Interactions between the Wiskott-Aldrich (WAS) protein (WASp), small GTPases, and the cytoskeletal organizing complex Arp2/3 appear to be critical for the transduction of signals from the cell membrane to the actin cytoskeleton in hematopoietic cells. This study shows that Fcγ-receptor (FcγR)-mediated phagocytosis is impaired in WASp-deficient peripheral blood monocytes, and that in macrophages, formation of the actin cup and local recruitment of tyrosine phosphorylated proteins is markedly attenuated. Results also show that, in normal macrophages, WASp itself is actively recruited to the cup, suggesting that assembly of this specialized cytoskeletal structure is dependent on its expression.
The Wiskott-Aldrich syndrome (WAS) protein (WASp) is a member of a family of cytoplasmic proteins that share similar domain structures, and that which are believed to be responsible for the transduction of signals from the cell membrane to the actin cytoskeleton.1,2 Interactions between WASp, the Rho family GTPase CDC42,3,4 and the cytoskeletal organizing complex Arp2/35 appear to be critical to this function, which when disturbed translates into measurable defects of cell polarization and motility.6,7 Patients with WAS contain a mutant copy of theWAS gene and in most cases there is no detectable expression of WASp in primary hematopoietic cells.8 Apart from studies that have demonstrated negative effects following injection of an isolated WASp CDC42-Rac interactive binding (CRIB) domain9into J774 mouse macrophages, the role of WASp in FcγR-mediated phagocytosis and linked signaling pathways is undefined.
Materials and methods
Patient samples
All patients with WAS exhibited a severe clinical phenotype, and none expressed detectable WASp in mononuclear cell lysates by Western blotting with rabbit polyclonal antisera SK3 as previously described (raised against C-terminal amino acids 487-501).8 Patient W-BL contains a G insertion at position 1357 in exon 10, resulting in a frameshift. Patient W-SS contains a T to C substitution that disrupts the splice donor site in intron 9. Patients W-JG and W-SG are siblings with an identical T to C missense mutation at nucleotide 284 in exon 2 (patients 3 and 4 in reference 8).
Purification of PBMC and phagocytosis assay
The PBMC were isolated by Histopaque-1077 (Sigma, Poole, England) gradient centrifugation and 2-5 × 105 cells were incubated at 37°C for various lengths of time with 15 to 20 mL fluorescein isothiocyanate (FITC)- Escherichia coli (Phagotest, Orpegen Pharma, Heidelberg, Germany), preopsonized with human IgG (Pierce). After incubation, cells were quenched, washed, and then stained with PE-CD14 monoclonal antibody (mAb) (Dako, Ely, England). FACS analysis (> 50 000 events acquired) was performed on a FacscaliburII (Beckton-Dickinson) by gating the monocytic fraction by forward and side scatter and analyzing the FL1 and FL2 channels by dotplot at each time point. The percentage of phagocytosis was calculated after gating the CD14 and fluorescein isothiocyanate (FITC) double positive events against a negative control sample incubated at 0°C. Experiments were performed in duplicate or triplicate, with normal control and WAS samples processed in parallel. Statistical analysis consisted of a 2-tail Student t test adjusted for unequal variances: ** = P < 0.002, * = P < 0.05, n = 3.
Primary macrophage cultures and adherent phagocytosis assay
The PBMC (5 × 105 cells) were seeded on to microchamber slides (Nunc, Rochester, NY) and nonadherent cells were then induced to differentiate into macrophages by incubation in Macrophage-SFM medium (Gibco-BRL, Paisley, Scotland) supplemented with recombinant human macrophage colony-stimulating factor (M-CSF, R&D System, Abingdon, England), as previously described.7 Latex beads (3μm diameter; Sigma) were incubated with 2% (w/v) bovine serum albumin (BSA) overnight at room temperature and opsonized by incubating with 1:200 dilution of rabbit anti-BSA IgG (Sigma) for 2 hours at 37°C. Beads were then washed and resuspended in serum-free RPMI medium. Opsonization was checked with FITC-conjugated antirabbit IgG. Cells were starved of serum in M-CSF-free RPMI medium for 2 hours before addition of IgG-opsonized latex beads at a ratio of 10 to 50 beads per cell. Beads were allowed to adhere to cells for 15 minutes on ice in cold RPMI medium. Unbound beads were washed off, and cells incubated for a further 15 minutes at 37°C in prewarmed medium. Phagocytosis was stopped by fixation in 4% (w/v) paraformaldehyde for 10 minutes, followed by quenching in 100 mmol/L ammonium acetate for 5 minutes. Cells were permeabilized by incubating for 4 minutes in 0.1% (v/v) Triton- × 100, followed by blocking with excess human IgG (Pierce, Chester, England). F-actin was visualized by incubation with rhodamine-labeled phalloidin (Molecular Probes, Leiden, Netherlands). Tyrosine-phosphorylated proteins were detected with mAb PY20 (FITC-PY20; Santa Cruz, Santa Cruz, CA) followed by FITC-conjugated sheep antimouse IgG (Boehringer Mannheim, Indianapolis, IN). Slides were mounted in Citifluor containing 10 nmol/L DAPI, and images recorded with a Nu200 CCD camera (Photometrics, Waterloo, ON) driven by IPLab Spectrum software (Signal Analytics, Vienna, VA). Images were handled with Adobe Photoshop.
Plasmid constructs and cell transfection
Plasmid vector pEGFP-WASp was constructed by amplifying the human WASp coding region by polymerase chain reaction and cloning it into pEGFP-C2 (Clontech, Basingstone, England), in frame and downstream of the EGFP coding region. Cos7 cells were transfected with Superfect (Quiagen, Crawley, England). Primary human macrophages were transfected with plasmid vector pEGFP-WASp by adenovirus-enhanced uptake14 with peptide 6.15
Results and discussion
To address this issue, we first assessed whether a measurable defect in the efficiency of FcγR-mediated phagocytosis could be detected in primary PBMC peripheral blood monocytes from 4 WAS patients (null mutants) (Figure 1). Freshly isolated mononuclear cells were incubated with fluorescein-conjugatedEscherichia coli preopsonized with human IgG. After cytofluorimetric analysis we measured the uptake in the CD14+ monocyte-gated fraction and found an approximately 2-fold reduction in the efficiency of phagocytosis in all WAS patients (Figure 1A). The difference between normal and WAS cells was most pronounced at early time points, although the defect was not fully restored even after 60 minutes, at which time the uptake in WAS cells was still only 70% of normal (Figure 1B). The attenuation of phagocytosis was also observed when measured at a single early time point for different bacteria to cell ratios (Figure 1C). These findings suggest that there is a defect in the early events of FcγR-mediated uptake that is intrinsic to WAS peripheral blood-derived monocytes.
Phagocytosis mediated by Fcγ-R. (A) Time course of phagocytosis of IgG-coated, FITC-labeled E coli by CD14-positive peripheral blood monocytes (PBM) isolated from 4 WAS patients (W-BL, W-SS, W-JG, W-SG; open bars) or from normal controls (filled bars). (B) Efficiency of phagocytosis by WAS PBM compared to normal control PBM. Pooled values from the 4 WAS patients are expressed as a percentage of pooled values from the normal controls. (C) Phagocytosis by normal (filled bars) and WAS (open bars) PBM, 15 minutes after addition of different amounts of IgG-coated, FITC-labeledE coli. WAS PBM were from patient W-SS. Cell type specificity of internalization was confirmed by demonstrating a negligible rate of uptake of IgG-coated, FITC-labeled E coli by the CD14− lymphocyte population (data not shown). Treatment of PBM with 10 mmol/L cytochalasin D for 30 minutes abrogated uptake of IgG-coated, FITC-labeled E coli to background levels (data not shown).
Phagocytosis mediated by Fcγ-R. (A) Time course of phagocytosis of IgG-coated, FITC-labeled E coli by CD14-positive peripheral blood monocytes (PBM) isolated from 4 WAS patients (W-BL, W-SS, W-JG, W-SG; open bars) or from normal controls (filled bars). (B) Efficiency of phagocytosis by WAS PBM compared to normal control PBM. Pooled values from the 4 WAS patients are expressed as a percentage of pooled values from the normal controls. (C) Phagocytosis by normal (filled bars) and WAS (open bars) PBM, 15 minutes after addition of different amounts of IgG-coated, FITC-labeledE coli. WAS PBM were from patient W-SS. Cell type specificity of internalization was confirmed by demonstrating a negligible rate of uptake of IgG-coated, FITC-labeled E coli by the CD14− lymphocyte population (data not shown). Treatment of PBM with 10 mmol/L cytochalasin D for 30 minutes abrogated uptake of IgG-coated, FITC-labeled E coli to background levels (data not shown).
FcγR-mediated internalization requires polymerization of actin at the site of ingestion and transient formation of an actin-rich phagocytic cup that is shed shortly after internalization is completed.10 Because the efficiency of FcγR-mediated phagocytosis in WAS monocytes appears to be most compromised at early time points, we analyzed cup formation at these times following incubation of adherent primary macrophages with IgG-coated latex beads. In normal macrophages, FcγR-mediated internalization was associated with a broad rim of actin polymerization around the engulfed particles (Figure 2A-C and D-F). For more than 80% of cell-associated beads, the F-actin could also be shown to co-localize with tyrosine phosphorylated proteins, reflecting assembly of the active cytoskeletal complex (Figure 2C and F). In the residual fraction, staining for F-actin or protein tyrosine phosphorylation was variable, presumably reflecting heterogeneity in the maturation state of the phagosome (data not shown).
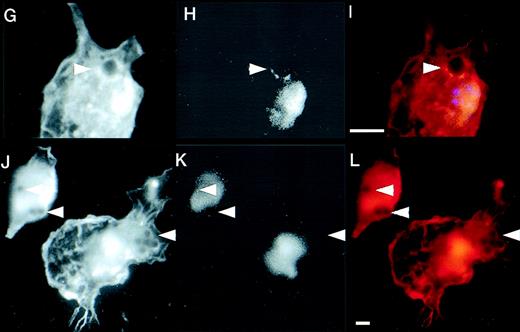
Defective formation of phagocytic cups in WAS macrophages and recruitment of WASp during IgG-mediated phagocytosis. (A-C and D-F) Two examples of phagocytic cup formation in normal macrophages incubated with IgG-coated latex beads (arrowheads). Cells were stained with rhodamine-phalloidin to show the distribution of F-actin (A and D, red) and FITC-PY20 to show tyrosine phosphorylation (B and E, green). Merged images (C and F) show co-localization (yellow) of the 2 signals. (G-I and J-L) Two examples of phagocytic cup formation (arrowheads) in WAS macrophages showing F-actin. (G and J, rhodamine phalloidin) and tyrosine phosphorylation (H and K, FITC-PY20). Merged images are also shown (I and L). In contrast to normal phagocytic cups, those of WAS macrophages are deficient in F-actin, giving the appearance of black disks surrounded by a less well-formed rim. Furthermore, there is no apparent accumulation of tyrosine phosphorylated proteins in the region of the phagocytic cup. (M and N) Expression of EGFP (M) and EGFP-WASp fusion protein (N) in Cos7 cells transiently transfected with plasmid vectors pEGFP-C2 and pEGFP-WASp, respectively. (O-U) Distribution of EGFP-WASp and actin in transfected normal human macrophages. Primary human macrophages transfected with plasmid vector pEGFP-WASp show accentuated cortical staining and co-localization of EGFP-WASp with F-actin (EGFP-rhodamine phalloidin merged image (O). (P-R and S-U) Two examples of transfected macrophages incubated with IgG-coated latex beads are shown. (P and S) EGFP-WASp fusion protein, visualized using a FITC filter. (Q and T) Rhodamine-phalloidin staining of F-actin. (R and U) The merged images and sites of co-localization of EGFP-WASp fusion protein and F-actin (yellow). An untransfected cell is also shown alongside a cell expressing EGFP-WASp (S-U). In cells containing phagocytosed latex beads, EGFP-WASp and F-actin both accumulate around the particles, although some remained co-localized with F actin-rich cortical areas in these cells. The scale bar represent 5μm.
Defective formation of phagocytic cups in WAS macrophages and recruitment of WASp during IgG-mediated phagocytosis. (A-C and D-F) Two examples of phagocytic cup formation in normal macrophages incubated with IgG-coated latex beads (arrowheads). Cells were stained with rhodamine-phalloidin to show the distribution of F-actin (A and D, red) and FITC-PY20 to show tyrosine phosphorylation (B and E, green). Merged images (C and F) show co-localization (yellow) of the 2 signals. (G-I and J-L) Two examples of phagocytic cup formation (arrowheads) in WAS macrophages showing F-actin. (G and J, rhodamine phalloidin) and tyrosine phosphorylation (H and K, FITC-PY20). Merged images are also shown (I and L). In contrast to normal phagocytic cups, those of WAS macrophages are deficient in F-actin, giving the appearance of black disks surrounded by a less well-formed rim. Furthermore, there is no apparent accumulation of tyrosine phosphorylated proteins in the region of the phagocytic cup. (M and N) Expression of EGFP (M) and EGFP-WASp fusion protein (N) in Cos7 cells transiently transfected with plasmid vectors pEGFP-C2 and pEGFP-WASp, respectively. (O-U) Distribution of EGFP-WASp and actin in transfected normal human macrophages. Primary human macrophages transfected with plasmid vector pEGFP-WASp show accentuated cortical staining and co-localization of EGFP-WASp with F-actin (EGFP-rhodamine phalloidin merged image (O). (P-R and S-U) Two examples of transfected macrophages incubated with IgG-coated latex beads are shown. (P and S) EGFP-WASp fusion protein, visualized using a FITC filter. (Q and T) Rhodamine-phalloidin staining of F-actin. (R and U) The merged images and sites of co-localization of EGFP-WASp fusion protein and F-actin (yellow). An untransfected cell is also shown alongside a cell expressing EGFP-WASp (S-U). In cells containing phagocytosed latex beads, EGFP-WASp and F-actin both accumulate around the particles, although some remained co-localized with F actin-rich cortical areas in these cells. The scale bar represent 5μm.
We next analyzed the formation of phagocytic cups in WAS mutant cells. In the absence of phagocytic stimuli, WAS macrophages lacked podosomes and were mostly devoid of filopodia.6 11 On addition of opsonized particles, typically 70% or more of WAS macrophages bound 1 or more beads in independent experiments, suggesting that particle attachment is normal. However, when compared to normal macrophages, the intensity of signal from polymerized actin in the immediate proximity of the engulfed particle was markedly reduced (Figure 2G and J). We then examined the local recruitment of tyrosine phosphorylated proteins. These proteins (including WASp) are associated with the regulated assembly and dissolution of the cytoskeletal complex. By contrast with normal cells, at time points well within the range of normal persistence of F-actin cups (in normal macrophages this extended beyond 30 minutes), the level of protein tyrosine phosphorylation around the engulfed particles was also markedly reduced (Figure 2H and K). The defects of actin polymerization and tyrosine phosphorylation were consistently observed in independent experiments on cells derived from 3 WAS patients. These findings indicate that the formation of an actin cup and assembly of an active cytoskeletal complex at the site of phagocytosis are dependent on the presence of a normally functional WASp.
Finally, to determine whether WASp is actively recruited to the phagocytic cup during this process, we constructed an expression vector in which wild-type WASp is fused at the N-terminus to enhanced green fluorescent protein (EGFP). When expressed in Cos7 cells, this fusion protein exhibited expression patterns identical to those shown previously for wild-type WASp, suggesting that the addition of EGFP does not interfere with normal function (Figure 2N). In transfected primary macrophages, where endogenous WASp is expressed, the EGFP-WASp fusion protein was instead homogeneously distributed in the cytoplasm in the absence of phagocytic stimuli, with areas of more pronounced cortical staining that co-localized with F-actin (Figure 2O). On addition of IgG-coated beads, the EGFP-fusion signal was mostly found to relocate around the engulfed particle, and also co-localized with F-actin (Figure 2P-R and S-U). These data indicate that WASp is actively recruited into the phagocytic cup during FcγR-mediated phagocytosis in normal primary macrophages and may therefore play a critical role in actin polymerization in situ during the early events of particle uptake.
Recent studies have shown that FcγR-mediated phagocytosis is at least in part dependent on CDC42 and can be disturbed by overexpression of the CRIB domain from WASp.9,12 Our findings are consistent with these because we have observed a decreased efficiency of FcγR-mediated phagocytosis in WAS PBMC peripheral blood monocytes and attenuation of localized actin polymerization during formation of the phagocytic cup in differentiated WAS macrophages. In addition, we have shown that EGFP-WASp fusion protein is actively relocated to the region of the evolving cup. However, the phenotypic differences between WAS mutant cells and those previously reported for rat basophilic leukemia cells stably expressing dominant negative N17CDC42 are more surprising.13 In these studies, expression of N17CDC42 was permissive for the formation of actin-rich phagocytic cups, which nevertheless failed to internalize the particles. In addition, the accumulation of tyrosine phosphorylated proteins around the particle was persistent. In marked contrast, our findings in primary human WAS macrophages are of reduced phagosome-specific actin polymerization and virtually absent tyrosine phosphorylation. The simplest interpretation of these combined observations is that WASp itself is responsible for recruitment of tyrosine phosphorylated proteins into the phagocytic cup. In addition to being a final effector for CDC42, WASp may therefore play a more central and proximal role in the assembly of a regulated cytoskeletal complex consisting of Rho family proteins, signaling molecules, and Arp2/3 complex at specialized sites of actin polymerization. The multidomain structure of WASp and other family members may well reflect this function. For the WAS itself, these findings suggest that phagocytic defects may contribute significantly to disease pathophysiology, both in terms of elimination of microorganisms, and perhaps also the phagocytic uptake of apoptotic cells.
Acknowledgments
We thank Laura Machesky for critical reading of the manuscript and the sharing of unpublished data, Robin May for valuable help in setting up the phagocytosis assay, Steve Hart for the kind gift of transfection peptides, Mike Blundell for construction of the EGFP-WASP fusion construct, Gareth Jones for advice on macrophage primary cultures, and Elaine O'Sullivan for critical reading of the manuscript.
Supported by grants from the Primary Immunodeficiency Association and the National Lottery Charities Board.
A.J.T. is a Wellcome Trust senior clinical fellow.
Reprints:Roberto Lorenzi, Department of Molecular Immunology, Institute of Child Health, 30 Guilford Street, London WC1N 1EH, England; e-mail: r.lorenzi@ich.ucl.ac.uk.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal