Abstract
The butyrate derivative isobutyramide (IBT) increases fetal hemoglobin (HbF) in patients with β-hemoglobinopathies, but little is known about its usefulness for prolonged therapeutic use. We treated 8 patients with transfusion-dependent β-thalassemia with 350 mg/kg of body weight per day of oral IBT for 126 to 384 days. During the trial period, the hemoglobin level was maintained between 85 g/L (range 82-87 g/L) (pretransfusion) and 115 g/L (range 110-119 g/L) (post-transfusion) (median, interquartile range), corresponding to 4-week transfusion intervals in all patients during the pretreatment phase. Adverse effects (bitter taste, epigastric discomfort) did not cause discontinuation of IBT. HbF increased in all patients from 3.1% (range 1.9%-4.8%) to 6.0% (range 3.3%-8.7) (P = .0017), while free Hb dropped from 0.48 g/L (range 0.39-0.81 g/L) to 0.19 g/L (range 0.16-0.24 g/L) (P < .0001). Transfusion intervals were consistently extended to 8 or 9 weeks in 1 patient, resulting in a decrease of daily iron load from 455 μg/kg per day (range 451-459 μg/kg per day) before therapy to 211μg/kg per day (range 203-286 μg/kg per day) during the 12-month treatment period. Prolongation of transfusion intervals achieved by IBT was less consistent in another patient, whose parenteral iron load nevertheless decreased from 683 μg/kg per day (range 618-748 μg/kg per day) to 542 μg/kg per day (340-596 μg/kg per day). In the other 6 patients, no prolongation of transfusion intervals was achieved. Response to treatment was associated with high pretreatment HbF (> 4.5%), high parental HbF, and increased erythropoietin levels (> 150 IU/L). We conclude that IBT prolongs transfusion intervals and reduces parenteral iron burden in some patients with transfusion-dependent β-thalassemia.
Introduction
The term β-thalassemia refers to a group of inherited hemoglobinopathies characterized by a reduced or absent synthesis of the β-globin chain. In homozygous β-thalassemia, the relative excess of insoluble α-globin chains precipitates within the red cell, which causes ineffective erythropoiesis and severe anemia.1 Increased γ-globin chain and fetal hemoglobin (HbF) synthesis is capable of compensating for the imbalance between α- and β-globin chains and may improve the clinical manifestations of β-thalassemia.2
Several observations have indicated that a number of short-chain fatty acids, and butyrate compounds in particular, are able to influence the developmental program of globin synthesis.3-6Subsequently, butyrate compounds were found to increase HbF levels in animal models and in erythroid cells of patients with β-hemoglobinopathies in vitro.7-9 These observations and the lack of major adverse effects in children with inherited urea-cycle disorders, who were treated with butyrates on a long-term basis,10,11 led to a small pilot study of intravenous arginine butyrate in children with sickle cell anemia and β-thalassemia.12,13 In this phase I/II study, an increase of fetal globin synthesis and Hb levels were reported, although a subsequent trial using a similar protocol could not confirm these previous findings.14
Sodium phenylbutyrate is an alternative compound that is absorbed enterally and has been reported to increase HbF levels in patients with sickle cell disease15 and to increase F reticulocytes in patients with β-thalassemia.16 However, phenylbutyrate is impalatable and displays a short biologic half-life. In contrast, oral isobutyramide (IBT) has been identified as a compound with a long plasma half-life.13,17 In a pilot study including patients with sickle cell anemia and β-thalassemia, IBT was reported to increase fetal globin synthesis and to cause a sustained rise in total Hb levels in one patient. However, signs of rapid drug tolerance and IBT-induced hemolysis were observed.18 A subsequent phase II study of IBT in nontransfused patients with thalassemia intermedia showed moderate increases of HbF without adverse effects.19
We report here a long-term clinical trial to examine the hematologic effects of orally administered IBT, particularly regarding changes in the transfusion requirement and subsequent changes in iron burden in patients with transfusion-dependent homozygous β-thalassemia.
Patients and methods
After written informed consent was obtained from the patients and their parents where applicable, 5 male and 3 female patients with homozygous β-thalassemia receiving regular red blood cell (RBC) transfusions were recruited for this study. The mean age was 17.5 years (range 8 to 25 years) on entry into the study. The mean age at diagnosis was 10 months (range 3 to 29 months), and transfusion therapy was started at a mean age of 16 months (range 6 to 29 months). Beginning in 1983, all patients (mean age 7 years; range 2 to 12 years) received deferoxamine (DFO) (25-75 mg/kg of body weight per day) as nightly 8- to 12-hour subcutaneous infusions 5 to 7 days a week using standard ambulatory pumps. Because of cardiac dysfunction, a venous access port to introduce high-dose DFO (120 mg/kg per day) was implanted in patient no. 3 about 1 year before the start of IBT and in patients no. 4 and 5 about 2 years before the start of IBT. However, because patients no. 3 and 4 developed hearing loss after 4 weeks of treatment, DFO had to be reduced to the initial dose of 55 and 75 mg/kg per day, respectively. In patient no. 5, high-dose DFO was continued for 1 year without severe adverse effects, resulting in distinct improvement of cardiac function. Three patients (nos. 3-5) had been splenectomized at ages of 10, 12, and 15 years, respectively. Five patients (nos. 3-6, 8) suffered from iron-related endocrinopathy (hypothyroidism, hypopararthyroidism, and hypogonadotrophic hypogonadism), necessitating hormone substitution. In addition, patients no. 4 and 6 had developed insulin-dependent diabetes mellitus. The patients' clinical profile, β-globin mutation, and parental percentage of HbF are shown in Table1. All patients were treated and followed up as outpatients at the Charité Children's Hospital, Berlin, where the treatment protocol was approved by the ethics committee.
Characterization of the 8 IBT-study patients with transfusion-dependent homozygous β-thalassemia
| Patient no. . | Duration of treatment (d) . | Sex . | Age (y) . | Country of origin . | DFO (mg/kg per day) . | Ferritin*(μg/L) . | anti-HCV +/− . | β-globin mutation . | Gγ-158 C → T . | Parental HbF (%) . | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mother† . | Father . | ||||||||||
| 1 | 224 | M | 8 | Turkey | 35 | 1969 (1618-2140) | − | IVS1 + 110 G → A/ IVS1 + 110 G → A | +/+ | <0.5 | <0.5 |
| 2 | 384 | M | 13 | Turkey | 25 | 3858 (3360-4520) | + | NS39/NS39 | −/− | 1.3 | <0.5 |
| 3 | 224 | F | 19 | Turkey | 55 | 1493 (1290-1772) | − | IVS1 + 110 G → A/ FS8/9(+G) | −/− | <0.5 | <0.5 |
| 4 | 224 | F | 25 | Greece | 75 | 2325 (1120-3409) | − | IVS1 + 110 G → A/ IVS2 + 745 C → G | −/− | <0.5 | <0.5 |
| 5 | 370 | M | 17 | Jordan | 75 | 3702 (2680-4293) | + | NS37/−86 C → G | −/− | 1.9 | 0.6 |
| 6 | 126 | M | 24 | Turkey | 75 | 9117 (6424-9864) | + | — | — | — | — |
| 7 | 224 | F | 14 | Turkey | 60 | 1453 (1270-1849) | + | IVS1 + 110 G → A/ IVS2 + 1 G → A | +/− | <0.5 | 0.9 |
| 8 | 224 | M | 20 | Turkey | 35 | 6881 (4730-9026) | + | NS39/IVS2 + 1 G → A | +/− | <0.5 | <0.5 |
| Patient no. . | Duration of treatment (d) . | Sex . | Age (y) . | Country of origin . | DFO (mg/kg per day) . | Ferritin*(μg/L) . | anti-HCV +/− . | β-globin mutation . | Gγ-158 C → T . | Parental HbF (%) . | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mother† . | Father . | ||||||||||
| 1 | 224 | M | 8 | Turkey | 35 | 1969 (1618-2140) | − | IVS1 + 110 G → A/ IVS1 + 110 G → A | +/+ | <0.5 | <0.5 |
| 2 | 384 | M | 13 | Turkey | 25 | 3858 (3360-4520) | + | NS39/NS39 | −/− | 1.3 | <0.5 |
| 3 | 224 | F | 19 | Turkey | 55 | 1493 (1290-1772) | − | IVS1 + 110 G → A/ FS8/9(+G) | −/− | <0.5 | <0.5 |
| 4 | 224 | F | 25 | Greece | 75 | 2325 (1120-3409) | − | IVS1 + 110 G → A/ IVS2 + 745 C → G | −/− | <0.5 | <0.5 |
| 5 | 370 | M | 17 | Jordan | 75 | 3702 (2680-4293) | + | NS37/−86 C → G | −/− | 1.9 | 0.6 |
| 6 | 126 | M | 24 | Turkey | 75 | 9117 (6424-9864) | + | — | — | — | — |
| 7 | 224 | F | 14 | Turkey | 60 | 1453 (1270-1849) | + | IVS1 + 110 G → A/ IVS2 + 1 G → A | +/− | <0.5 | 0.9 |
| 8 | 224 | M | 20 | Turkey | 35 | 6881 (4730-9026) | + | NS39/IVS2 + 1 G → A | +/− | <0.5 | <0.5 |
Median and interquartile range of ferritin values over a period of 1 year before the trial period.
None of the mothers were pregnant at the time the blood was drawn.
Before entry into the study, the patients were transfused with 10 to 20 mL/kg of body weight of packed RBCs with a hematocrit of 67% ± 3% (mean ± SD) at 4-week intervals. The baseline Hb level was 100 g/L or greater, and the post-transfusion Hb level was about 130 g/L, resulting in a mean Hb level of about 120 g/L. On entry into the study, the baseline Hb level was therefore reduced to 85 g/L and the mean post-transfusion level to 115 g/L, which was expected to activate endogenous RBC production. The regular 4-week blood transfusion regimen was discontinued during the study period. Instead, patients were transfused when the Hb declined to a level of 85 g/L or less, regardless of the time interval between 2 blood transfusions. However, when transfusions were necessary, the number of units of packed RBCs remained unchanged. The total duration of the study was 14 months, beginning with a prestudy phase consisting of 3 transfusion cycles (3 months), a treatment phase of 8 months, and a follow-up period of 3 months. An option to prolong treatment in patients obtaining clinical benefits by IBT was included in the study protocol.
IBT was prepared as a suspension immediately before use. The solid IBT was diluted 1:10 with water at room temperature. To cover the bitter taste of the substance, the carrier was natural grapefruit juice additionally flavored with grapefruit oil. IBT was administered starting at a dose of 250 mg/kg per day in 2 divided doses. The starting dose was maintained for approximately 8 weeks, at which point, in the absence of hepatic or renal toxicity, the dose was increased to 350 mg/kg per day and continued for an additional 24 weeks.
Safety parameters were monitored by physical examination, vital signs, and clinical laboratory evaluation (blood chemistry, blood counts, and differential counts). Efficacy was evaluated by changes in the transfusion interval, iron load, Hb, mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), lactate dehydrogenase (LDH), and plasma-free Hb. Assuming that 1 mL of packed RBCs contains 1 mg of iron, the transfusion-related daily iron loading was calculated from the volume of packed RBCs transfused and the actual transfusion interval. To obtain the exact volume, RBC bags were weighed before and after transfusion each time, and the hematocrit was determined. The measured weight was then divided by the specific weight (1.055 g/mL) and multiplied by the hematocrit, providing the volume transfused.
The reticulocyte count was obtained manually after staining with cresyl blue brilliant. The number and percentage of nucleated RBCs (nRBCs) was also determined in peripheral blood smears. The percentage of HbF was obtained by elution after cellulose acetate electrophoresis. The absolute HbF concentration was calculated as the product of the percentage of HbF and total Hb. Circulating erythropoietin (EPO) levels were measured by a commercially available radioimmunoassay.20 Mutations of the β-globin gene and the Gγ−158 C →‖T mutation were determined as previously described.21
The post-transfusion Hb level was determined 1 hour after the end of transfusion treatment. The decline of Hb level thereafter was first assessed in 2- to 3-week intervals. Upon approaching the baseline level of 85 g/L, the intervals between Hb measurements became increasingly shorter (1-3 times a week) until the pretransfusion Hb level of 85 g/L was reached. Each automated Hb measurement was paralleled by a manual count of nRBCs to control for possible artificial elevations in automated Hb measurements caused by the presence of nRBCs in the peripheral blood. MCV, MCH, LDH, free Hb, HbF, and reticulocyte counts were obtained 1 day before and 1 hour after completion of blood transfusion as well as once every 2 to 3 weeks within the transfusion interval. EPO levels were determined 1 day before transfusion.
For the purpose of this study, a clinical response was defined as a decrease in the daily body iron load of at least 20%, achieved by a prolongation of the transfusion interval during IBT therapy, based on an intention-to-treat analysis.
Because a normal distribution could not be assumed, all numeric data are presented as median and interquartile ranges unless stated otherwise. For the calculation of the median of Hb, HbF (% and absolute), MCV, MCH, free Hb, LDH, nRBCs, reticulocyte counts, and EPO, pretransfusion values were used. Groups were compared using the Mann-Whitney test.
Results
Pretransfusion and post-transfusion hemoglobin
In accordance with the study protocol, the patients' pretransfusion Hb level was kept at 85 g/L (range 82-87 g/L) during the prestudy phase, at 85 g/L (range 81-87 g/L) during IBT therapy, and at 85 g/L (83-87 g/L) during the post-study phase. The volume of RBCs given at each day of transfusion remained unchanged for all patients in the 3 phases of the trial: 13.4 mL/kg of body weight (range 11.7-18 mg/kg) before IBT, 13 mL/kg (range 12-17.4 mL/kg) during IBT, and 12.3 mL/kg (range 10.8-14.2 mL/kg) after IBT; P > .1. As a corollary, there was also no difference in the patients' post-transfusion Hb level: 115 g/dL (range 111-118 g/L) before IBT, 115 g/L (range 111-117 g/L) while on IBT, and 115 g/L (range 112-118 g/L) during the follow-up period; P > .1.
Transfusion interval and changes in iron load
Patients were grouped into responders and nonresponders depending on whether the transfusion interval could be prolonged and the average daily iron load decreased by at least 20% (Table2). By this definition, 2 patients (nos. 2 and 5) responded by a reduction of the daily iron burden by 21% and 54%, respectively. In patient no. 5, the transfusion intervals were extended from 4 weeks before the start of therapy to 8 and 9 weeks, respectively, during treatment (Figure1). The prolonged transfusion intervals were interrupted only once—when he developed an acute episode of catheter sepsis with hemolysis, during which time IBT was stopped. This was followed by an immediate relapse, with 2 transfusions being required at a 4-week interval. However, an extension of the transfusion intervals to 8 and 9 weeks, respectively, was possible after IBT treatment could be resumed. In this patient, the average daily iron load decreased from 455 μg/kg per day (range 451-459 μg/kg per day) before the start of therapy to 211 μg/kg per day (range 203-286 μg/kg per day) during the treatment period of 12 months.
Hematologic characterization of 8 patients with transfusion dependent β-thalassemia during 3 months of pretreatment and 8 months (nonresponders) to 13 months (responders) of treatment with oral IBT
| Component . | Patient type . | Values before IBT treatment* . | Values during IBT treatment† . | Pvalue . |
|---|---|---|---|---|
| Median (Interquartile range) . | Median (Interquartile range) . | |||
| HbF‡(%) | Responders | 5.2 (4.8-6.0) | 9.3 (7.1-16.4) | P = .0016 |
| Nonresponders | 2.4 (1.8-4.3) | 4.0 (2.9-6.6) | P = .0089 | |
| P = .0007 | P < .0001 | |||
| Absolute HbF‡(g/L) | Responders | 4.5 (4.0-5.1) | 7.1 (6.1-13.6) | P = .0018 |
| Nonresponders | 1.9 (1.5-2.4) | 3.4 (2.5-5.4) | P = .0071 | |
| P = .0011 | P < .0001 | |||
| MCV‡(fL) | Responders | 79.5 (76.5-81.3) | 83.8 (82.2-87.8) | P = .0029 |
| Nonresponders | 79.0 (77.0-81.0) | 86.1 (83.0-88.0) | P < .0001 | |
| P = .92 | P = .29 | |||
| MCH‡(pg) | Responders | 27.5 (26.7-28.1) | 28.8 (28.2-29.0) | P = .0056 |
| Nonresponders | 27.6 (27.2-27.9) | 29.2 (28.3-29.9) | P < .0001 | |
| P = .76 | P = .073 | |||
| Free Hb‡(g/L) | Responders | 0.51 (0.43-0.75) | 0.21 (0.17-0.27) | P = .0003 |
| Nonresponders | 0.45 (0.38-0.87) | 0.18 (0.15-0.22) | P < .0001 | |
| P = .59 | P = .20 | |||
| Erythropoietin‡(IU/L) | Responders | ND | 340 (180-480) | — |
| Nonresponders | ND | 66 (30-103) | — | |
| P < .0001 | ||||
| Iron load2-153 (μg/kg per day) | Responders | 539 (454-685) | 360 (214-564) | P = .0322 |
| Nonresponders | 444 (392-658) | 458 (385-647) | P = .96 | |
| P = .19 | P = .013 | |||
| Hemoglobin‡(g/L) | Responders | 84 (82-87) | 85 (82-86) | P > .1 |
| Nonresponders | 85 (81-87) | 85 (81-87) | P > .1 | |
| P > .1 | P > .1 | |||
| nRBCs/nL‡ | Responders | 0.67 (0.0-1.43) | 0.28 (0.0-0.74) | P > .1 |
| Nonresponders | 0.285 (0.075-3.77) | 0.18 (0.0-0.55) | P > .1 | |
| P > .1 | P > .1 |
| Component . | Patient type . | Values before IBT treatment* . | Values during IBT treatment† . | Pvalue . |
|---|---|---|---|---|
| Median (Interquartile range) . | Median (Interquartile range) . | |||
| HbF‡(%) | Responders | 5.2 (4.8-6.0) | 9.3 (7.1-16.4) | P = .0016 |
| Nonresponders | 2.4 (1.8-4.3) | 4.0 (2.9-6.6) | P = .0089 | |
| P = .0007 | P < .0001 | |||
| Absolute HbF‡(g/L) | Responders | 4.5 (4.0-5.1) | 7.1 (6.1-13.6) | P = .0018 |
| Nonresponders | 1.9 (1.5-2.4) | 3.4 (2.5-5.4) | P = .0071 | |
| P = .0011 | P < .0001 | |||
| MCV‡(fL) | Responders | 79.5 (76.5-81.3) | 83.8 (82.2-87.8) | P = .0029 |
| Nonresponders | 79.0 (77.0-81.0) | 86.1 (83.0-88.0) | P < .0001 | |
| P = .92 | P = .29 | |||
| MCH‡(pg) | Responders | 27.5 (26.7-28.1) | 28.8 (28.2-29.0) | P = .0056 |
| Nonresponders | 27.6 (27.2-27.9) | 29.2 (28.3-29.9) | P < .0001 | |
| P = .76 | P = .073 | |||
| Free Hb‡(g/L) | Responders | 0.51 (0.43-0.75) | 0.21 (0.17-0.27) | P = .0003 |
| Nonresponders | 0.45 (0.38-0.87) | 0.18 (0.15-0.22) | P < .0001 | |
| P = .59 | P = .20 | |||
| Erythropoietin‡(IU/L) | Responders | ND | 340 (180-480) | — |
| Nonresponders | ND | 66 (30-103) | — | |
| P < .0001 | ||||
| Iron load2-153 (μg/kg per day) | Responders | 539 (454-685) | 360 (214-564) | P = .0322 |
| Nonresponders | 444 (392-658) | 458 (385-647) | P = .96 | |
| P = .19 | P = .013 | |||
| Hemoglobin‡(g/L) | Responders | 84 (82-87) | 85 (82-86) | P > .1 |
| Nonresponders | 85 (81-87) | 85 (81-87) | P > .1 | |
| P > .1 | P > .1 | |||
| nRBCs/nL‡ | Responders | 0.67 (0.0-1.43) | 0.28 (0.0-0.74) | P > .1 |
| Nonresponders | 0.285 (0.075-3.77) | 0.18 (0.0-0.55) | P > .1 | |
| P > .1 | P > .1 |
ND indicates not done.
Data obtained prestudy, spanning 3 transfusion intervals (3 months).
Data obtained during 8 months of IBT treatment in nonresponders and during 12 to 13 months in responders.
For calculation of the median, pretransfusion values were used.
Assuming that 1 mL of RBC concentrate contains 1 mg of iron, daily iron load was calculated from the volume of RBC concentrate necessary to maintain total Hb not below 85 g/L before and during IBT therapy.
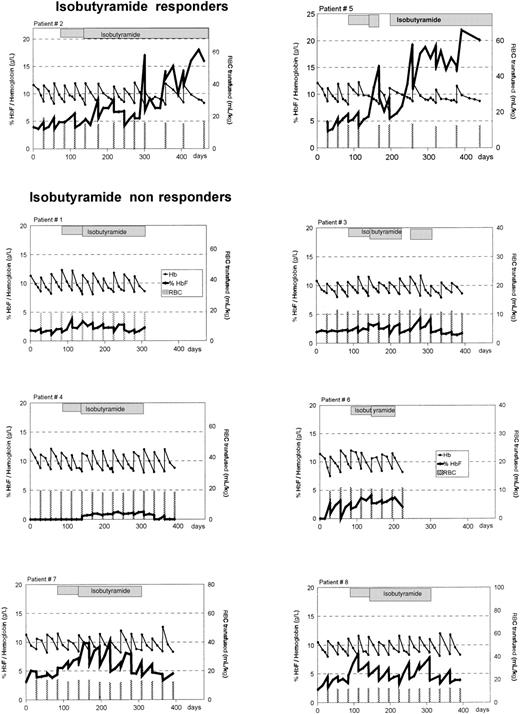
Alteration in percentage of HbF and total Hb in 8 patients with homozygous β-thalassemia treated with oral IBT.
Percentage of HbF, —; total Hb, —. Bars indicate the times of blood transfusions and the volume administered. The hatched boxes indicate administration of IBT. After 8 weeks of treatment, the dosage of IBT was increased from 250 mg/kg per day to 350 mg/kg per day in each patient. Treatment was interrupted in patient no. 5 from days 172 to 200 because of septicemia and in patient no. 3 from days 224 to 252 because of peripheral neuropathy.
Alteration in percentage of HbF and total Hb in 8 patients with homozygous β-thalassemia treated with oral IBT.
Percentage of HbF, —; total Hb, —. Bars indicate the times of blood transfusions and the volume administered. The hatched boxes indicate administration of IBT. After 8 weeks of treatment, the dosage of IBT was increased from 250 mg/kg per day to 350 mg/kg per day in each patient. Treatment was interrupted in patient no. 5 from days 172 to 200 because of septicemia and in patient no. 3 from days 224 to 252 because of peripheral neuropathy.
In patient no. 2, the effect of IBT was more protracted (Figure 1). During the pretreatment phase, this patient needed blood transfusions every 4 weeks. This did not change during the first 8 weeks after the initiation of IBT treatment, but transfusion intervals could be extended to 5 and 6 weeks thereafter. After a subsequent return to 4-week intervals for 3 cycles, a consistent extension to 8-week intervals ensued. In this patient, the average iron load declined from 683 μg/kg per day (range 618-748 μg/kg per day) prestudy to 542 μg/kg per day (range 340-596 μg/kg per day) during 13 months of IBT treatment.
In contrast to the 2 responders, in all of the 6 other patients the decline of post-transfusion Hb levels to the transfusion threshold of 85 g/L occurred within 4-week intervals irrespective of IBT treatment. These patients were therefore considered to represent nonresponders (Figure 1). Their daily iron load during the 3-month post-IBT phase was 449 μg/kg per day (range 480-586 μg/kg per day) and did not differ from iron load before or during IBT (Table 2).
Fetal hemoglobin production
Patient no. 5 showed a progressive increase in the HbF level over 300 days on IBT therapy (Figure 1). This was interrupted only once—by an acute episode of anemia related to septicemia from days 144 to 172, during which time IBT therapy was discontinued. This was paralleled by a rapid decrease of the HbF level. When IBT therapy was resumed, the HbF level increased again and peaked at 22% (22 g/L) on day 308. Patient no. 2 displayed a less marked response to IBT therapy than patient no. 5 (Figure 1). The HbF level increased to a maximum of 18% (17 g/L) on day 365.
It is notable that increases in HbF levels were not only seen in clinical responders but also in nonresponders (Figure 1; Table 2). Based on an intention-to-treat analysis, all 8 patients showed an increase in HbF production after oral IBT therapy was initiated. The median pretransfusion percentage of HbF increased from 3.1% (range 1.9%-4.8%) before therapy to 6.0% (range 3.3%-8.7%) (P = .0017) during oral IBT treatment. The median pretransfusion absolute HbF level rose from 2.7 g/L (range 1.6-4.1 g/L) to 5.1 g/L (range 2.7-6.6 g/L) (P = .0013). Upon discontinuation of IBT, the HbF level decreased rapidly and did not differ from pretreatment values; the absolute HbF concentration was 2.8 g/dL (range 0.83-4 g/L), and percent HbF was 2.4% (range 1.7%-4.5%);P > 0.1.
Interestingly, HbF concentrations were higher in clinical responders not only during but also before IBT treatment: Although at comparable baseline Hb levels, responders no. 2 and 5 displayed an HbF level of 5% (range 4.7%-5.7%) and 5.3% (range 4.9%-6.1%), respectively, before start of therapy, whereas the nonresponders' HbF was either not detectable or did not exceed 4.3%. Parental HbF levels of the responders were also higher than those of the nonresponders (Table 1), suggesting a genetic factor that influences the capacity to respond to IBT treatment.
Hematologic responses and indicators of hemolysis
During IBT therapy, all patients experienced an increase of pretransfusion MCV from 79 fL (range 77-81 fL) to 86 fL (range 82.6-88 fL) (P < .0001) and of pretransfusion MCH from 27.6 pg (range 27-28 pg) to 28.9 pg (range 28.3-29.7) (P < .0001) (Table 2). After cessation of IBT therapy, both MCV and MCH returned to pretreatment values within the 3-month follow-up period (data not shown). In addition, all 8 patients showed evidence of reduced hemolysis/ineffective erythropoiesis during IBT therapy. Median values for free Hb (pretransfusion) decreased from 0.48 g/L (range 0.39-0.81 g/L) to 0.19 g/L (range 0.16-0.24 g/L) (P < .0001) (Table2). Upon discontinuation of IBT, free Hb returned to pretreatment levels within the 3-month follow-up period (data not shown). In neither of the 8 patients were significant prestudy, study, or poststudy changes observed for the following parameters: LDH 232 IU/L (range 207-347 IU/L) prestudy, 218 IU/L (range 195-322 IU/L) during the study, and 241 IU/L (range 188-362 IU/L) poststudy—P > .1; reticulocyte counts 5% (range 3%-8.8%), 4% (range 2%-8%), and 3.5% (range 1%-7.8%), respectively—P > .1; nRBCs 0.29/nL (range 0.066-1.46/nL), 0.18/nL (range 0.0-0.58/nL), and 0.42/nL (range 0.19-9.0/nL), respectively— P > 0.1.
Circulating EPO concentrations were markedly higher in clinical responders than nonresponders (Table 2). Of the 6 patients with EPO levels below 150 IU/L, none responded to therapy, but the 2 responders displayed EPO levels above 150 IU/L. It is notable that EPO levels in the responders were significantly higher than in the nonresponders right from the start of IBT therapy. However, there were no further increases during the trial period thereafter (data not shown).
The β-globin genotype
Responder no. 5 was a compound heterozygote for the nonsense mutation of codon 37 and the −86 C → G mutation. The other responder (no. 2) was homozygous for the common nonsense mutation of codon 39, which results in the complete inactivation of the affected gene. In the 6 other patients who were classified as nonresponders, severe β-globin mutations that are common in the Mediterranean area could be identified (Table 1).
Compliance with IBT therapy
Four-week supplies of IBT were provided to the patients. Compliance was recorded by counting empty vials. Reported compliance with medication was 94% ± 3% (mean ± SD). Therapy in patient no. 6 was discontinued at day 126 after he had developed DFO-induced liver toxicity (see “Adverse events”). After elective cessation of therapy, patient no. 1 had no post-treatment phase because he could no longer visit the outpatient clinic. The 2 responders (nos. 2 and 5) continue to take IBT medication.
Adverse events
The most common adverse effect reported by all patients was a bitter taste and a full feeling immediately after the drug was taken,. However, this adverse effect was not severe enough to cause discontinuation of the treatment in any of the patients. Patients were allowed to vary the aqueous vehicle and, in the course of the trial, 6 of the 8 patients switched to cherry juice, which was felt to cover the bitter taste best. Only 1 patient continued taking grapefruit juice as the flavoring agent; 3 patients complained about recurrent epigastric discomfort, especially after the morning dose of the drug. Patient no. 6 complained of repeated episodes of nausea after taking IBT. By the 28th week of treatment, patient no. 3 developed bilateral numbness of the fingers and toes and weakness of the legs, with difficulties walking. She already had an episode of similar symptoms 1 year before the initiation of IBT. The symptoms were interpreted to represent peripheral neuropathy, which was supported by a decreased nerve conduction velocity. However, these findings persisted after the withdrawal of IBT. The patient then discontinued chelation therapy, and neuropathy slowly improved despite reintroduction of IBT after 4 weeks. After approximately 12 weeks of IBT, patient no. 5 developed septicemia caused by Staphylococcus cohnii in the implanted venous access port, which was treated with antibiotics. IBT therapy was discontinued for 4 weeks and resumed thereafter. Patient no. 6 developed jaundice and an increase in serum LDH levels causing discontinuation of IBT on day 126. However, these signs of liver toxicity only improved after the patient was taken off DFO. Several attempts to restart chelation therapy at a much lower dose were followed by a prompt rise in serum bilirubin levels, which returned to normal after cessation of DFO. Subject no. 7 developed a follicular pruritic rash covering the arms and extending to the trunk and thighs after 4 weeks of treatment. Within several days, the rash subsided spontaneously.
Discussion
The present study aimed to determine the safety and efficacy of a prolonged administration of oral IBT in a group of patients with homozygous β-thalassemia. In all of the patients enrolled, IBT increased HbF, with a prompt decline to baseline levels upon discontinuation of the drug. The bitter taste of the powder and epigastric discomfort represented common adverse effects during the 4- to 12-month course of IBT treatment. Many attempts to disguise the bitter taste proved unsatisfactory, although none of the patients actually discontinued IBT treatment because of its taste. One patient developed symptoms of peripheral neuropathy, which may possibly be related to IBT therapy. However, there was an episode of similar symptoms before IBT treatment. Furthermore, symptoms did not improve upon discontinuation of IBT. Instead, the patient recovered after DFO was stopped, which suggests that this adverse effect may be related to DFO rather than IBT. Another patient treated with 75 mg/kg per day of DFO for 12 years developed jaundice and an elevation in serum LDH during IBT. These symptoms persisted after the withdrawal of the study drug but improved immediately after stopping DFO. We assume that this liver toxicity represents an adverse effect of DFO therapy in this patient with hepatitis C infection and severe siderosis of the liver. However, IBT may have contributed to these symptoms.
A transfusion regimen with a baseline Hb of at least 100 g/L results in the down-regulation of endogenous EPO production and subsequently in the suppression of marrow activity.22 Assuming that a drug that activates endogenous HbF production cannot display its full effect in the presence of erythroid marrow inhibition, we lowered the pretransfusion Hb to 85 g/L during the study. Serial reticulocyte and nRBC counts assessed before, during, and after IBT treatment were performed to distinguish F-cell selection from a genuine increase of HbF synthesis. After initiation of IBT treatment, 2 of 8 patients demonstrated an improvement in the effectiveness of erythropoiesis as measured by a slower decrease of post-transfusion Hb to baseline levels. These effects were associated with an increased HbF production. The slower decline of Hb resulted in an extension of the transfusion intervals, with a corresponding reduction of the average daily iron loading by 21% to 54%. Such a reduction in excess body iron is expected to be beneficial because the mortality of patients with β-thalassemia is directly related to iron load.23-25 The intermittent decline of transfusion intervals to pretreatment levels in patient no. 2 may be explained by limited compliance, drug tolerance, or IBT-induced cellular growth arrest.18,26 27
A correlation between the β-globin mutation and the clinical response could not be identified. One responder (no. 5) was a compound heterozygote for the nonsense mutation of codon 37 and the rare −86 C → G mutation. This mutation affects the CACCC box of the promoter, which binds the ubiquitous transcription factor SP1 and the erythroid-specific transcription factor EKLF.28 Mutations of this sequence element are commonly associated with thalassemia intermedia.29,30 However, this particular mutation has previously been described in a single patient with thalassemia major.31 Similarily, patient no. 5 and his deceased elder brother clearly displayed the phenotype of transfusion-dependent thalassemia major.
Both dosage and duration of IBT therapy appear to be critical for sustained rises of HbF. In a previous short-term pilot study in patients with thalassemia intermedia, modest increases of HbF were attained by IBT in dosages not exceeding 150 mg/kg of body weight.19 The data of the study presented here suggest that dosages of 350 mg/kg or more and a longer treatment period may be needed to achieve functionally significant increases of Hb concentrations.
Although in all patients an increase of the HbF level was demonstrated during IBT treatment, a clinical benefit was achieved in only 2 patients. It is notable that the parental HbF level was increased in those 2 patients, suggesting a genetic factor responsible for a particular propensity to activate HbF synthesis under conditions of erythroid stress. Similarly, raised parental HbF levels have previously been associated with an increase of γ-globin synthesis in an Indian population with sickle cell disease.32 Furthermore, the pretreatment HbF levels of the 2 responders were significantly increased in comparison with the 6 nonresponders at a similar baseline Hb, which also suggests a particular capacity of the red cell precursors to synthesize γ-globin. Similar data have been obtained with the use of oral sodium phenylbutyrate in β-thalassemia.16 The hypothesis that the response to IBT therapy may be predicted by a higher baseline HbF is supported by observations in transgenic mice carrying γ-globin genes that differ in their level of expression.33 Those mice with highly preactivated γ-globin genes had a marked increase of γ-globin expression while on butyrate therapy, whereas no effect of butyrates occurred in those mice with a totally silenced γ-gene. Thus, the sensitivity of the γ-globin genes for butyrates may be genetically controlled.
A second feature that becomes apparent in the current study is the positive correlation between serum EPO levels and the likelihood to respond to IBT therapy. Although all patients were transfused at similar baseline Hb levels during the 3-month prestudy phase, circulating EPO levels were markedly higher in responders right from the start of therapy. This observation correlates with data obtained with oral sodium phenylbutyrate in β-thalassemia16 and with findings in baboons who received a treatment with butyric acid in combination with EPO.34 The explanation for the association between the capacity to respond to IBT therapy and EPO levels is unknown but may be related to a switch of EPO receptors from low to high affinity, as has been suggested in vitro.35Whatever the mechanism, future trials should assess whether a treatment of IBT in combination with EPO is beneficial in patients with low endogenous EPO levels.
In summary, the data presented here demonstrate that IBT monotherapy is capable of achieving a reduction of transfusion requirements in selected patients who were characterized by raised pretreatment and parental HbF levels and elevated endogenous EPO concentrations. Future trials will have to show whether these predicting parameters are valid in a larger number of patients and whether an IBT-EPO combination represents an option for patients with a low baseline level of γ-globin gene activity and low pretreatment EPO levels.
Acknowledgments
We are very grateful to Annette Bode, Ute Brueckner, and Karin Roth-Ostermann for technical assistance and the management of the patients. Special thanks go to the pediatric intensive care unit of the Charité for patient and helpful support at any time.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Andreas E. Kulozik, Department of General Pediatrics, Charité, Humboldt University, Augustenburger Platz 1, D-13353 Berlin, Germany; e-mail:andreas.kulozik@charite.de.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal