The critical role played by platelets in hemostasis, thrombosis, vascular remodeling, and healing is related to their function as exocytotic cells that secrete important effector molecules at the site of vascular injury. Recent insights into molecular mechanisms of secretion indicate that platelet granule secretion is homologous to exocytosis in neurons and other cells but involves a platelet-selective machinery that is uniquely coupled to cell activation. This review summarizes these recent insights in the context of the burgeoning literature on mechanisms of exocytosis in neurons and other cells—illustrating the fundamental homologies and the unique characteristics of platelet secretion.
Overview of platelet secretion
Physiologic role of exocytosis
Platelets are anuclear secretory cells that respond to substances produced by vascular injury (eg, thrombin, adenosine diphosphate [ADP], collagen, and so forth).1 Activation causes platelets to change shape, secrete their intracellular granules, and aggregate with each other.1 Platelets normally contain at least 3 types of large intracellular granules known as alpha, dense, and lysosomal granules.2 Although it was previously believed that only the alpha and dense granules were released in vivo, recent evidence indicates that lysosomal granules may also be secreted under physiologic circumstances.3 4 Platelet exocytosis provides the local, concentrated delivery of key effector molecules at the sites of vascular injury. These effector molecules amplify cell activation, initiate thrombus production, mediate intracellular adhesion, and trigger both cell proliferation and migration. Platelet secretion plays an important role in hemostasis because humans with defective platelet exocytosis suffer from a moderate bleeding tendency (see below).
Platelet granule development and contents
Platelet alpha granules contain polypeptides such as coagulation proteins (eg, fibrinogen, factor V), soluble adhesion molecules (eg, von Willebrand factor, vitronectin), growth factors (eg, platelet-derived growth factor, epidermal growth factor), protease inhibitors (eg, plasminogen activator inhibitor-1, α2-antiplasmin), and membrane adhesion molecules (eg, P-selectin, αIIbβ3). Platelet dense granules contain small molecules (eg, ADP, calcium, magnesium) that are important for activating cells.3
When platelets originate by segmentation from megakaryocytes, they contain a complex network of intact membrane structures that includes the plasmalemma, the granules, the surface-connected canalicular system (SCCS) and the dense tubular system. The development of platelet granules requires the activation of a platelet-selective genetic program because deletion of a transcription factor, NF-E2, blocks granule formation, as well as platelet release from the megakaryocyte.5 Membranes of the alpha granules contain proteins found on the plasma membrane (eg, αIIbβ36,7 CD36,8CD9, Rap1b9). Indeed as much as 10% of the glycoprotein (GP)Ib-IX-V complex can be found associated with the alpha granule membrane.7 This is consistent with the fusion of endocytic vesicles with alpha granules. Alpha granules also contain a mixture of proteins synthesized by the megakaryocyte (eg, β-thromboglobulin) as well as proteins endocytosed from the blood (eg, fibrinogen) by both the megakaryocyte and platelet.10-21 Platelets contain coated pits and coated vesicles21-25 and take up proteins by the process of receptor-mediated endocytosis; for example, fibrinogen is taken up by the integrin αIIbβ3.15,16,21,26,27 Thus the alpha granules appear to develop from the homotypic fusion of trans-Golgi vesicles in megakaryocytes as well as from heterotypic fusion of these vesicles with endocytic vesicles.10 28
The dense granules contain small molecules such as serotonin that are taken up and concentrated through specific transporting and storage mechanisms.29-31 Although the contents of the alpha and dense granules differ from each other, recent studies have shown the presence of the alpha granule protein P-selectin and the plasma membrane proteins αIIbβ3 and GPIb in the dense granule membrane.32-35 This suggests that dense granules, like alpha granules, arise from both endogenous syntheses in the megakaryocyte as well as from heterotypic fusion with endocytic vesicles budding from the plasma membrane.35
Ultrastructural features of secretion
Platelet activation causes dramatic cytoskeletal rearrangements that are important for shape change, adhesion, aggregation, exocytosis, and retraction.2,36,37 When platelet exocytosis occurs, granules fuse with the target plasmalemma or SCCS membranes to secrete their cargo into the extracellular space. Some experts favor a model in which granules individually fuse with the SCCS membrane,38,39 whereas others suggest that platelet secretion involves compound fusion of the granules with each other and then with the plasmalemma membrane.40-43 In bovine platelets, which lack an SCCS, the dense granules anchor in close physical apposition to the plasma membrane (in a manner similar to vesicle docking in neurons, see below). These anchored vesicles then fuse with the plasmalemma in response to increased intracellular Ca ++.44
At least 3 systems of cytoskeletal fibers are present in the platelet: the membrane skeleton, microtubules, and microfilaments (long actin filaments). When platelets are activated, the percentage of filamentous actin rapidly increases.36,45-47 The membrane skeleton is associated with the network of cytoplasmic actin filaments, various actin-binding proteins, and surface membrane glycoproteins (reviewed in Fox36 and Hartwig and Kwiatkowski48) and is preserved in Triton X-100 lysates of platelets as a continuous layer at the periphery of the cytoplasmic filaments.47 In resting platelets, circumferential bundles of microtubules play an important role in maintaining the discoid shape of platelets. Microtubule depolymerization leads to the loss of the discoid shape.49Microtubules also appear to play an important role in platelet secretion because colchicine (a tubulin ligand)50 and monoclonal antibodies to alpha and beta subunits of tubulin51 inhibit platelet secretion (when introduced into permeabilized cells). Microtubule-associated proteins regulate the stability and phosphorylation of the microtubules; these proteins may also play a role in microtubule reorganization.52 The contractile ring and stress fibers of platelets may also be involved in exocytosis and retraction.37 Cytoskeletal reorganization is mediated through actin polymerization and depolymerization regulated by phosphatidylinositol-4,5-bisphospate (PIP2), which binds to actin regulatory proteins such as scinderin and gelsolin.53 Consistent with these observations, recombinant scinderin potentiated Ca++-induced serotonin release in permeabilized platelets, whereas PIP2, or 2 peptides that interfere with scinderin-actin interactions, blocked exocytosis.54
In summary, changes in the platelet cytoskeleton are linked to the process of cell activation and appear to play a critical role in granule movement and exocytosis. Unfortunately the mechanisms through which cytoskeletal alterations moderate platelet secretion remain poorly understood.
Platelet secretory disorders: storage pool diseases
There are several recent reviews of platelet secretory defects.40-42,55,56 Congenital defects in platelet secretion are uncommon, and, the storage pool diseases (SPDs) are the best characterized abnormalities. Patients with the SPDs may have abnormal bleeding times and platelet aggregation defects.57 58 The SPDs have been categorized into 3 groups by electron microscopy: decreased or abnormal alpha granules (α-SPD), dense granules (δ-SPD), or both (αδ-SPD).
Patients with the δ-SPDs have diminished or absent platelet dense granules and frequently have vesicular defects in other cells besides platelets. For example, δ-SPD is found in patients with Chediak-Higashi syndrome (CHS) and Hermansky-Pudlak syndrome (HPS). CHS is characterized by immunologic deficiency, neutropenia, neurologic abnormalities, and large cellular inclusion bodies.59 In addition to abnormal secretion of ADP and platelet aggregation related to missing platelet dense granules, patients with CHS also have abnormalities of other storage organelles including melanosomes, cytolytic granules, and lysosomes.58 HPS (oculocutaneous albinism, platelet dysfunction, and lysosomal lipofuscin ceroid deposition) is another type of δ-SPD.60 HPS platelets lack dense granules, and there is abnormal lysosome morphology and function in other cells.61 A type of SPD has been found also in strains of minks, cattle, and mice (eg, the gunmetal, sandy, cocoa, muted, and mocha mouse).62-64
The δ-SPDs may represent the effect of different gene mutations at distinct steps in lysosomal vesicular trafficking. Studies of B- and T-cell lines in CHS have associated abnormal lysosomal-endosomal protein sorting with the immune abnormalities seen in patients with CHS.65,66 Defects in the LYST(lysosomal trafficking regulator) gene have been identified in patients with CHS and in beige mice.67-69 Introduction of a yeast artificial chromosome containing the LYST gene corrects the giant lysosome morphology of beige mouse fibroblasts.67 On the basis of these results and the homology of the carboxy terminal domain of LYST with VPS15, a kinase implicated in sorting of vacuolar proteins in yeast, it has been suggested that the LYST gene regulates some aspect of lysosomal trafficking.69
Mutations in different genetic loci have been identified in patients with HPS and in strains of animals demonstrating HPS-like storage pool defects such as the mocha and pale-ear mice.70 A duplication has been identified in a novel gene (HPS1) in 22 patients of Puerto Rican descent with HPS that was not found in normal controls of Puerto Rican descent or HPS patients who were not Puerto Rican.71 The HPS1 gene is predicted to encode a 700 amino acid polypeptide of unknown function.72 Pale-ear mice have defects in platelet dense granules and melanosomes and carry an insertional mutation in an exon of a 3′ coding sequence in the murine counterpart of the HPS locus.73 The mocha mouse has HPS-like storage pool defects involving platelet dense granules, melanosomes, and lysosomes. In these mice the gene for the δ-subunit of the AP-3 complex is mutated.74 AP-3 complex function appears critical for normal dense granule maturation because mutations have been found in the β-3A subunit of non-Puerto Rican HPS patients and the Pearl mouse.75 76
Although the relationships between the genetic defects of CHS, HPS, and the molecular pathogenesis of abnormal vesicle trafficking and function are not yet fully defined, these insights suggest that normal development of platelet dense granules is related to lysosomal vesicle trafficking. Defects in vesicle formation and trafficking markedly affect downstream processes such as exocytosis, even if the exocytotic machinery is intact.77
The α-SPD or gray platelet syndrome is characterized by enlarged platelets devoid of normal alpha granule staining as visualized by the gray color of a Wright-stained blood smear.78 These platelets have normal serotonin uptake and normal (to increased) numbers of dense granules and release lower amounts of serotonin in response to thrombin stimulation.79 The α-SPD platelets contain small abnormal vesicles that resemble alpha granule precursors associated with the Golgi apparatus in megakaryocytes.78,80 Although these platelets lack platelet factor 4 (a secreted protein), they have detectable amounts of the alpha granule protein P-selectin on the membranes of their internal vesicles and can translocate it to the plasma membrane in response to thrombin.81,82 These findings suggest that the defect in α-SPD is not in exocytosis itself but in the targeting of secretory proteins to the alpha granule.81 It is interesting to note that this defect was not seen in the targeting of von Willebrand factor or P-selectin to the Weibel-Palade bodies of endothelial cells in 2 patients with this disorder.83
The αδ-SPDs are deficient in both granule types and display a loss of primary and secondary aggregation responses to most common agonists.84,85 There is heterogeneity in P-selectin expression with most patients having little or no platelet P-selectin, whereas a small number have normal levels. The incomplete penetrance of the defect was confirmed by electron microscopy.82
The empty sack syndrome is another secretory disorder in which there is a reduced level of stored nucleotides and serotonin, but granule membrane proteins are present in normal numbers as measured by granulophysin content.86 Other patients with defective secretion have been identified with disorders that appear to involve impaired aggregation and cell signaling.87-89
Comparison of the physiology of platelet and neuronal secretion
Studies of vesicle trafficking and fusion in yeast, neurons, and neuroendocrine cells have provided a conceptual framework for elucidating regulated exocytosis in platelets. Regulated exocytosis is “triggered” by intracellular signal(s) produced by cell activation or membrane depolarization and is a subset of the vesicle trafficking and fusion processes that move molecules in and out of cells.
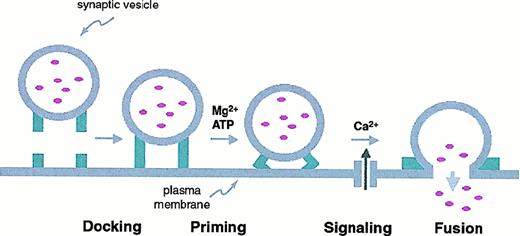
The triggered secretion of neurotransmitters is intensively studied and has been the subject of several recent reviews.90-97 There are also recent reviews of exocytosis in neuroendocrine98,99 and pancreatic cells.100It is generally agreed that neuronal exocytosis proceeds through a sequence of vesicle docking, priming, triggering, and fusion that leads to release of neurotransmitters into the synaptic cleft (Figure1). Electron microscopy has demonstrated the docking or close apposition of vesicles to the plasma membrane at the active zone of neurons.101 Although docking is necessary for exocytosis, only a subset of the vesicles present can be released rapidly indicating that additional “priming” or activation of the docked vesicle is necessary. The release of vesicles is “triggered” by increases in the intracellular Ca++concentration.
Steps in neuronal exocytosis.
Synaptic vesicles containing neurotransmitters dock with the plasma membrane. The docked vesicles are primed for secretion in an ATP/Mg++-dependent process that readies them for immediate release in response to local increases in Ca++ secondary to membrane depolarization. Subsequently the vesicle and plasma membranes fuse (through a SNARE-dependent mechanism) and the vesicle contents are released into the extracellular space.
Steps in neuronal exocytosis.
Synaptic vesicles containing neurotransmitters dock with the plasma membrane. The docked vesicles are primed for secretion in an ATP/Mg++-dependent process that readies them for immediate release in response to local increases in Ca++ secondary to membrane depolarization. Subsequently the vesicle and plasma membranes fuse (through a SNARE-dependent mechanism) and the vesicle contents are released into the extracellular space.
Recent studies of platelet secretion (see below) have indicated that it bears important similarities to neuronal exocytosis despite the fact that these cells have different origins (mesoderm versus ectoderm, respectively). Figure 2 highlights important similarities and differences between the 2 processes. Neurons contain small synaptic vesicles (SSV) loaded with neurotransmitters such as γ-aminobutyric acid (GABA) and acetylcholine. The SSV take up their neurotransmitters in the cell body and appear to recycle contents after exocytosis through endocytosis. Neurons also have small and large dense core vesicles that contain catecholamines and neuropeptides, respectively. Platelet dense granules resemble the neuronal small dense core vesicles because they contain small molecules such as serotonin that are taken up and concentrated from extracellular pools. Indeed, platelet dense granules have been used as a model system for understanding the uptake and transport of serotonin in amine-containing neurons.102 103 Similarly, the alpha granules resemble the large dense core vesicles of neurons because they contain a number of proteins that are synthesized in the megakaryocyte before platelet segmentation.
Physiology of regulated exocytosis in neurons and platelets.
The size and contents of vesicles, kinetics of exocytosis, secretory signals, Ca++ levels, magnitude of exocytosis, and role played by endocytosis in both cell types are shown. LDCV indicates large dense core vesicles; SSV, small synaptic vesicles.
Physiology of regulated exocytosis in neurons and platelets.
The size and contents of vesicles, kinetics of exocytosis, secretory signals, Ca++ levels, magnitude of exocytosis, and role played by endocytosis in both cell types are shown. LDCV indicates large dense core vesicles; SSV, small synaptic vesicles.
There is a marked difference in the kinetics of exocytosis and signal transduction between these 2 types of cells. Neurotransmitters are released from SSV within 200 μs, whereas platelet granule release typically takes 2 to 5 seconds—a 10 000-fold difference in rates. Although increases in Cai++ trigger both platelet and neuronal exocytosis, there are significant differences between these cells in the magnitude of the calcium signal and how it is generated. In neurons membrane depolarization down the axon elicits Ca++ influx via Ca++ channels. This leads to local increases in Ca++ concentration of ∼200 μmol/L, which triggers the exocytosis of vesicles in close proximity. Protein phosphorylation and other signaling mechanisms may also have modulating effects on neuronal exocytosis. In contrast, platelet secretion is triggered when extracellular receptors are activated by agonists (such as thrombin and ADP). This increases cytosolic intracellular Ca++ to 2 to 10 μmol/L and activates protein kinase C, a kinase whose activity is known to be important for platelet secretion (see below and Figure 3). In response to potent agonists, secretion occurs after a lag phase of ∼1.5 seconds and is nearly complete within 5 seconds.104 105 Finally, notable differences are apparent between these cells in the magnitude of exocytosis in response to a signal. The magnitude and rate of platelet exocytosis is related to the potency of the stimulus and strong agonists appear to release nearly all granules. In contrast, vesicles in neurons are released in a quantal fashion.
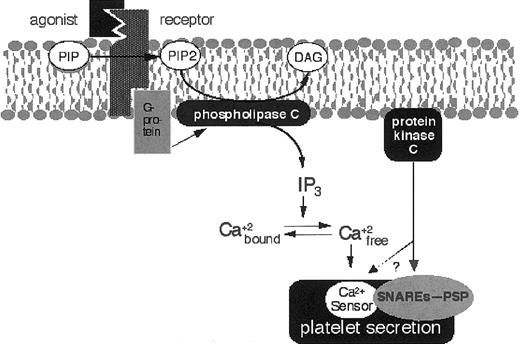
Platelet activation, intracellular signaling, and secretion.
When a ligand or agonist such as thrombin interacts with its cognate receptor on the platelet membrane, phospholipase C is activated through a G-protein–dependent mechanism. Alternatively, when collagen interacts with platelets, phospholipase C is also activated but through other mechanisms (not shown). Phospholipase C cleaves PIP2to DAG, which activates protein kinase C. Phospholipase C also produces IP3, which releases Ca++ from the platelet dense tubular system. Increased intracellular Ca++ and protein kinase C work synergistically to induce platelet exocytosis. Protein kinase C phosphorylates secretory molecules such as PSP.
Platelet activation, intracellular signaling, and secretion.
When a ligand or agonist such as thrombin interacts with its cognate receptor on the platelet membrane, phospholipase C is activated through a G-protein–dependent mechanism. Alternatively, when collagen interacts with platelets, phospholipase C is also activated but through other mechanisms (not shown). Phospholipase C cleaves PIP2to DAG, which activates protein kinase C. Phospholipase C also produces IP3, which releases Ca++ from the platelet dense tubular system. Increased intracellular Ca++ and protein kinase C work synergistically to induce platelet exocytosis. Protein kinase C phosphorylates secretory molecules such as PSP.
Conserved molecular mechanisms of exocytosis
SNARE machinery
The SNARE (soluble NSF attachment protein receptor) hypothesis has been an influential model of general vesicular fusion.106 The SNARE hypothesis states that the vesicle fusion apparatus contains 3 components: t-SNAREs, v-SNAREs, and a soluble SNAP/NSF component (Figure 4). The t-SNAREs are target receptors such as the syntaxins and SNAP-25 that are found on the target membrane (the plasma membrane in the case of exocytosis). The v-SNAREs are vesicle-associated membrane receptors (VAMP or synaptobrevins). The soluble components consist of NSF (N-ethylmaleimide-sensitive factor) and the soluble NSF-attachment proteins (SNAPs; α, β, γ isoforms). In yeast cells, vesicle membrane fusion requires 3 sequential steps of priming, docking, and fusion. Priming is thought to involve dissociation of the SNARE complex, “activation” of a t-SNARE, and adenosine triphosphate (ATP)-mediated release of SNAP catalyzed by NSF.107,108Docking, the stable association between vesicles and membranes (or between vacuoles), requires a low-molecular-weight GTPase or Rab-like molecule.109Fusion between membranes requires the formation of ternary core complexes between the t-SNAREs and a v-SNARE, but not NSF.109 The formation of a ternary complex between the t-SNAREs (SNAP-25 and syntaxin) and the v-SNARE (VAMP) may drive membrane fusion by overcoming the energy barrier that normally blocks it (see below).110 It is important to note that priming has a different meaning for neuroendocrine cells than it does for yeast. For neuroendocrine cells priming is a Mg-ATP–dependent step that follows docking that readies vesicles for rapid exocytosis in response to Ca++ signals (Figure 1), and probably involves the SNAREs.97,111 112
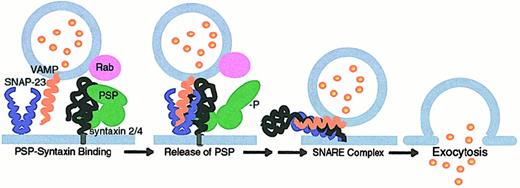
A hypothetical model for interactions of the SNARE machinery in platelet secretion.
90-97,106,120 158-162 Platelets contain the SNARE molecules syntaxin 2 and 4, SNAP-23, and VAMP. PSP (platelet Sec1 protein) binds to syntaxin 4 and can inhibit SNARE complex formation. Platelet granule membranes contain Rabs that may interact with PSP through effector molecules to play a role in vesicle docking and targeting. PSP is phosphorylated in a protein kinase C-dependent manner when platelets are activated by thrombin. This relieves the inhibitory effects of PSP on SNARE complex formation by blocking its binding with syntaxin. Alternatively, conformational changes induced in PSP by other molecules (potentially homologues of Doc2, Munc13, and so forth) may release it from syntaxin, permitting SNARE complex formation and the initiation of fusion and exocytosis.
A hypothetical model for interactions of the SNARE machinery in platelet secretion.
90-97,106,120 158-162 Platelets contain the SNARE molecules syntaxin 2 and 4, SNAP-23, and VAMP. PSP (platelet Sec1 protein) binds to syntaxin 4 and can inhibit SNARE complex formation. Platelet granule membranes contain Rabs that may interact with PSP through effector molecules to play a role in vesicle docking and targeting. PSP is phosphorylated in a protein kinase C-dependent manner when platelets are activated by thrombin. This relieves the inhibitory effects of PSP on SNARE complex formation by blocking its binding with syntaxin. Alternatively, conformational changes induced in PSP by other molecules (potentially homologues of Doc2, Munc13, and so forth) may release it from syntaxin, permitting SNARE complex formation and the initiation of fusion and exocytosis.
In neurons, syntaxin 1, SNAP-25, and VAMP 2 form a stable trimolecular SNARE complex.90-97,106 Syntaxin 1 belongs to a large family (at least 16 members) of type 1 integral membrane proteins that are classically found on the plasmalemma or acceptor membrane.97 SNAP-25 in neurons (or related molecules SNAP-23 and SNAP-29 in other cells) attaches to target membranes (ie, plasmalemma) via palmitoylated residues in the middle half of the molecule, making its NH2 and COOH terminal ends free to interact with syntaxin and VAMP. VAMPs 1 and 2 are also type I membrane proteins found in vesicle membranes. The VAMP family includes at least 8 known members.97 It has been postulated that the combinatorial complexity of the syntaxin–SNAP-25–VAMP system may play a role in directing vesicles to the correct target membranes for fusion, but in vitro experiments have not confirmed this hypothesis.113 Although the SNARE complex is sufficient in some systems for mediating vesicle-membrane mixing, the kinetics and regulation of this process in neurons, platelets, and other cells argues that other molecules are involved.97,114,115 The SNARE proteins may participate in the initial formation of a hemifusion stalk between membranes with subsequent generation of the fusion pore, lipid mixing, and exocytosis requiring other, as yet unidentified, molecular interactions.97
Sec1/Munc 18
Members of the Sec1/Munc 18 gene family regulate interactions between the SNARE proteins. The Sec1/Munc 18 proteins bind to the syntaxins and through this binding prevent formation of the core SNARE complex. At the same time the Sec1/Munc 18 molecules have been shown to be required for exocytosis. Sec 1, one of the genes found necessary for the final phase of exocytosis in yeast,116 has a homologue in worms (unc-18) that is required for normal acetylcholine release at the nerve terminal117 and for neurotransmitter release in flies.118 The murine homologue of Sec1/unc-18 (Munc-18-1) binds to syntaxin 1 in neurons and through this binding prevents formation of the SNARE core complex between syntaxin 1, SNAP-25, and VAMP119 that is required for fusion.
Deletion of the Munc 18-1 gene in mice is reported to be lethal because it prevents vesicle exocytosis even though it does not interfere with attachment of vesicles to the plasma membrane.97 The 3-dimensional structure of the neuronal Sec1-syntaxin complex has recently been solved.120 This structure has suggested that the Sec1 proteins may provide specificity for the pairing of vesicles with the correct target membranes in addition to modulating SNARE complex formation.
Excitation and secretion coupling molecules
Although yeast models are useful for understanding constitutive vesicular secretion, they do not account for the regulated exocytosis “triggered” in specialized secretory cells, such as neurons or neuroendocrine cells, by second messengers such as Ca++transients (reviewed in Calakos and Scheller91). The Ca++ sensors involved in regulated exocytosis are likely to differ between cell types and secretory processes.98Potential Ca++ sensor/effector molecules identified in neurons include synaptotagmin 1, Munc-13, and Doc2.121These proteins contain protein kinase C-C2 or potential Ca++-binding domains. Synaptotagmin 1 knockout mice show impaired fast Ca++-dependent vesicle exocytosis in neurons121,122 arguing that this protein plays a critical role in Ca++-triggered secretion in these cells. Munc13 (the murine homologue of unc13) contains a protein kinase C-C1 or diacylglycerol (DAG)-phorbol ester-binding domain, translocates to the plasma membrane, and enhances phorbol ester-induced release of neurotransmitters at neuromuscular junctions.123,124Unc-13 is necessary for normal synaptic function inCaenorhabditis elegans.125,126 Munc 13 can displace Sec1 (unc18) from syntaxin and deletion of theMunc13-1 gene in mice causes impaired synaptic vesicle release in response to action potentials.127,128 However, because Munc-13 does not appear to bind Ca++, its role as a direct Ca++ sensor has been challenged.127 The C2 domains of Doc2 interact with Ca++ and phospholipid, which may target it to synaptic vesicles and other membranes.129 One domain of Doc2 binds the murine Sec1 protein (Munc18) and competes with Munc18-syntaxin binding, whereas another domain of Doc2 interacts with Munc13 in a manner that is enhanced by Ca++ and phorbol ester.130,131 In genetic experiments, overexpression of Doc2 increases secretion in PC12 cells, suggesting that this protein plays a critical role in exocytosis.132 Several other potential Ca++sensors have been proposed including the annexins, calcyclin, calmodulin, unc31, frequenin, rim, and scinderin.133Although these molecules may prove to modulate other Ca++-dependent steps in the exocytotic pathway, the late-acting or triggering Ca++ sensor is likely to interact directly with the SNARE proteins because of their critical role in the initiation of vesicle fusion.
Other modulators of exocytosis
Abundant evidence implicates the Rab proteins, a large family of Ras-related low-molecular-weight GTPases in vesicle trafficking and fusion. At present the precise role played by the Rab proteins in exocytosis remains unclear despite evidence that these molecules interact with the SNAREs in yeast 134-136 and are involved in vesicle docking, in a step between NSF/SNAP action and fusion.107 The Rab proteins may exert their effects through effector molecules that specifically interact with the guanosine triphosphate (GTP)- or guanosine diphosphate (GDP)-bound form of the molecule. Deletion of the rab3a gene in mice causes a mild phenotype with an increase in the number of vesicle fusion events elicited by an action potential137; this suggests that Rab3A may play an inhibitory role in neuronal exocytosis.
Molecular mechanisms of platelet exocytosis
Activation-secretion coupling
The platelet is activated (Figure 3) when specific physiologic ligands (eg, ADP, thrombin, thromboxane A2, platelet activating factor, collagen, epinephrine) interact with cognate receptors on the plasma membrane. Receptor engagement activates phospholipase C either via G-protein–coupled mechanisms (eg, thrombin, reviewed in Brass et al138) or via other signaling mechanisms (eg, collagen reviewed in Barnes et al139). Phospholipase C cleaves PIP2 to inositol-1,4,5-trisphosphate (IP3) and DAG. In turn IP3 increases cytosolic Ca++ from 40 to 100 nmol/L to 2 to 10 μmol/L, which initiates granule secretion.140-145 There is strong evidence in platelets that protein kinase C also plays an important role in inducing secretion. Platelets contains several isoforms of protein kinase C (α,βΙ,βΙΙ,δ,ζ,η,θ), many of which are activated by DAG and Ca++.146 After cellular activation by thrombin, protein kinase C phosphorylates pleckstrin, myristoylated-alanine rich C kinase substrate (MARCKS), and several other substrates within seconds, with kinetics that parallel the kinetics of platelet secretion and aggregation.147-149Phorbol esters mimic DAG in their stimulation of protein kinase C; they induce platelet secretion and aggregation and potentiate the effects of calcium ionophores or agents that increase Cai++.150-152 Inhibitors of protein kinase C block dense granule secretion153 and have been used to demonstrate synergism between Cai++ and protein kinase C activity in platelet secretion.154 Although the protein kinase C isoform(s) required for platelet secretion is not known, protein kinase Cβ has been shown recently to be necessary for exocytosis in mast cells.155
A potential role for low-molecular-weight GTP-binding proteins (eg, the Rabs and other GTPases) in exocytosis has been suggested because the exocytotic effects of Ca++ are potentiated by GTP-γ-S (which stimulates both heterotrimeric and low-molecular-weight G-proteins), whereas AlF4 (which only activates heterotrimeric G-proteins) does not induce secretion.156 157
Current knowledge
Recent studies have revealed that the secretory machinery in platelets has important homologies to the machinery found in neurons and other cells. Platelets can form core SNARE complexes in vitro that support SNAP-dependent NSF-ATPase activity158 and this αSNAP-dependent NSF ATPase activity has been shown to be critical for exocytosis of both alpha and dense granules.159 Platelet membranes contain syntaxins 2 and 4, which play distinctive roles in granule exocytosis; syntaxin 2 is involved in dense granule release, whereas syntaxin 4 is necessary for platelet alpha granule secretion.158,160-162 Platelets contain abundant amounts of SNAP-23, and a VAMP, which interact and form SNARE complexes that reportedly dissociate after cell activation (Figure4).160,161 SNAP-23 is required for dense granule exocytosis, but it is not yet clear which VAMP is functionally involved in secretion.162,163 A platelet Sec1 protein (PSP) has been cloned, which is orthologous to Munc-18c.161 PSP forms a tight complex with syntaxin 2 and syntaxin 4 that can prevent the formation of the SNARE complex (Figure 4)161,164; antibodies against PSP inhibit dense granule secretion in permeabilized cells (J. Polgar and G. L. Reed, 1998, unpublished observations). PSP is phosphorylated in platelets activated by thrombin through a protein kinase C-dependent mechanism. Protein kinase C-phosphorylation of PSP inhibits syntaxin 4 binding, which relieves the inhibitory effect of this molecule on SNARE complex formation.161Alternatively, other as yet uncharacterized platelet molecules, such as homologues of Doc2, Munc 13, and so on, may induce a conformational change that releases PSP from syntaxin (Figure 4).120
Platelets also contain a number of different Rab molecules including Rab 3b, 6, 8, 11, 31.77,165 Rabs 3b, 6, and 8 are phosphorylated when cells are activated by thrombin. Rab 6 phosphorylation proceeds through protein kinase C mechanism and is associated with alterations in the GTP/GDP binding affinities and cell localization.165 166 To date, no direct functional role has been established for the Rabs in platelet secretion.
Mechanisms of exocytosis in other blood cells
At present very little information is available about the mechanisms of secretion in leukocytes or endothelial cells. It has been argued that leukocytes use lysosomes for both storage and exocytosis, unlike typical secretory cells, which use separate organelles for storage and release of their secretory products.167 SNARE proteins have been found in neutrophils, and phagocytosis appears to occur through a SNARE-dependent mechanism.168-172 In mast cells, SNAP-23 relocation from the plasmalemma to the granule membrane is required for compound granule-granule, granule-plasmalemma fusion typical of these cells.173 Overexpression of Rab 3d in rat basophilic leukemic cells (RBL-2H3 cells) inhibits exocytosis of hexosaminidase stimulated by interactions with the high-affinity IgE receptors.174 Evidence indicates that endothelial cell secretion also occurs through an NSF–SNARE-dependent mechanism175 though NSF-dependence has not been found by other investigators.176
Summary and future directions
Despite their unique characteristics, platelets use a molecular machinery for exocytosis that is homologous to that used in other types of secretory cells (eg, neurons) and different organisms (yeast to humans). However, many of the important physiologic differences between platelets and neurons in the regulation and kinetics of exocytosis are due to the fact that platelet secretory molecules arise from different genes and have distinctive features.
The core SNARE-related molecules responsible for platelet secretion have recently been identified. Now the regulatory linkages or signaling mechanisms that link these molecules and the process of platelet activation need to be elucidated more completely. Because the coupling of cell activation to exocytosis differs significantly between platelets and neurons, it is likely that the molecules that transduce these processes in platelets will be quite different from their counterparts in neurons. Unlike neuronal exocytosis, secretion in platelets is triggered by extracellular ligand-receptor interactions that lead to cell activation. These receptors are coupled to second messengers, such as Ca++, DAG, and protein kinase C, that signal to the exocytotic machinery. Recent studies have demonstrated that thrombin activation of platelets induces protein kinase C-mediated phosphorylation of PSP, providing important linkages between thrombin activation and the secretory machinery.161 Increased Cai++ is a critical determinant of secretion in platelets but the molecular sensor(s) in platelets that transduces or couples the Cai++ signal to secretion must still be discovered.
Platelets represent a unique opportunity for examining exocytosis in a cellular system that is not confounded by the membrane trafficking events found in actively synthetic cells. Beyond studies of the exocytotic machinery, important insights into secretion have come from physiologic, pharmacologic, ultrastructural, and clinical investigations. It is clear from ultrastructural analyses and from studies of patients with secretory deficiencies, that platelet exocytosis is the net result of several molecular and cellular processes acting in sequence. These processes include the formation, packaging, “differentiation,” and maintenance of secretory granules. The molecular mechanisms underlying these processes are important areas for future research. Valuable insights into this process are likely to come from identification of the genes responsible for secretory pool disorders in humans and animals. A particularly striking difference between platelets and nonhematopoietic cells is the strong functional association between changes in platelet shape (mediated by the cytoskeleton) and the exocytotic process. Further studies are necessary to define these linkages at the molecular level. Although many of the signaling pathways involved in platelet activation have been identified, the linkages of these signals to the secretory machinery are poorly understood, even though patients with impaired platelet activation-secretion coupling mechanisms have been described. The fact that some of the patients with platelet storage pool disorders do not appear to have functional defects in their other secretory cells suggests that there may be a megakaryocyte- and platelet-selective molecular machinery for granule formation, packaging, maintenance, and exocytosis that is responsible for the special function of the alpha and dense granules.
Insights into the molecular mechanisms responsible for platelet exocytosis will help define the causes of human platelet secretory disorders. These discoveries will provide general clues for understanding exocytosis in other vascular cells. Insights into these mechanisms may also be useful for designing a new class of therapeutic molecules to reduce the role played by platelet secretion in thrombosis and vascular remodeling—processes that contribute to cardiovascular and cerebrovascular disease.
Acknowledgments
The authors are grateful to the other members of the laboratory, particularly Sul-Hee Chung, Aiilyan Houng, and Lin Liu. Given the scope of this review, it has not been possible to discuss many important contributions to the field of platelet secretion, and the authors apologize to researchers whose work has not been cited.
Supported in part by National Institutes of Health grant HL-64057.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Guy L. Reed, Cardiovascular Biology Laboratory, Harvard School of Public Health, II-127, 677 Huntington Ave, Boston, MA 02115; e-mail: reed@cvlab.harvard.edu.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal