Abstract
The role of plasminogen activator inhibitor-1 (PAI-1) in the plasma, blood platelets, and vessel wall during acute arterial thrombus formation was investigated in gene-deficient mice. Photochemically induced thrombosis in the carotid artery was analyzed via transillumination. In comparison to thrombosis in C57BL/6J wild-type (wt) mice (113 ± 19 × 106 arbitrary light units [AU] n = 15, mean ± SEM), thrombosis in PAI-1−/− mice (40 ± 10 × 106 AU, n = 13) was inhibited (P < .01), indicating that PAI-1 controls fibrinolysis during thrombus formation. Systemic administration of murine PAI-1 into PAI-1−/− mice led to a full recovery of thrombotic response. Occurrence of fibrinolytic activity was confirmed in 2-antiplasmin (2-AP)–deficient mice. The sizes of thrombi developing in wt mice, in 2-AP+/− and 2-AP−/− mice were 102 ± 35, 65 ± 8.1, and 13 ± 6.1 × 106 AU, respectively (n = 6 each) (P < .05), compatible with functional plasmin inhibition by 2-AP. In contrast, thrombi in wt mice, t-PA−/− and u-PA−/−mice were comparable, substantiating efficient inhibition of fibrinolysis by the combined PAI-1/2-AP action. Platelet depletion and reconstitution confirmed a normal thrombotic response in wt mice, reconstituted with PAI-1−/− platelets, but weak thrombosis in PAI-1−/− mice reconstituted with wt platelets. Accordingly, murine (wt) PAI-1 levels in platelet lysates and releasates were 0.43 ± 0.09 ng/109 platelets and plasma concentrations equaled 0.73 ± 0.13 ng/mL. After photochemical injury, plasma PAI-1 rose to 2.9 ± 0.7 ng/mL (n = 9, P < .01). The plasma rise was prevented by ligating the carotid artery. Hence, during acute thrombosis, fibrinolysis is efficiently prevented by plasma 2-AP, but also by vascular PAI-1, locally released into the circulation after endothelial injury.
Thrombi dissolve as a consequence of activation of the fibrinolytic system. The physiologic plasminogen activators tissue-type plasminogen activator (t-PA) and urokinase-type plasminogen activator (u-PA) are serine proteinases activating the proenzyme plasminogen to the broad specificity enzyme plasmin.1 The serpin plasminogen activator inhibitor-1 (PAI-1) is the main inhibitor of both t-PA and u-PA and constitutes a critical regulator of plasminogen activation.2 Several clinical studies have associated elevations in plasma PAI-1 with increased risk for thrombosis,3,4 whereas a drop in plasma PAI-1 levels may be a cause of recurrent bleeding.5,6 Animal studies substantiated that increased circulating PAI-1 levels are associated with a prothrombotic tendency,7 whereas inhibition of PAI-1 with monoclonal anti–PAI-1 antibodies8,9 or low-molecular weight compounds10 has antithrombotic consequences. Plasmin is very rapidly and specifically inhibited by alpha2-antiplasmin (α2-AP), which circulates in plasma at a high concentration of 1 μmol/L.11 The physiologic importance of α2-AP is illustrated by the significant bleeding tendency in patients with homozygous α2-AP deficiency; heterozygotes have no or only mild bleeding complications.12
Whereas α2-AP is synthetized in the liver and released into the circulation, the origin of active PAI-1 in the circulation is less well-defined. PAI-1 is produced by several cell types, including endothelial cells, smooth muscle cells, fibroblasts, and hepatocytes.13 Two distinct pools of PAI-1 exist in the circulation, 1 in platelets and 1 in plasma. Human platelets are a major reservoir of PAI-1, with up to 90% of the circulating human PAI-1 contained within platelet α-granules,14 yielding up to 700 ng PAI-1 per 109 platelets.15 However, platelet PAI-1 exists predominantly in a latent or inactive form,15 suggesting its effect on fibrinolysis to be rather limited. Nevertheless, the inhibitory effect of platelets on clot lysis16,17 was proposed to be mediated partly by platelet PAI-1, a conclusion supported by a differential clot lysis efficiency in the presence of normal platelets or platelets derived from PAI-1–deficient patients.18
Different experimental conditions in various in vitro studies may explain discordant results on the role of platelet PAI-1 in fibrinolysis.16,17,19 In addition, important species differences were reported with respect to the relative concentrations of PAI-1 in plasma, platelets, inside thrombi, and in tissues.20-23 Furthermore, species-dependent differences in the proportion of active versus latent PAI-1 have been observed.23 The study of mouse gene knock-outs has made clear that a combined t-PA and u-PA gene deficiency leads to extensive spontaneous fibrin deposition,24 but also that in PAI-1−/− mice, lysis of pulmonary plasma clots is significantly enhanced.25
In a murine model of ferric chloride-induced arterial thrombosis, significantly smaller residual thrombosis was shown 24 hours after injury induction in PAI-1−/− mice than in wild-type (wt) mice.26 In addition, from the shorter reperfusion times observed during thrombolytic therapy of platelet-rich arterial thrombi in PAI-1−/− mice, Zhu et al27 concluded that PAI-1 is a major determinant of the resistance to thrombolysis by pharmacologic t-PA concentrations. In more acute studies of photochemically induced arterial thrombosis, a significant prolongation of the time required to occlude the vessels with thrombi was observed in PAI-1−/− mice, whereas u-PA and t-PA deficiencies had no effect on closure time,28 highlighting the importance of PAI-1 during thrombus formation.
In this study, we have investigated the role of PAI-1 for the control of fibrinolysis during acute thrombosis, by focusing on PAI-1 present in different compartments. By performing PAI-1 reconstitution experiments in plasma and platelets, this study provides the first evidence that PAI-1 released from the vasculature participates in the control of endogenous fibrinolysis in mice, at sites of arterial injury initiating acute thrombosis.
Materials and methods
Reagents
Recombinant t-PA (Actilyse) was from Boehringer Ingelheim (Ingelheim, Germany). The chromogenic plasmin substrate S-2403 was purchased from Chromogenix (Antwerp, Belgium) and equine tendon collagen from Nycomed (München, Germany). Busulfan was acquired from Sigma (St Louis, MO). Recombinant murine and human PAI-1 were prepared as described elsewhere.25 Other reagents were obtained commercially.
Animals
α2-AP, PAI-1, t-PA, or u-PA gene-deficient mice were generated via homologous recombination in embryonic stem cells, as described.24,25,29,30 To avoid potential effects of strain differences, consecutive generations of mice carrying the null gene allele were backcrossed repeatedly to C57BL/6J mice. Only mice generated after 5 or more backcrosses were used in this study. Genotyping of mice was performed by Southern blot analysis of tail tip DNA.29
Experimental thrombosis model
All animal experiments were reviewed and approved by the Institutional Review Board of the University of Leuven and were performed in accordance with protocols approved by the Institutional Animal Care and Research Advisory Committee. Thrombus was induced as recently described.31 Mice (10 to 12 weeks old) of both sexes weighing 19 to 31 g were anesthetized by intraperitoneal injection of sodium pentobarbital (60 mg/kg) and fixed on a heated operating table. Atropine sulfate was also injected and endotracheal intubation carried out. A 2F venous cathether was inserted into the right jugular vein for injection of reagents and rose-bengal. The left carotid artery was carefully exposed from the surrounding tissue and mounted on a transilluminator. The exposed artery was irradiated with the green light (wavelength 540 nm) of a Xenon lamp (L4887, Hamamatsu Photonics, Hamamatsu, Japan), equipped with a heat-absorbing filter and a green filter. Irradiation was directed via a 3-mm diameter optic fiber attached to a manipulator. Just after the injection of rose-bengal (20 mg/kg) via the intravenous catheter, irradiation was started for various time intervals (1-4 minutes).
The analytical procedures for the quantitation of mural thrombi in the mouse carotid artery have been described.32 The total light intensity versus time curve was established over 40 minutes, and thrombus formation was measured by comparing the area under the curve and expressed in arbitrary light units (AU).
Administration of recombinant murine PAI-1
Immediately after a 3-minute photochemical injury, recombinant murine PAI-1, diluted with saline just before use, was injected into PAI-1−/− mice (n = 6) as a single bolus (35 ng), followed by a continuous infusion (76 ng/h) for 40 minutes via a microinfusion pump (Perfusor, Braun, Melsungen, Germany) and an intravenous catheter. Blood samples for platelet counting and plasma PAI-1 measurements were collected at the end of the experiment via the vena cava into a syringe containing 3.8% sodium citrate as an anticoagulant (1/10 volume). After centrifugation at 3000 rpm for 10 minutes, the citrated plasma was stored at −70°C.
Platelet reconstitution experiments
Thombocytopenia was induced in wt mice and PAI-1−/− mice via a triple intraperitoneal injection of busulfan at 20 mg/kg,33 dissolved in polyethylene glycol 400 diluted with saline to 25% just before the injection, on day 0, 3, and 9. On day 21, circulating platelets sampled via tail bleeding were counted on a Cell Dyn 1300 (Abbott, Ottignies/Louvain-la-Neuve, Belgium), and mice were subdivided in groups to test thrombus induction without and after reconstitution with murine washed platelets. Thrombocytopenic animals were anesthetized as outlined above and reconstituted with 5 × 108platelets, prepared from wt or PAI-1−/− mice and injected as a bolus in a volume of 200 μL into the jugular vein. For this purpose, platelets were prepared after sampling blood from the vena cava of donor mice on acid-citrate dextrose (ACD). Platelet-rich plasma (PRP) was prepared via 2 subsequent centrifugations at 800 rpm for 5 minutes. Pooled PRP was then mixed with an equal volume of ACD and centrifuged at 2000 rpm for 10 minutes to pellet platelets. The platelet pellet was resuspended in saline at 2.5 × 106 platelets/μL. Platelets were counted at the end of the thrombosis experiment both in wt mice and in platelet-reconstituted thrombocytopenic mice.
Amidolytic assay
Blood samples were collected from PAI-1+/+ and PAI-1−/− mice (n = 2 each) as described above, and washed platelets (prepared as above) were lysed with 1% Triton X-100 and 5 minutes later 10-fold diluted with phosphate-buffered saline (PBS). Active PAI-1 was evaluated by first adding human t-PA (23 pmol/L) to the platelet lysate (or dilutions) for 10 minutes at room temperature, and then by measuring residual t-PA activity via the activation of plasminogen. Therefore, human Lys-plasminogen (0.2 μmol/L) was incubated with the treated human t-PA sample at 37°C in the presence of CNBr-digested human fibrinogen fragments (0.1 μmol/L) as described.34Generated plasmin was measured by the simultaneous addition of S-2403 (0.5 mmol/L) and A(405 nm) was measured up to 40 minutes with a multiscan spectrophotometer ELx808 (Bio-Tek Instruments, Highland Park, Winooski, VT). t-PA activities were derived from plots of A(405 nm) versus (time),2 which were proportional to the t-PA activity. Initial activation rates of plasminogen by t-PA in the absence or presence of platelet lysates/releasates were monitored in the same way. Measurements were performed in duplicate.
Preparation of lysates and releasates from murine platelets for PAI-1 dosage
Washed platelets prepared from vena cava blood of C57BL/6J mice (n = 6) were resuspended up to 5 × 108platelets/mL in PBS containing 0.002% Tween 80, 5 mmol/L EDTA, and 0.1% bovine serum albumin (BSA). After centrifugation, platelets in the pellet were lysed with 1% Triton X-100 in 100 μL and, after 5 minutes of incubation, 10-fold diluted with buffer. Alternatively, platelets were incubated for 10 minutes at 37°C with equine tendon collagen (20 μg/mL; this concentration induced murine platelet aggregation during classical aggregometry). After centrifugation, platelet lysates and releasates were immediately analyzed via enzyme-linked immunosorbent assay (ELISA) (see below).
Tissue and plasma sampling after photochemical injury
Blood samples were collected from the vena cava from C57BL/6J controls and from mice 40 minutes after the 3-minute photochemical injury in wt mice (n = 18), respectively, in thrombocytopenic mice, reconstituted with wt platelets or PAI-1−/−platelets (3 groups of n = 6). To delineate the origin of the plasma PAI-1 elevation after injury, blood sample controls were prepared after injection of rose-bengal, but without injuring light irradiation (n = 6) or after carotid artery ligation, with (n = 6) or without (n = 6) vessel wall injury. Citrated plasma was stored at −70°C. Carotid arteries of both injured and noninjured sites were carefully dissected and rinsed with saline. Tissues (pooled from 3 arteries to raise the sensitivity) were homogenized with 100 μL of PBS containing 1% Triton X-100, diluted with 900 μL PBS, and stored at −70°C. Tissue protein content was determined using a dye-binding assay kit (Bio-Rad, Hercules, CA) with BSA as a standard. Measurements were performed in duplicate.
PAI-1 antigen and activity determinations
Murine PAI-1 antigen levels in tissue and platelet extracts and in plasma withdrawn before/after photochemical injury were measured by a sensitive combined monoclonal-polyclonal antibody assay, calibrated with recombinant murine PAI-1.35 For this purpose, the murine antimurine PAI-1 monoclonal antibody H34G6 (4 μg/mL) was coated on microtiter plates for 48 hours at 4°C. Bound PAI-1 in the samples was revealed using a biotinylated and diluted (1:250) rabbit polyclonal antiserum raised in the laboratory against murine PAI-1, incubated for 1 hour at 37°C, and then by a peroxidase-labeled avidin-biotin complex, according to standard protocols. In this assay, a linear relationship exists between A(492 nm) and antigen concentrations between 0.03 ng/mL and 3 ng/mL PAI-1. Recovery experiments validating the precision of the PAI-1 antigen determination in lysed platelets consisted of the addition of known amounts of murine PAI-1 (0-3 ng/mL) to the platelets before sample preparation via lysis.
For PAI-1 activity determinations, the monoclonal antihuman PAI-1 antibody 16F11 (4 μg/mL), which cross-reacts with murine PAI-1, was coated on microtiter plates, for 48 hours at 4°C.29Samples containing t-PA–PAI-1 complexes were incubated as described above for the antigen-ELISA, followed by addition of horseradish peroxidase (HRP)-conjugated monoclonal antibody 62E8 (directed against human t-PA; dilution 1:8000) for 2 hours at room temperature. For calibration, purified active recombinant human PAI-1 was used,36 after preincubation with an excess of t-PA. All activity data are expressed in nanograms per milliliter (ng/mL) complex and all ELISA measurements were performed in duplicate. With the use of human t-PA-PAI-complexes, a linear dose-response was observed between 0.03 and 10 ng/mL of complex.
Complex formation between t-PA and PAI-1 extracted or released both from human and murine platelets was performed as follows. t-PA (30 ng/mL) was added to washed platelets (0-5 × 108 platelets/mL) and extraction was performed with 1% Triton X-100 for 5 minutes, followed by a 10-fold dilution to reduce the Triton X-100 concentration, avoiding detergent-induced conformational changes in the extracted PAI-1.37 Separate control experiments in which recombinant murine PAI-1 was preincubated with 1% Triton X-100 for 5 minutes confirmed that this treatment was without effect on the PAI-1 activity. The addition of t-PA to the platelets before initiation of lysis with Triton X-100 was likewise performed to shorten the exposure time of released PAI-1 to the detergent. Recovery experiments validating the precision of the PAI-1 activity determination were performed by adding known amounts of murine PAI-1 to the platelets (0-3 ng/mL) before addition of t-PA, sample preparation via lysis and ELISA.
Plasma samples were measured 10-fold diluted, at which dilution no plasma matrix-dependent effects were observed. Platelet lysates, platelet releasates, and tissue extracts were measured 2-fold diluted, both in the antigen and in the activity ELISA.
Statistical analysis
Data are presented as mean ± SEM. Comparison between groups was performed using the Dunnett multiple range test, Student t test, Mann-Whitney test, Dunn's multiple comparison test, or Tukey-Kramer multiple comparison test, as described in the text. P values of < .05 are considered significant.
Results
Thrombus generation in PAI-1+/+, PAI-1+/−, and PAI-1−/− mice
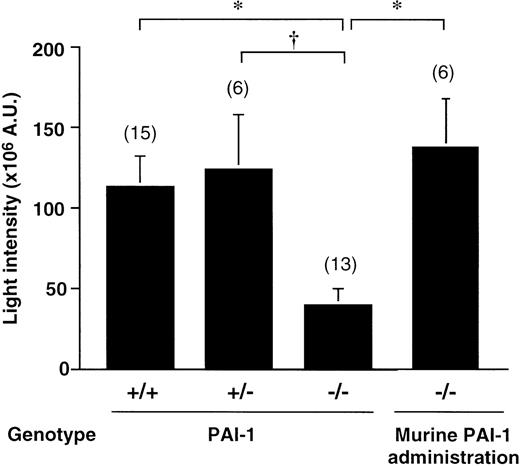
As shown in Figure 1, the absence of PAI-1 was associated with an apparent antithrombotic effect. The average thrombus size in wt mice after a 3-minute photochemical injury equaled 113 ± 19 × 106 AU (n = 15). Thrombus formation in PAI-1−/− mice was significantly decreased (40 ± 10 × 106 AU, n = 13, P < .01 by Dunnett multiple range test). The average thrombus size in heterozygotes (124 ± 33 × 106 AU, n = 6) did not differ from that in wt mice (P < .05 by Dunnett multiple range test, versus the thrombus size in PAI-1−/−mice). Injection into PAI-1−/− mice of a bolus of recombinant murine PAI-1 of 35 ng, followed by a continuous infusion at 76 ng/h until the end of the 40-minute experiment, normalized thrombus formation (Figure 1). The average thrombus size (137 ± 30 × 106 AU, n = 6,P < .01 by Student t test versus thrombus size in nontreated PAI-1−/− mice) was comparable to that in PAI-1+/+ mice. Plasma PAI-1 antigen levels at the end of the infusion were 10 ± 2.5 ng/mL (n = 6).
Thrombus generation in PAI-1+/+, PAI-1+/−, and PAI-1−/− mice.
Cumulative thrombus formation over 40 minutes after a 3-minute carotid artery photochemical injury, expressed as total light intensity;+/+: wild-type mice; +/−: PAI-1 heterozygotes;−/−: PAI-1–deficient homozygotes; thrombus formation in PAI-1−/−mice after an intravenous bolus (35 ng) with recombinant murine PAI-1, administered immediately after photochemical injury, followed by continuous infusion (76 ng/h) for 40 minutes via an intravenous catheter (n = 6). Data are the mean ± SEM for the number of mice indicated in parentheses. *P < .01 by Dunnett multiple comparison test and †P < .05.
Thrombus generation in PAI-1+/+, PAI-1+/−, and PAI-1−/− mice.
Cumulative thrombus formation over 40 minutes after a 3-minute carotid artery photochemical injury, expressed as total light intensity;+/+: wild-type mice; +/−: PAI-1 heterozygotes;−/−: PAI-1–deficient homozygotes; thrombus formation in PAI-1−/−mice after an intravenous bolus (35 ng) with recombinant murine PAI-1, administered immediately after photochemical injury, followed by continuous infusion (76 ng/h) for 40 minutes via an intravenous catheter (n = 6). Data are the mean ± SEM for the number of mice indicated in parentheses. *P < .01 by Dunnett multiple comparison test and †P < .05.
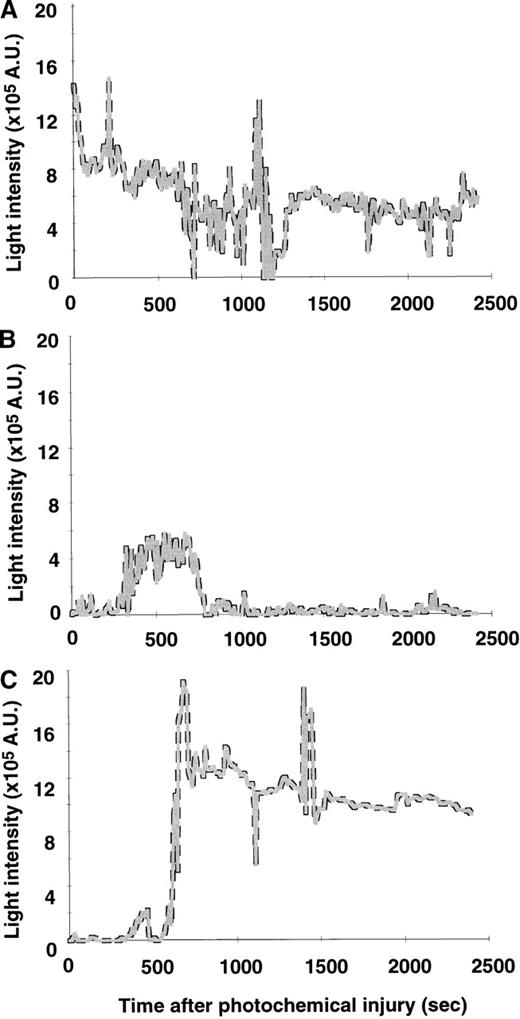
Figure 2 shows representative tracings of the light intensity versus time curves during thrombus formation in wt mice, in PAI-1−/− mice, and recombinant murine PAI-1–treated gene-deficient PAI-1−/− mice. In wt mice, a cyclic flow pattern of massive thrombus formation was induced immediately after the photochemical injury, leading to a slowly decreasing thrombus (Figure 2A). In contrast, in PAI-1−/− mice, thrombi only developed just after the photochemical injury and no stable thrombus was observed at later time points (Figure 2B). The injection of recombinant murine PAI-1 restored injury induced thrombus formation in the later phase of the observation period (Figure 2C).
Thrombus progression after photochemical injury.
Typical patterns of light intensity recorded during 40 minutes after photochemical injury of the carotid artery of PAI-1+/+ mice (A), PAI-1−/− mice (B), and recombinant murine PAI-1 treated PAI-1−/− mice (C).
Thrombus progression after photochemical injury.
Typical patterns of light intensity recorded during 40 minutes after photochemical injury of the carotid artery of PAI-1+/+ mice (A), PAI-1−/− mice (B), and recombinant murine PAI-1 treated PAI-1−/− mice (C).
Thrombus generation in 2-AP+/+, 2-AP+/−, and 2-AP−/− mice
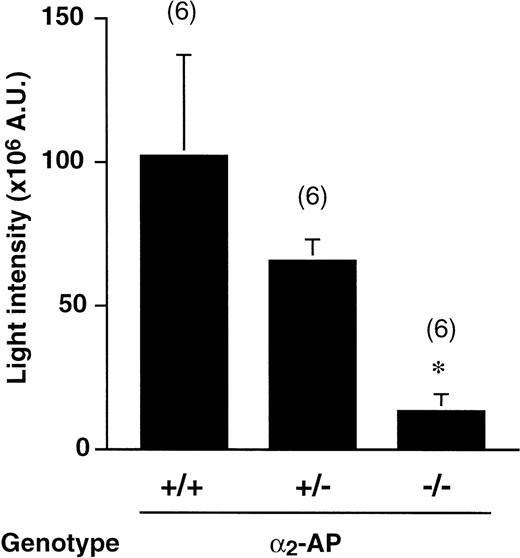
As shown in Figure 3, the absence of plasma α2-AP was also associated with an apparent antithrombotic effect. The average thrombus size in wt mice after a 3-minute photochemical injury (102 ± 35 × 106AU, n = 6) did not differ from that in wt controls in Figure 1. The thrombus size, however, was gene dose-dependent. In α2-AP−/− mice, it was greatly and significantly decreased (13 ± 6.1 × 106 AU, n = 6, P < .05 by Dunnett multiple range test). In heterozygote α2-AP+/− mice, an intermediate intensity of thrombus generation (65 ± 8.1 × 106 AU, n = 6) was observed, in agreement with the expression of intermediate plasma levels of α2-AP.38
Thrombus generation in 2-AP+/+, 2-AP+/−, and 2-AP−/− mice.
Cumulative thrombus formation over 40 minutes after a 3-minute carotid artery photochemical injury, expressed as total light intensity;+/+: wild-type mice; +/−: α2-AP heterozygotes; −/−: α2-AP–deficient homozygotes. Data are the mean ± SEM for the number of mice indicated in parentheses (*P < .05 by Dunnett multiple comparison test in comparison with the α2-AP+/+ group).
Thrombus generation in 2-AP+/+, 2-AP+/−, and 2-AP−/− mice.
Cumulative thrombus formation over 40 minutes after a 3-minute carotid artery photochemical injury, expressed as total light intensity;+/+: wild-type mice; +/−: α2-AP heterozygotes; −/−: α2-AP–deficient homozygotes. Data are the mean ± SEM for the number of mice indicated in parentheses (*P < .05 by Dunnett multiple comparison test in comparison with the α2-AP+/+ group).
Thrombus generation in t-PA−/− and u-PA−/− mice
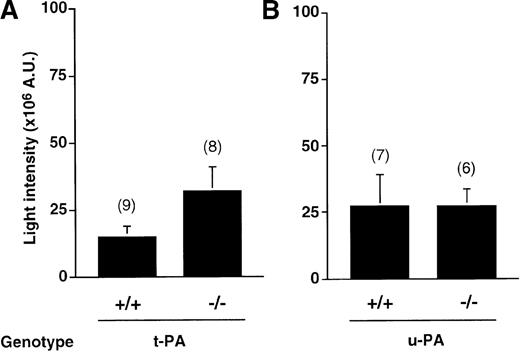
Thrombogenicity in these gene-deficient mice was investigated after induction of a milder photochemical injury, consisting of a 2-minute irradiation, because of the potential increase in thrombogenicity. As shown in Figure 4, t-PA−/− mice were inclined to more intense thrombosis (32 ± 8.6 × 106 AU, n = 8) than wt mice (15 ± 4 × 106 AU, n = 9,P = .078). In contrast, the average thrombus size in u-PA−/− mice (27 ± 12 × 106 AU, n = 7) was almost identical to that in wt mice (27 ± 6.6 × 106AU, n = 6). Comparison of all data revealed no significant differences between wt mice and t-PA−/− or u-PA−/− mice.
Thrombus generation in t-PA+/+ and t-PA−/− mice versus u-PA+/+ and u-PA−/− mice.
Cumulative thrombus formation over 40 minutes after a mild 2-minute carotid artery photochemical injury, expressed as total light intensity; +/+: wild-type mice;−/−: t-PA– or u-PA–deficient homozygotes. Data are the mean ± SEM for the number of mice indicated in parentheses.
Thrombus generation in t-PA+/+ and t-PA−/− mice versus u-PA+/+ and u-PA−/− mice.
Cumulative thrombus formation over 40 minutes after a mild 2-minute carotid artery photochemical injury, expressed as total light intensity; +/+: wild-type mice;−/−: t-PA– or u-PA–deficient homozygotes. Data are the mean ± SEM for the number of mice indicated in parentheses.
Effect of PAI-1 on plasminogen activation by t-PA
To investigate whether physiologic t-PA concentrations are inhibited by murine platelet PAI-1, a sensitive kinetic and coupled assay of plasminogen activation was carried out, necessitating only picomolar concentrations of t-PA. In the presence of CNBr-fibrinogen fragments, Lys-plasminogen (0.2 μmol/L) was activated in a time-dependent manner by t-PA (final concentration, 20 pmol/L), leading to an exponential increase with time of A(405 nm), after hydrolysis of the plasmin substrate S-2403 (not shown). Control incubations of t-PA with recombinant human PAI-1 at 30 pmol/L inhibited subsequent plasminogen activation completely (not shown). In the presence of platelet lysates (derived from 7.5-30 × 104platelets/μL), plasminogen activation was inhibited in a dose-dependent manner, but to a comparable degree for lysates from PAI-1+/+ and PAI-1−/− mice (not shown). Because under these conditions t-PA activity was influenced by other factors present in the platelet lysate than the presence of PAI-1, this approach was abandoned and further analyses were exclusively made by ELISA.
Murine PAI-1 antigen and activity levels in plasma and platelets
With the use of a sensitive sandwich ELISA (monoclonal-polyclonal antibodies), PAI-1 antigen values measured in plasma obtained from 9 wt C57BL/6J mice averaged 0.73 ± 0.13 ng/mL. Recovery experiments, during which murine PAI-1 was added to washed platelets before lysis, indicated that platelet membrane components did not interfere with the detection of the PAI-1 antigen in ELISA and that added PAI-1 was fully detected. In PAI-1−/− mice (n = 4), PAI-1 antigen remained undetectable, confirming the specificity of the ELISA, at least when analyzing 10-fold diluted plasma samples. In plasma from endotoxin-treated mice (n = 6, pooled), PAI-1 antigen levels rose to 136 ± 20 ng/mL (n = 4), in agreement with intense vascular release of PAI-1 into the circulation after endotoxin treatment.21 PAI-1 antigen levels in platelet lysates and releasates were 0.56 ± 0.19 ng/109 platelets (n = 6) and 0.34 ± 0.08 ng/109 platelets (n = 9), respectively, yielding an average value of 0.43 ± 0.09 ng/109 platelets (n = 15).
The feasibility of activity measurements for platelet PAI-1 in platelet lysates prepared via detergent extraction was first tested with human platelets. Analysis via the activity-ELISA yielded 22 ± 3.9 ng t-PA–PAI-1 complex per 109 platelets for lysed platelets (n = 4) and 11 ± 1.8 ng/109 platelets for the releasate of collagen stimulated platelets (n = 4), in agreement with known activity levels for human platelet PAI-1. Further recovery experiments during which murine PAI-1 was added to the platelets before the addition of t-PA and lysis with Triton X-100, however, revealed that only about 30% of the added reactive PAI-1 was detected in complex with t-PA. Hence, similar to their effect during the t-PA–induced plasminogen activation, platelet membrane components interfere slightly with t-PA inhibition by PAI-1. However, no t-PA–PAI-1 complexes could be detected in t-PA–treated murine platelet lysates. In view of the total antigen content of the platelet and the sensitivity of the t-PA–PAI-1 complex assay (0.03 ng/109 platelets to be multiplied with a correction factor of 3-4 for the presence of platelet membranes), this finding implies that at best 20% of platelet PAI-1 is active. On the contrary, in plasma obtained from 7 wt C57BL/6J mice, the ELISA estimated PAI-1 activities equivalent to 0.40 ± 0.10 ng/mL, whereas in PAI-1−/− mice (n = 3), PAI-1 activity remained undetectable. In plasma obtained from endotoxin-treated mice (n = 6, pooled), estimated PAI-1 activity values were 175 ± 20 ng/mL (3 separate measurements). These data imply that most of the circulating plasma PAI-1 is active.
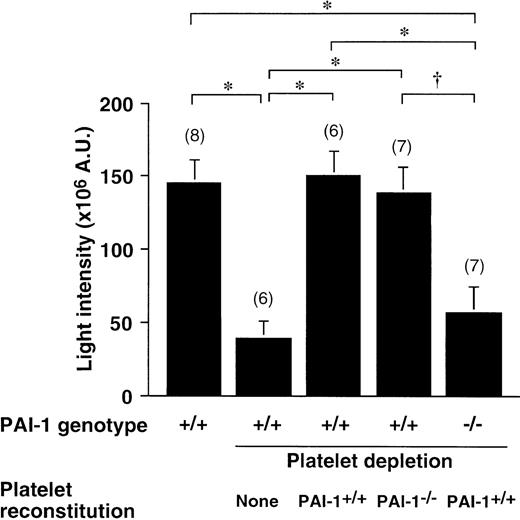
Thrombosis after platelet reconstitution
After treatment with busulfan,33 circulating platelet numbers dropped progressively as a result of inhibition of megakaryocyte differentiation and simultaneous clearance of circulating platelets. Platelet counts in wt mice averaged 8.3 ± 0.4 × 105/μL blood, but 21 days after initiation of treatment, platelet numbers had dropped to 1.4 ± 0.12 × 105/μL (n = 19) in the treated wt mice and to 1.7 ± 0.23 × 105/μL in the PAI-1−/− group (n = 7). Figure5 shows that as a consequence of the thrombocytopenia, the thrombotic response was greatly decreased in comparison to the response in untreated wt mice. Furthermore, reconstituting wt platelets in thrombocytopenic wt mice could restore thrombus formation completely. However, thrombus formation could be restored equally well in thrombocytopenic wt mice with PAI-1−/− platelets. In contrast, wt platelets were not capable of normalizing the thrombotic response in PAI-1−/− mice. The thrombus size in this group (39% ± 12% of control thrombus) did not differ statistically from the average thrombus size measured in the PAI-1−/− group in Figure 1 (35% ± 8.8% of corresponding control group). These experiments confirmed that platelet PAI-1 is not the determinant of the fibrinolytic control in vivo. Platelet numbers measured at the end of the thrombosis experiment averaged 2.6 ± 0.24 × 105/μL (n = 8) in PAI-1−/− mice reconstituted with wt platelets, and 2.5 ± 0.08 × 105/μL (n = 7) in PAI-1+/+ mice reconstituted with PAI-1−/− platelets, respectively, 2.4 ± 0.19 × 105/μL (n = 8) in PAI-1+/+ mice reconstituted with PAI-1+/+platelets. Platelet numbers in wt controls equaled 4.3 ± 0.34 × 105/μL (n = 8) at the end of the thrombosis experiment. Therefore, reconstituted platelet numbers equal about 60% of the normal count (about 5 × 105/μL initially), in agreement with the injected amount of 5 × 108 platelets per mouse.
Thrombus generation in platelet reconstituted thrombocytopenic PAI-1+/+ and PAI-1−/−mice.
Cumulative thrombus formation over 40 minutes after a 3-minute carotid artery photochemical injury, expressed as total light intensity;+/+: wild-type mice;−/−: PAI-1–deficient homozygotes. Thrombocytopenia was induced with busulfan in the groups indicated; mice were reconstituted with 5 × 108 PAI-1+/+ or PAI-1−/− platelets and thrombosis was quantitated after injury induction. Data are the mean ± SEM for the number of mice indicated in parentheses. *P < .01 by Tukey-Kramer multiple comparison test in comparison with the groups indicated by the horizontal brackets; †P < 05.
Thrombus generation in platelet reconstituted thrombocytopenic PAI-1+/+ and PAI-1−/−mice.
Cumulative thrombus formation over 40 minutes after a 3-minute carotid artery photochemical injury, expressed as total light intensity;+/+: wild-type mice;−/−: PAI-1–deficient homozygotes. Thrombocytopenia was induced with busulfan in the groups indicated; mice were reconstituted with 5 × 108 PAI-1+/+ or PAI-1−/− platelets and thrombosis was quantitated after injury induction. Data are the mean ± SEM for the number of mice indicated in parentheses. *P < .01 by Tukey-Kramer multiple comparison test in comparison with the groups indicated by the horizontal brackets; †P < 05.
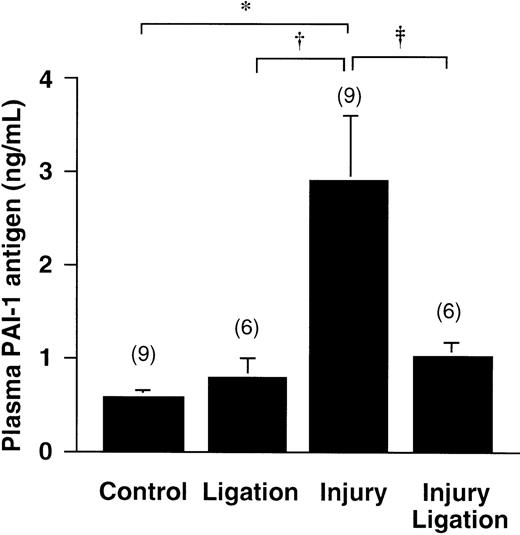
PAI-1 antigen levels in tissue and plasma after photochemical injury
PAI-1 antigen levels in plasma of wt mice 40 minutes after the photochemical injury were 2.9 ± 0.7 ng/mL, compared with 0.57 ± 0.09 ng/mL in the control group, exposed to the dye without injury (n = 9 each, P < .01 by Dunn's multiple comparison test) (Figure 6). Because this increase could not be explained by release of the very low PAI-1 content in platelets (less than 1 ng per mouse), we investigated whether PAI-1 would be released by the injured carotid artery. Figure 6shows that a double ligation (without photochemical injury) around the carotid artery hardly triggers any increase of plasma PAI-1 by itself. However, preventing contact between the injured area and the circulation, by ligating the injured vessel area, almost completely abolishes the observed rise of plasma PAI-1 after injury. Hence, these results substantiate that significant amounts of PAI-1 are released from the vessel wall in the vicinity of the developing thrombus. Likewise, PAI-1 levels in the injured carotid artery, removed 40 minutes after the photochemical reaction, were 1.3 ± 0.42 ng/mg protein extracted, compared with 4.9 ± 1.5 ng/mg for control carotid arteries (n = 6 for samples consisting of 3 pooled arteries, P < .05 by Student t test).
Release of PAI from the vessel wall.
PAI-1 antigen levels in plasma 40 minutes after a 3-minute carotid artery photochemical injury, compared with control values (rose-bengal injection without injury); effect of ligation without injury (second bar) and of ligation in combination with injury (fourth bar) on PAI-1 plasma concentrations is also shown. Data are mean ± SEM for the number of animals indicated between parenthesis. *P < .01 by Dunn's multiple comparison test in comparison with the groups indicated by the horizontal brackets; †P < .05; ‡P < .05 by alternative t test for the direct comparison between the groups indicated; these groups had unequal SD distribution.
Release of PAI from the vessel wall.
PAI-1 antigen levels in plasma 40 minutes after a 3-minute carotid artery photochemical injury, compared with control values (rose-bengal injection without injury); effect of ligation without injury (second bar) and of ligation in combination with injury (fourth bar) on PAI-1 plasma concentrations is also shown. Data are mean ± SEM for the number of animals indicated between parenthesis. *P < .01 by Dunn's multiple comparison test in comparison with the groups indicated by the horizontal brackets; †P < .05; ‡P < .05 by alternative t test for the direct comparison between the groups indicated; these groups had unequal SD distribution.
Separate measurements of plasma PAI-1 in thrombocytopenic mice at the end of the thrombosis induction confirmed that the rise of PAI-1 did not depend on the presence of platelets. In comparison to plasma PAI-1 in nonthrombocytopenic and noninjured wt mice injected with rose-bengal (0.35 ± 0.04 ng/mL; n = 6), plasma PAI-1 rose to 1.76 ± 0.1 ng/mL after injury in thrombocytopenic wt mice (n = 6) and to 1.79 ± 0.12 ng/mL in thrombocytopenic wt mice reconstituted with wt platelets (n = 6), respectively, 2 ± 0.23 ng/mL in thrombocytopenic wt mice reconstituted with PAI-1−/− platelets (n = 6). Although these numbers are slightly lower than those in Figure 6, these experiments confirm that the rise of plasma PAI-1 after vessel wall injury is not influenced by the PAI-1 content of the circulating platelets.
Discussion
Several models in mice have been reported, in which thrombosis is induced via a vascular injury, inflicting damage to endothelial cells, elastic lamina, and smooth muscle cells.39,40 The model used in this study causes only mild damage to endothelial cells; thrombi are platelet-rich and resemble clinical thrombi by electron microscopic analysis.41 By combining transillumination and photochemical vessel wall injury in the mouse, it has become possible to link the degree of vessel wall injury to the intensity of thrombosis, which develops as a consequence of endothelial cell destruction.31 This methodology was used in this study to investigate the consequences for thrombus generation of gene inactivation of inhibitors of fibrinolysis, and more in particular to study the contribution of PAI-1 present in plasma, endothelial cells and platelets.
The role of PAI-1 in controlling fibrinolysis in vivo has already been investigated extensively. Thus, Fay et al42 recently reported a significant decrease in residual thrombi 24 hours after vessel wall injury with FeCl3 in PAI-1−/− mice, compared with wt mice. However, they reported similar initial thrombus formation in PAI-1−/− and PAI-1+/+mice.26,42 In contrast, Matsuno et al28recently identified a delay in acute thrombus formation in PAI-1−/− mice, suggesting that PAI-1 controls fibrinolysis also in the early phase of thrombus development. However, this interpretation is at variance with a normal tail bleeding and blood loss after amputation of the cecum in PAI-1−/− mice.25 The spontaneous lysis of 125I-fibrin–labeled pulmonary emboli in PAI-1+/− mice was not different from that in PAI-1+/+ mice after 4 hours, but significantly weaker fibrinolysis was found in PAI-1+/− mice after 8 hours.25 During recent t-PA–induced thrombolysis studies in mice, reperfusion occurred in all mice that received t-PA, but reperfusion times were significantly shorter in PAI-1−/− mice.27
Our current results indicate that both α2-AP and PAI-1 play a determinant role in controlling acute arterial thrombus formation. Inactivation of α2-AP indeed leads to a potent and gene dose-dependent antithrombotic effect. Gene inactivation of PAI-1 is associated with a somewhat weaker antithrombotic effect, which was not gene dose-dependent; ie, heterozygous PAI-1+/− mice showed normal arterial thrombus formation. It has recently been shown that α2-AP cross-links to fibrin.43 Therefore, theoretically, the reduced thrombus formation observed in PAI-1 and α2-AP gene-deficient mice could be postulated to result from a reduced contribution to thrombus formation of PAI-1, respectively α2-AP. However, the efficiency of spontaneous thrombolysis seems to be controlled primarily by circulating α2-AP and not by the amount of α2-AP cross-linked to fibrin38 and α2-AP cross-linking does not seem to affect the lysability of fibrin clots by the murine fibrinolytic system. These data support our interpretation that α2-AP plays a relevant role in regulating thrombus formation, in relation to its high plasma content, via controlling fibrinolysis, but not via influencing fibrin clot architecture. Because of the lack of a strict gene dose-dependent antithrombotic effect for PAI-1, its role in acute thrombus formation appeared to be more complex, which findings were the basis for this study, that investigates contributions of PAI-1 present in different compartments to the control of thrombosis. Our finding that thrombus generation in t-PA−/− and u-PA−/−mice was equal to that in wt mice agrees with results of Matsuno et al.28 It therefore seems that during an acute thrombotic response, t-PA– and u-PA–induced plasmin formation in wt mice is efficiently inhibited by the combined action of PAI-1 and α2-AP.
The intravenous injection of recombinant murine PAI-1, in combination with a continuous PAI-1 infusion, fully restored thrombus development in PAI-1−/− mice, at steady-state plasma PAI-1 levels (at the end) equal to 10 ± 2.5 ng/mL (equivalent to 15-fold the wt plasma concentration). These findings confirm that soluble plasma PAI-1 is responsible for t-PA inhibition during thrombus formation. In contrast to work performed by others, studying the effects of exogenous PAI-1 in thrombosis models at high doses of PAI-144,45 because of the short half-life in vivo,46this study shows that low plasma concentrations of active PAI-1 suffice to restore inhibition of fibrinolysis. To investigate whether platelet PAI-1 would be involved in t-PA inhibition during the continuous deposition of platelets in a growing thrombus, crossover design experiments were performed, in which PAI-1−/− mice were reconstituted with PAI-1+/+ platelets and PAI-1+/+ mice with PAI-1−/− platelets. These experiments confirmed that thrombocytopenia could be induced in mice using busulfan and that thrombocytopenia resulted in loss of a thrombotic response to vascular injury. Furthermore, reconstitution with wt platelets in wt mice led to a full recovery of thrombus development, whereas reconstitution of PAI-1−/− mice with wt platelets could not restore thrombus development. Because a normal thrombotic response was measured in wt mice reconstituted with PAI-1–deficient platelets, it became clear that thrombosis was not controlled via platelet PAI-1.
Histologic and immunohistochemical studies confirmed endothelial cell destruction by the photochemical injury, in agreement with earlier findings. Because large amounts of granulocytes were found in the vicinity of the injured site (not shown), we conclude that PAI-1 is released into the circulation after injury and the accompanying inflammatory reaction, generating high concentrations of PAI-1 in the vicinity of the developing thrombus. This conclusion is supported by the 4- to 5-fold increase in plasma PAI-1 after injury in wt mice but also in thrombocytopenic mice. Accordingly, vascular tissue PAI-1 antigen concentrations were reduced after injury, presumably as a consequence of the release from the vessel into the circulation. Furthermore, ligating the injured area could completely abrogate the rise of plasma PAI-1. A mechanism for the regulation of thrombolysis in vivo has been proposed in which PAI-1 released from endothelial cells is incorporated into developing fibrin clots.47 Our own immunofluorescent PAI-1 staining of carotid artery cross sections was too diffuse to conclude decisively whether PAI-1 would predominantly be released from endothelial or from smooth muscle cells. Several studies indicate, however, that mouse endothelial cells do not contain PAI-1 messenger RNA (mRNA)48 49 and even take up PAI-1 from the circulation. Because these authors found faint PAI-1 expression by resting murine medial smooth muscle cells, those findings are indicative of PAI-1 release from the media in this study. Nevertheless, we can conclude that at sites of vascular injury, thrombi develop in an antifibrinolytic microenvironment, due to local secretion of PAI-1. The potent inhibition of plasmin by circulating α2-AP completes this antifibrinolytic action.
To understand the lack of effect on thrombosis of platelet PAI-1, we adapted existing ELISA assays to accurately measure PAI-1 antigen and activity levels in mouse platelets. The low murine PAI-1 antigen concentrations measured in plasma (below 1 ng/mL) are consistent with data described elsewhere.29,35 These levels are more than 10-fold lower than in human plasma15,50 but comparable to rat plasma.22 At least half of this concentration was estimated to represent active PAI-1, as also observed in man.15,50Murine platelet PAI-1 antigen levels measured in lysates and releasates were around 0.5 ng/109 cells; ie, about 50-fold and 500-fold lower than in rat and human platelets, respectively.15,22,50,51 We could easily detect active PAI-1 in human platelets, but not in murine platelets. From the lack of reactivity with human t-PA, and correcting for interferences during the detection, we estimate that at best 20% of the PAI-1 antigen in murine platelets represents active PAI-1, as in human platelets,40-42 corresponding to an active PAI-1 pool circulating in platelets equivalent to 100 pg only. These numbers explain in our cross-over thrombosis studies that platelet PAI-1 antigen levels and activity are too low to exert any effect during platelet accumulation in developing thrombi. They also support our findings that it is the local release of vascular PAI-1 that controls fibrinolysis in the developing thrombus. They further explain the lack of a gene-dose effect in the PAI-1+/− mice, because when released from the vessel wall, locally high PAI-1 concentrations can be reached, even in heterozygous animals.
The currently described results do not detract from conclusions in other animal models in which small amounts of active PAI-1 in platelets played a role in regulating fibrinolysis. The 500-fold lower levels of PAI-1 in mouse platelets compared with human platelets rather provided a unique opportunity to study the role of PAI-1 from other sources in regulating fibrinolysis. Rather than diminish the role of platelet PAI-1 for human biology, this study raises the possibility that vascular release of PAI-1 may play a similar role in humans.
In conclusion, this study confirms that, during acute thrombosis, fibrinolysis is efficiently prevented by the combined action of plasma α2-AP and PAI-1. PAI-1 is released locally into the circulation from the vessel wall, as a consequence of the injury, and participates in the control of thrombus formation. Thus, our findings show a role for the vessel wall in controlling the fibrinolytic system during thrombosis in vivo.
Acknowledgments
We thank Dr P. Declerck (Laboratory for Pharmaceutical Biology and Phytopharmacology, Faculty of Pharmaceutical Sciences, KU Leuven) for generously providing some of the antibodies and the recombinant murine PAI-1 used in this study. The help of I. Vreys (immunohistochemical studies) and H. Moreau (ELISAs for PAI-1) is greatly appreciated.
Reprints:Marc F. Hoylaerts, Center for Molecular and Vascular Biology, Herestraat 49, B-3000 Leuven, Belgium; e-mail: Marc.Hoylaerts@med.kuleuven.ac.be.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal