Severe congenital neutropenia (SCN) or Kostmann syndrome is a disorder of myelopoiesis characterized by a maturation arrest at the stage of promyelocytes or myelocytes in bone marrow and absolute neutrophil counts less than 200/μL in peripheral blood. Treatment of these patients with granulocyte colony-stimulating factor (G-CSF) leads to a significant increase in circulating neutrophils and a reduction in infection-related events in more than 95% of the patients. To date, little is known regarding the underlying pathomechanism of SCN. G-CSF-induced neutrophils of patients with SCN are functionally defective (eg, chemotaxis, superoxide anion generation, Ca++mobilization). Two guanosine triphosphatases (GTPases), Rac2 and RhoA, were described to be involved in many neutrophil functions. The expression of these GTPases and their regulation in patients' neutrophils were of interest. This study determined that the guanosine diphosphate (GDP)-dissociation inhibitor RhoGDI is overexpressed at the protein level in patients' neutrophils and that overexpression is a result of G-CSF treatment. RhoA and LyGDI are expressed at similar levels, whereas Rac2 shows a decreased expression. In addition, association of Rac2 and RhoGDI or LyGDI is abrogated or not detectable based on the low Rac2 expression in patients' neutrophils.
Severe congenital neutropenia (SCN) or Kostmann syndrome, first described by Kostmann in 1956,1 is a disorder of myelopoiesis characterized by a block in differentiation of myeloid progenitor cells at the promyelocytic stage in bone marrow and absence or low levels of mature neutrophils in peripheral blood.1,2 Patients with SCN suffer from severe and recurrent bacterial infections. Treatment of these patients with pharmacologic doses of granulocyte colony-stimulating factor (G-CSF) leads to an increase in the neutrophil counts to above 1000/μL associated with a reduction in infection-related events in more than 95% of the patients,3-5 suggesting a defect in the response to G-CSF. G-CSF preferentially stimulates the proliferation and differentiation of neutrophil progenitor cells6 and the function of mature neutrophils. In 1992 and 1993, Elsner et al7,8 demonstrated that neutrophils from patients with SCN are functionally defective (eg, chemotaxis, superoxide anion generation, Ca++ mobilization). Some of the functional features seen in neutrophils from these patients were also detected in neutrophils from healthy individuals or patients with cancer under treatment with G-CSF (for review, see Spiekermann et al9).
The Ras-related small guanosine triphosphate (GTP)-binding proteins (GTPases) are signaling molecules involved in a number of cellular processes such as cell growth, cytoskeletal organization, and secretion (for review, see Hall10). RhoA is involved in the formation of actin stress fibers and focal contact sites,11 whereas Rac proteins are involved in the activation of nicotinamide adenine dinucleotide phosphate (NADPH) oxidase12-14 and play a role in growth factor-induced membrane ruffling.15 The GTPases are active only in the GTP-bound state. Cycling between GTP-bound and guanosine diphosphate (GDP)-bound states is therefore tightly regulated. GTPase-activating proteins (GAPs) accelerate the intrinsic GTP hydrolytic activity of the GTPases and thereby down-regulate their activities.16 17 Other regulatory proteins, termed GDP/GTP exchange factors (GEFs), alter the activity of the GTPases by controlling the rate of spontaneous GDP dissociation from the GTPases; guanidine nucleotide-releasing factors (GRFs) increase the dissociation rate and GDP-dissociation inhibitors (GDIs) decrease the dissociation rate.
RhoGDI (RhoGDI-1 or RhoGDIα) is expressed in all cell types and can interact with several Ras-like GTP-binding proteins, including RhoA, RhoB, and Rac.18-20 RhoGDI is also found in a cytosolic complex with Rac2 and NADPH oxidase and thus may regulate superoxide anion formation.14 LyGDI (RhoGDI-2 or RhoGDIβ) is preferentially expressed in hematopoietic cells.21 This indicates that LyGDI likely plays some significant role in the growth and differentiation processes of hematopoietic cells. This significance is underscored by increasing evidence for the involvement of regulators of G-proteins in clinical diseases.
Recently, Rac2 knock-out mice have been described.22 In these animals an increase of myelopoiesis was observed. Neutrophils from Rac2 knock-out mice displayed defective functions, similar to defects seen in neutrophils from patients with SCN (eg, reduced superoxide anion generation, decreased chemotaxis). Therefore, we were interested in the expression and regulation of GTPases and GDIs in neutrophils from these patients.
To identify differences in the expression of cytosolic proteins (eg, G-proteins) in neutrophils from SCN patients as compared to healthy donors, we performed 2-dimensional gel electrophoresis.23Analyzing differentially expressed proteins in normal neutrophils as compared to neutrophils from SCN patients, we identified LyGDI by amino acid sequencing. RhoGDI, which shows a higher expression in patients' neutrophils, could be identified by Western blot analysis with a specific antibody. To investigate the role of the GTPases (RhoA, Rac2), which are regulated by GDIs (LyGDI, RhoGDI) in human neutrophils, we performed immunoprecipitations and Western blot analyses. As a control we tested neutrophils from healthy individuals treated with G-CSF (10 μg/kg/d) for 4 days.
Material and methods
Samples
Neutrophils from 3 different patients who were clinically diagnosed as having SCN or Kostmann syndrome were tested according to the following criteria: absolute neutrophil counts (ANC) below 200/μL in the peripheral blood, maturation arrest at the promyelocyte level in the bone marrow, absence of antineutrophil antibodies, and onset of severe bacterial infections during the first 12 months of life.1,2,24 All 3 patients being tested were treated with r-metHuG-CSF (filgrastim; 1-10 μg/kg/d)5 and they revealed no signs of bacterial infections at the time when the study was performed. As controls neutrophils from 3 healthy individual treated for 4 days with G-CSF (10 μg/kg/d) and from 6 untreated healthy donors were tested.
Antibodies and chemicals
All chemicals were purchased from Sigma (Deisenhofen, Germany) or Merck (Darmstadt, Germany). The primary antibodies against LyGDI (sc-6047), RhoGDI (sc-360), RhoA (sc-418), and Rac2 (sc-96) were purchased from Santa Cruz (Heidelberg, Germany), and the Rac2 antibody is not cross-reactive to Rac1. Horseradish peroxidase (HRP)-conjugated antiphosphotyrosine (anti-PY) antibody (RC20) was from Dianova (Hamburg, Germany). The HRP-conjugated secondary antibodies goat-antimouse IgG and swine-antirabbit IgG were from Dako (Hamburg, Germany), and the antigoat antibody was from Santa Cruz.
Isolation of neutrophils
Neutrophils were separated from heparinized (100 U/mL Heparin Novo; Novo Industrie, Mainz, Germany) peripheral blood by dextrin sedimentation (Plasmasteril; Fresenius, Oberursel, Germany) and Ficoll-Paque (Pharmacia, Freiburg, Germany) density centrifugation. The pellet was immediately resuspended in 5 mL ice-cold distilled water and subsequently in 2.5 mL of a 2.7% w/v solution of NaCl to lyse contaminating erythrocytes. Then the cells were washed twice in ice-cold phosphate-buffered saline (PBS) pH 7.2 (137 mmol/L NaCl, 2.7 mmol/L KCl, 10 mmol/L Na2HPO4, 5.5 mmol/L KH2PO4). More than 98% of the cells were viable as assayed by trypan blue dye exclusion and the percentage of neutrophils was more than 95% as assayed by hematoxylin staining.
In vivo stimulation of normal neutrophils
Three healthy individuals were treated for 4 days with G-CSF (10 μg/kg/d) for peripheral stem cell separation. At day 5 neutrophils were isolated as described above.
Cell lysis and immunoprecipitation
The neutrophils (2 × 107 cells) from peripheral blood were pulse centrifuged and resuspended in 100 μL (for Western blotting) or 500 μL (for immunoprecipitation) lysis-buffer (50 mmol/L Tris-base pH 7.4, 150 mmol/L NaCl, 1 mmol/L EGTA, 1% v/v NP-40, 2 mmol/L Na3VO4, 1 mmol/L NaF, 4 μg/mL leupeptin, 4 μg/mL pepstatin, 10 μg/mL aprotinin, 4 mmol/L Pefabloc SC, 250 U Benzonase, 2 mmol/L MgCl2), and shaken for 30 minutes at 4°C. The lysates were then centrifuged at 13,000g for 15 minutes at 4°C to remove insoluble materials. Proteins were determined by the Bradford method.25 A protein assay solution and bovine serum albumin (BSA) as a standard were used (Biorad, München, Germany). For Western blotting 50 μg protein was diluted in 3× sample buffer (1× sample buffer: 62.5 mmol/L Tris-base pH 6.8, 2% w/v sodium dodecyl sulfate [SDS], 5% v/v 2-mercaptoethanol, 20% v/v glycerol, traces of bromophenol blue), and the samples were boiled for 5 minutes before electrophoresis. For immunoprecipitations the cell lysates (500 μg protein) were precleared by rotating for 30 minutes at 4°C with 10 μL of a 50% w/v slurry of protein A-agarose beads (Biomol, Hamburg, Germany). After centrifugation to pellet the protein A-agarose beads, lysates were incubated with antibodies (2 μg each) against LyGDI, RhoGDI, Rac2, or RhoA, respectively, for 16 hours at 4°C with rotation. Immunocomplexes were collected with protein A- or protein G-agarose beads (UBI, Hamburg, Germany) at 4°C with rotation for 4 hours. Immunoprecipitates were washed 3 times in lysis buffer and then eluted by boiling in 30 μL 2× sample buffer.
Western blot analysis
Cell lysates and immunoprecipitates were prepared as described above. The proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE)26using a 8% w/v polyacrylamide gel, and were transferred onto a nitrocellulose membrane (Biorad) with a semidry transfer unit (Forschungswerkstatt from the Medical School Hannover) in a buffer containing 50 mmol/L boric acid pH 9 and 20% v/v methanol. Then the membranes were blocked with TBS-T (10 mmol/L Tris-base pH 7.5, 100 mmol/L NaCl, and 0.1% v/v Tween-20) containing 5% w/v nonfat dry milk (TBS-TM) for 1 hour at room temperature, and incubated with primary antibody (anti-RhoA, anti-Rac2, anti-LyGDI, or anti-RhoGDI were 1:1000 diluted in TBS-TM) for an additional 1 hour at room temperature. After that, the membranes were washed with 4 changes of TBS-T for a total of 20 minutes, and incubated with HRP-conjugated goat-antimouse IgG, HRP-conjugated swine-antirabbit IgG (1:3000 diluted in TBS-TM), or HRP-conjugated antigoat IgG (1:15,000 diluted in TBS-TM) for 1 hour at room temperature. Next the membranes were washed again with 6 changes of TBS-T for a total of 30 minutes and the immunoblot was developed by the enhanced chemiluminescence method following the manufacturer's guideline (ECL; Amersham, Braunschweig, Germany). For detection of protein tyrosine phosphorylation the membrane was blocked in TBS-T containing 1% w/v BSA (TBS-TB), and detection was performed with HRP-conjugated anti-PY antibody (1:2500 diluted in TBS-TB), and ECL system.
For reprobing, the membranes were stripped in 62.5 mmol/L Tris-base pH 6.7, 100 mmol/L 2-mercaptoethanol, 2% w/v SDS, for 30 minutes at 50°C, followed by reblocking and reprobing with the appropriate antibodies.
RNA isolation
Total RNAs were extracted from the cells with the single-step isolation method described by Chomczynsky and Sacchi27using TRIzol (Gibco BRL, Gaithersburg, MD). In brief, 1 to 3 × 107 cells were lysed with 1 mL of TRIzol. After addition of 0.1 mL chloroform, the lysates was mixed thoroughly and incubated on ice for 5 minutes. Centrifugation of the lysate forms 2 phases. RNA remains exclusively in the upper aqueous phase and was precipitated with an equal volume of isopropanol and washed once with 70% v/v ethanol. The air-dried RNA pellet was dissolved in water treated with diethylpyrocarbonate (DEPC), and concentration was determined by measuring extinction at 260 nm.
Semiquantitative reverse transcriptase-polymerase chain reaction (RT-PCR) analysis
RNA has been transcribed into complementary DNA (cDNA) with reverse transcriptase (10 U Superscript II reverse transcriptase/μg RNA; Gibco BRL) using random primers (0.5 μg/μg RNA) in 50 mmol/L Tris-HCl, 50 mmol/L KCl, 10 mmol/L MgCl2, 10 mmol/L DTT, 0.5 mmol/L spermidine, and 1 mmol/L dNTPs at 42°C for 45 minutes.
Polymerase chain reaction was performed with specific primers for Rac2, RhoGDI or β-actin, respectively. Primers for Rac2 (5′-ACCGTGTTTGACAACTATTCAG-3′ and 5′-ACGGCCCGGATGGCCTCG-3′) amplify a 425 base pair (bp) fragment, primers for RhoGDI (5′-CATCCAGGAGATCCAGGAGC-3′ and 5′-GACTTGATGCTGTAGCTGCC-3′) amplify a 435 bp fragment, and primers for β-actin (5′-TACATGGCTGGGGTGTTGAA-3′ and 5′-AAGAGAGGCATCCTCACCCT-3′) amplify a 218 bp fragment.
The cDNA amplifications were carried out in 25 μL of 10 mmol/L Tris-HCl, 50 mmol/L KCl, 1 mmol/L MgCl2, 0.1 μmol/L primer, and 1 U of Taq DNA Polymerase (Gibco BRL). Each cycle of PCR consisted of denaturation for 60 seconds at 94°C, annealing for 60 seconds at 60°C for Rac2, 62°C for RhoGDI, and 64°C for β-actin, respectively, and extension for 60 seconds at 72°C and was repeated 22 times (Rac2), 25 times (RhoGDI), or 20 times (β-actin), respectively. PCR-amplified DNA fragments were visualized by ethidium bromide staining after agarose gel electrophoresis.
Hybridization with an internal oligonucleotide
Gel-separated PCR products were blotted onto a positively charged nylon membrane (Boehringer Mannheim, Mannheim, Germany) by capillary transfer in 20× SSC (3 mol/L NaCl, 0.3 mol/L sodium citrate, pH 7.0). Prehybridization (30-45 minutes) and hybridization (1-2 hours) occurred at 42°C in DIG Easy-Hyb solution (Boehringer Mannheim). As a hybridization probe, internal oligonucleotides specific for Rac2 (5′-CATCATCCTGGTGGGCACCAAG-3′), RhoGDI (5′-CATGAAGTACATCCAGCATACG-3′) or β-actin (5′-ATCGAGCACGGCATCGTCAC-3′), respectively, were labeled with Digoxigenin (DIG) using the DIG 3′ End labeling Kit (Boehringer Mannheim). Washes were repeated twice in 2× SSC for 5 minutes at room temperature and in 0.5× SSC for 5 minutes at 42°C. The membrane was blocked (1% w/v blocking reagent in maleic acid buffer) for 30 minutes before incubation with anti-DIG antibodies coupled to alkaline phosphatase (30 minutes). After washing twice in maleic acid buffer, detection was performed by incubating with the chemiluminescent substrate CDP-Star (Tropix, Bedford, MA). Autoradiographic film (X-OMAT/AR by Kodak, Rochester, NY) was exposed to the blot for several seconds up to 30 minutes.
Results
Expression of GTPases, RhoA and Rac2, in neutrophils from healthy individuals and SCN patients
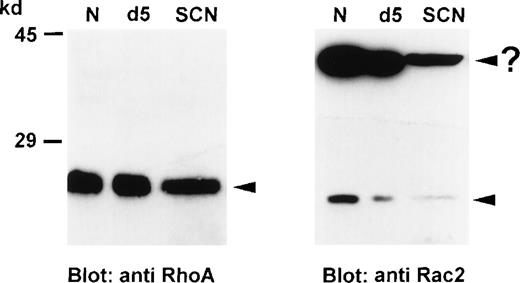
We examined the expression patterns of 2 GTPases (RhoA and Rac2), which were reported to be involved in different neutrophil functions, in neutrophils from healthy individuals and SCN patients in Western blot analysis with specific antibodies. RhoA is expressed at a similar level in normal (N) and patients' neutrophils (SCN), whereas Rac2 shows a decreased expression in SCN patients (Figure1). To test whether this decreased expression is a result of G-CSF treatment, we tested neutrophils from healthy individuals treated with G-CSF for 4 days (10 μg/kg/d). At day 5 (d5) neutrophils from these G-CSF-treated healthy individuals revealed RhoA expression similar to normal neutrophils without G-CSF stimulation, and comparable to SCN patients. Rac2 expression, however, was decreased as compared to normal unstimulated neutrophils, but similar to neutrophils from SCN patients. These results were representative for 6 independent experiments with cells from 3 SCN patients, 3 G-CSF-treated individuals, and 6 untreated healthy individuals.
Expression of GTPases, RhoA and Rac2, in neutrophils from healthy individuals and SCN patients.
Neutrophil lysates (50 μg protein each) were separated on a 8% SDS-PAGE, blotted onto nitrocellulose membranes, and detected with anti-RhoA or anti-Rac2 antibodies. These results were representative for 6 independent experiments with cells from 3 SCN patients, 3 G-CSF-treated individuals, and 6 untreated healthy individuals. N indicates normal neutrophils; SCN, patients' neutrophils; and d5, in vivo-stimulated neutrophils from a healthy donor.
Expression of GTPases, RhoA and Rac2, in neutrophils from healthy individuals and SCN patients.
Neutrophil lysates (50 μg protein each) were separated on a 8% SDS-PAGE, blotted onto nitrocellulose membranes, and detected with anti-RhoA or anti-Rac2 antibodies. These results were representative for 6 independent experiments with cells from 3 SCN patients, 3 G-CSF-treated individuals, and 6 untreated healthy individuals. N indicates normal neutrophils; SCN, patients' neutrophils; and d5, in vivo-stimulated neutrophils from a healthy donor.
Interestingly, in Rac2 immunoblots in addition to the 21-kd protein, we also detected a protein with an estimated molecular mass of 42 to 44 kd (Figure 1; right part, arrow with question mark). This protein shows the highest expression in normal neutrophils, too. In neutrophils from a healthy individual treated with G-CSF, the signal was weaker, and in SCN patients we found the weakest signal. Our hypothesis is that this protein might represent a Rac2-LyGDI, Rac2-RhoGDI, or Rac2-Rac2 complex, respectively.
Expression of GDIs, LyGDI and RhoGDI, in neutrophils from healthy individuals and SCN patients
Next, we investigated the expression patterns of GDIs (LyGDI and RhoGDI), which were described to associate with Rac2 or RhoA or both. These GDIs negatively regulate GTPases by inhibiting the dissociation of GDP and therefore the binding of GTP. LyGDI, which is preferentially expressed in hematopoietic cells, was detectable at similar levels in neutrophils from healthy donors (N) and SCN patients (SCN), whereas RhoGDI, a ubiquitously expressed GDI, showed a higher expression in patients' neutrophils (Figure 2). To test whether this increased expression of RhoGDI is a result of G-CSF treatment, we tested neutrophils from a healthy individual treated with G-CSF for 4 days (10 μg/kg/d). At day 5 neutrophils were investigated (d5) and we could show that on G-CSF treatment in a healthy individual the RhoGDI expression is up-regulated. These results were representative for 6 independent experiments with cells from 3 SCN patients, 3 G-CSF treated individuals, and 6 untreated healthy individuals.
Expression of GDIs, LyGDI and RhoGDI, in neutrophils from healthy individuals and SCN patients.
Neutrophil lysates (50 μg protein each) were separated on a 8% SDS-PAGE, blotted onto nitrocellulose membranes, and detected with anti-LyGDI or anti-RhoGDI antibodies. These results were representative for 6 independent experiments with cells from 3 SCN patients, 3 G-CSF-treated individuals, and 6 untreated healthy individuals. N indicates normal neutrophils; SCN, patients' neutrophils; and d5, in vivo stimulated neutrophils from a healthy donor.
Expression of GDIs, LyGDI and RhoGDI, in neutrophils from healthy individuals and SCN patients.
Neutrophil lysates (50 μg protein each) were separated on a 8% SDS-PAGE, blotted onto nitrocellulose membranes, and detected with anti-LyGDI or anti-RhoGDI antibodies. These results were representative for 6 independent experiments with cells from 3 SCN patients, 3 G-CSF-treated individuals, and 6 untreated healthy individuals. N indicates normal neutrophils; SCN, patients' neutrophils; and d5, in vivo stimulated neutrophils from a healthy donor.
RNA expression of Rac2 and RhoGDI in neutrophils from healthy individuals and SCN patients
To test the expression of Rac2 and RhoGDI at the messenger RNA (mRNA) level, semiquantitative RT-PCR analyses were performed (Figure3). Rac2, which demonstrated a decreased protein expression in SCN patients and G-CSF-treated healthy individuals as well, also showed decreased expression in SCN patients (SCN) at the mRNA level. Under treatment with G-CSF a decreased Rac2 expression could also be observed in healthy individuals (d5). Interestingly, RhoGDI, which demonstrated increased protein expression in SCN patients and G-CSF-treated healthy individuals as well, showed decreased RhoGDI mRNA expression, too. These results were representative for 5 independent experiments with cells from 2 SCN patients, 2 G-CSF-treated individuals, and 3 untreated healthy individuals.
Semiquantitative RT-PCR analysis of Rac2 and RhoGDI mRNA expression in neutrophils from healthy individuals and SCN patients.
RNA was isolated from neutrophils27 and RT-PCR was performed using specific primers for Rac2 or RhoGDI, respectively. PCR products were separated by agarose gel electrophoresis and blotted onto nylon membranes. Specific products were hybridized with DIG-labeled internal oligonucleotides specific for Rac2 or RhoGDI, respectively, and detection was performed with anti-DIG antibodies and the chemiluminescent substrate CDP-Star. As a control β-actin expression was tested. These results were representative for 5 independent experiments with cells from 2 SCN patients, 2 G-CSF- treated individuals, and 3 untreated healthy individuals. N indicates normal neutrophils; SCN, patients' neutrophils; and d5, in vivo stimulated neutrophils from a healthy donor.
Semiquantitative RT-PCR analysis of Rac2 and RhoGDI mRNA expression in neutrophils from healthy individuals and SCN patients.
RNA was isolated from neutrophils27 and RT-PCR was performed using specific primers for Rac2 or RhoGDI, respectively. PCR products were separated by agarose gel electrophoresis and blotted onto nylon membranes. Specific products were hybridized with DIG-labeled internal oligonucleotides specific for Rac2 or RhoGDI, respectively, and detection was performed with anti-DIG antibodies and the chemiluminescent substrate CDP-Star. As a control β-actin expression was tested. These results were representative for 5 independent experiments with cells from 2 SCN patients, 2 G-CSF- treated individuals, and 3 untreated healthy individuals. N indicates normal neutrophils; SCN, patients' neutrophils; and d5, in vivo stimulated neutrophils from a healthy donor.
Association of GTPases and GDIs in neutrophils from healthy individuals and SCN patients
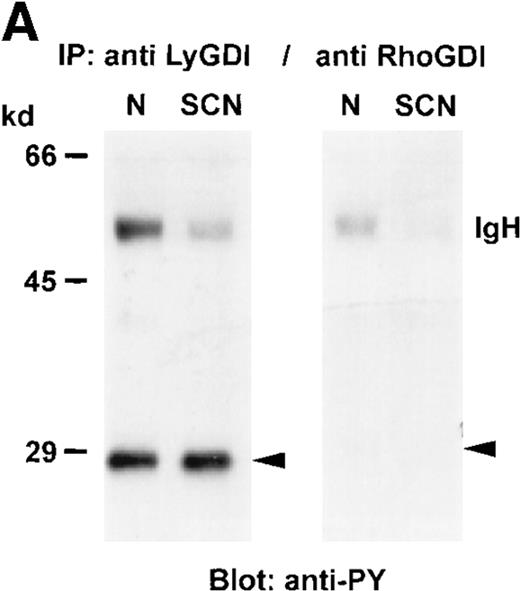
Interactions of GTPases with GDIs are a possibility in the regulation of GTPase activity. We investigated these associations in co-immunoprecipitation experiments. As shown in Figure 2 we found that RhoGDI was overexpressed in patients' neutrophils and that overexpression was a result of G-CSF treatment. RhoA and LyGDI were expressed at similar levels, whereas Rac2 showed a decreased expression (Figure 1). In LyGDI and RhoGDI immunoprecipitates Rac2 (Figure4A) and RhoA (Figure 4B) could be detected in normal neutrophils (N) but not in patients' neutrophils (SCN). In patients' neutrophils (SCN) only RhoA was detectable in LyGDI and RhoGDI immunoprecipitations (Figure 4B). As a control, in Figure 4C the immunoprecipitated proteins are shown and reveal the same results as shown in Figure 2. These results were representative for 3 independent experiments with cells from 3 SCN patients and 3 healthy individuals.
Association of GTPases and GDIs in neutrophils from healthy individuals and SCN patients.
GDIs were immunoprecipitated from neutrophil cytosol (500 μg protein each), separated on a 8% SDS-PAGE, blotted onto nitrocellulose membranes and detected with (A) anti-Rac2 antibody, (B) anti-RhoA antibody, or (C) anti-LyGDI or anti-RhoGDI antibodies. These results were representative for 3 independent experiments with cells from 3 SCN patients and 3 healthy individuals. N indicates normal neutrophils, and SCN, patients' neutrophils.
Association of GTPases and GDIs in neutrophils from healthy individuals and SCN patients.
GDIs were immunoprecipitated from neutrophil cytosol (500 μg protein each), separated on a 8% SDS-PAGE, blotted onto nitrocellulose membranes and detected with (A) anti-Rac2 antibody, (B) anti-RhoA antibody, or (C) anti-LyGDI or anti-RhoGDI antibodies. These results were representative for 3 independent experiments with cells from 3 SCN patients and 3 healthy individuals. N indicates normal neutrophils, and SCN, patients' neutrophils.
Tyrosine phosphorylation patterns of GDIs and GTPases in neutrophils from healthy individuals and SCN patients
Immunoprecipitations were performed to test the tyrosine phosphorylation of GDIs and GTPases in neutrophils from normal individuals and patients. GDIs or GTPases were immunoprecipitated and the detection was performed with anti-PY antibodies. LyGDI was constitutively tyrosine phosphorylated in normal (N) and patients' neutrophils (SCN), whereas RhoGDI was not (Figure 5A). As a control, in Figure 5B the immunoprecipitated proteins are shown and the same results as shown in Figure 2 were obtained. These results were representative for 3 independent experiments with 3 SCN patients and 3 healthy individuals.
Tyrosine phosphorylation of GDIs in neutrophils from healthy individuals and SCN patients.
GDIs were immunoprecipitated from neutrophil cytosol (500 μg protein each), separated on a 8% SDS-PAGE, blotted onto nitrocellulose membranes, and detected with (A) anti-PY antibody, or (B) anti-LyGDI or anti-RhoGDI antibodies. These results were representative for 3 independent experiments with cells from 3 SCN patients and 3 healthy individuals. N indicates normal neutrophils, and SCN, patients' neutrophils.
Tyrosine phosphorylation of GDIs in neutrophils from healthy individuals and SCN patients.
GDIs were immunoprecipitated from neutrophil cytosol (500 μg protein each), separated on a 8% SDS-PAGE, blotted onto nitrocellulose membranes, and detected with (A) anti-PY antibody, or (B) anti-LyGDI or anti-RhoGDI antibodies. These results were representative for 3 independent experiments with cells from 3 SCN patients and 3 healthy individuals. N indicates normal neutrophils, and SCN, patients' neutrophils.
In RhoA or Rac2 immunoprecipitations no tyrosine phosphorylation of the GTPases (p21) could be demonstrated (Figure 6A), whereas other co-immunoprecipitated tyrosine phosphorylated proteins could be detected. In Figure 6B the immunoprecipitated proteins are shown and the same results as shown in Figure 1 were obtained. These results were representative for 3 independent experiments with cells from 3 SCN patients and 3 healthy individuals.
Tyrosine phosphorylation of GTPases in neutrophils from healthy individuals and SCN patients.
GTPases were immunoprecipitated from neutrophil cytosol (500 μg protein each), separated on a 8% SDS-PAGE, blotted onto nitrocellulose membranes, and detected with (A) anti-PY antibody, or (B) anti-RhoA or anti-Rac2 antibodies. These results were representative for 3 independent experiments with cells from 3 SCN patients and 3 healthy individuals. N indicates normal neutrophils, and SCN, patients' neutrophils.
Tyrosine phosphorylation of GTPases in neutrophils from healthy individuals and SCN patients.
GTPases were immunoprecipitated from neutrophil cytosol (500 μg protein each), separated on a 8% SDS-PAGE, blotted onto nitrocellulose membranes, and detected with (A) anti-PY antibody, or (B) anti-RhoA or anti-Rac2 antibodies. These results were representative for 3 independent experiments with cells from 3 SCN patients and 3 healthy individuals. N indicates normal neutrophils, and SCN, patients' neutrophils.
Taken together, our results demonstrate that the GTPase RhoA and the GDI LyGDI are expressed at similar levels in normal and patients' neutrophils, whereas Rac2 shows a decreased expression and RhoGDI an increased expression at the protein level. The higher expression of RhoGDI and the decreased expression of Rac2 may be a result of G-CSF treatment in SCN patients. At the mRNA level both Rac2 and RhoGDI expression are decreased in SCN patients and G-CSF treated healthy individuals. We suggest that G-CSF may act as a negative regulator for Rac2 mRNA and protein expression. Treatment with G-CSF also leads to decreased mRNA expression of RhoGDI, and possibly to a diminished degradation or an increased stability of the RhoGDI protein. LyGDI is constitutively tyrosine phosphorylated in normal, unstimulated, and patients' neutrophils, but RhoGDI is not. In addition, association of Rac2 and RhoGDI or LyGDI is abrogated or not detectable based on the low Rac2 expression in patients' neutrophils.
Discussion
Severe congenital neutropenia is a disorder of myelopoiesis characterized by severe neutropenia, secondary to a maturation arrest at the promyelocyte/myelocyte stage in bone marrow and an ANC below 200/μL in the peripheral blood of the affected patients, and the onset of severe bacterial infections during the first 12 months of life.1,2 The etiology of SCN is still unknown. Although G-CSF serum levels are elevated in SCN patients,28 daily subcutaneous administration of pharmacologic dosages of recombinant human G-CSF (filgrastim or lenograstim) in these patients leads to a significant increase in circulating neutrophils up to 1000/μL, associated with clinical benefit.3-5
In previous studies, the functions of G-CSF-induced neutrophils from SCN patients were investigated. These cells were normal with respect to their ability to produce reactive oxygen intermediates (ROI) after PMA (phorbol 12–myristate 13–acetate) stimulation. However, they demonstrated a decreased superoxide anion generation and chemotaxis in response to fMLP (n-formyl methionyl leucyl phenylalanin), an impaired Ca++ mobilization after fMLP stimulation, an increased adhesion to plastic surfaces, and an altered surface marker expression (eg, CD64, CD14, CD16).7,8,29 Some functional features seen in neutrophils from SCN patients could also be detected in healthy individuals and cancer patients under G-CSF treatment, for example, reduced chemotaxis, increased adhesion, and altered surface marker expression (for review, see Spiekermann et al9). In 1998, Leavey et al30 demonstrated that in healthy individuals G-CSF administration imparts only modest effects on neutrophil function and molecular events and results in a diverse pattern of activities not always consistent with increased function. Given the diverse functional and molecular effects of neutrophils, the clinical benefit of G-CSF most likely lies in an increased absolute number of neutrophils with enhanced survival characteristics (B Kasper, own observations in SCN neutrophils) providing improved host defense.
Two GTPases, Rac2 and RhoA, were described to be involved in many neutrophil functions.12-14 We were interested in the expression of these GTPases and their regulation in patients' G-CSF-induced neutrophils. We found that the GTPase Rac2 is only weakly expressed and the GDP-dissociation inhibitor RhoGDI is overexpressed in patients' neutrophils at the protein level. These differences are most likely the result of G-CSF treatment, because similar changes in the protein expressions could also be observed in neutrophils from healthy individuals treated with G-CSF. However, it cannot be excluded that these differences are due to the underlying pathomechanism of SCN. The GTPase RhoA and the GDP-dissociation inhibitor LyGDI are expressed at similar levels in neutrophils from SCN patients and healthy individuals.
It has been postulated that GDIs may serve as a chaperon-like molecule to transport Rho proteins through the cytosol to their site of function at a target membrane.31 It could be suggested that the G-CSF-stimulated decreased expression of Rac2 protein in the cytoplasm may be due to the translocation of Rac2 to the plasma membrane or membrane cytoskeleton or due to the overexpression of RhoGDI. In intact neutrophils stimulation with fMLP or PMA results also in part in a translocation of Rac2 to the plasma membrane (0.9% or 10% of total cytosolic Rac2 protein, respectively).14 In contrast, el Benna et al32 found a small percentage of Rac2 was transferred to the membrane cytoskeleton on activation of neutrophils, but not to the plasma membrane. Altogether, the detection of decreased Rac2 levels in patients' neutrophils is most likely not only due to membrane or cytoskeleton bound Rac2 protein, because it is not very likely that on stimulation with G-CSF nearly 95% of the total Rac2 would have been translocated. The differences in the Rac2 protein expression in patients' neutrophils were really significant as compared to normal neutrophils.
Next, we investigated the mRNA expression of Rac2 and RhoGDI. As shown in Figure 3, both Rac2 mRNA and RhoGDI mRNA expression decreased during G-CSF treatment, as well as in SCN patients. We suggest that G-CSF treatment negatively regulates Rac2 mRNA and protein expression, whereas RhoGDI protein demonstrated a diminished degradation or an increased stability under G-CSF treatment.
Leffers et al33 demonstrated that overexpression of both GDIs in human keratinocytes produces rounding up of the cells and disruption of the actin cytoskeleton. These changes were described to be associated with inactive Rho protein(s).11 The authors33 conclude, that overexpression of GDIs may inactivate the Rho protein(s). In SCN patients, RhoGDI overexpression may be due to decreased degradation or an increased stability under G-CSF treatment. In addition Elsner et al8 found increased amounts of total actin and basal F-actin content. Elevated basal F-actin limits the magnitude of fMLP-induced change in F-actin content. The authors8 conclude that the abnormalities in microfilamentous cytoskeletal organization in response to fMLP could translate into a decreased chemotaxis of neutrophils from SCN patients. Another mechanism, that leads to inactivation of GTPases was found by Zhang and Zheng34 who described that Rho family GTPases (eg, Cdc42 or Rac2) form reversible homodimers in both the GTP- and GDP-bound states. The homophilic interaction of GTPases in the GTP-bound state (eg, Cdc42-GTP or Rac2-GTP) caused a significant stimulation of the intrinsic GTPase activity and the authors34 concluded that dimerization may play a role in the negative regulation of specific Rho family GTPases. Interestingly, as shown in Figure 1, we have detected a protein with a molecular mass compatible with a Rac2 homodimer, when anti-Rac2 antibody was used for detection.
In U937 cells, a myelomonocytic cell line, stimulation with PMA results in differentiation into monocyte-/macrophage-like cells and phosphorylation of LyGDI, but not RhoGDI.35 Purification of GTPase-GDI complexes from cytosol in these cells demonstrated that LyGDI is not able to form stable complexes with RhoA, Rac1, or Rac2, respectively. Gorvel et al35 concluded that LyGDI is associated with yet uncharacterized Rho proteins. A new GTPase belonging to the Rho family has recently been identified: TTF (Translocation Three Four).36 TTF is expressed in a restricted manner in hematopoietic cells and represents a potential interacting partner of LyGDI. Our experiments were performed with neutrophils, and we could demonstrate associations between LyGDI and Rac2 or RhoA in normal human neutrophils, respectively. In patients' neutrophils no LyGDI-Rac2 or RhoGDI-Rac2 associations could be demonstrated most likely due to the lack or low level of Rac2 expression.
In 1999, Roberts et al22 presented their results obtained in Rac2 knock-out mice. Interestingly, neutrophils from Rac2-deficient mice displayed defective functions, similar to defects seen in neutrophils from SCN patients, expressing low levels or no Rac2 protein and Rac2 mRNA. We suggest that G-CSF treatment negatively regulates Rac2 mRNA expression and that the low level of Rac2 protein might be responsible for at least some of the impaired functions in patients' neutrophils. The clinical benefit of G-CSF in these patients is probably not due to an enhancement of neutrophil functions, but lies most likely in an increased absolute number of neutrophils with enhanced survival characteristics providing improved host defense. The pathomechanism for the defective Rac2 expression remains to be investigated. One possible mechanism is that overexpression of the RhoGDI protein secondarily leads to a decreased expression of its binding partner Rac2. In addition to the effect on neutrophil function, Rac2 might also be involved in the G-CSF receptor signaling pathway via mitogen-activated protein kinases. Indeed, recent reports document the functional role of Rac proteins in distinct signaling pathways,37 especially on p21-activated kinase activation, which is involved in several functions associated with cell cycle regulation, cell transformation, and apoptosis. It could be postulated that decreased Rac2 expression is one of the pathomechanisms involved in defective G-CSF receptor signaling in SCN.
Supported in part by grant DFG WE942/4-3 from the Deutsche Forschungsgemeinschaft (Bonn, Germany), and by AMGEN Inc. (Thousand Oaks, CA).
Reprints:Brigitte Kasper, Forschungszentrum Borstel, Department of Immunology and Cell Biology, Parkallee 22a, 23845 Borstel, Germany; e-mail: bkasper@fz-borstel.de.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.









This feature is available to Subscribers Only
Sign In or Create an Account Close Modal