A recent study in dogs suggested that erythropoietin (EPO) not only promotes the synthesis of increased numbers of reticulated platelets but that these newly produced platelets are hyperreactive compared with controls. Because of the increasing use of EPO in the perioperative setting, we characterized the effects of EPO on platelet reactivity in healthy human volunteers. In a randomized, controlled trial, we studied the effects of EPO on platelet reactivity, thrombopoiesis, and endothelial activation in circumstances similar to those of autologous blood donation. Thirty healthy male volunteers received placebo or EPO (100 or 500 U/kg of body weight given intravenously) three times a week for 2 weeks and underwent phlebotomy on days 8 and 15. Thrombin receptor–activating peptide induced expression of P-selectin, and CD63 increased 2- to 3-fold during EPO treatment. The enhanced platelet reactivity was also reflected by a 50% increase in soluble P-selectin in plasma. Plasma E-selectin levels increased in a dose-dependent fashion by more than 100% during EPO treatment, indicating substantial activation of endothelial cells. A 10% to 20% increase in platelet counts was observed in both EPO groups on day 5. In the placebo group, platelets increased only several days after the first phlebotomy. The increase in platelet counts was not reflected by changes in the amounts of reticulated platelets or circulating progenitor cells. In summary, we found that EPO markedly enhances endothelial activation and platelet reactivity, which may adversely affect patients at cardiovascular risk. However, the increased platelet reactivity could be exploited in patients with platelet dysfunction.
Erythropoietin (EPO) is the primary regulator of erythrocyte production and is required for the survival and proliferation of committed erythroid progenitor cells. EPO enhances the rate of formation of red blood cells in the bone marrow, and several lines of evidence suggest that it also affects thrombopoiesis and platelet function. In studies in vitro, EPO potentiated mouse megakaryocyte maturation1 and acted synergistically with thrombopoietin to promote the production of megakaryocyte precursors from CD34-positive cells.2 In studies in vivo, EPO increased the number of megakaryocytes in the bone marrow of splenectomized mice3 and elevated platelet counts in these mice3 and in rats.4,5 In a 1997 study in dogs, Wolf et al6 found that EPO increased the relative number of young (reticulated) platelets. They also observed functional hyperreactivity in total and reticulated platelets in comparison with platelets from control animals.6 Wolf et al7have also reported that EPO potentiated thrombus development in a canine model of arteriovenous shunting, reflecting a thrombogenic effect of EPO in the animals.
In patients, EPO is approved for treatment of anemia in chronic renal failure, infection with human immunodeficiency virus, and cancer, and for facilitating autologous blood donation.8,9 In patients with uremia, EPO treatment caused an increase in circulating bone marrow progenitor cells,10 including megakaryocytic progenitor cells,11 thereby demonstrating an effect of EPO on a broad spectrum of human hematopoietic progenitor cells. The long-term use of recombinant EPO does not induce thrombocytosis, although platelet number may increase by 10% to 20%.12 In contrast, EPO caused a pronounced increase in platelet counts in patients with chronic liver disease.13 Moreover, EPO improves platelet function, as indicated by shortened bleeding times and improved platelet aggregation in uremic patients given the agent.14 15
When used in autologous blood–donation programs, EPO treatment significantly reduces requirements for allogeneic blood transfusions in anemic patients.16 Furthermore, perioperative treatment with EPO reduces the risk of allogeneic transfusions in patients for whom preoperative blood donation is not feasible.17 18However, the effects of EPO on platelet function in autologous blood donors or surgical patients have never been addressed. Yet the effect of EPO on platelet reactivity or activation is an important issue, particularly for older patients undergoing major orthopedic surgery or coronary bypass grafting, because potential procoagulant effects of EPO could be dangerous for patients with cardiovascular disease.
Hence, we studied whether EPO enhances platelet reactivity in a dose-dependent manner in subjects undergoing blood donation. We also aimed to characterize whether an increase in platelet reactivity was due to enhanced thrombopoiesis as measured by peripheral platelet counts and assessments of reticulated platelets and circulating colony-forming units–megakaryocyte (CFU-MK). Finally, we examined whether an increase in platelet reactivity was accompanied by increased platelet activation, increased endothelial activation, or both.
Materials and methods
Study design
Our double-blind, placebo-controlled, randomized study was conducted in 3 parallel groups of 30 healthy male volunteers (10 in each group). Informed consent was obtained from all subjects after approval of the study protocol by the Ethics Committee of the Vienna University School of Medicine.
Inclusion criteria were age between 19 and 45 years, body weight 60 to 80 kg, body mass index below the 85th percentile, blood pressure lower than 140/90 mmHg, hematocrit value between 0.39 and 0.47, and serum ferritin level above 50 μg/L. Health status was determined by medical history and a battery of laboratory and clinical tests, including physical examination, clinical chemistry studies, and virologic and drug screenings, as previously described.20Exclusion criteria were history of thromboembolic or cardiovascular disease or hypertension, history of convulsion, liver or kidney dysfunction, regular use of medication, abuse of alcoholic beverages, use of any medication (prescription or over-the-counter) in the 3 weeks before the first study day, and symptoms of a clinically relevant illness in the 3 weeks before the first study day.
Study protocol
Subjects in a fasting state came to the Department of Clinical Pharmacology at 8:00 am on all study days. After the subjects rested for 15 minutes, blood was drawn with use of a butterfly needle into tubes (Vacutainer Systems; Becton Dickinson, Rutherford, NJ). Because subcutaneous injection of EPO produces a burning sensation,21 intravenous infusions were used to avoid unblinding; thus, bolus infusions of EPO (either 100 U/kg or 500 U/kg of body weight) or placebo (physiologic saline) were given. Another blood sample was obtained from the contralateral arm 1 hour after EPO infusion. Subjects then received the first tablet of oral iron supplementation and were instructed to take 2 tablets a day until day 21 of the study. Additional EPO or placebo infusions were scheduled on days 3, 5, 8, 10, and 12 . On days 8 and 15, all subjects underwent phlebotomy (450 mL) after the blood sampling but before the EPO or placebo infusion. The phlebotomies were performed to avoid possible adverse effects of EPO on blood viscosity and blood pressure and to mimic typical circumstances of an autologous blood–donation setting. Subjects did not receive any EPO treatment after day 12 but still had blood samples taken on days 18 and 23.
Sampling and analysis of primary outcome variables
Measurement of platelet reactivity with thrombin receptor–activating peptide (TRAP).
Venous whole blood was collected into tubes containing trisodium citrate (0.129 mol/L) (Vacutainer Systems; Becton Dickinson). The sample of whole blood was mixed gently, and 100 μL was diluted with 900 μL of phosphate-buffered saline (PBS). Ten microliters of this dilution was incubated with 10 μL of fluorescein isothiocyanate (FITC)–labeled anti-P-selectin antibody, or with 10 μL of FITC-labeled anti-CD63 (Immunotech; Instrumentation Laboratories, Vienna, Austria) and with 10 μL of a TRAP dilution at 23°C for 15 minutes. Two concentrations of TRAP (TRAP-6 [molecular wt, 748.9 g/mol]; Bachem, Switzerland) were used: 7.3 μmol/L of TRAP (final concentration), which promoted approximately half of the maximal platelet stimulation at baseline, and 3.7 μmol/L of TRAP, which promoted approximately 20% of the maximal platelet stimulation before EPO administration. After incubation, 500 μL of PBS was added, and acquisition on a flow cytometer (fluorescence-activated cell sorter scan; Becton Dickinson, San Jose, CA) was started immediately afterward.22 23 For the TRAP dilution, 5 mg of the powder was dissolved in 1.5 mL of bidistilled water, and 10 μL of this solution was further diluted with 2 mL of PBS.
Determination of circulating adhesion molecules, EPO, and CFU-MK.
Plasma concentrations of circulating P-selectin (cP-selectin), circulating E-selectin (cE-selectin), circulating intercellular adhesion molecule 1 (cICAM-1), circulating vascular cell adhesion molecule 1 (cVCAM-1), and EPO were measured with commercially available enzyme immunoassays (R&D Systems, United Kingdom) as previously described.20 24
Complete blood counts and reticulocyte counts in blood treated with EDTA were determined by using an automatic blood cell counter (Sysmex, Japan). Venous whole blood was collected into tubes containing trisodium citrate (0.129 mol/L; Vacutainer Systems; Becton Dickinson). Reticulated platelets were measured from whole blood as described previously.25 Circulating CFU-MK were assessed as described previously by Geissler et al.26
Data analysis
Results are expressed as the mean and 95% confidence interval (CI) in the text and the mean and range in the table. Because of the nonnormal distribution of data, all comparisons were made with use of nonparametric statistics. All statistical analyses within groups were done with the Friedman analysis of variance (ANOVA), and post hoc comparisons were done with the Wilcoxon signed rank test. To compare treatment effects between study groups, the Kruskal-Wallis ANOVA was used, with the Mann-Whitney U test used for post hoc comparisons. A 2-tailed P value of less than .05 was considered to represent significance.
Results
Trough EPO levels
Demographic data and baseline values are shown in Table1. All subjects had predose EPO levels within the expected normal physiologic range27 (Figure1). Trough EPO levels increased in a dose-dependent manner and peaked 2 days after the first 500-U/kg-EPO infusion (P < .001 compared with baseline value). In the placebo group, plasma EPO levels gradually increased from day 12, reaching their maximum value 3 days after the second phlebotomy (Figure 1; P = .005 compared with baseline).
Baseline data in 30 volunteers in 3 study groups
| . | Placebo (n = 10) . | Erythropoietin, 100 U/kg (n = 10) . | Erythropoietin, 500 U/kg (n = 10) . |
|---|---|---|---|
| Body mass index, kg/m2 | 23 (20-25) | 23 (21-25) | 22 (19-25) |
| Age, y | 27 (20-34) | 28 (20-38) | 27 (20-37) |
| Ferritin, μg/L | 122 (66-387) | 115 (67-202) | 105 (64-172) |
| Erythropoietin, IU/L | 5.7 (2.4-10.4) | 5.2 (2.4-13.3) | 4.0 (2.4-8.1) |
| Hemoglobin, g/L | 143 (129-157) | 140 (128-153) | 146 (130-166) |
| Mean corpuscular volume, fl | 85 (79-89) | 88 (80-95) | 87 (83-90) |
| Reticulocytes, 109/L | 57 (32-88) | 42 (25-61) | 52 (27-89) |
| Highly fluorescent reticulocytes, % | 0.4 (0.1-1) | 0.4 (0.1-0.9) | 0.5 (0.1-1.1) |
| Platelets, 109/L | 203 (150-266) | 217 (175-260) | 206 (153-273) |
| Reticulated platelets, % | 2.2 (0.9-3.6) | 2.1 (1.0-3.7) | 2.1 (0.9-2.6) |
| CFU-GM, cells/mL | 342 (83-819) | 279 (46-606) | 378 (90-854) |
| BFU-E, 103 cells/mL | 1.0 (0.3-2.2) | 0.8 (0.1-1.3) | 1.2 (0.3-2.7) |
| CFU-GEMM, cells/mL | 38 (6-116) | 30 (0-62) | 59 (0-177) |
| CFU-MK, cells/mL | 37 (0-143) | 28 (0-112) | 23 (4-43) |
| Circulating P-selectin, μg/L | 28 (18-44) | 36 (20-73) | 35 (21-46) |
| Circulating E-selectin, μg/L | 30 (18-51) | 45 (23-71) | 32 (18-45) |
| . | Placebo (n = 10) . | Erythropoietin, 100 U/kg (n = 10) . | Erythropoietin, 500 U/kg (n = 10) . |
|---|---|---|---|
| Body mass index, kg/m2 | 23 (20-25) | 23 (21-25) | 22 (19-25) |
| Age, y | 27 (20-34) | 28 (20-38) | 27 (20-37) |
| Ferritin, μg/L | 122 (66-387) | 115 (67-202) | 105 (64-172) |
| Erythropoietin, IU/L | 5.7 (2.4-10.4) | 5.2 (2.4-13.3) | 4.0 (2.4-8.1) |
| Hemoglobin, g/L | 143 (129-157) | 140 (128-153) | 146 (130-166) |
| Mean corpuscular volume, fl | 85 (79-89) | 88 (80-95) | 87 (83-90) |
| Reticulocytes, 109/L | 57 (32-88) | 42 (25-61) | 52 (27-89) |
| Highly fluorescent reticulocytes, % | 0.4 (0.1-1) | 0.4 (0.1-0.9) | 0.5 (0.1-1.1) |
| Platelets, 109/L | 203 (150-266) | 217 (175-260) | 206 (153-273) |
| Reticulated platelets, % | 2.2 (0.9-3.6) | 2.1 (1.0-3.7) | 2.1 (0.9-2.6) |
| CFU-GM, cells/mL | 342 (83-819) | 279 (46-606) | 378 (90-854) |
| BFU-E, 103 cells/mL | 1.0 (0.3-2.2) | 0.8 (0.1-1.3) | 1.2 (0.3-2.7) |
| CFU-GEMM, cells/mL | 38 (6-116) | 30 (0-62) | 59 (0-177) |
| CFU-MK, cells/mL | 37 (0-143) | 28 (0-112) | 23 (4-43) |
| Circulating P-selectin, μg/L | 28 (18-44) | 36 (20-73) | 35 (21-46) |
| Circulating E-selectin, μg/L | 30 (18-51) | 45 (23-71) | 32 (18-45) |
CFU-GM indicates colony-forming units-granulocyte, macrophage; BFU-E, burst-forming units-erythroid; CFU-GEMM, colony-forming units-granulocyte, erythrocyte, megakaryocyte, and macrophage; and CFU-MK, colony-forming units-megakaryocytes. Data are expressed as mean (range).
Effects of erythropoietin (EPO) treatment (3 times a week [arrows]) on hemoglobin levels, highly fluorescent reticulocytes (% HFR), reticulocyte counts, and trough EPO levels (top to bottom) in subjects undergoing phlebotomy (P) on days 8 and 15.
Young, healthy male volunteers (total n = 30) received bolus infusions of either 500 U/kg of EPO (black squares; n = 10), 100 U/kg of EPO (open triangles; n = 10), or placebo (open circles; n = 10) in a randomized, double-blind trial. Data are presented as mean ± SEM.
Effects of erythropoietin (EPO) treatment (3 times a week [arrows]) on hemoglobin levels, highly fluorescent reticulocytes (% HFR), reticulocyte counts, and trough EPO levels (top to bottom) in subjects undergoing phlebotomy (P) on days 8 and 15.
Young, healthy male volunteers (total n = 30) received bolus infusions of either 500 U/kg of EPO (black squares; n = 10), 100 U/kg of EPO (open triangles; n = 10), or placebo (open circles; n = 10) in a randomized, double-blind trial. Data are presented as mean ± SEM.
Reticulocytes, highly fluorescent reticulocytes (HFR), and hemoglobin
As described previously,28 EPO increased reticulocyte counts in a dose-dependent fashion (Figure 1). Reticulocyte counts rose 2-fold and 4-fold, respectively, in response to the 100-U/kg- and 500-U/kg-EPO dose (P = .005 compared with baseline). In both EPO groups, reticulocytes returned to baseline levels on day 23. In response to 2 phlebotomies, reticulocytes started to increase on day 12 in the placebo group (P = .017) and reached 2-fold higher levels on day 23 (P = .005). Concomitantly, the percentage of HFR had peaks on day 5 (8%; P = .005) and on day 12 (5%) in the 500-U/kg-EPO group and returned to the baseline level of 1% on day 18. There was a similar time course for HFR values in subjects who received 100 U/kg of EPO, although peaks were significantly lower (P = .005 compared with the 500-U/kg-EPO group). No changes in HFR occurred in the placebo group (P > .05).
Hemoglobin levels increased on day 8 (P = .02) in the 500-U/kg-EPO group, decreased by 4 g/L (95% CI, −8 to 1 g/L) after the first blood donation, and increased again until the second blood donation (P = .01). Hemoglobin levels were still above baseline levels on day 23 despite 2 blood donations. Again, there was a similar time course in the 100-U/kg-EPO group, although hemoglobin levels were lower from day 12 through day 18 (P = .003 compared with the 500-U/kg-EPO group). In the placebo group, hemoglobin levels fell by 6 g/L (95% CI, 12 to 1 g/L) after the first blood donation (P = .01) and again after the second blood donation by 7 g/L (CI, 11 to 3 g/L; P = .005).
Platelet reactivity in response to TRAP
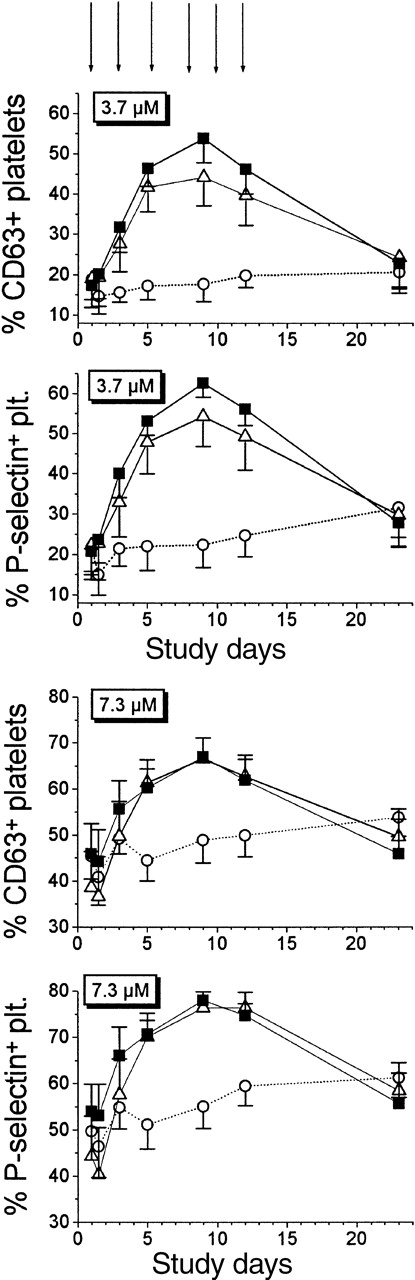
To measure platelet reactivity, whole blood was stimulated with TRAP, and results expressed as percentages of P-selectin–positive and CD63-positive platelets. Before beginning this study, we determined with blood from unrelated healthy male volunteers the concentrations of TRAP-6 that promoted half of the maximal stimulation of platelets (7.3 μmol/L) and 20% of the maximal platelet stimulation (3.7 μmol/L) (Figure 2). These 2 TRAP doses were then used in all subsequent experiments. One hour after the first EPO infusion, TRAP-induced platelet activation was not altered, demonstrating that EPO did not directly affect platelet activation of circulating platelets. This was also confirmed by in vitro incubation studies; even EPO concentrations up to 15 IU/L did not enhance platelet reactivity (data not shown).
Time-dependent effects of EPO treatment on platelet reactivity in whole blood as measured by P-selectin–positive and CD63-positive platelets in response to stimulation with thrombin receptor–activating peptide (TRAP).
Two doses of TRAP were used: 3.7 μmol/L and 7.3 μmol/L, which elicited approximately 20% and 50%, respectively, of the maximal stimulation at baseline. Healthy male volunteers (n = 30) received bolus infusions of either 500 U/kg of EPO (black squares; n = 10), 100 U/kg of EPO (open triangles; n = 10), or placebo (open circles; n = 10). EPO did not affect platelet reactivity 1 hour after infusion (symbols adjacent to baseline values) but maximally increased platelet reactivity after 9 days (ie, in a period similar in length to platelet life time). Data are mean ± SEM.
Time-dependent effects of EPO treatment on platelet reactivity in whole blood as measured by P-selectin–positive and CD63-positive platelets in response to stimulation with thrombin receptor–activating peptide (TRAP).
Two doses of TRAP were used: 3.7 μmol/L and 7.3 μmol/L, which elicited approximately 20% and 50%, respectively, of the maximal stimulation at baseline. Healthy male volunteers (n = 30) received bolus infusions of either 500 U/kg of EPO (black squares; n = 10), 100 U/kg of EPO (open triangles; n = 10), or placebo (open circles; n = 10). EPO did not affect platelet reactivity 1 hour after infusion (symbols adjacent to baseline values) but maximally increased platelet reactivity after 9 days (ie, in a period similar in length to platelet life time). Data are mean ± SEM.
With both TRAP concentrations, the percentages of P-selectin–positive and CD63-positive platelets increased from day 3, peaked on day 9 (P = .005 compared with baseline; P = .002 for each EPO group compared with placebo), and returned to baseline values on day 23. No differences between the 2 EPO groups were observed (P > .05). In the placebo group, platelet reactivity increased only slightly on day 23 (P < .05), indicating the presence of an adaptive physiologic response to blood loss.
Plasma levels of soluble P-selectin
Plasma levels of cP-selectin increased in a dose-dependent fashion and reached the maximum value on day 8 (P = .001 compared with baseline after 500 U/kg of EPO). Soluble P-selectin levels were still 10% to 20% higher than baseline values on day 18 in both EPO groups (P < .05 compared with placebo). Plasma levels of soluble P-selectin were higher in the 500-U/kg-EPO group than in the 100-U/kg-EPO group from day 8 to day 15 (P < .05). No significant changes were observed in the placebo group (Figure3).
Effects of EPO treatment (3 times a week [arrows]) on the relative increase in circulating P-selectin and circulating E-selectin levels in men undergoing phlebotomy (P) on days 8 and 15.
Subjects (n = 30) received bolus infusions of either 500 U/kg of EPO (black squares; n = 10), 100 U/kg of EPO (open triangles; n = 10), or placebo (open circles; n = 10). Data are mean ± SEM.
Effects of EPO treatment (3 times a week [arrows]) on the relative increase in circulating P-selectin and circulating E-selectin levels in men undergoing phlebotomy (P) on days 8 and 15.
Subjects (n = 30) received bolus infusions of either 500 U/kg of EPO (black squares; n = 10), 100 U/kg of EPO (open triangles; n = 10), or placebo (open circles; n = 10). Data are mean ± SEM.
Correlations between cP-selectin and P-selectin expression on platelets
Baseline P-selectin expression on platelets did not correlate with plasma levels of cP-selectin. However, after 8 days of EPO treatment, basal P-selectin expression on platelets, as well as the change in TRAP-stimulated P-selectin expression on platelets, correlated with cP-selectin levels (r = 0.45; P = .01 andr = 0.54; P = .002, respectively; data not shown).
Reticulated platelets, platelet counts, and circulating CFU-MK
To determine whether the increased platelet reactivity could be due to newly synthesized hyperreactive platelets, we measured reticulated platelets. No changes in reticulated platelets were detectable in any of the 3 groups (Figure 4). In contrast, platelet counts started to increase on day 3 in both EPO-treated groups (P < .05 compared with baseline) and were about 13% higher than baseline values on day 5. In the placebo group, platelet counts started to rise on day 12 (ie, several days after the first phlebotomy). Platelet counts in the 100-U/kg-EPO group and the placebo group were still 18% above baseline values on day 23 (P < .01; Figure4).
Effects of EPO treatment (3 times a week [arrows]) on platelet counts and reticulated platelets in men undergoing phlebotomy (P) on days 8 and 15.
Subjects (n = 30) received bolus infusions of either 500 U/kg of EPO (black squares; n = 10), 100 U/kg of EPO (open triangles; n = 10), or placebo (open circles; n = 10). Data are mean ± SEM.
Effects of EPO treatment (3 times a week [arrows]) on platelet counts and reticulated platelets in men undergoing phlebotomy (P) on days 8 and 15.
Subjects (n = 30) received bolus infusions of either 500 U/kg of EPO (black squares; n = 10), 100 U/kg of EPO (open triangles; n = 10), or placebo (open circles; n = 10). Data are mean ± SEM.
We were interested in whether EPO might affect colony-forming units in our subjects, as it did in uremic patients,10 but no significant changes in CFU-MK were observed in any of the 3 study groups (data not shown).
Plasma levels of cE-selectin, cICAM-1, and cVCAM-1
Because endothelial cells have EPO receptors,12 we studied whether EPO infusion induced endothelial activation. We found that plasma levels of cE-selectin increased by more than 100% in the 500-U/kg-EPO group on day 12 and were still 63% above baseline levels on day 18 (P = .005 compared with baseline; Figure 3). There was a similar time course for cE-selectin levels in the 100-U/kg-EPO group (P = .005 compared with baseline), although peak values were lower (P = .004 compared with the 500-U/kg-EPO group). These effects were significantly different from results in the placebo group (P < .005 compared with both EPO groups). After the end of the study and analysis of all variables, we were surprised by these marked changes in E-selectin levels. Hence, we decided to measure plasma levels of cICAM-1 and cVCAM-1 in volunteers given 500 U/kg of EPO or placebo. Infusion of 500 U/kg of EPO increased cVCAM-1 levels from day 5 on (P = .012); they were 22% higher (95% CI, 13% to 32%) than baseline levels on day 12. In the placebo group, however, cVCAM-1 levels remained stable (P > .05). Plasma levels of cICAM-1 were not affected by 500 U/kg of EPO or placebo (data not shown).
Tolerability
Similar to the experience in a previous study,29transient influenza-like symptoms were reported by 2 of our volunteers—1 who received the 100-U/kg-EPO dose and 1 who received the 500-U/kg-EPO dose. The latter volunteer also reported short-lasting chills after each of the bolus infusions.
Discussion
A recent study in dogs showed that EPO not only promotes erythropoiesis but also affects platelet quality by increasing platelet reactivity.6 Thus, we characterized this putative effect in humans. We found that EPO enhanced platelet reactivity in men, as measured by TRAP-stimulated α-granule secretion. Hence, we duplicated the results of the animal study6 in humans and also demonstrated CD63 mobilization from the lysosomal compartment of human platelets.
Platelet reactivity did not differ significantly between the 2 EPO doses (Figure 2), suggesting that we reached a near-maximal effect with the 100-U/kg dose. Furthermore, platelet reactivity was not enhanced 1 hour after EPO infusion or after in vitro incubation, which indicates that EPO does not directly affect the thrombogenicity of circulating platelets. Similar to the results in dogs,6 platelet reactivity in humans increased slowly and reached the maximum level on day 9. Because platelet life time is 8 to 10 days,23 30 our data indicate a thrombogenic effect of EPO on the newly synthesized platelets.
EPO moderately but transiently stimulated thrombopoiesis: platelet counts increased by about 15%. A similar increase in platelets occurred in the placebo group but only with a time lag, ie, after the phlebotomies. Our findings regarding the degree of platelet increase are in good agreement with data previously obtained in healthy men undergoing repeated phlebotomies.19 Furthermore, EPO induced a transient 10% to 20% increase in platelet numbers in uremic patients12, 31,32 and an even greater increase in patients with chronic liver disease.13 Patients with liver disease could potentially profit most from increased platelet counts, especially when undergoing invasive procedures such as percutaneous liver biopsies.
Despite an increase in platelet counts, no increase in reticulated platelets or circulating progenitor cells was observed in response to EPO or to phlebotomies (Figure 4). This was unexpected, because Ingram and Coopersmith33 originally described reticulated platelets after blood loss, and EPO increased circulating progenitor cells in uremic patients.10,11 Possibly, neither quantification of progenitor cells nor reticulated platelet counts may currently be sensitive enough to detect small changes within the normal range. Thus, we could not confirm a previous study in dogs6that showed a 2.7-fold increase in the percentage of reticulated platelets after infusion of EPO doses that produced a 4-fold increase in reticulocyte counts, similar to our study (Figure 1). Surprisingly, platelet counts decreased during EPO treatment in the dogs.4 6 The researchers hypothesized that a premature loss of the hyperreactive platelets in vivo might have occurred, as has been observed in consumptive thrombocytopenia.
Because EPO increased ex vivo platelet reactivity, we wondered whether EPO infusion would also enhance in vivo platelet activation. P-selectin is stored in endothelial cells and α granules of platelets and is released from those locations on platelet activation.23,34,35 Thus, a soluble form of P-selectin is found in the circulation, and increased levels of cP-selectin have been observed in various cardiovascular disorders.36,37-39 In this study, we demonstrated a dose-dependent increase in soluble P-selectin after EPO infusion (Figure 3). These results indicate that EPO not only enhances platelet reactivity ex vivo but also increases platelet activation in vivo, which could promote a prothrombic effect in humans.40
However, soluble P-selectin could also derive from endothelial cells because P-selectin expression is not confined to platelets but is also found on endothelial cells. Because endothelial cells express EPO receptors,12 we examined whether endothelial activation also occurs. We found that EPO increased cE-selectin levels by more than 100% (Figure 3). cE-selectin may be a risk factor for cardiovascular disease,41 so this finding could have clinical implications, particularly for patients with atherosclerosis.
Our findings are of clinical interest for several reasons. First, clinical trials have shown that EPO therapy combined with autologous blood donations can significantly reduce the need for allogeneic blood in patients at high risk of transfusion.18,42,43Furthermore, perioperative use of EPO decreases the need for blood transfusions.44 In orthopedic surgery, platelet activation may play a crucial role in promoting coagulation and shortening bleeding times. Subramaniam et al45 showed that mice deficient in P-selectin have a 40% longer bleeding time, suggesting that P-selectin may be involved in primary hemostasis. Thus, enhanced platelet expression of P-selectin may translate into decreased bleeding but may possibly put patients undergoing surgery at risk of thrombosis and thromboembolism.
Second, EPO is effective in treating anemia of prematurity46,47 because this condition is due to a transient developmental abnormality in EPO production.48Furthermore, preterm infants also have dysfunctional platelets.49 50 Thus, EPO might be particularly beneficial in patients with anemia of prematurity because it would both increase production of red blood cells and possibly reverse or ameliorate the platelet dysfunction. This idea merits future study because no evidence of a beneficial effect of EPO in preterm infants has been published.
In summary, we showed that EPO markedly enhances platelet and endothelial activation in humans. Thus, we postulate that a patient-oriented approach to the use of EPO entails its administration not only to patients who are likely to respond to EPO but also to anemic patients, who additionally benefit from platelet activation. Whether heightened platelet reactivity and endothelial activation may increase the risk of thromboembolism warrants further investigation.
Reprints:Bernd Jilma, Department of Clinical Pharmacology–TARGET, Vienna University Hospital School of Medicine, Waehringer Guertel 18-20 A-1090 Wien, Austria; e-mail:bernd.jilma@univie.ac.at.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

![Fig. 1. Effects of erythropoietin (EPO) treatment (3 times a week [arrows]) on hemoglobin levels, highly fluorescent reticulocytes (% HFR), reticulocyte counts, and trough EPO levels (top to bottom) in subjects undergoing phlebotomy (P) on days 8 and 15. / Young, healthy male volunteers (total n = 30) received bolus infusions of either 500 U/kg of EPO (black squares; n = 10), 100 U/kg of EPO (open triangles; n = 10), or placebo (open circles; n = 10) in a randomized, double-blind trial. Data are presented as mean ± SEM.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/95/9/10.1182_blood.v95.9.2983.009k27_2983_2989/5/m_bloo00927001x.jpeg?Expires=1767719028&Signature=BPoalsN9uSTv6ZIBmw3b4jRcZQYri3LDbtdRe-vdSms0ci4tiQAQQFjagtNXJHQ966NsqVBR3a5WVWknIooSIY1u-~UX7b6Zzy9bcisQVvc1SdoD521sxoRpCT~fbc0vC3VcY1lHae3n6fRTH1ThM9335tDV9n8l9CKGIURkZLXZYInDKfZpGrOAkKmLxNY4lE~UT7~jHZUbIan1I200p5TtuEoTel3slG1bArLNutgBfRhGX1VP6uejHW-JOZICUxeCPhMc4GYii6SeUGjs-SG6y9zlQWPE6-VUEx63bKLlQXHuF7HwoAuIKpJwYSlmjqsH6fjhCKevGq23s1ahWg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 3. Effects of EPO treatment (3 times a week [arrows]) on the relative increase in circulating P-selectin and circulating E-selectin levels in men undergoing phlebotomy (P) on days 8 and 15. / Subjects (n = 30) received bolus infusions of either 500 U/kg of EPO (black squares; n = 10), 100 U/kg of EPO (open triangles; n = 10), or placebo (open circles; n = 10). Data are mean ± SEM.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/95/9/10.1182_blood.v95.9.2983.009k27_2983_2989/5/m_bloo00927003x.jpeg?Expires=1767719028&Signature=e1H9xvZQzDJRv0VFwzfgTM1tMmqZa0nInYwszZ4bjUB~rXpOYdaqvCQpOuElJmLGzhbIVOVSqCwY7TUpNrcjL2Eb5ZkJGs247EncUndEY-dlefQaiCyvaFP2CblZwo0BiFbz-iuxE~zOwVAJwpY~AAx2gS762CFg8hHVf7qHGGrl0k~IQNpHDG6F47hj0GZw7bumqSKyH8wlaYBYbahMbjsubUfgkjUEULQQuMH9ExXaveZsFIsLCWWEKWH~Dx~v8KkO5UmX~MwbiTD765NJr699s5wRq~Kt77F1OM~XThs~egPPZ1PA22WPfLCqQt9K8BxUd-plkpoETtAdAIOBiA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 4. Effects of EPO treatment (3 times a week [arrows]) on platelet counts and reticulated platelets in men undergoing phlebotomy (P) on days 8 and 15. / Subjects (n = 30) received bolus infusions of either 500 U/kg of EPO (black squares; n = 10), 100 U/kg of EPO (open triangles; n = 10), or placebo (open circles; n = 10). Data are mean ± SEM.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/95/9/10.1182_blood.v95.9.2983.009k27_2983_2989/5/m_bloo00927004x.jpeg?Expires=1767719028&Signature=VwJgkNEfZ5PfLG64AVQwtybBP6Ppb~08Lrxk92DK4rpR3FjgyVvIGlKoFpdU4mwzECGn-bh5YmYQwJrSpFEHAbVG-oYTqOm4sfzw4QCSpD3ACoiSVoMaglaECVLKCteoqthCZfLkhWuRRDa6zyuVnztuqQnACLbXSBRV5y3DFYVbyfJYqpZ3yS1HnNKeic3PpGwxoHhxDKzKEy3Q~nA6qgD3huqaktHZP2TzdFAl9zo9zU1CaEsidd4tKi7fbWVZG0vAC-J97qGvjtlc3EjLVVWzI9B~UCIkyfNpu2Azl48lYt0-KyshIb~3VOJ9BL2t5E4dUiMxne4gZn7GXk-UQw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal