events (Figure 5). Crucially, we should be able to picture this scenario relying on BCR-ABL alone because, at least until now, there is no unequivocal evidence that additional genetic lesions are present during chronic phase. We do not know how long it takes to move from the initial genetic event to fully established chronic-phase CML, but there is good reason to believe that the proliferative advantage of CML over normal cells is limited. Together with the largely normal differentiation capacity and function of CML blood cells, one feels that Ph-positive hematopoiesis cannot be so much different from normal hematopoiesis until the disease accelerates. Thus, Bcr-Abl is likely to hijack pathways that normally increase blood cell output in response to physiologic stimuli rather than to interrupt or replace them with pathways that are not normally used in hematopoietic cells. Indeed, there is plenty of experimental evidence to support this notion. Importantly, Bcr-Abl is capable of activating survival pathways along with proliferative stimuli without the need for a second cooperating genetic lesion; in this way, the apoptotic response that would otherwise follow an isolated proliferative stimulus is avoided. The sustained dependence on growth factors is an indication that Bcr-Abl is not a complete substitute; rather, it tips the balance to provide a limited growth advantage in vivo. This growth advantage is also dependent on specific survival conditions: transient regeneration of Ph-negative hematopoiesis is often observed after autografting, even when the autograft seems to be comprised exclusively of Ph-positive stem cells, and long-term cultures initiated from patients with chronic-phase CML become dominated by BCR-ABL–negative cells after some time.177 Thus, there appears to be a specific interaction (or noninteraction) of CML progenitor cells with their microenvironment that is crucial to maintain their proliferative advantage. Whether this interaction is stimulatory for CML over normal progenitor cells or inhibitory for normal over CML progenitor cells remains to be seen. Similarly, we can look at extramedullary hematopoiesis as a loss of function (ie, loss of the capacity to respond to negative signals) or a gain of function (ie, acquisition of a capacity to respond to positive signals that are not provided in the bone marrow) phenomenon. Much of the evidence implicates integrins in mediating these abnormal interactions, but other proteins may also play a role. Overall, it appears that the organization of cell membrane and cytoskeleton is more profoundly perturbed in CML progenitor cells than might be anticipated from the largely normal function of their progeny. Furthermore, Bcr-Abl may interfere with the “wiring” between integrin receptors on the cell surface and the nucleus and so disturb the communication of the cell with its environment. Another mechanism may also be important: Bcr-Abl appears to induce the degradation of certain inhibitory proteins. This might thwart cellular counter-reactions that would otherwise be activated, rather like cutting the telephone cable before the police can be called in.
Many questions remain unanswered. Why is there a predominantly myeloid expansion when all 3 lineages carry the translocation? What is the biologic basis for the extraordinary variability in the clinical course of a disease that appears to carry just a single genetic lesion? What is the molecular basis for the genomic instability that we see clinically as relentless progression to blast crisis?
Where do we go from here? The more we learn about the pathogenesis of CML, the more we realize its extraordinary complexity. Perhaps one should not be too surprised because it has become clear that cellular processes tend to rely on integrated networks rather than on straight unidirectional pathways. Only in this way can the cell achieve the flexibility required to respond to the various stimuli within a multicellular organism. Clearly, some components must be more important, and some less so, in the transformation network operated by Bcr-Abl. Absolutely essential features may be restricted to functional domains and to certain residues of the Bcr-Abl protein itself, and downstream effectors may be able to substitute for each other, at least to some extent. In this respect, the use of knockout mice that lack specific downstream molecules will allow one to define their precise relevance for Bcr-Abl–mediated cellular transformation. It may turn out that the combined elimination of several components abrogates transformation by Bcr-Abl, whereas each component individually is of limited significance. Chronic phase CML operates very much by exploiting physiologic pathways, perhaps by gently “coaxing” hematopoiesis toward the classical CML phenotype; nevertheless it prepares the ground for blast crisis. Thus, to understand CML, we must study its chronic phase. We must move away from artificial systems, such as transduced fibroblasts, and take on the demanding task of studying signal transduction in primary progenitor cells.
Supported by grants from Leukaemia Research Fund (UK) and the Dr Ernst und Anita Bauer Stiftung (Germany).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Michael W. N. Deininger, Department of Hematology/Oncology, University of Leipzig, Johannisallee 32, Leipzig 04103, Germany; e-mail:deim@medizin.uni-leipzig.de.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal