Abstract
Infusions of donor peripheral blood T cells can induce durable remissions of Epstein-Barr virus (EBV) lymphomas complicating marrow grafts, but they contain alloreactive T cells capable of inducing graft-versus-host disease. EBV-specific T-cell lines or clones avoid this problem but require 30 to 40 days of culture to establish. To accelerate the generation of EBV-specific T cells, we tested whether retroviral vectors, which only integrate in dividing cells, could be used to transduce and select antigen-reactive T cells early after sensitization to autologous EBV-transformed B cells. T cells were transduced with a dicistronic retroviral vector, NIT, which encodes low-affinity nerve growth factor receptor as an immunoselectable marker and herpes simplex virus thymidine kinase as a suicide gene, at different time points after sensitization. EBV-specific cytotoxic T lymphocyte precursor (CTLp) frequencies in purified NIT+T-cell fractions transduced on day 8 of culture were comparable to those of EBV-specific T-cell lines cultured for 30 days or more. Alloreactive CTLp frequencies were markedly reduced in the NIT+ fraction relative to the untransduced T-cell population. NIT+ fractions transduced on day 8 possessed more CD4+ T cells than the cell lines at day 30 and exhibited the same selective pattern of reactivity against immunodominant antigens presented by specific HLA alleles. In contrast, T cells transduced with NIT 5 days after stimulation with mitogen and interleukin-2 were relatively depleted of T cells specific for autologous EBV-transformed cells. Thus, retroviral vectors may be used for rapid selection of viral antigen-reactive T cells depleted of alloreactive T cells.
Lymphomas associated with Epstein-Barr virus (EBV) are a potentially lethal complication of marrow and organ allografts.1-3 The risk of developing an EBV lymphoma is increased in recipients of HLA-disparate related or unrelated unmodified marrow grafts receiving prolonged immunosuppression or T-cell–depleted marrow grafts.3,4 Because of the increasing use of such transplants, the need for consistently effective, logistically practicable approaches for the treatment of EBV lymphomas has increased. In 1994, our group reported that infusions of small numbers of peripheral blood mononuclear cells (PBMCs) derived from a seropositive marrow donor could induce durable and complete regression of EBV lymphomas emerging as a complication of related or unrelated T-cell–depleted marrow grafts.5 This finding has now been confirmed by several centers.6-9 Furthermore, the role of T cells as the principle effectors of the regressions observed has been demonstrated by Rooney et al,6 who have used genetically marked EBV-specific T-cell lines to treat 2 patients developing this complication and have reported that infusions of such in vitro–derived EBV-specific T cells may prevent EBV lymphomas in high-risk groups.
Infusions of donor-derived PBMCs, even in small numbers, may also transfer alloreactive T cells in numbers sufficient to cause severe or even lethal graft-versus-host disease (GVHD), particularly if the donor and host differ at 1 or more HLA alleles.3,5,8 Conversely, the generation of EBV-reactive T-cell lines adequately depleted of contaminating alloreactive T cells necessitates culture of donor T cells in the presence of irradiated autologous EBV-transformed B cells for at least 4 weeks.10 While cloning of EBV-reactive T cells can delete alloreactive T cells, the time required to expand these clones to sufficient numbers for therapy remains substantial.11,12 Because EBV lymphomas progress rapidly and are lethal in up to 50% of cases within 20 days,4these approaches require the establishment of such T-cell lines or clones prior to or at the time of transplantation rather than at the time of disease presentation. This presents significant logistical and economic problems, because T-cell lines need to be established and generated for all patients at significant risk but may be needed for only the 10% to 15% of patients who develop this complication.
An alternative strategy proposed by Sadelain and Mulligan13 and initially employed by Bonini et al9 is the use of unselected T cells transduced after mitogenic stimulation with a retroviral vector encoding a selectable marker and a drug sensitivity gene such as thymidine kinase. Because such T cells may contain subpopulations of alloreactive cells capable of inducing GVHD, incorporation of herpes simplex virus thymidine kinase (HSV-TK) permits eradication of alloreactive T cells expressing HSV-TK and reversal of GVHD through treatment of the host with ganciclovir.8,13 14 However, such treatment may also eliminate desired effectors such as transduced EBV-reactive T cells. Thus, the therapeutic advantage of suicide vector–modified unselected T cells rests with the balance existing between desired antigen-reactive T-cell populations and alloreactive T cells capable of inducing severe GVHD.
In this study, we have investigated whether retroviral vectors, which selectively integrate in dividing target cells,15 16 can be used to preferentially transduce those T cells within a polyclonal population that are specifically proliferating in response to an antigen. Our results suggest that antigen-specific T cells can be selected by this strategy early after in vitro sensitization and that alloreactive T cells can be substantially depleted by this approach.
Materials and methods
Production and culture of EBV-lymphoblastic cell lines
PBMCs at a concentration of 1 × 106/mL were incubated for 24 hours after isolation by Ficoll-Hypaque density centrifugation with the EBV-containing supernatant of the marmoset cell line 95-8 in the presence of 0.5 μg phytohemagglutinin (PHA)-16 (Murex-Diagnostik, Norcross, GA) in RPMI 1640 (GIBCO, Life Technologies, Grand Island, NY), 10% heat-inactivated fetal calf serum (FCS), 10 U/mL penicillin, 10 μg/mL streptomycin, and 1% L-glutamine. After 24 hours, cells were washed and recultured in EBV-containing medium without PHA in 24-well plates at a concentration of 1 × 106/mL. Cells were fed with RPMI 1640, 10% FCS, L-glutamine, penicillin, and streptomycin twice a week and expanded according to the growth and cell number. The cells were finally characterized by fluorescence-activated cell sorter (FACS) analysis using CD3, CD19, and CD20 monoclonal antibodies (Becton Dickinson, San Jose, CA). Aliquots of the immortalized B-lymphoblastoid cell lines (BLCLs) were frozen and the remaining cells maintained in culture.
Homozygous BLCLs for the HLA-A and HLA-B alleles, generously provided by Dr Bo Dupont, were maintained in the same medium. PHA blasts were generated by culturing 1 × 106/mL PBMC with 0.5 μg/mL PHA-16 for 3 days. The cells were washed and further cultured for 4 days in the presence of 5 IU/mL interleukin (IL-2) (Collaborative Biomedical Products, Bedford, MA).
Generation and culture of EBV-specific CTLs
PBMCs were isolated by Ficoll-Hypaque density centrifugation of anticoagulated whole blood. T lymphocytes were positively selected by staining with an anti-CD3 phycoerythrin monoclonal antibody (Becton Dickinson) on a MoFlo cell sorter (Cytomation, Fort Collins, CO), achieving a purity of more than 98%. EBV-specific cytotoxic T lymphocytes (CTLs) were generated by stimulating 1 × 106/mL CD3+ cells with 2.5 × 104/mL autologous BLCLs, which were irradiated with 60 Gy in Iscove's modified Dulbecco's medium supplemented with 10% heat-inactivated human AB serum (Gemini, Calabasas, CA), 35-μg/mL transferrin, 5-μg/mL insulin, 2 × 10-5 -M ethanolamine, 1-μg/mL palmitic acid, 1-μg/mL linoleic acid, and 1-μg/mL oleic acid (all from Sigma, St. Louis, MO) for 6 days in 25-cm2 flasks. Cells were washed, recultured at a concentration of 1 × 106/mL, and restimulated with 2 × 105/mL BLCL at day 7. Cells were either prepared for gene transfer on day 8 (early gene transfer) or kept in culture with restimulations weekly at an effector-to-target ratio of 5:1. After the third restimulation, T cells were prepared for gene transfer on day 23 (late gene transfer). A total of 5 IU of IL-2 (Collaborative Biomedical Products) were added for the first time at day 10 to the cultures and 2 to 3 times weekly thereafter.
For generation of alloreactive cells, donor T cells were stimulated with fully mismatched allogeneic EBV BLCL.
Generation of mitogen-activated cells
Nontissue culture–treated 12-well plates (Becton Dickinson Labware, Franklin Lakes, NJ) were coated with 1 μg/mL anti-CD3 and 1 μg/mL anti-CD28 monoclonal antibodies (PharMingen, San Diego, CA) for 3 to 4 hours at 37°C. Coated wells were incubated with 1% human serum albumin for 20 minutes and washed twice with phosphate-buffered saline (PBS). Freshly isolated PBMCs were plated at a concentration of 1 × 106/mL for 3 days at 37°C and transferred to 25-cm2 flasks (Corning, Corning, NY). IL-2 (10 IU/mL) was added to fresh culture medium for 2 days before gene transfer.
Vector system and gene transfer
Vector system.
The construct used in this study is a dicistronic vector, termed NIT, encoding a mutated and truncated human low-affinity nerve growth factor receptor (LNGFR) as an inactive cell surface marker that permits purification of transduced cells by immunoselection and the monitoring of gene expression by FACS analysis, and HSV-TK, which renders the cells sensitive to ganciclovir. The vector has been previously described by Gallardo et al.17 The PG13/NIT7 supernatants were used as cell-free viral stocks with a viral titer of multiplicity of infection (MOI) 1-2, as measured by FACS and Southern blot analyses on human A549 cells.
Gene transfer.
EBV-activated T cells (day 5, day 8, or day 23 of culture) or αCD3/αCD28–immobilized monoclonal antibody stimulated cells were placed in fibronectin-coated wells according to the technique described by Pollok et al18; 5 μg/mL of fibronectin fragments (TaKaRa Biomedicals, Shiga, Japan) were coated on nontissue culture–treated plates for 2 hours at room temperature in 6-well plates. Plates were blocked with 1% human serum albumin for at least 30 minutes and washed twice with PBS. Cells were plated at a concentration of 1 × 106/mL for 24 hours. Fifty percent of the supernatant was replaced with fresh medium containing 10% heat-inactivated human AB serum and 10 IU/mL IL-2. Cells were maintained in culture at a concentration of 1 × 106/mL to 1.5 × 106/mL. Three days later, cells were analyzed for gene expression by flow cytometry using the cell surface marker anti-NGFR monoclonal antibody.
Proliferation assay
To determine optimal time points for gene transfer into EBV-activated T lymphocytes, a proliferation assay was performed on days 1, 5, 7, 14, 21, and 28. Purified T cells were initially stimulated with irradiated autologous BLCLs on day 0 at an effector-to-stimulator ratio of 40:1. One day before the assay, T cells were restimulated at a ratio of 5:1. (For the day 5 and day 7 assays, separate flasks were initially set up to avoid a repeated stimulation on day 4 and day 6 for the day 7 proliferation assay.) A total of 2 × 105 cells/well were plated 1 day after restimulation in triplicate in 96-well plates. Equal amounts of stimulators only were plated separately to assess the background proliferation. Tritium thymidine (18.5 kBq) (Dupont, NEN Products, Boston, MA) was added per well for 24 hours. Cells were harvested, and proliferation was determined using a Wallac-counter (WALLAC, Gaithersburg, MD). Background values were subtracted from the experimental data. A total of 2 × 106 cells of the same bulk culture were transduced with the dicistronic vector on the same days proliferation assays were performed, and gene transfer efficiency was assessed by FACS analysis.
Flow cytometric analysis
Monitoring of the gene expression of NGFR of T lymphocytes was performed by 2-color flow cytometry using FACScan (Becton Dickinson) by labeling the cells with an anti-NGFR monoclonal antibody (20.4) (American Type Culture Collection, Rockville, MD) on ice for 45 minutes. Goat antimouse fluorescein isothiocyanate was added for 15 minutes as secondary antibody. After blocking with normal mouse serum (ICN/CAPPEL, Aurora, OH) for 10 minutes, anti-CD3 phycoerythrin (Becton Dickinson) was added for 15 minutes. Cells were washed twice with PBS after each step and before analysis.
Phenotyping of EBV-specific CTL lines was performed by gating lymphocytes using forward sight scatter and sideward sight scatter. Cells were stained with anti-CD3, anti-CD4, and anti-CD8 for T-cell subpopulations. Although cells had been purified initially for T lymphocytes, the transduced cells were reanalyzed for the presence of natural killer (NK) cells, defined as CD3−CD16+CD56+, using anti-CD16 and anti-CD56 monoclonal antibodies (Becton Dickinson).
Cell purification by FACS sorting
Gene-modified cells were prepared for purification using a FACStarPlus cell sorter (Becton Dickinson). A dual-color cell staining was performed with anti-NGFR and anti-CD3 as described for FACS analysis. Dual-color positive cells (NIT positive fraction) and single-color CD3 cells (NIT negative fraction) were sorted to high purity (more than 95%). One unsorted fraction was retained. The 3 cell fractions were subsequently used separately for cytotoxicity assays and limiting dilution analyses as described below.
Cytotoxicity assay
Cytolytic activity of effector cells was assayed against51Cr-labeled targets in standard 4-hour release assays. Target cells included autologous BLCLs, HLA class I mismatched allogeneic BLCLs, and K562 for major histocompatibility complex (MHC)–unrestricted lysis as a parameter for NK cell lysis and PHA blasts. For each donor HLA class I allele, a BLCL expressing the HLA-A and HLA-B allele homozygously could be included to determine the HLA restriction of the EBV-specific CTLs. Briefly, 1 × 106 target cells were incubated with 3700 kBq51Cr for 1 hour, washed 3 times, and plated in 96 wells. Cytotoxicity was analyzed using 0.8 × 105 effector cells: 4 × 103 target cells per well in a total volume of 200 μL, at an effector-to-target ratio of 20:1. All targets were plated in triplicate.
After an incubation of 4 hours, supernatants were harvested and the specific cytotoxicity determined using a microplate scintillation counter (Packard Instruments, Downer's Grove, IL). The percentage of specific lysis was calculated as 100% × (experimental release − spontaneous release)/(maximum release − spontaneous release). Maximum release was obtained by adding 100 μL of 5% Triton X-100 to the 100-μL medium containing target cells. Spontaneous release was consistently below 15% of maximum release in all assays.
Limiting dilution analysis
To evaluate CTL precursor (CTLp) frequencies in EBV-BLCL–activated or mitogen-activated T cells, limiting dilution analyses were performed using a modification of the methods of Bourgault et al19and Langhorne and Lindahl,20 as described by Lucas et al.21 Briefly, the cultured T-cell fractions were seeded in final volumes of 200 μL in 24 replicate wells per dilution of T cells in 96-well round bottom microplates. A decreasing number of effector cells (2 × 104, 1.25 × 104, 7813, 4883, 3052, 1907, 1192, and 745) were stimulated with 1 × 104 6000-cGy–irradiated autologous BLCL. A total of 1 × 104 autologous PBMCs, irradiated with 3000cGy, were added as feeders. On days 0, 2, and 5, 10 IU/mL of IL-2 were added to the cultures. On day 8, the plates were split and tested against autologous BLCLs and allogeneic targets. Wells were scored positive when 51Cr release exceeded the average plus 3 SD of control wells. The CTLp frequencies were calculated by the method of Taswell22 using a computer program provided by Dr Y. Kawanishi (Medical College of Wisconsin, Milwaukee, WI).
Results
Transduction conditions for selection of antigen-specific T cells
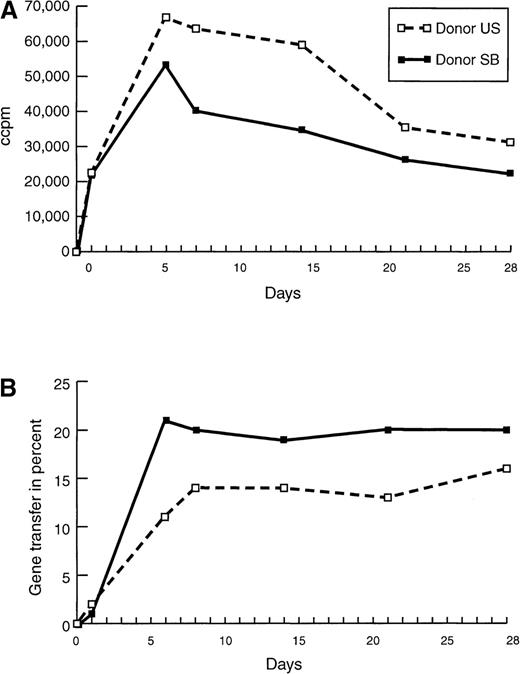
To define conditions that maximize selection of antigen-reactive cells, we initially examined the transduction of purified T-cell populations at increasing time intervals after stimulation with irradiated autologous EBV-transformed B cells. To measure vector gene expression, we took advantage of the mutant LNGFR encoded by the dicistronic NIT vector. Expression of this gene induces a high level of LNGFR protein on the surface of transduced cells. Thus, by using 2-color fluorescence, the proportion of T cells expressing the LNGFR can be accurately assessed and quantitated (Figure1). The experiments evaluating the time after stimulation with autologous BLCL at which transduction is maximized are presented in Figure 2. These results demonstrate that efficiency of transduction, under the conditions used, paralleled the level of proliferation of the stimulated T cells, measured by tritium thymidine incorporation at the time of retroviral transfer. Thus, maximum transduction of the stimulated T cells was observed after 5 days of stimulation for donor U.S. and 7 days for donor S.B.
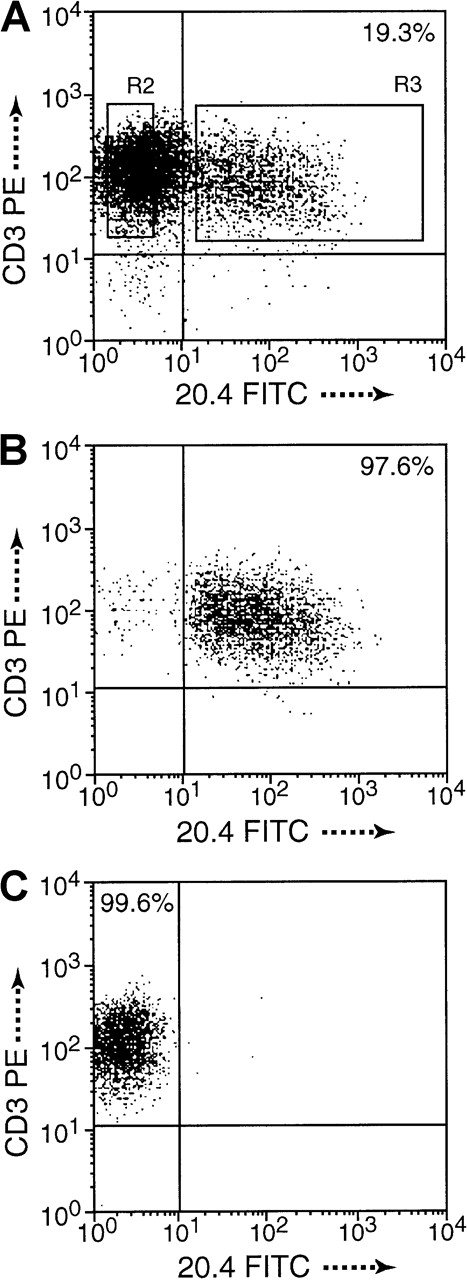
Cell purification by dual-color FACSort.
(A) Monitoring of gene expression (19.3%) 3 days after transduction of NIT. (B) CD3+ and NGFR+ T cells, termed NIT+ fraction, and (C) CD3+ and NGFR−, termed NIT− fraction, were sorted to high purity, 97.52% and 99.54%, respectively, in this example. Purity of sorted cell fractions was above 95% in all experiments. Cells of the unsorted fractions were included in the functional assays.
Cell purification by dual-color FACSort.
(A) Monitoring of gene expression (19.3%) 3 days after transduction of NIT. (B) CD3+ and NGFR+ T cells, termed NIT+ fraction, and (C) CD3+ and NGFR−, termed NIT− fraction, were sorted to high purity, 97.52% and 99.54%, respectively, in this example. Purity of sorted cell fractions was above 95% in all experiments. Cells of the unsorted fractions were included in the functional assays.
Proliferation and gene transfer efficiency of purified T-cell populations.
(A) Proliferation of and (B) gene transfer of NIT into T cells of donor U.S. and donor S.B. were compared at different time points after initiation with irradiated autologous EBV-transformed B cells. Cultures were restimulated 24 hours before the assays. Background proliferation of the irradiated BLCL was subtracted from the proliferation value of the stimulated T cells. To standardize the gene transfer procedure, previously frozen supernatant was used, explaining the relatively low gene transfer efficiency in this experiment. Cells, transduced with fresh supernatant as controls, showed a 30% to 50% higher gene expression at all time points.
Proliferation and gene transfer efficiency of purified T-cell populations.
(A) Proliferation of and (B) gene transfer of NIT into T cells of donor U.S. and donor S.B. were compared at different time points after initiation with irradiated autologous EBV-transformed B cells. Cultures were restimulated 24 hours before the assays. Background proliferation of the irradiated BLCL was subtracted from the proliferation value of the stimulated T cells. To standardize the gene transfer procedure, previously frozen supernatant was used, explaining the relatively low gene transfer efficiency in this experiment. Cells, transduced with fresh supernatant as controls, showed a 30% to 50% higher gene expression at all time points.
Enrichment of EBV-reactive T cells by retroviral transduction and selection early after in vitro sensitization
To determine the proportion of EBV-reactive T cells transduced after different periods of in vitro stimulation, T cells from 2 seropositive donors were transduced 5, 8, and 23 days after initial stimulation. The frequencies of EBV-reactive and alloreactive cytotoxic T cells were assessed in the unselected, NIT+, and NIT− T-cell populations. The T cells were sorted by dual-color staining for CD3 and LNGFR (monoclonal antibody 20.4) (Figure 1B and 1C). As shown in Table1, NIT+ T cells transduced after 5 days of culture exhibited frequencies of EBV-specific and allospecific cytotoxic T cells that did not differ significantly from the unsorted population. In contrast, sorted NGFR+ T cells transduced 8 days after initial sensitization contained EBV-specific T cells at frequencies that were 4- to 6-fold greater than in the unsorted T-cell populations and alloreactive T cells at frequencies that were 5- to 15-fold lower than those detected in the unsorted fraction. Furthermore, the frequencies of EBV-specific and allospecific cytotoxic T cells in the NIT+ T cells transduced at day 8 of culture were comparable to those detected in unsorted populations of EBV-stimulated T cells in culture for 30 days after initial sensitization.
Anti-EBV and anti-allo frequencies, including confidence intervals of unsorted, NIT+, and NIT− T cells of donor S.B. and U.S. after gene transfer on days 5, 8, or 23 and further processing as described
| . | Frequency unsorted . | Confidence interval . | Frequency NIT+ . | Confidence interval . | Frequency NIT− . | Confidence interval . |
|---|---|---|---|---|---|---|
| Donor S.B., day 5 | ||||||
| Anti-EBV | 1/1362 | 1/1156-1/1659 | 1/1236 | 1/1063-1/1477 | 1/4432 | 1/3654-1/5878 |
| Anti-allo | 1/1452 | 1/1282-1/1674 | 1/3888 | 1/3124-1/4976 | 1/6132 | 1/5413-1/7069 |
| Ratio | 1:1 | 3:1 | 1.4:1 | |||
| Donor S.B., day 8 | ||||||
| Anti-EBV | 1/9630 | 1/7483-1/13 694 | 1/1577 | 1/1372-1/1854 | 1/13 645 | 1/9327-1/14 163 |
| Anti-allo | 1/9507 | 1/8241-1/11 232 | 1/114 716 | 1/71 118-1/296 446 | 1/12 431 | 1/10 833-1/15 494 |
| Ratio | 1:1 | 72:1 | 1:1 | |||
| Donor S.B., day 23 | ||||||
| Anti-EBV | 1/2314 | 1/1864-1/3328 | 1/1289 | 1/1074-1/1563 | 1/7633 | 1/5974-1/9126 |
| Anti-allo | 1/120 886 | 1/74 634-1/310 089 | < 1/500 000 | < 1/500 000 | 1/144 613 | 1/88 365-1/397 045 |
| Ratio | 52:1 | > 400:1 | 19:1 | |||
| Donor U.S., day 5 | ||||||
| Anti-EBV | 1/2393 | 1/1980-1/2656 | 1/1243 | 1/1019-1/1593 | 1/6314 | 1/5937-1/6980 |
| Anti-allo | 1/5929 | 1/5194-1/6947 | 1/8271 | 1/7603-1/8931 | 1/5260 | 1/4643-1/6065 |
| Ratio | 2.4:1 | 6:1 | 1:1 | |||
| Donor U.S., day 8 | ||||||
| Anti-EBV | 1/2883 | 1/2396-1/3418 | 1/755 | 1/609-1/1086 | 1/8462 | 1/6245-1/11 479 |
| Anti-allo | 1/14 621 | 1/11 278-1/20 186 | 1/66 773 | 1/48 832-1/118 494 | 1/14 800 | 1/11 978-1/20 465 |
| Ratio | 5:1 | 88:1 | 1.7:1 | |||
| Donor U.S., day 23 | ||||||
| Anti-EBV | 1/1470 | 1/1302-1/1687 | 1/1599 | 1/1411-1/1864 | 1/4954 | 1/3823-1/6105 |
| Anti-allo | 1/62 650 | 1/43 518-1/111 803 | < 1/500 000 | < 1/500 000 | 1/126 615 | 1/78 081-1/334 587 |
| Ratio | 42:1 | > 400:1 | 25:1 |
| . | Frequency unsorted . | Confidence interval . | Frequency NIT+ . | Confidence interval . | Frequency NIT− . | Confidence interval . |
|---|---|---|---|---|---|---|
| Donor S.B., day 5 | ||||||
| Anti-EBV | 1/1362 | 1/1156-1/1659 | 1/1236 | 1/1063-1/1477 | 1/4432 | 1/3654-1/5878 |
| Anti-allo | 1/1452 | 1/1282-1/1674 | 1/3888 | 1/3124-1/4976 | 1/6132 | 1/5413-1/7069 |
| Ratio | 1:1 | 3:1 | 1.4:1 | |||
| Donor S.B., day 8 | ||||||
| Anti-EBV | 1/9630 | 1/7483-1/13 694 | 1/1577 | 1/1372-1/1854 | 1/13 645 | 1/9327-1/14 163 |
| Anti-allo | 1/9507 | 1/8241-1/11 232 | 1/114 716 | 1/71 118-1/296 446 | 1/12 431 | 1/10 833-1/15 494 |
| Ratio | 1:1 | 72:1 | 1:1 | |||
| Donor S.B., day 23 | ||||||
| Anti-EBV | 1/2314 | 1/1864-1/3328 | 1/1289 | 1/1074-1/1563 | 1/7633 | 1/5974-1/9126 |
| Anti-allo | 1/120 886 | 1/74 634-1/310 089 | < 1/500 000 | < 1/500 000 | 1/144 613 | 1/88 365-1/397 045 |
| Ratio | 52:1 | > 400:1 | 19:1 | |||
| Donor U.S., day 5 | ||||||
| Anti-EBV | 1/2393 | 1/1980-1/2656 | 1/1243 | 1/1019-1/1593 | 1/6314 | 1/5937-1/6980 |
| Anti-allo | 1/5929 | 1/5194-1/6947 | 1/8271 | 1/7603-1/8931 | 1/5260 | 1/4643-1/6065 |
| Ratio | 2.4:1 | 6:1 | 1:1 | |||
| Donor U.S., day 8 | ||||||
| Anti-EBV | 1/2883 | 1/2396-1/3418 | 1/755 | 1/609-1/1086 | 1/8462 | 1/6245-1/11 479 |
| Anti-allo | 1/14 621 | 1/11 278-1/20 186 | 1/66 773 | 1/48 832-1/118 494 | 1/14 800 | 1/11 978-1/20 465 |
| Ratio | 5:1 | 88:1 | 1.7:1 | |||
| Donor U.S., day 23 | ||||||
| Anti-EBV | 1/1470 | 1/1302-1/1687 | 1/1599 | 1/1411-1/1864 | 1/4954 | 1/3823-1/6105 |
| Anti-allo | 1/62 650 | 1/43 518-1/111 803 | < 1/500 000 | < 1/500 000 | 1/126 615 | 1/78 081-1/334 587 |
| Ratio | 42:1 | > 400:1 | 25:1 |
The anti-auto:anti-allo frequency ratio is indicated below each experiment.
The potential of retroviral transduction at day 8 of culture to select for EBV-specific T cells and against alloreactive T cells was assessed in studies of an additional 3 seropositive donors. Composite results for the 5 normal donors tested are presented in Table2. In this table, frequencies of EBV-specific and allospecific T cells in the unsorted, NGFR+, and NGFR− populations are presented together with confidence intervals for the limiting dilution analyses performed. In 4 of 5 donors studied, there was a significant enrichment of EBV-specific T cells in the NIT+ transduced T-cell population. This enrichment ranged from 1.5- to 6.1-fold over the unsorted fraction. Similarly, in each case there was a significant reduction in the frequency of alloreactive T cells, ranging from 2- to 15-fold, in the transduced and selected NIT+ T-cell fraction.
Anti-EBV and anti-allo frequencies, including confidence intervals of unsorted, NIT+, and NIT− T cells of 5 donors after gene transfer on day 8
| . | Frequency unsorted . | Confidence interval . | Frequency NIT+ . | Confidence interval . | Frequency NIT− . | Confidence interval . |
|---|---|---|---|---|---|---|
| Donor S.B. | ||||||
| Anti-EBV | 1/9630 | 1/7483-1/13 694 | 1/1577 | 1/1372-1/1854 | 1/13 645 | 1/9327-1/14 163 |
| Anti-allo | 1/9507 | 1/8241-1/11 232 | 1/114 716 | 1/71 118-1/296 446 | 1/12 431 | 1/10 833-1/15 494 |
| Ratio | 1:1 | 72:1 | 1:1 | |||
| Donor U.S. | ||||||
| Anti-EBV | 1/2883 | 1/2396-1/3418 | 1/755 | 1/609-1/1086 | 1/8462 | 1/6245-1/11 479 |
| Anti-allo | 1/14 621 | 1/11 278-1/20 186 | 1/66 773 | 1/48 832-1/118 494 | 1/14 800 | 1/11 978-1/20 465 |
| Ratio | 5:1 | 88:1 | 1.7:1 | |||
| Donor J.A. | ||||||
| Anti-EBV | 1/6116 | 1/5417-1/7021 | 1/4876 | 1/4121-1/5970 | nd | |
| Anti-allo | 1/2995 | 1/2650-1/3443 | 1/45 397 | 1/32 175-1/77 069 | nd | |
| Ratio | 1:2 | 10:1 | ||||
| Donor J.K. | ||||||
| Anti-EBV | 1/9548 | 1/7886-1/12 098 | 1/5728 | 1/4908-1/6879 | 1/14 800 | 1/12 987-1/16 734 |
| Anti-allo | 1/24 770 | 1/18 693-1/37 182 | 1/52 704 | 1/35 668-1/88 366 | 1/11 596 | 1/10 324-1/13 453 |
| Ratio | 2.5:1 | 10:1 | 1:1.2 | |||
| Donor M.A. | ||||||
| Anti-EBV | 1/2692 | 1/1963-1/3106 | 1/738 | 1/584-1/1002 | 1/3623 | 1/3234-1/4119 |
| Anti-allo | 1/17 247 | 1/14 202-1/21 952 | 1/91 024 | 1/59 436-1/194 271 | 1/15 226 | 1/12 770-1/18 850 |
| Ratio | 6:1 | 123:1 | 4:1 |
| . | Frequency unsorted . | Confidence interval . | Frequency NIT+ . | Confidence interval . | Frequency NIT− . | Confidence interval . |
|---|---|---|---|---|---|---|
| Donor S.B. | ||||||
| Anti-EBV | 1/9630 | 1/7483-1/13 694 | 1/1577 | 1/1372-1/1854 | 1/13 645 | 1/9327-1/14 163 |
| Anti-allo | 1/9507 | 1/8241-1/11 232 | 1/114 716 | 1/71 118-1/296 446 | 1/12 431 | 1/10 833-1/15 494 |
| Ratio | 1:1 | 72:1 | 1:1 | |||
| Donor U.S. | ||||||
| Anti-EBV | 1/2883 | 1/2396-1/3418 | 1/755 | 1/609-1/1086 | 1/8462 | 1/6245-1/11 479 |
| Anti-allo | 1/14 621 | 1/11 278-1/20 186 | 1/66 773 | 1/48 832-1/118 494 | 1/14 800 | 1/11 978-1/20 465 |
| Ratio | 5:1 | 88:1 | 1.7:1 | |||
| Donor J.A. | ||||||
| Anti-EBV | 1/6116 | 1/5417-1/7021 | 1/4876 | 1/4121-1/5970 | nd | |
| Anti-allo | 1/2995 | 1/2650-1/3443 | 1/45 397 | 1/32 175-1/77 069 | nd | |
| Ratio | 1:2 | 10:1 | ||||
| Donor J.K. | ||||||
| Anti-EBV | 1/9548 | 1/7886-1/12 098 | 1/5728 | 1/4908-1/6879 | 1/14 800 | 1/12 987-1/16 734 |
| Anti-allo | 1/24 770 | 1/18 693-1/37 182 | 1/52 704 | 1/35 668-1/88 366 | 1/11 596 | 1/10 324-1/13 453 |
| Ratio | 2.5:1 | 10:1 | 1:1.2 | |||
| Donor M.A. | ||||||
| Anti-EBV | 1/2692 | 1/1963-1/3106 | 1/738 | 1/584-1/1002 | 1/3623 | 1/3234-1/4119 |
| Anti-allo | 1/17 247 | 1/14 202-1/21 952 | 1/91 024 | 1/59 436-1/194 271 | 1/15 226 | 1/12 770-1/18 850 |
| Ratio | 6:1 | 123:1 | 4:1 |
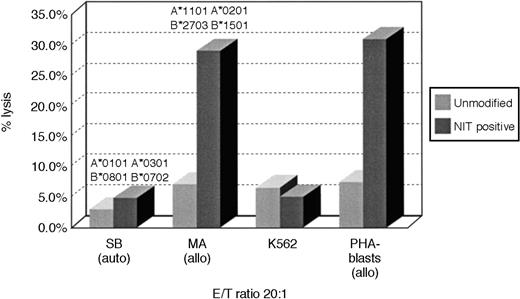
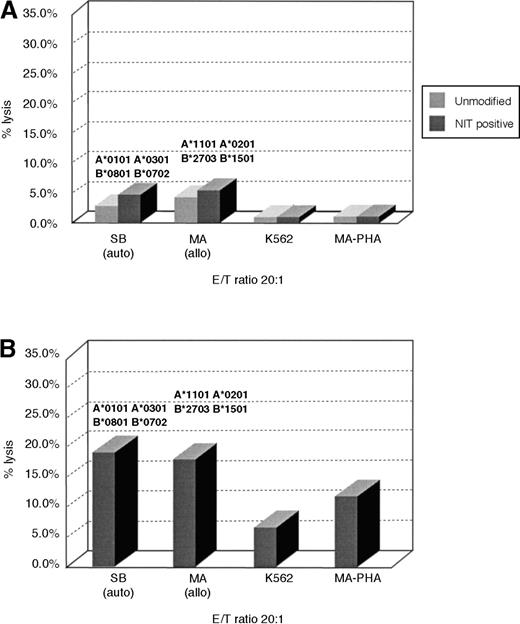
To determine whether this strategy of retrovirus transduction and selection could also permit early enrichment of T cells reactive against other determinants, we concurrently sensitized T cells with irradiated allogeneic EBV-transformed B cells and assessed T cells transduced 8 days after sensitization for allospecific cytotoxicity in51Cr cytotoxicity assays and for the frequencies of allospecific cytotoxic cells in unsorted and NIT+-transduced T-cell populations. As shown in Figure3, unsorted T cells from donor S.B. sensitized to fully allogeneic EBV BLCL from donor M.A. exhibit low but appreciable specific reactivity against EBV BLCL or PHA blasts from donor M.A. In contrast, the NIT+-transduced T-cell fraction sorted on the basis of NGFR expression exhibits strong and specific reactivity against the PHA blasts and EBV BLCL of donor M.A. In limiting dilution analyses, the NIT+ T-cell fractions exhibited a 13-fold higher frequency of T cells reactive against the allogeneic EBV BLCL stimulating cells and, concurrently, a 3- to 8-fold depletion of EBV-specific T cells reactive against autologous EBV-transformed B-cell targets (Table 3).
Cytotoxicity of transduced and unmodified T cells after activation with allogeneic EBV BLCL.
Purified T cells of donor S.B. were stimulated with fully MHC-mismatched allogeneic BLCLs of donor M.A. MA-BLCL, not recognized by the NIT+ T-cell fraction after stimulation with autologous BLCLs of S.B., exhibited strong reactivity against EBV BLCL and PHA blasts of M.A., after initiation and restimulation with the allogeneic BLCLs and gene transfer on day 8 as described, showing the preferential selection of anti-allogeneic T cells. The corresponding anti-allo frequencies are described in Table 3.
Cytotoxicity of transduced and unmodified T cells after activation with allogeneic EBV BLCL.
Purified T cells of donor S.B. were stimulated with fully MHC-mismatched allogeneic BLCLs of donor M.A. MA-BLCL, not recognized by the NIT+ T-cell fraction after stimulation with autologous BLCLs of S.B., exhibited strong reactivity against EBV BLCL and PHA blasts of M.A., after initiation and restimulation with the allogeneic BLCLs and gene transfer on day 8 as described, showing the preferential selection of anti-allogeneic T cells. The corresponding anti-allo frequencies are described in Table 3.
Anti-EBV and anti-allo frequencies of donor S.B. after sensitization of T cells with fully allogeneic BLCLs in the unsorted and NIT+ cell fraction
| . | Frequency unsorted . | Confidence interval . | Frequency NIT+ . | Confidence interval . | . |
|---|---|---|---|---|---|
| Anti-EBV | 1/156 233 | 1/91 233-1/543 342 | 1/469 928 | 1/158 751- < 1/600 000 | |
| Anti-allo | 1/4242 | 1/3679-1/5009 | 1/324 | 1/233-1/531 | |
| Auto/allo ratio | 1:37 | 1:1450 |
| . | Frequency unsorted . | Confidence interval . | Frequency NIT+ . | Confidence interval . | . |
|---|---|---|---|---|---|
| Anti-EBV | 1/156 233 | 1/91 233-1/543 342 | 1/469 928 | 1/158 751- < 1/600 000 | |
| Anti-allo | 1/4242 | 1/3679-1/5009 | 1/324 | 1/233-1/531 | |
| Auto/allo ratio | 1:37 | 1:1450 |
Representation of T-cell subsets in NIT-transduced T-cell populations
Extended culture of T cells reactive against EBV-transformed B cells has been shown to favor expansion of CD8+ EBV-specific cytotoxic T-cell populations. However, CD4+ T cells may provide important signals for expansion of virus-specific T-cell populations. For this reason, we were interested in comparing the representation of CD4+ and CD8+ subsets in T-cell populations transduced early and late postsensitization in vitro.
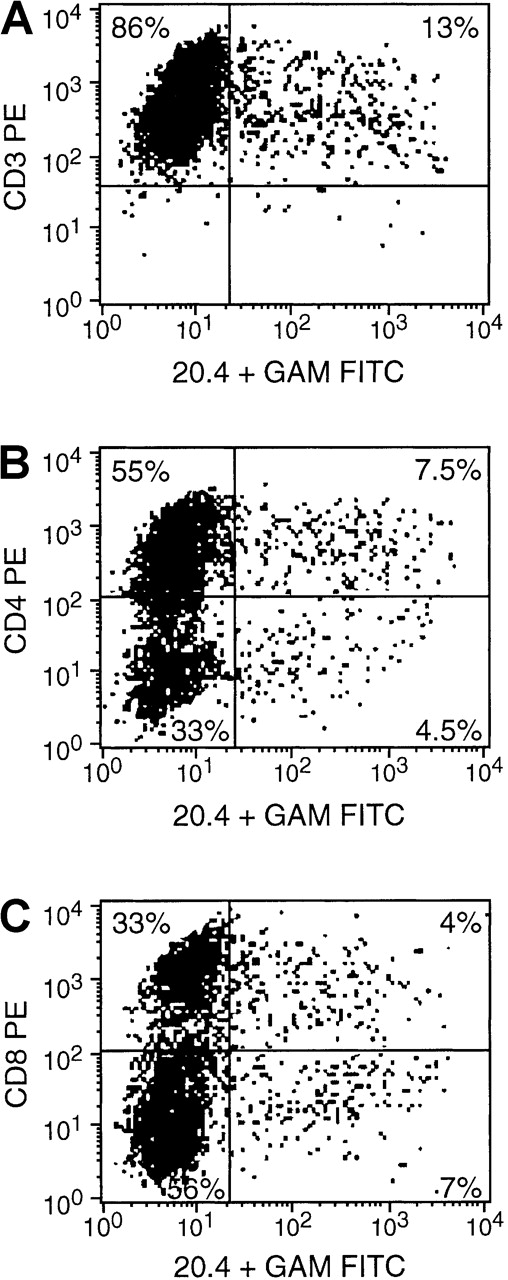
T cells isolated from normal seropositive donors were sensitized in vitro to irradiated autologous EBV BLCL and transduced with the NIT vector supernatant at day 8. Three days later, the unsorted populations were assessed by immunocytofluorometry for NIT+ and NIT− cells in the T-cell populations. A representative FACS analysis is presented in Figure4. Thirteen percent of the isolated T cells were transduced; the distribution of CD4+ and CD8+ T cells in the transduced NIT+ fraction (7.5% and 4.5%, respectively; CD4/CD8 ratio, 1.6) did not differ from the relative proportions of CD4+ and CD8+ T cells in the nontransduced NIT− fraction (55% and 33%, respectively; CD4/CD8 ratio = 1.6).
FACS analysis obtained 3 days after gene transfer.
Cells were stained with anti-CD3, anti-CD4, or anti-CD8 and anti-NGFR (monoconal antibody 20.4). The results indicate an equal transvection of CD4 and CD8 lymphocyte subsets, reflected by the CD4/CD8 ratio of 1.6 in both the transduced NIT+ T cells and the nontransduced NIT− T-cell fraction.
FACS analysis obtained 3 days after gene transfer.
Cells were stained with anti-CD3, anti-CD4, or anti-CD8 and anti-NGFR (monoconal antibody 20.4). The results indicate an equal transvection of CD4 and CD8 lymphocyte subsets, reflected by the CD4/CD8 ratio of 1.6 in both the transduced NIT+ T cells and the nontransduced NIT− T-cell fraction.
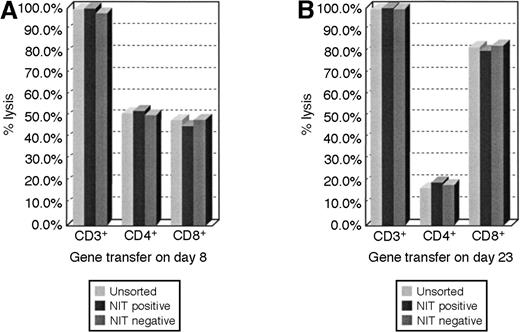
We analyzed the representation of NIT+ T cells in cultures of EBV BLCL-sensitized T cells transduced 8 or 23 days after initial sensitization in vitro. These results indicate that the proportional representation of CD4+ and CD8+ T cells in transduced populations closely parallels that detected in the sensitized T-cell population as a whole and that CD4+ T cells are represented in a significantly higher proportion of the T cells transduced early after sensitization (Figure5).
FACS analyses of lymphocyte subsets in the unsorted and sorted T-cell fractions.
The proportional representation of CD4+ and CD8+ cells in the NIT+ T-cell fraction parallels the unsorted T-cell population after early and after late transduction. (A) The results after gene transfer on day 8 showed a proportional higher representation of CD4+ T cells in the cell populations, whereas in panel B, after gene transfer on day 23 of cultures, T cells consisted of 80% CD8+ cells and 20% CD4+ T lymphocytes.
FACS analyses of lymphocyte subsets in the unsorted and sorted T-cell fractions.
The proportional representation of CD4+ and CD8+ cells in the NIT+ T-cell fraction parallels the unsorted T-cell population after early and after late transduction. (A) The results after gene transfer on day 8 showed a proportional higher representation of CD4+ T cells in the cell populations, whereas in panel B, after gene transfer on day 23 of cultures, T cells consisted of 80% CD8+ cells and 20% CD4+ T lymphocytes.
HLA restriction patterns of EBV-reactive T cells transduced early after in vitro stimulation with autologous EBV LCL
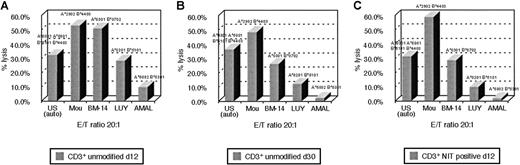
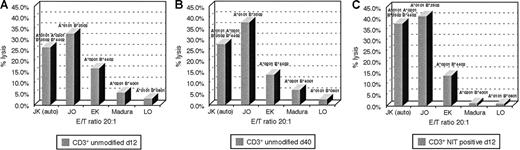
An advantage hypothesized for suicide vector–modified T cells transduced early after activation is the potentially broad spectrum of antigen-reactive cells represented in such T-cell populations, as compared with the more restricted reactivities represented in T-cell lines.9 To examine this hypothesis, we analyzed the patterns of HLA restriction exhibited by unsorted and sorted LNGFR+ T cells from in vitro cultures transduced with the NIT vector on day 8 after sensitization with EBV BLCL and compared them with the patterns of HLA-restricted cytotoxicity exhibited by EBV BLCL-sensitized T cells from the same donor after 30 days of in vitro culture and expansion. In these experiments, the different EBV-sensitized T-cell fractions were assayed for their capacity to lyse autologous EBV LCL and EBV LCL derived from allogeneic donors homozygous for HLA-A, -B, and -Dr alleles shared by or allogeneic to the donor. Results of analyses of T-cell fractions from 2 donors are presented in Figures6 and 7. As seen in Figure 6, unsorted T cells from cultures transduced with NIT on day 8 induced 33% lysis of autologous EBV LCL (HLA A*0301, B*5101/A*6801, B*4403) and were also cytotoxic for targets sharing HLA B*4403 (53%) and HLA A*0301 (51%) and HLA B*5101 (28%) but exhibited little reactivity against targets expressing HLA A*6802. In contrast, after 30 days of culture, cytotoxicity against EBV LCL–expressing HLA B*4403 was dominant (48%), with less activity detected against targets bearing HLA A*0301 (26%) and minimal reactivity against targets bearing HLA B*5101 and HLA A*6802. Strikingly, an almost identical pattern of a dominant cytolytic response against an HLA B*4403 target was also observed in testings of the LNGFR-expressing T cells transduced with NIT 8 days after sensitization. A similar enrichment of T cells reactive against EBV antigens presented by an immunodominant allele (ie, HLA B*3502) was also observed in the sorted LNGFR-expressing fraction of T cells from donor J.K., transduced on day 8 and in the unsorted EBV-specific T cells tested after 40 days of in vitro culture. This pattern contrasts with the broader pattern of HLA restriction exhibited by the unsorted T cells tested after transduction at day 8. These results suggest that immunodominant EBV-reactive T cells, ie, T cells that recognize immunodominant EBV antigens that are presented by specific HLA alleles, are preferentially transduced early after in vitro stimulation with autologous EBV BLCL.
HLA restriction pattern of EBV-reactive T cells of donor U.S.
The pattern is shown (A) after 12 days in culture, (B) after 30 days in culture, and (C) in the NIT+ fraction after gene transfer on day 8 and FACSort on day 11. In the 51Cr release assay, each donor MHC class I allele could be paired with a BLCL, expressing that allele homozygously. HLA-B*4403 is the allele, presenting predominantly EBV peptide to the T cells in the late culture, as opposed to the broader HLA spectrum of T cells cultured for 12 days only. The more restricted pattern to HLA-B*4403 can be demonstrated in the NIT+ fraction after early selection.
HLA restriction pattern of EBV-reactive T cells of donor U.S.
The pattern is shown (A) after 12 days in culture, (B) after 30 days in culture, and (C) in the NIT+ fraction after gene transfer on day 8 and FACSort on day 11. In the 51Cr release assay, each donor MHC class I allele could be paired with a BLCL, expressing that allele homozygously. HLA-B*4403 is the allele, presenting predominantly EBV peptide to the T cells in the late culture, as opposed to the broader HLA spectrum of T cells cultured for 12 days only. The more restricted pattern to HLA-B*4403 can be demonstrated in the NIT+ fraction after early selection.
HLA restriction of EBV-reactive T cells of donor J.K.
(A) The unsorted EBV-reactive T cells. (B) After 40 days in culture. (C) The NIT+ fraction on day 12 of donor J.K. HLA-B*3502 is the dominantly presenting allele in the purified NIT+ T-cell population and the established EBV-specific T-cell line after 40 days of culture.
HLA restriction of EBV-reactive T cells of donor J.K.
(A) The unsorted EBV-reactive T cells. (B) After 40 days in culture. (C) The NIT+ fraction on day 12 of donor J.K. HLA-B*3502 is the dominantly presenting allele in the purified NIT+ T-cell population and the established EBV-specific T-cell line after 40 days of culture.
Assessment of EBV-specific and alloreactive T cells in mitogen-activated NIT-transduced T-cell populations
Published reports of clinical trials using suicide vector–modified T cells for treatment of EBV lymphomas or leukemic relapse after transplantation have employed T cells transduced after nonspecific stimulation with the mitogen PHA. Accordingly, we sought to characterize and quantify EBV-reactive and alloreactive T cells in T-cell populations transduced after stimulation with PHA or immobilized αCD3 and αCD28. In Figure 8A, we compared the cytotoxic activities of unsorted and NIT-transduced LNGFR-expressing T cells with autologous and allogeneic EBV BLCL and with allogeneic uninfected PHA blasts and K562 targets. As can be seen, the transduced cells exhibit negligible activity against any of these targets. This lack of reactivity was confirmed in limiting dilution analyses. The frequencies of CTLp reactive against autologous or allogeneic EBV BLCL were less than 3 × 10−5 in the mitogen-stimulated unsorted and the sorted NIT-transduced LNGFR-expressing T cells derived from donors S.B. and U.S. These frequencies are markedly lower than the frequencies of EBV-specific or alloreactive T cells detected in the unsorted or transduced T-cell population specifically sensitized with autologous or allogeneic EBV BLCL.
Cytotoxic activity of NIT+ T cells transduced after mitogen stimulation.
T cells of donor S.B. were stimulated with αCD3/αCD28–immobilized monoclonal antibodies for 3 days and subsequently with 10 IU/mL IL-2 for 2 days in 6-well plates before gene transfer. NIT+cells were purified as described and used for functional assays. (A) The cytotoxic activity of the NIT+ T cells is less than 10% against autologous EBV BLCL and allogeneic targets. Equally activated but unmodified T cells were used as controls, showing no difference between the 2 T-cell populations. (B) The result of the same NIT+ T cells 8 days later after secondary stimulation with autologous BLCLs. The cells lysed both autologous and allogeneic EBV BLCL with comparable activity.
Cytotoxic activity of NIT+ T cells transduced after mitogen stimulation.
T cells of donor S.B. were stimulated with αCD3/αCD28–immobilized monoclonal antibodies for 3 days and subsequently with 10 IU/mL IL-2 for 2 days in 6-well plates before gene transfer. NIT+cells were purified as described and used for functional assays. (A) The cytotoxic activity of the NIT+ T cells is less than 10% against autologous EBV BLCL and allogeneic targets. Equally activated but unmodified T cells were used as controls, showing no difference between the 2 T-cell populations. (B) The result of the same NIT+ T cells 8 days later after secondary stimulation with autologous BLCLs. The cells lysed both autologous and allogeneic EBV BLCL with comparable activity.
Given the low frequency of EBV-reactive and alloreactive T cells in the mitogen-stimulated and NIT-transduced populations, we were interested to determine whether and to what degree EBV-specific T cells could be generated from these transduced fractions after secondary stimulation with autologous EBV BLCL. Results of such analyses for 1 of 3 donors tested are presented in Figure 8B. As can be seen, cytotoxicity of 20% against autologous EBV BLCL and 18% against allogeneic EBV BLCL was detected 8 days following stimulation of these LNGFR+ cells with autologous BLCLs. We were unable to sustain these cells beyond an additional 14 days in vitro. Similarly activated cells from 2 additional donors failed to generate populations that exhibited significant cytotoxicity to autologous or allogeneic EBV-transformed targets after 8 days of sensitization to autologous EBV-transformed B cells.
Discussion
As seen in our study population and also recorded by other groups, the frequencies of CTLp reactive against EBV in seropositive adults are similar to the frequencies of CTLp directed against HLA alloantigens in normal individuals.19,21,23 Current approaches involving adoptive cell therapy with EBV-specific T-cell lines require 28 to 40 days of in vitro expansion to achieve a comparable depletion of alloreactive T cells capable of inducing severe GVHD. Unfortunately, EBV lymphomas evolve rapidly. Indeed, the median time from diagnosis to death due to EBV lymphomas developing early after transplantation has been reported in 1 international compilation to be as short as 20 days.4 For this reason, such T cells must be generated prior to or at the time of transplantation to be available for treatment during the 2- to 8-month period posttransplant during which such patients are at maximal risk of developing an EBV lymphoproliferative disorder.3 4
In this study, we examined whether the ability of retroviral vectors to selectively integrate in dividing cells could be exploited as a method for early selection of virus-specific or allospecific T cells replicating in response to irradiated EBV-transformed B cells. To facilitate in vitro separation of transduced T cells, we employed a dicistronic vector, termed NIT, which encodes a mutant nerve growth factor receptor, LNGFR, that is expressed on the surface of transduced cells at levels permitting their isolation byimmunoadsorption, and HSV-TK, to permit depletion of transduced cells in vivo in the event that transduced cells infused induced clinically severe acute GVHD. Our experiments indicate that such a strategy does indeed permit early selection and enrichment of virus-specific T cells while at the same time markedly reducing populations of alloreactive T cells in the transduced fraction. EBV-specific CTLp frequencies in populations transduced 8 days after in vitro stimulation with autologous irradiated EBV BLCL and selected by immunoadsorption were comparable to those detected in cultures of T cells sensitized to autologous EBV BLCL and expanded in vitro for 30 days. Furthermore, early transduction and selection also resulted in depletion of alloreactive T cells to concentrations similar to those detected in T-cell lines cultured for 30 days. Thus, this approach reduces the time required to generate virus-specific T cells depleted of alloreactive T cells by more than 3 weeks. For the logistics of a program of adoptive cell therapy, this may represent significant advantages. Furthermore, it provides a treatment option for patients who present with this complication who do not have prepared T cells.
The number of EBV-specific T cells required to induce remissions of widespread lymphoma is small. For example, our previous studies demonstrated that total doses providing as few as 1 × 103 CTLp could be infused and would expand rapidly to induce durable regression of disease. Based on our results, the sensitization and early selection of NIT+T cells could provide doses of NIT+ EBV CTLp that do not require further expansion in vitro prior to infusion. For example, among the donors we studied, PBMCs from donor U.S. had an EBV CTLp frequency of 15 × 10−3 at day 0. A dose of donor leukocytes providing 106 T cells/kg from this seropositive donor (doses of 0.5 × 106 to 1.0 × 106 have induced durable regressions in each patient in our series) would thus provide 70 kg × 106 T cells/kg × 15 × 10−3 = 4666 EBV CTLp. If 1 × 108 T cells were isolated from about 150 mL of blood from this donor, sensitized with autologous EBV BLCL, and then transduced with the vector on day 8, by day 11, the time when we isolate the NIT+ cells, the total T-cell population would be about 3 × 108. Assuming 20% are transduced (6 × 107 T cells) and 50% of the NIT+ cells are recovered after isolation by immunoadsorption, the yield would be 3 × 107 NIT+ T cells. As noted in Table 2, the EBV CTLp frequency in the NIT+ T-cell fraction of donor U.S. is 1 in 755. Thus, the total dose of NIT+ EBV CTLp that would be provided, were all of these cells infused, would be 4 × 104 EBV CTLp or 8.5-fold greater than the dose that would have been provided with the conventional 106 T cells/kg dose used by us in dosing donor leukocytes. Were we to use the dose of T cells used by Rooney et al6 28 in their trials of adoptively transferred EBV-specific T-cell lines (3 × 105 T cells/kg), the dose of CTLp provided by the NIT+ T cells isolated from 1 × 108 starting T cells would be 1.5-fold the dose used by Rooney, assuming CTLp frequencies are equivalent. Thus, the technique proposed does not necessitate further expansion of the NIT+ T cells to achieve a dose adequate for treatment.
Although retroviral vector–mediated selection of antigen-reactive T cells may markedly reduce the time required to generate EBV-specific T-cell populations appropriately depleted of alloreactive cells, it does not alter the time required to generate autologous EBV-transformed B cells for T-cell sensitization. The use of mitogens rather than EBV-transformed B cells to stimulate the T cells prior to transduction circumvents this limitation and has been hypothesized to have the advantage of fostering transduction of a large array of antigen-reactive T cells.9 In fact, our experiments suggest that mitogen stimulation may induce a striking depletion of both EBV-specific and allospecific T cells. Indeed, the frequencies of CTLp reactive against either EBV or allogeneic targets were markedly lower than those detected in unstimulated donor T-cell populations. This finding may in part explain the delayed onset of acute GVHD noted by Contassot et al in murine allograft recipients treated with mitogen-stimulated T cells transduced with a vector encoding HSV-TK and neomycin phosphotransferase.24
Our findings that the frequencies of alloreactive CTLp in the isolated NIT+-transduced T cells are as low as those detected in EBV-reactive T-cell lines cultured for 30 to 40 days suggests the possibility that such cells, administered at doses of 1 × 107/m2 to 5 × 107/m2 may contain numbers of alloreactive T cells that are lower than the threshold dose required to initiate GVHD in HLA-matched unrelated or HLA single allele disparate hosts, because administration of such doses by Rooney et al derived from T-cell lines cultured for 30 to 40 days has not been associated with clinically significant GVHD.25 However, because the transduced NIT+ populations contain CD4+ T cells well in excess of those that would be detected in T-cell lines derived for 30 to 40 days, it is also possible that these transduced T cells would have a greater potential for GVHD induction, necessitating the inclusion of the HSV-TK gene as a safety element. While unmodified donor-derived PBMCs can be used to induce durable regression of EBV lymphomas emerging in HLA-matched recipients, infusion of as few as 106 T cells/kg in the PBMC mixtures can induce significant GVHD in up to 30% of cases if administered less than 6 months posttransplant.3 5 Thus, this approach may also have advantages for such patients as well.
Despite the clear enrichment of EBV-reactive T cells achieved in the NIT+ fractions, the CTLp frequencies detected would suggest that a large proportion of the vector-modified T cells are either not reactive against EBV antigens or are not clonogenic. Recent studies, in which HLA tetramers presenting specific EBV peptides have been used to quantitate EBV-reactive T cells, have suggested that the proportion of T cells in the circulation reactive against EBV antigens may exceed the proportion of reactive T cells detectable by limiting dilution analyses by 20- to 100-fold.26 27 If this were also to apply to the NIT+ populations, EBV-reactive cells would likely constitute the major antigen-reactive populations among the transduced cells. Nevertheless, under the conditions used in these experiments, small numbers of T cells reactive to other antigens, including alloantigens, were also transduced. It is possible that these T cells are part of the small fraction of unstimulated T cells that are in active division, or they are T cells nonspecifically activated by culture conditions or by cytokines released by irradiated EBV-transformed B cells. Such activation may explain the lack of selectivity noted in T-cell populations transduced on day 5. Indeed, it was not until 8 days of culture, when antigen-specific proliferation had achieved a plateau, that maximal transduction of antigen-sensitized T cells was regularly observed. These observations were not unique to the selection of EBV-reactive T cells but also held true for retroviral-mediated selection of alloantigen-specific T cells early after sensitization in mixed leukocyte culture.
In conducting these studies, we were particularly interested to compare the characteristics of virus-specific T cells transduced early after sensitization with T cells generated after extended periods (30 days) of sensitization and expansion in vitro. One striking difference is the higher proportion of CD4+ T cells observed in the T lymphocytes transduced early after sensitization in comparison with the T cells transduced after extended in vitro culture. This difference could be ascribed to differences in the proportionate representation of CD4+ and CD8+ T cells in the T-lymphocyte population at the time of transduction with the NIT vector, because there was no apparent difference in the capacity of the NIT vector to transduce CD4+ versus CD8+ T cells. The higher proportionate representation of CD4+ T cells in NIT+ fraction transduced early after sensitization could constitute an advantage for such cells when used for adoptive therapy. In clinical trials evaluating adoptive transfer of T cells predominantly of the CD8+ phenotype derived from long-term EBV-reactive cell lines or cytomegalovirus-specific CD8+T-cell clones, expansion of the transformed T cells in vivo has been limited.11,28 In contrast, infusions of fresh, unselected donor lymphocytes that contain a predominance of CD4+ T cells have led to dramatic expansion of virus-reactive cells.21 Similarly, in studies exploring the use of tumor-antigen reactive T cells for adoptive cell therapy, CD4+ T cells have been found to provide stimuli important to the expansion of tumor-reactive T cells posttransfer and to the clinical expression of an antitumor effect.29
It is well recognized that EBV seropositive adults maintain populations of EBV-specific T cells in the blood, which often exhibit relatively restricted reactivity against immunodominant EBV peptides presented by specific HLA alleles.30 31 Strikingly, this selection of immunodominant T cells also marks the population of T cells transduced early after sensitization to autologous EBV BLCL. Indeed, in each patient studied, the dominant HLA-restricting alleles targeted by the transduced NIT+ cells mirrored these targeted by the EBV-specific T-cell lines expanded over 30 to 40 days in vitro. These findings suggest either that the frequency of T cells restricted by these alleles is strikingly higher than that of other EBV reactive T cells in the circulation or that their potential for activation by the EBV antigens presented by these alleles is significantly greater than that of other EBV-reactive T-cell populations. Comparisons of T cells binding immunodominant and subdominant peptides presented on HLA tetramers for their sensitivity to retroviral transduction after sensitization may discriminate between these possibilities.
In summary, the results of this study suggest that retroviral vectors, by virtue of their selective integration into dividing cells, can be used to selectively transduce T cells activated to proliferate in response to antigens. Use of retroviral vectors encoding a distinctive cell surface marker such as LNGFR, which permits isolation of transduced cells expressing the vector at high purity, permits rapid selection of such cells early after in vitro sensitization. This approach may significantly expedite isolation of antigen-reactive T cells and depletion of contaminating alloreactive T cells for use in adoptive cell immunotherapy.
Acknowledgments
We are grateful for the persistent and reliable support of Patrick Anderson, Diane Domingo, and Tom Delohery of the Flow Cytometry Core Facility for many FACSorts and FACS analyses. We thank Theresa Diaz-Barrientos and Linda Hirschberger for excellent technical assistance.
Supported in part by grants CA-59350 National Cancer Institute, MD; CA23766 National Cancer Institute, MD; HL53752 National Heart, Lung, and Blood Institute, MD; The Larry H. Smead Fund, The Aubrey Fund for Pediatric Cancer Research, The Andrew Gaffney Foundation, the Scholars Award of the McDonnell Foundation for Molecular Medicine, and The Vincent Astor Chair Clinical Research Fund.
Reprints:Richard J. O'Reilly, Bone Marrow Transplantation Service, Memorial Sloan-Kettering Cancer Center, 1275 York Ave, Room H1409, New York, NY 10021; e-mail: oreillyr@mskcc.org.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal