Abstract
Reconstitution of the peripheral T-cell compartment is a critical aspect for the success of bone marrow transplantation and is also dependent on the reestablishment of normal thymic structure and function. Graft-versus-host disease (GVHD), however, exacerbates posttransplant immunodeficiency through a deleterious effect on thymic function. To investigate the mechanisms of GVHD-mediated thymic disease, 2 murine parent→F1transplantation models of acute and chronic GVHD, respectively, were studied. Acute GVHD was associated with changes in thymic architecture and a reduction in cellularity mainly because of the decrease in CD4+CD8+, or double-positive (DP) thymocytes, to less than 15% of values found in mice without GVHD. Simultaneously, mature donor-derived T cells expanded in the confines of the allogeneic thymic microenvironment, leading to local inflammation. Through analysis of in vivo cell proliferation, we demonstrated that the ensuing depletion of DP thymocytes was secondary to a decreased commitment of resident pro-T and pre-T cells to enter the cell cycle. Moreover, DP cells themselves showed altered proliferative capacities in the presence of acute GVHD. These findings suggested that thymic atrophy in acute GVHD is effected by impaired cellular proliferation of immature host thymocytes and that the failure of these cells to enter the cell cycle is dependent on an interferon (IFN)-γ–driven immune response. In contrast, interleukin-4–driven chronic GVHD was not accompanied by a sustained thymic infiltration of donor T cells. Consequently, there was a lack of apparent structural changes, a restricted in situ transcription of inflammatory cytokines, and a virtually unchanged cell cycle progression in vivo.
After bone marrow transplantation (BMT), the peripheral T-cell pool is regenerated through thymic and extrathymic pathways.1-3 The first pathway stipulates that either autologous or allogeneic lymphoid precursor cells seed to the recipient's thymus and undergo differentiation and selection to functionally mature T cells. The thymus-independent mechanism of T-cell reconstitution involves the peripheral expansion of donor-derived mature T cells cotransfused with the hematopoietic stem cells. However, prevailing evidence suggests that the thymus-independent pathway of T-cell regeneration is limited in its ability to restore complete host immune competence.1 Indeed, efficient reconstitution of the host T-cell compartment is dependent on the presence of a functional thymus.4-6
The newly generated T lymphocytes recapitulate normal thymic ontogeny, a process that, in turn, is severely affected by graft-versus-host disease (GVHD). GVHD constitutes a major transplant-related complication initiated by host-reactive donor T cells.7,8Histologic analyses and functional studies of clinical and experimental BMT have revealed that thymic epithelial cells, bone marrow–derived thymic stroma cells, and thymocytes are targets of acute GVHD.2,9-12 Moreover, experimental GVHD-induced thymic disease leads to the loss of normal thymic repertoire selection.11,13-15 Thymic dysfunction as a consequence of GVHD is thus likely to contribute to the profound immunodeficiency period regularly observed after BMT.2 3
A typical property of thymic GVHD is the change in the composition of the different thymocyte subpopulations.2 However, the precise mechanism(s) responsible for the depletion of immature (in particular CD4+CD8+ coreceptor double-positive [DP]) thymocytes have not yet been fully established. Increased programmed cell death of DP cells in nonirradiated parent→F1 recipients16 may be caused by cell-mediated cytotoxicity effected by natural killer cells or donor-derived allospecific T cells.17-19 Other studies have suggested that antigen-nonspecific effector mechanisms such as the production of corticosteroids are operational in DP elimination.20-22 Moreover, because intrathymic lymphopoiesis is regulated by intensive cross-talk between thymocytes and different stromal cells,23 24 any injury to or destruction of thymic epithelial cells would have a substantial impact on thymic development.
At present it is unclear whether additional mechanisms contribute to the depletion of the pool of DP thymocytes during GVHD. Here we addressed the prospect that cell cycle progression of the most immature CD3−CD4−CD8−triple-negative (TN) pro-T and pre-T cells is affected by disease. Under normal conditions, the differentiation process within the TN thymocyte subset corresponds to a strong expansion phase,23,25,26 which is an important prerequisite for proper development to DP cells. In the current study, an unirradiated murine P→F1 haploidentical transplantation model was used.27-34 This model provides several advantages. First, it is especially suited to study graft-versus-host reaction (GVHR)-mediated effects on the thymus because it is independent of the injury to thymic epithelial cells by γ-radiation conditioning.35 Second, acute or chronic forms of GVHD can be generated in allogeneic recipients, depending on the haplotype of the parental strain injected. Acute and chronic GVHD are elicited through distinct mechanisms characterized by a differential cytokine production. In acute GVHD (C57BL/6→B6D2F1), the preferential production of IL-2 and interferon (IFN)-γ by expanding donor T cells leads to the generation of antihost cytotoxic T lymphocytes (CTL)28-30 and to the induction of inflammatory cytokines.31 In contrast, chronic GVHD after DBA/2→B6D2F1 transplantation is characterized by the activation and expansion of predominantly IL-4–secreting donor T cells, by the lack of an antihost CTL response, and by the stimulation of host B cells to secrete autoantibodies.30,32-34 36
Our data illustrate that acute GVHD but not chronic GVHD results in severe changes of thymic architecture and leads to aberrant T-cell development. Thymic atrophy during acute GVHD can be explained by at least 2 parallel mechanisms. Here we demonstrate that, in addition to enhanced apoptosis,16 the failure of pro-T cells and pre-T cells to enter the cell cycle and to progress to the next developmental stage similarly contributes to the depletion of DP thymocytes. These profound changes are associated with an intrathymic inflammatory response likely initiated by thymus-infiltrating donor T cells.
Materials and methods
Mice
Female DBA/2 (H-2d, Ly5.2+), C57BL/6 (B6; H-2b, Ly5.2+), and [B6 × DBA/2]F1 (B6D2F1, H-2bd, Ly5.2+) mice were obtained from Biological Research Laboratories (Füllinsdorf, Switzerland). Female B6.SJL-PtprcaPep3b/BoyJ (Ly5.1) congenic mice (B6.Ly5.1, H-2b, Ly5.1+) were purchased from the Jackson Laboratories (Bar Harbor, ME). Animals between 6 and 10 weeks of age were kept under pathogen-free conditions and in accordance with the institute's guidelines and federal regulations.
Reagents
For 3- and 4-color flow cytometric (FACS) analyses, the following monoclonal antibodies (mAbs) conjugated to biotin, fluorescein isothiocyanate, phycoerythrin, or CyChrome were used: anti-CD3 (clone 145-2C11), anti-CD8 (53-6.7), anti-CD4 (RM4-5), anti-TCRβ (H57-592), anti-H-2Kd (SF1-1.1), anti-H2Kb (AF6-88.5), anti-CD44 (clone IM7), anti-CD25 (PC61), anti-Ly5.1 (CD45.1; A20), and anti-CD16/CD32 (2.4G2) (Pharmingen, San Diego, CA), streptavidin–tricolor (Caltag, Burlingame, CA), and streptavidin–Cy5 (Zymed Laboratories, San Francisco, CA). 5′-Bromo-2′-deoxyuridine (BrdU) was obtained from Sigma (Buchs, Switzerland). Fluorescein isothiocyanate–conjugated anti-BrdU mAb 3D4 was purchased from Becton Dickinson (Mountain View, CA). Purified and biotinylated mAbs for the detection of cytokines and immunoglobulins were purchased from Pharmingen. Primers for semiquantitative PCR were designed using oligo 4.0 primer analysis software (National Biosciences, Plymouth, MN) and were manufactured by Life Technologies (Paisley, UK).
Induction and characterization of acute and chronic GVHD
Acute GVHD was induced in unirradiated B6D2F1 mice by the transplantation of 50 × 106 unseparated parental B6 splenocytes, whereas chronic GVHD was induced in B6D2F1mice by the transplantation of 80 × 106 parental DBA/2 splenocytes, as described.16,30 In preparatory experiments we confirmed previous data28-33 that acute GVHD in the B6→B6D2F1 model is characterized by the spontaneous production of IFN-γ by donor T cells and by the generation of antihost CTL. In contrast, the development of chronic GVHD in the DBA/2→B6D2F1 model is characterized by enhanced serum autoantibody levels and by the absence of spontaneous IFN-γ secretion and antihost CTL. The common events shared by these 2 diseases are the expansion of donor-derived CD4+ and CD8+ T cells in secondary lymphoid organs and the development of T-cell immunodeficiency, as assessed by the proliferation of recipient splenocytes in response to various T-cell mitogens in vitro (greater than 90% inhibition; data not shown). Cytokine secretion, CTL activity, and serum immunoglobulins in transplanted hosts were measured, as described elsewhere.28 30
In vivo BrdU labeling
Transplanted and untransplanted mice were injected intraperitoneally with BrdU (1 mg in 0.2 mL phosphate-buffered saline [PBS]) twice at 2-hour intervals, as described.37 38 For the detection and characterization of proliferating cells in situ, mice were killed 1 hour after the second BrdU pulse, and cells were stained and analyzed by flow cytometry.
Flow cytometric analysis
Cells (0.05-1 × 106) were washed, resuspended in 1% fetal calf serum–PBS–sodium azide, and incubated for 15 minutes at 4°C with unlabeled 2.4G2 mAb to prevent unspecific binding to Fcγ receptors. For 3-color flow cytometry, cells were first stained with fluorochrome- and biotin-conjugated mAbs and were subsequently labeled with streptavidin-tricolor or –Cy5. Washed cells were immediately analyzed by flow cytometry (FACScan; Becton Dickinson). For the detection of DNA-synthesizing cells, 4-color flow cytometry was used. Thymocytes were isolated from BrdU-treated mice and surface stained with the appropriate biotin- or fluorochrome-conjugated mAbs. Subsequently, cells were treated with ice-cold 0.15 mol/L NaCl/95% EtOH for 30 minutes at 4°C and fixed for another 30 minutes with PBS containing 1% paraformaldehyde and 0.01% Tween-20. Cells were then incubated for 10 minutes at room temperature in a buffer containing 50 U/mL DNAase I in 0.15 mol/L NaCl/4.2 mmol/L MgCl2, as described.38 After washing, 3D4 mAb was added, and cells were incubated for an additional 30 minutes at room temperature, washed, and finally resuspended in 1% bovine serum albumin–PBS–sodium azide. All 4 color analyses were conducted on a FACScalibur dual laser (Becton Dickinson).
Detection of donor–host chimerism
To discern donor-derived T cells from host T cells in thymus and secondary peripheral lymphatic organs, 2 complementary approaches were used. First, cells from transplanted mice were tested for the simultaneous expression of TCRβ, H-2Kb, and H-2Kd. In acute GVHD, donor T cells were distinguished from resident mature thymocytes by their lack of H-2Kd. In chronic GVHD, TCRβhigh and H-2Kd+, but not H-2Kb+, cells were considered to be of donor origin. Second, splenocytes (50 × 106) from the B6.Ly5.1 (CD45.1) congenic mouse strain were transplanted into B6D2F1 recipients. Donor T cells were distinguished from resident thymocytes by their Ly5.1 expression.
Heterotopic thymus transplantation
A single thymic lobe from neonatal B6 mice was transplanted aseptically under the left kidney capsule of 6- to 8-week-old B6D2F1 mice. Two days later, GVHD was induced in thymus-transplanted mice by the infusion of 50 × 106 splenocytes from B6 donor mice. The orthotopic thymus was allogeneic, whereas the heterotopic thymus was syngeneic to the donor inoculum. Mice were analyzed on day 14 after GVHD induction.
Semiquantitative polymerase chain reaction
Whole thymic tissue or thymocyte subpopulations (where indicated) were isolated 2 weeks after transplantation for polymerase chain reaction (PCR) analysis of cytokine gene expression. To obtain distinct thymocyte subpopulations, single-cell suspensions with specific phenotypes were sorted using a FACSvantage (Becton Dickinson). Purity of Ly5.1+CD4+CD8− and Ly5.1−CD4+CD8− cells was greater than 85%, and purity of Ly5.1+CD4−CD8+ and Ly5.1−CD4−CD8+ thymic cells exceeded 95%. Total RNA from frozen thymic tissue and sorted cells, respectively, was isolated and reverse transcribed, and the resultant cDNA was amplified for 30 cycles. The following primers were used: IFN-γ sense, TACTGCCACGGCACAGTCAT; IFN-γ antisense, GCATCCTTTTTCGCCTTGCT; IL-4 sense, CACCTTGGAAGCCCTACAGA; IL-4 antisense, ATCATCGGCATTTTGAACGA; tumor necrosis factor (TNF)-α sense, AGGTCTACTTTGGAGTCA TTGC; TNF-α antisense, ACATTCGAGGCTCCAGTGAATTCGG; GAPDH sense, CATCAAGAAGGTGGTGAAGC; GAPDH antisense, CCTGTTGCTGTAGCCGTATT. For semiquantitative analysis of cytokine mRNA expression and for comparisons, GAPDH expression was standardized and a ratio of the band intensities of cytokine to GAPDH amplicons was calculated using Quantity-1 software (Bio-Rad, Hercules, CA).
Histopathology
For histologic analysis, thymuses were isolated and embedded in OCT (Tissue-Tek, Sakura Finetec, The Netherlands). Frozen samples were cut into 5-μm thick sections and stained with hematoxylin and eosin.
Statistical analysis
Values were mean ± SEM. For 2-group comparisons, the nonparametric 2-tailed Mann–Whitney U test was used, whereas for multiple group comparisons, analysis of variance (ANOVA) was used.
Results
Loss of thymic architecture is a result of acute GVHD but not chronic GVHD
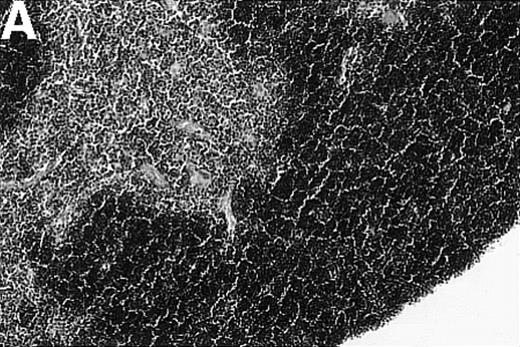
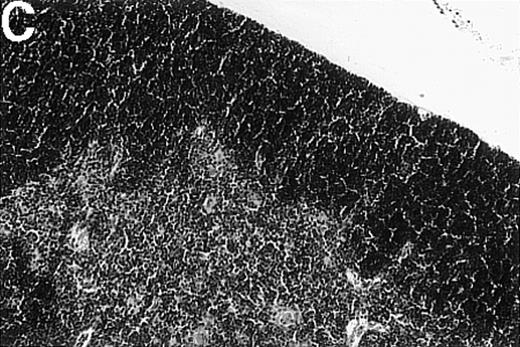
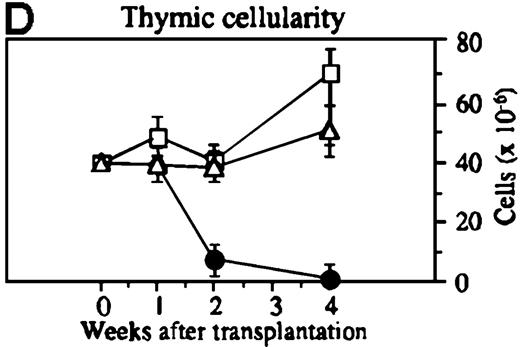
To investigate whether acute or chronic GVHD affects the thymic architecture, an unirradiated P→F1 hybrid model was chosen. After B6→B6D2F1 transplantation, acute GVHD developed, and the thymuses of recipient mice displayed severe morphologic changes (Figure 1). Two weeks after transplantation, the size of the thymus was decreased, and the loss of a regular thymic architecture was apparent with a clear lack of a demarcation between cortex and medulla. In the course of the disease, thymic cellularity progressively decreased and did not recover until the animal's death approximately 4 weeks after transplantation (Figure1D). In contrast, the thymic architecture was not overtly altered during chronic GVHD (DBA/2→B6D2F1). Correspondingly, thymic cellularity was not diminished from this disease when it was analyzed between 1 and 4 weeks after transplantation.
Thymic disease is a consequence of acute but not chronic GVHD.
Acute GVHD was induced by the transfer of 50 × 106parental B6 splenocytes to unirradiated B6D2F1 mice (B), whereas chronic GVHD was induced by the transfer of 80 × 106 parental DBA/2 splenocytes to unirradiated B6D2F1 mice (C). Syngeneically transplanted B6D2F1 mice served as controls (A). Frozen thymic sections (5 μm) were analyzed for histopathology at 2 weeks after transplantation. Magnification ×200. (D) Thymic cellularity as a function of time (total cells per thymus × 10−6) in syngeneically transplanted mice (▵) and in mice with acute (•) and chronic (□) GVHD, respectively. The figure represents combined data (mean ± SEM) of individual mice from at least 8 separate experiments; 20 to 42 mice were analyzed for each group.
Thymic disease is a consequence of acute but not chronic GVHD.
Acute GVHD was induced by the transfer of 50 × 106parental B6 splenocytes to unirradiated B6D2F1 mice (B), whereas chronic GVHD was induced by the transfer of 80 × 106 parental DBA/2 splenocytes to unirradiated B6D2F1 mice (C). Syngeneically transplanted B6D2F1 mice served as controls (A). Frozen thymic sections (5 μm) were analyzed for histopathology at 2 weeks after transplantation. Magnification ×200. (D) Thymic cellularity as a function of time (total cells per thymus × 10−6) in syngeneically transplanted mice (▵) and in mice with acute (•) and chronic (□) GVHD, respectively. The figure represents combined data (mean ± SEM) of individual mice from at least 8 separate experiments; 20 to 42 mice were analyzed for each group.
Aberrant thymic development in acute GVHD
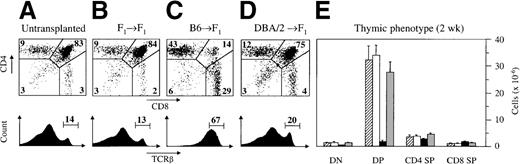
The alterations in thymic architecture suggested that thymocyte development was not regular in the presence of acute GVHD. To assess intrathymic T-cell development, cells were examined by flow cytometry according to their developmental stages.26 39 At 2 weeks after the induction of acute GVHD, the relative distribution of thymocyte subpopulations was abnormal (Figure2). The proportion of DP cells was greatly diminished, whereas the relative size of the TCRβhighpopulation was concomitantly increased by 4- to 5-fold. Absolute cell numbers within the DP population declined from a normal value of 33.7 ± 3.9 × 106 cells in syngeneically transplanted mice to 1.8 ± 0.6 × 106 cells in GVHD+ mice (Figure 2E). Therefore, the strong reduction in thymic cellularity was caused primarily by a decrease in DP thymocytes to 14% of the value found in mice without GVHD. Onset of these changes occurred approximately 8 to 9 days after transplantation. At this point, the frequency of DP cells in GVHD+ mice was 67.4% ± 2.8%, corresponding to a total number of 27.0 ± 4.8 × 106 thymocytes. Concomitantly, we detected 6.8 ± 1.3 million CD4 single-positive (SP) and 2.5 ± 0.4 × 106 CD8 SP mature thymocytes, corresponding to an approximately 3-fold increase over values in syngeneically transplanted mice (data not shown).
Acute GVHD results in aberrant intrathymic T-cell development.
Acute (C) or chronic (D) GVHD was induced in unirradiated B6D2F1 mice, as described in Figure 1. Untransplanted (A) or syngeneically transplanted (B) B6D2F1 mice served as controls. Two weeks after transplantation, the surface expression of CD4 and CD8 (upper panels) and of TCRβ (lower panels) on thymocytes were analyzed by flow cytometry. Cell analysis was restricted to live thymocytes, as defined by forward scatter and side scatter. Numbers depicted in each quadrant of the dot plot represent mean frequencies (%) of the respective populations. (E) Quantification of the 4 major thymocyte subsets (cells × 10−6) was performed in thymuses isolated from untransplanted (▨) or syngeneically transplanted (□) B6D2F1 mice and from mice with acute (▪) and chronic ( ) GVHD, respectively. The figure represents combined data (mean ± SEM) of individual mice from at least 8 separate experiments; 12 to 41 mice were analyzed for each group.
) GVHD, respectively. The figure represents combined data (mean ± SEM) of individual mice from at least 8 separate experiments; 12 to 41 mice were analyzed for each group.
Acute GVHD results in aberrant intrathymic T-cell development.
Acute (C) or chronic (D) GVHD was induced in unirradiated B6D2F1 mice, as described in Figure 1. Untransplanted (A) or syngeneically transplanted (B) B6D2F1 mice served as controls. Two weeks after transplantation, the surface expression of CD4 and CD8 (upper panels) and of TCRβ (lower panels) on thymocytes were analyzed by flow cytometry. Cell analysis was restricted to live thymocytes, as defined by forward scatter and side scatter. Numbers depicted in each quadrant of the dot plot represent mean frequencies (%) of the respective populations. (E) Quantification of the 4 major thymocyte subsets (cells × 10−6) was performed in thymuses isolated from untransplanted (▨) or syngeneically transplanted (□) B6D2F1 mice and from mice with acute (▪) and chronic ( ) GVHD, respectively. The figure represents combined data (mean ± SEM) of individual mice from at least 8 separate experiments; 12 to 41 mice were analyzed for each group.
) GVHD, respectively. The figure represents combined data (mean ± SEM) of individual mice from at least 8 separate experiments; 12 to 41 mice were analyzed for each group.
Essentially normal thymic development during chronic GVHD
Thymocyte development was strikingly different in mice with chronic GVHD from that in mice with acute GVHD (Figure 2). The relative frequencies of the different immature and mature thymocyte subsets were similar to those seen in untransplanted or syngeneically transplanted mice. There was, however, a modest increase in the absolute cell number of CD4 SP thymocytes (to 4.4 ± 0.6 × 106cells) at 2 weeks after transplantation. Time-course experiments did not reveal any changes in the relative frequencies of thymocyte subpopulations, either at earlier time points or as late as 4 weeks after transplantation (data not shown). These minimal numeric changes were in stark contrast to the profound functional T-cell deficiency observed in this model because concanavalin A or anti-CD3–induced proliferative responses of isolated recipient splenocytes (measured at 1 or 2 weeks) were severely diminished despite the presence of phenotypically mature T cells in the periphery (data not shown).
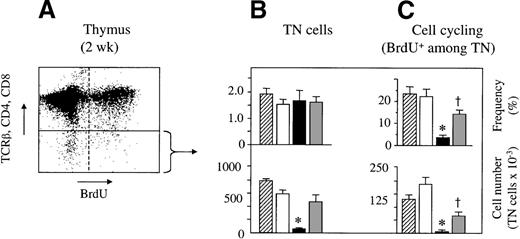
Impaired cell cycle progression of resident TCR−CD4−CD8− thymocytes in the presence of acute and chronic GVHD
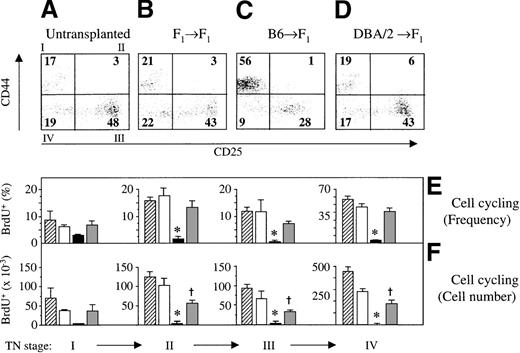
A range of mechanisms may contribute to the observed loss of DP thymocytes in acute GVHD. It is conceivable that GVHD perturbs the development of TN thymocytes to their next stage of maturation, the DP cells. Because the developmental transition from the TN to the DP stage is accompanied by a 20- to 50-fold cellular expansion,25,39resident TN cells were analyzed for cell cycle progression (as tested by BrdU incorporation into DNA; Figure 3A). Two weeks after transplantation, TN cells represented approximately 2% of all thymocytes in both experimental GVHD models, and their frequencies were therefore not different from those of control mice (Figure 3B).39 Because of the decrease in thymic cellularity, however, absolute TN cell numbers were significantly lower in acute GVHD. Significantly, the frequencies of viable BrdU+ TN cells were greatly diminished in acute GVHD when compared to those in syngeneically transplanted mice (Figure 3C). No overt impairment in the frequency (11.2% ± 0.7% vs. 12.6% + 2.2%) or absolute number of cycling cells was observed among the TN thymocytes 1 week after GVHD induction. In the presence of chronic GVHD, both the frequency and the number of cycling TN cells were also decreased in comparison to those in syngeneically transplanted F1 mice. Nevertheless, this reduction was modest in comparison to that for acute GVHD (Figure 3C). TN cells are further subdivided into 4 distinct subsets based on their surface expression of CD44 and CD25. Maturation within the TN compartment occurs in the sequence CD25−CD44+ (stage I) → CD25+CD44+ (stage II) → CD25+CD44− (stage III) → CD25−CD44− (stage IV).26 Cell proliferation occurs mainly in stages II and IV of TN cells.25 39 To investigate at which developmental point GVHD affects cell cycle progression, the relative abundance and BrdU incorporation of the 4 stages were assessed. During acute GVHD, the frequency of stage I pro-T cells was increased in lieu of cells at the usually most populous stage III (Figure4A-C). Cell cycle analysis in acute GVHD revealed that viable TN cells of stages II, III, and IV were severely inhibited in their proliferative capacity (Figure 4E,F). In addition, pro-T cells of stage I also displayed a tendency toward decreased proliferation, though this was not significantly different (P = 0.17) than that in syngeneically transplanted mice. A relative increase of CD44−CD25low thymocytes was noted in thymuses affected by acute GVHD, as measured by the 4-fold lower median CD25 fluorescence intensity of stage III cells (data not shown). This observation suggested a maturation delay in TN cells at the point at which they usually transit swiftly to stage IV.
Cell cycle progression of host-derived TN thymocytes is impaired in GVHD.
Acute or chronic GVHD was induced in unirradiated B6D2F1mice. Untransplanted or syngeneically transplanted B6D2F1mice served as controls. Thymocytes were analyzed at 2 weeks after transplantation for surface expression of CD4, CD8, and TCRβ and for the incorporation of BrdU into cellular DNA.(A) Representative flow cytometric analysis of thymocytes from a mouse with acute GVHD. (B, C) Frequencies of TN thymocytes (%; mean ± SEM) was assessed by flow cytometry of thymuses from untransplanted (▨) or syngeneically transplanted (□) B6D2F1 mice and from mice with acute (▪) and chronic ( ) GVHD, respectively. Lower panels show absolute numbers of cycling cells (mean ± SEM; ×10−3) among TN thymocytes and represent pooled data from 2 independent experiments; 3 to 5 mice were analyzed for each group. *P < .01 versus mice with chronic GVHD and syngeneic controls, respectively. †P < .01 versus syngeneic controls (ANOVA).
) GVHD, respectively. Lower panels show absolute numbers of cycling cells (mean ± SEM; ×10−3) among TN thymocytes and represent pooled data from 2 independent experiments; 3 to 5 mice were analyzed for each group. *P < .01 versus mice with chronic GVHD and syngeneic controls, respectively. †P < .01 versus syngeneic controls (ANOVA).
Cell cycle progression of host-derived TN thymocytes is impaired in GVHD.
Acute or chronic GVHD was induced in unirradiated B6D2F1mice. Untransplanted or syngeneically transplanted B6D2F1mice served as controls. Thymocytes were analyzed at 2 weeks after transplantation for surface expression of CD4, CD8, and TCRβ and for the incorporation of BrdU into cellular DNA.(A) Representative flow cytometric analysis of thymocytes from a mouse with acute GVHD. (B, C) Frequencies of TN thymocytes (%; mean ± SEM) was assessed by flow cytometry of thymuses from untransplanted (▨) or syngeneically transplanted (□) B6D2F1 mice and from mice with acute (▪) and chronic ( ) GVHD, respectively. Lower panels show absolute numbers of cycling cells (mean ± SEM; ×10−3) among TN thymocytes and represent pooled data from 2 independent experiments; 3 to 5 mice were analyzed for each group. *P < .01 versus mice with chronic GVHD and syngeneic controls, respectively. †P < .01 versus syngeneic controls (ANOVA).
) GVHD, respectively. Lower panels show absolute numbers of cycling cells (mean ± SEM; ×10−3) among TN thymocytes and represent pooled data from 2 independent experiments; 3 to 5 mice were analyzed for each group. *P < .01 versus mice with chronic GVHD and syngeneic controls, respectively. †P < .01 versus syngeneic controls (ANOVA).
Cell cycle progression of pro-T cells and pre-T cells is impaired during GVHD.
Acute (C) or chronic (D) GVHD was induced in unirradiated B6D2F1 mice. Untransplanted (A) or syngeneically transplanted (B) B6D2F1 mice served as controls. TN thymocytes were analyzed at 2 weeks after transplantation for the surface expression of CD44 and CD25 and for the incorporation of BrdU into cellular DNA. Numbers depicted in each quadrant of the dot plot represent mean frequencies (%) of the respective populations. (E, F) Frequencies (%; mean ± SEM) and absolute cell numbers (×10−3) of cycling cells among TN thymocytes was assessed by flow cytometry of thymuses from untransplanted (▨) or syngeneically transplanted (□) B6D2F1mice and from mice with acute (▪) and chronic ( ) GVHD, respectively. The graph represents pooled data from 2 independent experiments; 3 to 5 mice were analyzed for each group.*P < .01 versus mice with chronic GVHD and syngeneic controls, respectively. †P < .02 versus syngeneic controls (ANOVA).
) GVHD, respectively. The graph represents pooled data from 2 independent experiments; 3 to 5 mice were analyzed for each group.*P < .01 versus mice with chronic GVHD and syngeneic controls, respectively. †P < .02 versus syngeneic controls (ANOVA).
Cell cycle progression of pro-T cells and pre-T cells is impaired during GVHD.
Acute (C) or chronic (D) GVHD was induced in unirradiated B6D2F1 mice. Untransplanted (A) or syngeneically transplanted (B) B6D2F1 mice served as controls. TN thymocytes were analyzed at 2 weeks after transplantation for the surface expression of CD44 and CD25 and for the incorporation of BrdU into cellular DNA. Numbers depicted in each quadrant of the dot plot represent mean frequencies (%) of the respective populations. (E, F) Frequencies (%; mean ± SEM) and absolute cell numbers (×10−3) of cycling cells among TN thymocytes was assessed by flow cytometry of thymuses from untransplanted (▨) or syngeneically transplanted (□) B6D2F1mice and from mice with acute (▪) and chronic ( ) GVHD, respectively. The graph represents pooled data from 2 independent experiments; 3 to 5 mice were analyzed for each group.*P < .01 versus mice with chronic GVHD and syngeneic controls, respectively. †P < .02 versus syngeneic controls (ANOVA).
) GVHD, respectively. The graph represents pooled data from 2 independent experiments; 3 to 5 mice were analyzed for each group.*P < .01 versus mice with chronic GVHD and syngeneic controls, respectively. †P < .02 versus syngeneic controls (ANOVA).
In chronic GVHD, the frequencies of proliferating cells among each of the 4 TN populations were not statistically different from those of controls without GVHD, even though these fractions were consistently reduced in number (Figure 4F). The frequencies of TN stage II to IV cells undergoing cell proliferation were thus significantly lower in acute GVHD than in chronic GVHD. Taken together, these data suggest that acute GVHD may affect TN thymocytes in their progression to the stage of more mature DP cells secondary to a failure to enter cell cycle at TN stages II to IV.
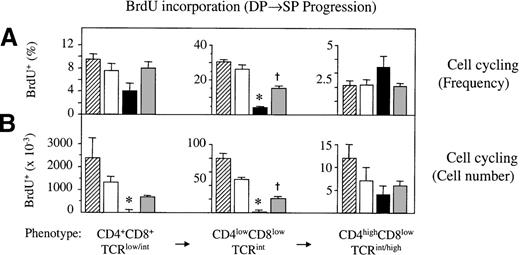
During acute GVHD, DP cells display altered proliferative capacities
Double-positive thymocytes undergo TCR-mediated selection, and survivors of this stringent process differentiate, dependent on their major histocompatibility class specificity, into mature CD4 or CD8 SP thymocytes, respectively. These cells are then competent to emigrate to the periphery.39 To test whether the few DP thymocytes present in an atrophied thymus are able to progress normally to their subsequent SP stage, we analyzed thymocytes at distinct developmental stages along the DP→SP differentiation pathway.40During acute GVHD, the frequency of proliferating CD4lowCD8low TCRint thymocytes was strongly diminished (Figure 5A). Two weeks after transplantation, fewer than 2 × 103 cells incorporated BrdU as opposed to 49 × 103 cells in syngeneically transplanted mice. In the CD4+CD8+TCRlow/int and CD4highCD8lowTCRint/hi populations, the frequencies of BrdU+ cells were not statistically different from those of mice without GVHD, though absolute cell numbers were decreased. In chronic GVHD, incorporation of BrdU was similar to that in non-GVHD controls except for the CD4lowCD8low TCRint cells that proliferated by approximately 50% less. This was a modest decrease in comparison to that in acute GVHD (Figure 5).
Impaired cell cycle progression of DP thymocytes during GVHD.
Acute and chronic GVHD was induced in unirradiated B6D2F1mice. Syngeneically transplanted or naive B6D2F1 mice served as non-GVHD controls. Three developmental stages along the DP→SP differentiation pathway were analyzed at 2 weeks after transplantation for the incorporation of BrdU into cellular DNA. (A) Frequencies (%; mean ± SEM) and (B) absolute cell numbers (×10−3) of cycling cells among DP thymocytes were assessed by flow cytometry of thymocytes from untransplanted (▨) or syngeneically transplanted (□) B6D2F1 mice and from mice with acute (▪) and chronic ( ) GVHD, respectively. The graph represents pooled data from 3 independent experiments; 4 to 7 mice were analyzed for each group. *P < .2 versus mice with chronic GVHD and syngeneic controls, respectively.†P < .02 versus syngeneic controls (ANOVA).
) GVHD, respectively. The graph represents pooled data from 3 independent experiments; 4 to 7 mice were analyzed for each group. *P < .2 versus mice with chronic GVHD and syngeneic controls, respectively.†P < .02 versus syngeneic controls (ANOVA).
Impaired cell cycle progression of DP thymocytes during GVHD.
Acute and chronic GVHD was induced in unirradiated B6D2F1mice. Syngeneically transplanted or naive B6D2F1 mice served as non-GVHD controls. Three developmental stages along the DP→SP differentiation pathway were analyzed at 2 weeks after transplantation for the incorporation of BrdU into cellular DNA. (A) Frequencies (%; mean ± SEM) and (B) absolute cell numbers (×10−3) of cycling cells among DP thymocytes were assessed by flow cytometry of thymocytes from untransplanted (▨) or syngeneically transplanted (□) B6D2F1 mice and from mice with acute (▪) and chronic ( ) GVHD, respectively. The graph represents pooled data from 3 independent experiments; 4 to 7 mice were analyzed for each group. *P < .2 versus mice with chronic GVHD and syngeneic controls, respectively.†P < .02 versus syngeneic controls (ANOVA).
) GVHD, respectively. The graph represents pooled data from 3 independent experiments; 4 to 7 mice were analyzed for each group. *P < .2 versus mice with chronic GVHD and syngeneic controls, respectively.†P < .02 versus syngeneic controls (ANOVA).
Donor T cells migrate to the thymus during acute GVHR
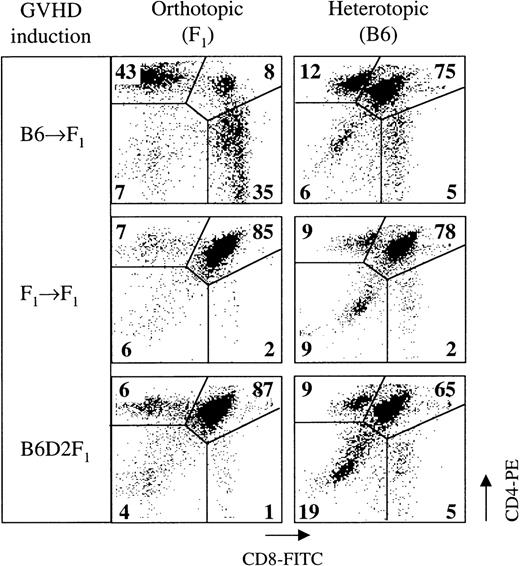
Our results and previous data2,3,16,18 19demonstrate that the thymus is a target of acute GVHD in the P→F1 model. To test whether the observed thymic changes were a consequence of alloantigen-specific recognition, mice were transplanted with thymic lobes and then with allogeneic splenocytes. To this end, neonatal B6 thymuses were grafted under the kidney capsule of naive B6D2F1 mice, which in turn were infused 2 days later with B6 splenocytes to induce GVHD. Thus, the orthotopic thymus was allogeneic whereas the heterotopic thymus was syngeneic to the infused T cells. Orthotopic and heterotopic thymic tissues were analyzed in the double-transplanted mice at the height of the GVHR at 2 weeks after T-cell transfer. Flow cytometric analysis revealed that DP cells were eliminated from the orthotopic thymus, whereas the cellular composition of the transplanted thymus appeared indistinguishable from that of mice without GVHD (Figure6). These data infer that the depletion of DP cells during acute GVHD is a direct consequence of allorecognition of host DP thymocytes.
A heterotopically transplanted thymus syngeneic to donor splenocytes is not a target of acute GVHD.
A single thymic lobe from neonatal B6 mice was transplanted aseptically under the left kidney capsule of B6D2F1 mice. Forty-eight hours later, GVHD was induced in organ-transplanted mice by the infusion of 50 × 106 B6 splenocytes. In this setting, the orthotopic thymus is allogeneic, whereas the heterotopic thymus is syngeneic to the donor inoculum. Thymocytes were analyzed for surface expression of CD4 and CD8 by flow cytometry on day 14 after GVHD induction (day 16 after thymus transplantation). Numbers within the flow cytometry plots represent mean frequencies (%) of the respective populations among all live thymocytes. The graph is representative of 1 experiment; 2 to 3 mice were analyzed for each group.
A heterotopically transplanted thymus syngeneic to donor splenocytes is not a target of acute GVHD.
A single thymic lobe from neonatal B6 mice was transplanted aseptically under the left kidney capsule of B6D2F1 mice. Forty-eight hours later, GVHD was induced in organ-transplanted mice by the infusion of 50 × 106 B6 splenocytes. In this setting, the orthotopic thymus is allogeneic, whereas the heterotopic thymus is syngeneic to the donor inoculum. Thymocytes were analyzed for surface expression of CD4 and CD8 by flow cytometry on day 14 after GVHD induction (day 16 after thymus transplantation). Numbers within the flow cytometry plots represent mean frequencies (%) of the respective populations among all live thymocytes. The graph is representative of 1 experiment; 2 to 3 mice were analyzed for each group.
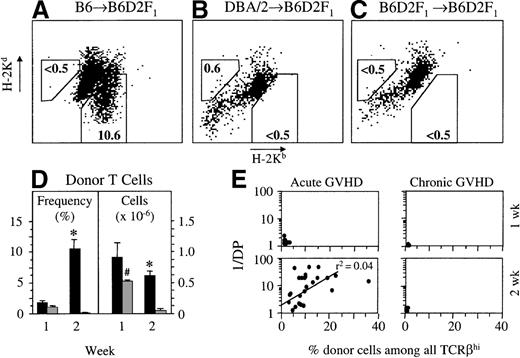
We therefore tested whether and to what extent donor-derived mature T cells infiltrated the thymus during GVHD. In the acute GVHD model, donor-derived T cells were distinguished from mature host thymocytes because of the lack of H-2Kd expression by the former (Figure 7A). Donor-derived T cells could first be detected at 1 week after transplantation (Figure 7D). At this time, splenic donor–host chimerism was already substantial (more than 30% of splenic CD4+ and CD8+ T cells were of donor origin; data not shown). The frequency of intrathymic donor T cells increased to 11% cells among TCRβhighcells 2 weeks after transplantation (corresponding to an absolute number of 0.62 ± 0.09 × 106 donor T cells). The relative increase was primarily caused by the progressive decrease in host-type thymocytes. Comparable results were obtained with the transfer of splenocytes from B6.Ly5.1 congenic donors to B6D2F1 recipients. On average, 22% of CD4 and 43% of CD8 SP mature thymic T cells were Ly5.1+ at 2 weeks after transplantation, corresponding to approximately 106donor-derived intrathymic T cells (data not shown). Taking the loss of DP thymocytes (1/DP) as a measure of thymic injury, the number of infiltrating donor T cells was directly correlated with the phenotypic changes in thymocyte subsets (Figure 7E).
During acute GVHD, donor-derived T cells infiltrate the thymus.
Acute (A) or chronic (B) GVHD was induced in unirradiated B6D2F1 mice. Syngeneically transplanted B6D2F1mice served as controls (C), and donor–host chimerism was measured at 2 weeks after transplantation. In the B6→B6D2F1model, mature donor T cells were TCRβhighH-2Kb+Kd−, whereas in the DBA/2→B6D2F1 model, TCRβhighH-2Kb-H-2Kd+ T cells were of donor origin. The background in syngeneically transplanted mice was 0.5% or less. (D) Quantification of donor-derived mature T cells in thymuses of transplanted hosts with acute (▪) or chronic ( ) GVHD. Donor cell infiltration is given either as a percentage of all TCRβhigh thymocytes or as an absolute cell number (×10−6) of donor-derived T cells among TCRβhigh cells in the thymus at 1 and 2 weeks after transplantation (mean ± SEM). (E) Loss of DP thymocytes (1/DP) is plotted against the percentage of TCRβhigh infiltrating donor T cells at 1 and 2 weeks after transplantation for both GVHD models. The graph represents pooled data from 2 (chronic GVHD) to 9 (acute GVHD) independent experiments; 5 to 25 mice were analyzed for each group. *P < .01 versus mice with chronic GVHD.#P < .01 versus chronic GVHD at 2 weeks (2-tailed Mann–Whitney U test).
) GVHD. Donor cell infiltration is given either as a percentage of all TCRβhigh thymocytes or as an absolute cell number (×10−6) of donor-derived T cells among TCRβhigh cells in the thymus at 1 and 2 weeks after transplantation (mean ± SEM). (E) Loss of DP thymocytes (1/DP) is plotted against the percentage of TCRβhigh infiltrating donor T cells at 1 and 2 weeks after transplantation for both GVHD models. The graph represents pooled data from 2 (chronic GVHD) to 9 (acute GVHD) independent experiments; 5 to 25 mice were analyzed for each group. *P < .01 versus mice with chronic GVHD.#P < .01 versus chronic GVHD at 2 weeks (2-tailed Mann–Whitney U test).
During acute GVHD, donor-derived T cells infiltrate the thymus.
Acute (A) or chronic (B) GVHD was induced in unirradiated B6D2F1 mice. Syngeneically transplanted B6D2F1mice served as controls (C), and donor–host chimerism was measured at 2 weeks after transplantation. In the B6→B6D2F1model, mature donor T cells were TCRβhighH-2Kb+Kd−, whereas in the DBA/2→B6D2F1 model, TCRβhighH-2Kb-H-2Kd+ T cells were of donor origin. The background in syngeneically transplanted mice was 0.5% or less. (D) Quantification of donor-derived mature T cells in thymuses of transplanted hosts with acute (▪) or chronic ( ) GVHD. Donor cell infiltration is given either as a percentage of all TCRβhigh thymocytes or as an absolute cell number (×10−6) of donor-derived T cells among TCRβhigh cells in the thymus at 1 and 2 weeks after transplantation (mean ± SEM). (E) Loss of DP thymocytes (1/DP) is plotted against the percentage of TCRβhigh infiltrating donor T cells at 1 and 2 weeks after transplantation for both GVHD models. The graph represents pooled data from 2 (chronic GVHD) to 9 (acute GVHD) independent experiments; 5 to 25 mice were analyzed for each group. *P < .01 versus mice with chronic GVHD.#P < .01 versus chronic GVHD at 2 weeks (2-tailed Mann–Whitney U test).
) GVHD. Donor cell infiltration is given either as a percentage of all TCRβhigh thymocytes or as an absolute cell number (×10−6) of donor-derived T cells among TCRβhigh cells in the thymus at 1 and 2 weeks after transplantation (mean ± SEM). (E) Loss of DP thymocytes (1/DP) is plotted against the percentage of TCRβhigh infiltrating donor T cells at 1 and 2 weeks after transplantation for both GVHD models. The graph represents pooled data from 2 (chronic GVHD) to 9 (acute GVHD) independent experiments; 5 to 25 mice were analyzed for each group. *P < .01 versus mice with chronic GVHD.#P < .01 versus chronic GVHD at 2 weeks (2-tailed Mann–Whitney U test).
In chronic GVHD, donor-derived T cells were distinguished from mature host thymocytes by their lack of H-2Kb expression (Figure7B). Despite the limited infiltration at 1 week after transplantation, there was only a small number of donor T cells after an additional week (less than 1% of total thymocytes, or 0.20 ± 0.05 × 106 cells; Figure 7D). Thus, donor T cells demonstrate in chronic GVHD a limited migration to or expansion in the thymus.
During acute GVHD, thymus-infiltrating T cells expand and express inflammatory cytokines in situ
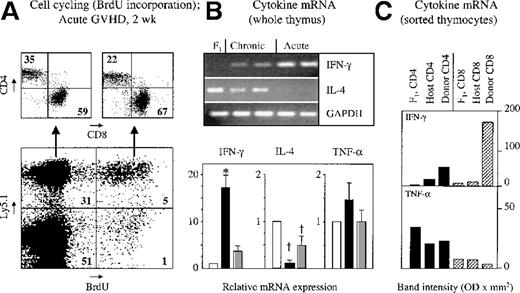
To investigate the function of donor T cells in the thymus of mice with acute GVHD, cell proliferation was assessed in vivo, simultaneously using a donor-specific marker (Ly5.1) with the detection of intracellular BrdU incorporation. Two weeks after the transfer of B6.Ly5.1 splenocytes, 13% of Ly5.1+ T cells were proliferating, and there was a preferential expansion among CD8+ T cells (Figure 8A). To further detail their function, thymus-infiltrating T cells were analyzed for cytokine expression because the development of acute GVHD is linked to the secretion by T cells of the Th1 signature cytokine IFN-γ.30,41 42 Transcription of this cytokine was detected using semiquantitative PCR analysis. A strong up-regulation of IFN-γ–specific message was found in the unseparated thymic tissue of mice with acute GVHD in comparison with untransplanted B6D2F1 mice (Figure 8B). The origin of IFN-γ was subsequently determined separately for donor and host SP T cells, respectively. Among Ly5.1+ T cells, IFN-γ was preferentially expressed in CD8+ T cells (Figure 8C). In contrast, IFN-γ transcripts were detected neither in mature CD4 SP nor in CD8 SP thymocytes of host origin (Ly5.1−). Additional evidence for intrathymic inflammation was provided by the observation that increased transcripts for TNF-α were found in unseparated thymic tissue of mice with acute GVHD when compared to unmanipulated or syngeneically transplanted animals (Figure 8B). Analyzing TNF-α expression in SP thymic cells, specific transcripts were either unchanged or diminished among donor and recipient cells when they were compared to naive B6D2F1 thymocytes (Figure8C). In view of an overall increase of TNF-α mRNA in unseparated thymic tissue, the results obtained with sorted thymic T cells suggested that TNF-α was produced by cells other than thymocytes.
Thymus-infiltrating donor T cells proliferate in situ and express inflammatory cytokines during acute GVHD.
Acute or chronic GVHD was induced in unirradiated B6D2F1mice. Cell cycling and cytokine mRNA expression of thymic cells were analyzed 2 weeks after transplantation. (A) Representative flow cytometric plots of thymocytes from a mouse with acute GVHD. Numbers depicted in each quadrant of the dot plots represent mean frequencies (%) of the respective populations. (B) For analysis of cytokine production, semiquantitative PCR of cDNA from frozen whole thymic tissue was used. PCR products were separated by gel electrophoresis, and the bands depict amplicons from 1 naive B6D2F1 mouse and from 2 representative mice with acute or chronic disease on day 15 after transplantation. Bands were further analyzed by densitometry, and the intensities of each amplicon cytokine mRNA from mice with acute (▪) or chronic ( ) GVHD were expressed as ratios compared with syngeneically transplanted controls (□). Expression levels for the different genes in the B6D2F1 control were arbitrarily set at 1, and differences in initial cDNA input levels were corrected based on band intensities measured for GAPDH products. (C) Freshly isolated thymocytes from naive B6D2F1 mice (F1) and from transplanted mice with acute GVHD (day 15) were separated on a FACSvantage into donor- and host-derived CD4 SP (▪) and CD8 SP (▨) cell subsets based on their expression of Ly5.1. PCR amplification products (IFN-γ and TNF-α) were analyzed by densitometry, and optical densities of bands were compared. The graph represents pooled data from 5 independent experiments; 3 to 8 mice were analyzed for each group. *P < .001 versus mice with chronic GVHD and syngeneic controls, respectively. †P < .03 versus syngeneic controls (ANOVA).
) GVHD were expressed as ratios compared with syngeneically transplanted controls (□). Expression levels for the different genes in the B6D2F1 control were arbitrarily set at 1, and differences in initial cDNA input levels were corrected based on band intensities measured for GAPDH products. (C) Freshly isolated thymocytes from naive B6D2F1 mice (F1) and from transplanted mice with acute GVHD (day 15) were separated on a FACSvantage into donor- and host-derived CD4 SP (▪) and CD8 SP (▨) cell subsets based on their expression of Ly5.1. PCR amplification products (IFN-γ and TNF-α) were analyzed by densitometry, and optical densities of bands were compared. The graph represents pooled data from 5 independent experiments; 3 to 8 mice were analyzed for each group. *P < .001 versus mice with chronic GVHD and syngeneic controls, respectively. †P < .03 versus syngeneic controls (ANOVA).
Thymus-infiltrating donor T cells proliferate in situ and express inflammatory cytokines during acute GVHD.
Acute or chronic GVHD was induced in unirradiated B6D2F1mice. Cell cycling and cytokine mRNA expression of thymic cells were analyzed 2 weeks after transplantation. (A) Representative flow cytometric plots of thymocytes from a mouse with acute GVHD. Numbers depicted in each quadrant of the dot plots represent mean frequencies (%) of the respective populations. (B) For analysis of cytokine production, semiquantitative PCR of cDNA from frozen whole thymic tissue was used. PCR products were separated by gel electrophoresis, and the bands depict amplicons from 1 naive B6D2F1 mouse and from 2 representative mice with acute or chronic disease on day 15 after transplantation. Bands were further analyzed by densitometry, and the intensities of each amplicon cytokine mRNA from mice with acute (▪) or chronic ( ) GVHD were expressed as ratios compared with syngeneically transplanted controls (□). Expression levels for the different genes in the B6D2F1 control were arbitrarily set at 1, and differences in initial cDNA input levels were corrected based on band intensities measured for GAPDH products. (C) Freshly isolated thymocytes from naive B6D2F1 mice (F1) and from transplanted mice with acute GVHD (day 15) were separated on a FACSvantage into donor- and host-derived CD4 SP (▪) and CD8 SP (▨) cell subsets based on their expression of Ly5.1. PCR amplification products (IFN-γ and TNF-α) were analyzed by densitometry, and optical densities of bands were compared. The graph represents pooled data from 5 independent experiments; 3 to 8 mice were analyzed for each group. *P < .001 versus mice with chronic GVHD and syngeneic controls, respectively. †P < .03 versus syngeneic controls (ANOVA).
) GVHD were expressed as ratios compared with syngeneically transplanted controls (□). Expression levels for the different genes in the B6D2F1 control were arbitrarily set at 1, and differences in initial cDNA input levels were corrected based on band intensities measured for GAPDH products. (C) Freshly isolated thymocytes from naive B6D2F1 mice (F1) and from transplanted mice with acute GVHD (day 15) were separated on a FACSvantage into donor- and host-derived CD4 SP (▪) and CD8 SP (▨) cell subsets based on their expression of Ly5.1. PCR amplification products (IFN-γ and TNF-α) were analyzed by densitometry, and optical densities of bands were compared. The graph represents pooled data from 5 independent experiments; 3 to 8 mice were analyzed for each group. *P < .001 versus mice with chronic GVHD and syngeneic controls, respectively. †P < .03 versus syngeneic controls (ANOVA).
In chronic GVHD, thymic transcripts for TNF-α and IFN-γ were smaller than in the acute form of the disease. The abundance of IFN-γ–specific mRNA was still higher than the concentrations noted in syngeneically transplanted control mice. Taken together, these results demonstrated the dominance of a Th1-like response in thymic tissue of mice with acute GVHD, whereas thymic inflammation in chronic GVHD was minimal and was reflective of a “Th0” cytokine response.
Discussion
Effective reconstitution of the donor-derived peripheral T-cell compartment after BMT is contingent on 2 independent pathways—the intrathymic differentiation of donor stem cells and the peripheral expansion of donor T cells.1-6,43 Thymic function is also important for T-cell reconstitution in adolescent and adult recipients of BMT because, even though thymic lymphoid development declines with time, substantial cellular output is maintained to old age.3,44,45 Relevant to any age, thymic injury is enhanced in the presence of GVHD (reviewed in Hakim and Mackall2). The current study was designed to increase our understanding of the mechanisms by which experimental GVHD causes thymic atrophy. Here we demonstrate that after B6→B6D2F1 transplantation, thymic atrophy is distinguished by a prominent decrease in relative and absolute numbers of DP thymocytes (Figure 2). This change is associated with the intrathymic accumulation of donor-derived T cells that express IFN-γ (Figures 7 and 8). Cell cycle progression among viable resident TN thymocytes is highly reduced in the presence of acute GVHD when compared to syngeneically transplanted animals (Figures 3 and 4). In contrast, the development of chronic GVHD after DBA/2→B6D2F1 transplantation is characterized by almost normal thymic cellularity, phenotype, and cell cycle progression. The scarcity of any changes correlates with a low level of intrathymic inflammation and IFN-γ mRNA expression.
These data led us to conclude that extensive morphologic and functional alterations of the thymus are dependent on a Th1-driven immune response after transplantation. Significantly, the marked loss of DP cells was effected by impaired cellular proliferation of host pro-T and pre-T cells. This finding is relevant because only TN thymocytes that have cycled and have successfully rearranged and expressed 1 TCRβ gene locus are allowed to progress to the DP stage.5,26 39 Thus, acute GVHD prevents the normal TN maturation from stage II to the late stage IV. This lack of a strong clonal expansion results in the failure to generate sufficient amounts of DP thymocytes, leading to thymic atrophy.
The mechanisms responsible for the failure of TN thymocytes to enter cell cycle have not been defined. It is possible that a lack of growth and survival factors by thymic epithelial cells or other cells of the microenvironment causes impairment in TN expansion. In normal thymic development, the cytokine IL-7 provides a crucial survival and proliferation signal to TN thymocytes.46 A lack of stromal cell–derived IL-7 secretion might, therefore, explain the TN defect during acute GVHD. We have measured thymic IL-7 production by semiquantitative PCR in the B6→B6D2F1 model. However, we have not found any evidence for a decrease in stromal IL-7 transcripts in the presence of acute GVHD (data not shown). In contrast, the abundance of IL-7 message was even enhanced when the relative increase of stromal cells was taken into consideration (data not shown). It is conceivable then that the defect in proliferation lies within the population of viable TN thymocytes. This understanding is further supported by the fact that the attendant condition of an acute GVHD-induced intrathymic inflammatory response generates several other cytokines (eg, IL-2, TNF-α) that are known to promote, either alone or in combination, thymocyte proliferation at distinct developmental stages.47,48 Prolonged disruption of the normal thymic microenvironments may nevertheless contribute to a defective cellular expansion. Thymocytes normally develop in direct physical contact with thymic stromal cells, which, in turn, form a 3-dimensional meshwork of primarily thymic cortical and medullary epithelial cells. As defined by separate phenotypes and functions, those stromal cells provide distinct microenvironments critical for the development and selection of thymocytes.23,49 In the course of acute GVHD, the ensuing changes of the thymic microenvironments have been demonstrated to promote aberrant thymic education.11 13-15
It is unlikely that the failure of TN cells to proliferate is the only mechanism responsible for the depletion of the DP pool. In this respect, we have recently demonstrated that GVHD causes enhanced programmed cell death among DP thymocytes.16 Hence, we speculate that both mechanisms, increased apoptosis and diminished cell cycle, are a direct consequence of alloantigen-specific T-cell recognition in the course of GVHD. This contention is favored by the data shown in Figure 6. Indeed, donor T cells that have gained access to the thymus in the course of acute GVHD18,19 represent activated effector cells, as judged by their in situ proliferation and cytokine secretion (Figures 7 and 8). It is unclear whether these donor-derived cells become activated in the periphery and subsequently migrate to the thymus or whether the initial alloantigen recognition occurs in the thymus. In acute GVHD, the cytokine phenotype of donor-derived cells clearly corresponds to a Th1-like pattern (ie, the preferential production of IL-2 and IFN-γ). This response may affect thymic function by 2 (interrelated) pathways, cell-mediated cytotoxicity and induction of inflammatory cytokines such as TNF-α. Infiltrating donor CTLs can directly target host DP thymocytes because these immature cells express intermediate levels of major histocompatibility class I antigens (data not shown). In addition, a role for TNF-α–mediated immunopathology may be inferred from results using cultured DP thymocytes as lysis targets50 and from the observation of elevated serum TNF-α levels31 and increased intrathymic TNF-α transcripts during acute GVHD in the F1-hybrid model (Figure 8). Our data argue, however, against the involvement of systemic TNF-α in DP depletion because the elimination of syngeneic thymocytes bearing this phenotype in the heterotopic thymus during acute GVHD (Figure 6) is expected. A causative role for TNF-α in the programmed cell death of DP thymocytes in acute GVHD remains to be established.
The distinct disease patterns of acute and chronic murine GVHD observed in parent→F1 transplantation models reflect the differential immune responses mediated by Th1 and Th2 donor T cells, respectively.29-33,42 Although T-cell activation and expansion in secondary lymphoid organs are shared between acute and chronic GVHD,30 the respective consequences of these events differ significantly. In contrast to acute GVHD, there was no morphologic evidence of thymic disease during chronic GVHD (Figure 1). Moreover, lymphoid development was normal, as evidenced by the normal frequency and numbers of all thymocyte subpopulations (Figure 2). Consistent with the lack of overt thymic disease, donor T-cell infiltration and the ensuing intrathymic inflammation were minimal in the presence of chronic GVHD (Figures 7 and 8). Although donor-derived T cells were present in thymuses early in this disease, their numbers quickly decreased from approximately 0.5 × 106cells to virtually undetectable levels by 2 weeks after transplantation. This finding is in obvious contrast to the substantial and sustained numbers of activated donor T cells in secondary lymphoid organs (our unpublished data and Rus et al30). These results further imply that thymus-infiltrating donor T cells in chronic GVHD are less efficient in inducing a local inflammatory response. Expression of thymic IL-4 but not IFN-γ mRNA was indeed prominent in this disease model (Figure 8). The limited numbers of donor T cells present in the thymus early after chronic GVHD induction may nevertheless be capable of causing a milder form of thymic GVHD because cell cycle progression among TN cells was moderately diminished relative to syngeneically transplanted mice (Figure 4).
Because donor-derived T cells constitute only a minority of SP cells (Figure 7) and because host DP cells display a defect in their capacity to enter the cell cycle (Figure 5), additional mechanisms are expected in acute GVHD to contribute to the observed increase in mature SP cells. Under physiological conditions, only activated T cells recirculate to the thymus.51 During GVHD, T cells of host origin may gain access to the inflamed thymus regardless of their antigenic specificities. These cells may thus contribute to the relative increase in mature host T cells. To test this issue, HSA (CD24) and Qa-2 cell surface expression was analyzed on CD3high, H-2Kd(high) host thymocytes. SP thymocytes are usually HSA+, whereas peripheral T cells lose expression of this marker and acquire Qa-2 expression.52 Our data suggest that during acute GVHD, peripheral host-derived mature T cells indeed recirculate to an extended degree to the thymus (not shown).
We have demonstrated that the thymus is a target of acute GVHD and, to a limited extent, of chronic GVHD. In-depth analysis of the mechanisms interfering with normal thymic T-cell development may provide the basis for novel strategies in the prevention or reduction of GVHD-associated immunodeficiency. Of relevance to peripheral lymphoid homeostasis will be an assessment of the impact of GVHD on thymocyte output. In this respect, a recent study used a lethal irradiation BMT model to demonstrate that thymic cellular output in acute GVHD was reduced to approximately 25% of normal.53 It remains to be established whether such a limited thymocyte output is still sufficient to sustain a phenotypically normal peripheral T-cell compartment.
Acknowledgments
We thank Tracy N. Hayden, Verena Jäggin Verin, and Verena Wyss for expert technical assistance.
Supported by grants 3100-046-936.96 (W.K., G.A.H.) and 3100-43600.95 (G.A.H.) from the Swiss National Science Foundation.
Reprints:Werner Krenger, Laboratory of Pediatric Immunology, Department of Research, Basel University Medical School, Hebelstrasse 20, 4031 Basel, Switzerland; e-mail: werner.krenger@unibas.ch.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal