Abstract
Coagulation factor XIIIa is a transglutaminase that catalyzes covalent cross-link formation in fibrin clots. In this report, we demonstrate that factor XIIIa also mediates adhesion of endothelial cells and inhibits capillary tube formation in fibrin. The adhesive activity of factor XIIIa was not dependent on the transglutaminase activity, and did not involve the factor XIIIb-subunits. The adhesion was inhibited by 99% using a combination of monoclonal antibodies directed against integrin vβ3 and β1-containing integrins, and was dependent on Mg2+ or Mn2+. Soluble factor XIIIa also bound to endothelial cells in solution, as detected by flow cytometry. In addition, factor XIIIa inhibited endothelial cell capillary tube formation in fibrin in a dose-dependent manner. Furthermore, the extent of inhibition differed in 2 types of fibrin. The addition of 10 to 100 μg/mL factor XIIIa produced a dose-dependent reduction in capillary tube formation of 60% to 100% in γA/γA fibrin, but only a 10% to 37% decrease in γA/γ′ fibrin. These results show that factor XIIIa supports endothelial cell adhesion in an integrin-dependent manner and inhibits capillary tube formation.
Factor XIIIa-cross-linked fibrin is essential for normal wound healing,1 and is also involved in certain pathologic conditions such as solid tumor growth.2 During both wound repair and neovascularization of tumor stroma, fibrin is formed by thrombin cleavage of fibrinopeptides A and B from fibrinogen, exposing cryptic polymerization sites.3 Fibrinogen is a 340 000-d dimeric protein composed of 2 sets of 3 chains, α, β, and γ,4 and circulates in the plasma in 2 major forms.5,6 The predominant form, γA/γA fibrinogen, has 2 γA chains, whereas the minor form, γA/γ′ fibrinogen, has 1 γA and 1 γ′ chain and comprises 10% to 15% of the total fibrinogen population.7,8 The γ′ chain results from alternative processing of the mRNA,9,10 and differs from γA in that the γA chain carboxy terminal amino acids 408-411 are replaced by an anionic 20 amino acid sequence11 that contains sulfotyrosine residues.12 γ′ chains lack the platelet binding site, γA 400-411, and do not support significant platelet aggregation or adhesion.13-15 γA/γ′ fibrinogen binds noncovalently to the zymogen form of factor XIII.16 In addition, γA/γ′ fibrin clots formed in the presence of active factor XIIIa lyse more slowly than γA/γA clots.17 Plasma factor XIII is a 320 000-d clotting factor composed of 2 a- and 2 b-subunits, and circulates in plasma as an inactive zymogen with the stoichiometry a2b2. Platelet factor XIII, in contrast, consists only of the a2dimer.18,19 After activation of the coagulation cascade, thrombin cleaves the Arg37-Gly38 bond in the a-subunits of factor XIII, and fibrin induces dissociation of the resultant a2′ dimer from the b-subunits in the presence of calcium to form active factor XIIIa.20 Active factor XIIIa is a transglutaminase that covalently cross-links fibrin α and γ chains to form a more stable clot.18 21
In addition to its well-characterized role in coagulation, the factor XIIIa-subunit supports the adhesion of platelets22 and fibroblasts.23 The cellular binding of factor XIIIa may play a role in tissue remodeling after vascular injury, especially in light of the fact that individuals with genetic defects in factor XIII have impaired wound healing.1 The role of the transglutaminase activity of the a-subunit in cell adhesion is a subject of controversy, with some studies indicating that enzymatic activity is necessary for adhesion,24 and other studies indicating that activity is not required.25 The binding of factor XIIIa to platelets is mediated by integrin αIIbβ3 (glycoprotein IIb-IIIa),26 whereas binding to fibroblasts is mediated by integrin αvβ3 and β1-containing integrins.24
Integrin αvβ3 on vascular endothelial cells is believed to play an essential role in angiogenesis.27Although angiogenesis still occurs in mice lacking either the αv28 or β3subunit,29 extensive pharmacologic and biochemical data support a role for αvβ3 in angiogenesis. Monoclonal antibodies and synthetic peptides antagonists directed against αvβ3 inhibit angiogenesis27 and capillary tube formation in fibrin (Dallabrida and Farrell, manuscript submitted). A humanized monoclonal antibody directed against αvβ3, Vitaxin,30 is in clinical trials as an angiogenesis antagonist. Because factor XIIIa binds to αvβ3, and αvβ3is present on vascular endothelial cells,31 32 we investigated the role of factor XIII in angiogenesis. In this report, we demonstrate that immobilized factor XIIIa mediates the adhesion of microvascular endothelial cells in an αvβ3- and β1-dependent manner, and binds to cells in solution as well. Endothelial cells also adhere to active-site inactivated factor XIIIa, but not factor XIIIb. Furthermore, factor XIIIa inhibits endothelial cell capillary tube formation with a different concentration dependence in γA/γA fibrin compared with γA/γ′ fibrin.
Materials and methods
Factor XIII
Factor XIII (Enzyme Research Laboratories, South Bend, IN) was activated in 0.1 mol/L Tris-HCl, pH 8.5, by adding 11.2 mmol/L CaCl2 and 5.7 NIH units/ml (3000 NIH U/mg) α-thrombin (provided by Dr Walter Kisiel, University of New Mexico, Albuquerque, NM) and incubating for 2 hours at 37°C.33 A 0.1 μg/mL of D-Phe-Pro-Arg-chloromethyl ketone (PPACK) (Calbiochem, La Jolla, CA) was added and incubated for 10 minutes to inhibit α-thrombin. In some experiments, factor XIIIa was then inactivated 3 times with 2.2 mmol/L iodoacetamide for 15 minutes at 37°C. Factor XIIIa transglutaminase activity was measured by the incorporation of 5-(biotinamido)pentylamine into N, N′-dimethylcasein (Sigma Chemical Corp, St Louis, MO).33
Factor XIIIb subunits were purified from a factor XIII preparation (provided by Dr Jun Mizuguchi, KAKETSUKEN, Kumamoto, Japan) as described previously.18 Purified factor XIIIb was stored at −70°C in 50 mmol/L Tris-HCl, pH 8.5, 100 mmol/L NaCl, 1 mmol/L EDTA, 10 KIU/mL aprotinin (Bayer, Kankakee, IL)/20% glycerol.
γA/γA and γA/γ′ fibrinogen
Plasminogen-free human fibrinogen (Calbiochem, La Jolla, CA) was further purified using DEAE-cellulose as described previously.7,17 For some assays, factor XIIIa activity was removed from fibrinogen by mild urea treatment as described previously.34 Fibrinogen samples were subjected to electrophoresis on 10% polyacrylamide gels under reducing conditions.35 Fibrinogen preparations were tested for clottability and fibrinolysis as described previously.17 In some experiments, the amount of factor XIIIa-mediated cross-linking in the fibrin clots was assayed using a D-dimer agglutination assay (Sigma) as described previously.17
Cell culture
The human dermal microvascular endothelial cell line HMEC-136 (provided by Dr Edwin W. Ades, Centers for Disease Control and Prevention, Atlanta, GA) was grown in MCDB-131, 10% fetal bovine serum (FBS), 2 mmol/L L-glutamine, 100 U/mL penicillin, 100 μg/mL streptomycin (Life Technologies, Grand Island, NY), 5 ng/mL epidermal growth factor (Collaborative Biomedical Products, Bedford, MA), 1 μg/mL hydrocortisone (Sigma), and cultured at 37°C with 5% CO2.
To prepare factor XIII-deficient medium, a factor XIII immunoaffinity resin was made by reacting goat antihuman factor XIII IgG (American Diagnostica, Greenwich, CT) with Affi-gel 10 (BioRad, Hercules, CA) according to the manufacturer's protocol. DMEM containing 20% FBS was incubated with the resin to remove contaminating factor XIII. Fractions containing less than 10% of the initial factor XIIIa activity were supplemented with 2 mmol/L L-glutamine, 200 KIU/mL aprotinin and sterile-filtered.
Cell adhesion assays
Factor XIII preparations or fibrinogen preparations were coated on microtiter wells at 4°C overnight, then blocked with 2% bovine serum albumin (BSA) (Sigma) in phosphate-buffered saline (PBS; 120 mmol/L NaCl, 2.7 mmol/L KCl, 10 mmol/L sodium phosphate, pH 7.4) (Sigma). In some experiments, fibrinogen was converted to fibrin by adding α-thrombin (1 NIH U/mL) and incubating at 37°C for 30 minutes, then incubating with PPACK (130 μg/mL) for 10 minutes to inhibit α-thrombin. HMEC-1 cells were detached with 0.25% trypsin/1 mmol/L EDTA and suspended in DMEM, 10% FBS, 2 mmol/L L-glutamine. Detached cells were resuspended in serum-free adhesion assay buffer, DMEM, 2 mmol/L L-glutamine, 2% BSA, 10 mmol/L HEPES, pH 7.4, and allowed to adhere to coated wells for 40 minutes at 37°C in 5% CO2. For some assays, anti-integrin antibodies MAB1976 (Chemicon, Temecula, CA) directed against integrin αvβ3 (aka LM609),31 MAB1965 (Chemicon) directed against the β1 integrin subunit,37 or TSI/18 (provided by Dr Edward F. Plow, The Cleveland Clinic, Cleveland, OH) directed against the β2integrin subunit,38 or RGD-based synthetic peptides (Life Technologies) were added to cells for 30 minutes before the adhesion assay. For adhesion assays performed in the presence of different divalent cations, a modification of the procedure of Smith et al39 was followed, in which trypsinized cells were resuspended in 1 mg/mL soybean trypsin inhibitor and assayed for adhesion in Hank's balanced salt solution containing 0.1 mmol/L, 0.5 mmol/L, or 2.0 mmol/L divalent cation. In all assays, adherent cells were fixed with 10% formalin (Sigma), stained with 1% toluidine blue in formalin, solubilized with 2% sodium dodecyl sulfate (SDS) in PBS, and assayed for absorbance at 650 nm. Values were corrected for background binding to BSA-blocked wells.
Flow cytometry
HMEC-1 cells were detached with 5 mmol/L EDTA in PBS, resuspended in adhesion assay buffer, and incubated with factor XIIIa (15 μg/mL) for 30 minutes. Goat antihuman factor XIII antibody (American Diagnostica) was the primary antibody, and the secondary antibody was fluorescein-5-isothiocyanate (FITC)-conjugated rabbit F(ab′)2 fragment to goat IgG (ICN, Aurora, OH). HMEC-1 cells were fixed in 2% paraformaldehyde in PBS, and 10 000 cells per sample were analyzed by flow cytometry with a FACScan (Becton Dickinson).
3-dimensional fibrin-based capillary tube formation assay
The method of Nehls and Drenckhahn40 was modified to use HMEC-1 cells grown on Cytodex-3 microcarriers (Pharmacia) at 37°C with 5% CO2 in complete MCDB-131 medium. Approximately 15 to 20 confluent HMEC-1 cell-coated microcarriers were added per microtiter well to 50 μL sterile-filtered solutions containing 1.5 mg/mL γA/γA or γA/γ′ fibrinogen in PBS with 200 KIU/mL aprotinin and 30 ng/mL bFGF (R & D Systems, Minneapolis, MN). α-thrombin (0.625 NIH U/mL) was immediately added and incubated for 30 minutes to induce fibrin clot formation. Fifty microliters of factor XIII-depleted tube formation assay medium, DMEM, 20% FBS, 2 mmol/L L-glutamine, 200 KIU/mL aprotinin, was added and clots were incubated at 37°C with 5% CO2 for 1 hour. For some experiments, sterile factor XIIIa was included in the assay medium. An additional 50 μL of tube formation assay medium was then added, and plates were incubated at 37°C with 5% CO2 for 2 to 4 days. Cell nuclei were stained with 50 μg/mL bis-benzimide (Sigma). Cell sprouts, defined as projections containing a minimum of 3 nuclei,40 were counted using fluorescence microscopy. Photomicrographs were taken using an Olympus B-Max 50 epifluorescence microscope with a Paultek (Grass Valley, CA) cooled charge-coupled device camera interfaced with a Scion LG3 framestore board.
Results
Endothelial cell adhesion to factor XIIIa does not require transglutaminase activity
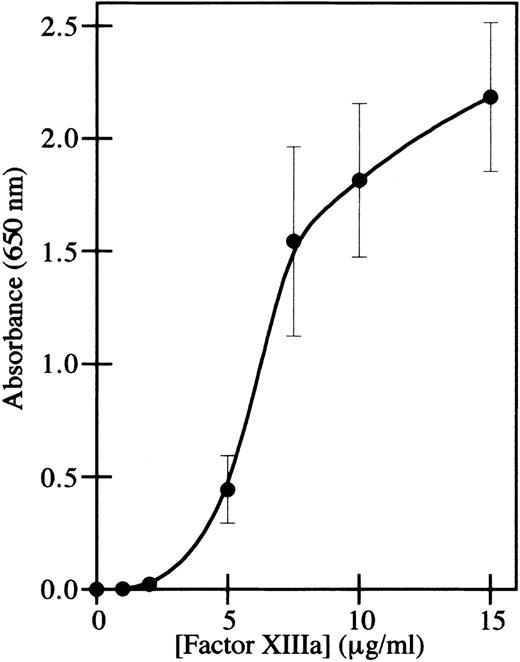
The adhesion of endothelial cells to factor XIIIa was assayed by immobilizing thrombin-activated factor XIIIa on plastic wells and measuring the adhesion of endothelial cells. The human microvascular endothelial cell line HMEC-136 was used for the adhesion assay rather than primary endothelial cells, because these immortalized cells retain the characteristics of microvascular endothelial cells during prolonged culture in vitro and do not dedifferentiate.41 This was a particular concern in order to be able to compare endothelial cell adhesion with endothelial cell capillary tube formation, using an assay in which extended culture of the cells in fibrin was necessary. Adhesion of HMEC-1 cells to factor XIIIa was dose-dependent (Figure 1), similar to the adhesion of fibroblasts and bovine aortic endothelial cells.25 Although thrombin was used to activate factor XIII, and thrombin has an RGD site that can mediate cell adhesion,42 the amount of binding in wells containing only thrombin was less than 1% of the binding to factor XIIIa, and this amount was subtracted from the data points obtained with factor XIIIa.
HMEC-1 cells adhere to factor XIIIa in a dose-dependent manner.
Cell adhesion assays were conducted using immobilized thrombin-activated factor XIIIa. HMEC-1 cells were allowed to attach for 40 minutes at 37°C in 5% CO2 to wells coated with the indicated concentrations of factor XIIIa. Adherent HMEC-1 cells were fixed, stained with toluidine blue, solubilized with 2% SDS, and assayed for absorbance at 650 nm. Background binding to uncoated wells was subtracted from each value. Each point represents the mean of 6 determinations ± SD.
HMEC-1 cells adhere to factor XIIIa in a dose-dependent manner.
Cell adhesion assays were conducted using immobilized thrombin-activated factor XIIIa. HMEC-1 cells were allowed to attach for 40 minutes at 37°C in 5% CO2 to wells coated with the indicated concentrations of factor XIIIa. Adherent HMEC-1 cells were fixed, stained with toluidine blue, solubilized with 2% SDS, and assayed for absorbance at 650 nm. Background binding to uncoated wells was subtracted from each value. Each point represents the mean of 6 determinations ± SD.
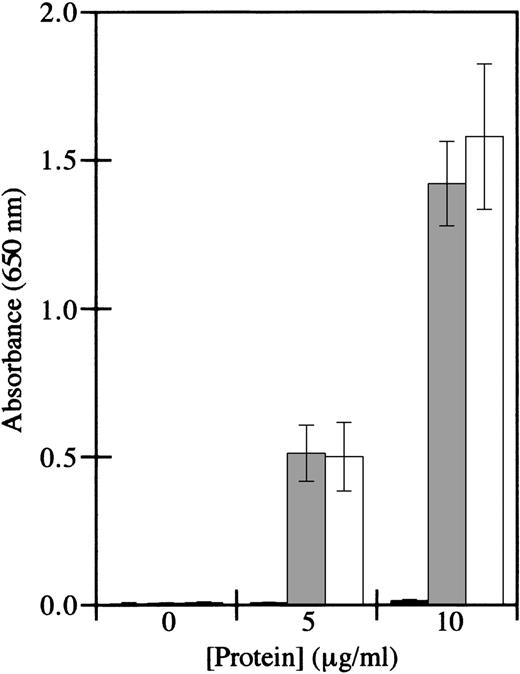
To determine the identity of the factor XIII subunit that mediated binding, factor XIIIb subunits were tested in cell adhesion assays. Factor XIIIb was devoid of adhesive activity, whereas factor XIIIa supported cell adhesion (Figure 2). Furthermore, the transglutaminase activity of factor XIIIa was not required for adhesion, since iodoacetamide-inactivated factor XIIIa supported cell adhesion similarly to active factor XIIIa. These results are consistent with those of Greenberg and Shuman,24 who showed that platelet adhesion to factor XIIIa is not dependent on the transglutaminase activity of factor XIIIa. These results indicate that HMEC-1 adhesion is mediated by factor XIIIa, but not by factor XIIIb, by a mechanism that is independent of enzyme activity.
HMEC-1 cells adhere to iodoacetamide-inactivated factor XIIIa, but not factor XIIIb.
Cell adhesion assays were conducted using immobilized factor XIIIb (black bars), iodoacetamide-inactivated factor XIIIa (gray bars), or thrombin-activated factor XIIIa (white bars). HMEC-1 cells were allowed to attach for 40 minutes at 37°C in 5% CO2 to wells coated with the indicated concentrations of the different factor XIII preparations. Adherent HMEC-1 cells were fixed, stained with toluidine blue, solubilized with 2% SDS, and assayed for absorbance at 650 nm. Background binding to uncoated wells was subtracted from each value. Each bar represents the mean of 6 determinations ± SD.
HMEC-1 cells adhere to iodoacetamide-inactivated factor XIIIa, but not factor XIIIb.
Cell adhesion assays were conducted using immobilized factor XIIIb (black bars), iodoacetamide-inactivated factor XIIIa (gray bars), or thrombin-activated factor XIIIa (white bars). HMEC-1 cells were allowed to attach for 40 minutes at 37°C in 5% CO2 to wells coated with the indicated concentrations of the different factor XIII preparations. Adherent HMEC-1 cells were fixed, stained with toluidine blue, solubilized with 2% SDS, and assayed for absorbance at 650 nm. Background binding to uncoated wells was subtracted from each value. Each bar represents the mean of 6 determinations ± SD.
Endothelial cell adhesion to factor XIIIa is dependent on particular divalent cations and inhibited by antibodies to β1integrins and vβ3
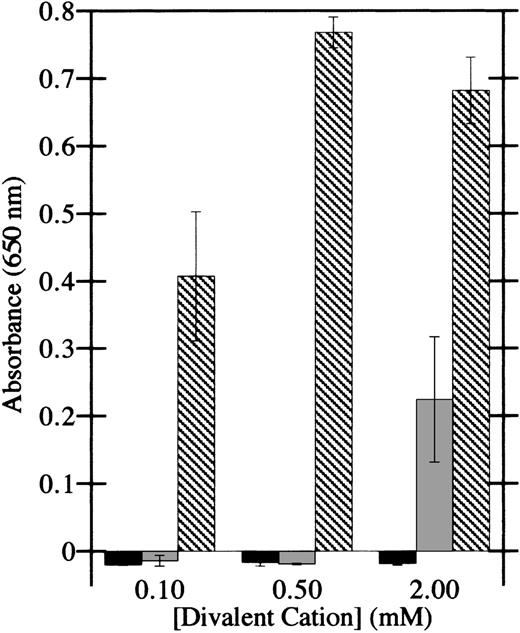
To determine whether integrins are involved in the adhesion of HMEC-1 cells to factor XIIIa, as they are in platelet and fibroblast adhesion to factor XIIIa, the dependence of adhesion on divalent cations was investigated. Ca++ was unable to support adhesion of HMEC-1 cells to factor XIIIa at all doses tested (Figure3), whereas Mg2+ was modestly effective at 2 mmol/L. However, Mn2+ at doses of 100 μmol/L supported significant adhesion of HMEC-1 cells. In addition, Mn2+ induced appreciable cell spreading on factor XIIIa. Together, these results display the hallmarks of integrin-mediated cell adhesion.39
HMEC-1 cell adhesion to factor XIIIa is dependent on particular divalent cations.
Cell adhesion assays were conducted in Hank's balanced salt solution in the presence of the indicated concentrations of CaCl2(black bars), MgCl2 (gray bars), or MnCl2(crosshatched bars). HMEC-1 cells were allowed to attach for 40 minutes at 37°C to wells coated with 15 μg/mL thrombin-activated factor XIIIa. Adherent HMEC-1 cells were fixed, stained with toluidine blue, solubilized with 2% SDS, and assayed for absorbance at 650 nm. Background binding to uncoated wells was subtracted from each value. Each bar represents the mean of 6 determinations ± SD.
HMEC-1 cell adhesion to factor XIIIa is dependent on particular divalent cations.
Cell adhesion assays were conducted in Hank's balanced salt solution in the presence of the indicated concentrations of CaCl2(black bars), MgCl2 (gray bars), or MnCl2(crosshatched bars). HMEC-1 cells were allowed to attach for 40 minutes at 37°C to wells coated with 15 μg/mL thrombin-activated factor XIIIa. Adherent HMEC-1 cells were fixed, stained with toluidine blue, solubilized with 2% SDS, and assayed for absorbance at 650 nm. Background binding to uncoated wells was subtracted from each value. Each bar represents the mean of 6 determinations ± SD.
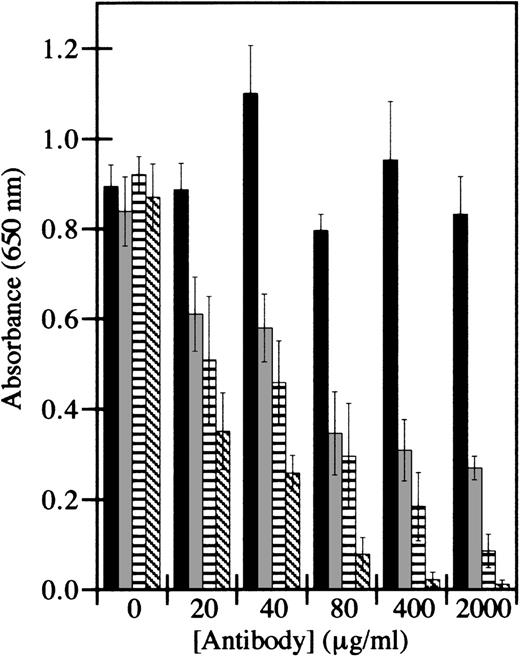
Synthetic peptide integrin antagonists and monoclonal antibodies directed against specific integrins were therefore used to inhibit HMEC-1 adhesion. The RGD motif is an amino acid sequence commonly recognized by integrins.43 However, the maximum inhibition of adhesion by the peptide GRGDSP at 250 μmol/L was only 50%, whereas the control peptide GRGESP had no detectable effect (data not shown). Control antibodies directed against β2-containing integrins, which are not present on HMEC-1 cells,41 did not inhibit HMEC-1 adhesion to factor XIIIa (Figure 4). However, antibodies directed against β1-containing integrins or against αvβ3 inhibited adhesion in a dose-dependent manner. The inhibition by these antibodies was additive, such that the combination of both antibodies inhibited cell adhesion by 99% at the highest doses tested. These results suggest that both β1-containing integrins and αvβ3 are involved in HMEC-1 adhesion to factor XIIIa.
HMEC-1 cell adhesion to factor XIIIa is dependent on β1-containing integrins and integrin vβ3.
Cell adhesion assays were conducted using immobilized thrombin-activated factor XIIIa. HMEC-1 cells were preincubated with the indicated concentration of antibodies directed against β2-containing integrins (black bars), αvβ3 (gray bars), β1-containing integrins (striped bars), or αvβ3 plus β1 (hatched bars) for 30 minutes at 37°C in 5% CO2, and allowed to attach for 40 minutes to wells coated with 7.5 μg/mL factor XIIIa. Adherent HMEC-1 cells were fixed, stained with toluidine blue, solubilized with 2% SDS, and assayed for absorbance at 650 nm. Background binding to uncoated wells was subtracted from each value. Each bar represents the mean of 6 determinations ± SD.
HMEC-1 cell adhesion to factor XIIIa is dependent on β1-containing integrins and integrin vβ3.
Cell adhesion assays were conducted using immobilized thrombin-activated factor XIIIa. HMEC-1 cells were preincubated with the indicated concentration of antibodies directed against β2-containing integrins (black bars), αvβ3 (gray bars), β1-containing integrins (striped bars), or αvβ3 plus β1 (hatched bars) for 30 minutes at 37°C in 5% CO2, and allowed to attach for 40 minutes to wells coated with 7.5 μg/mL factor XIIIa. Adherent HMEC-1 cells were fixed, stained with toluidine blue, solubilized with 2% SDS, and assayed for absorbance at 650 nm. Background binding to uncoated wells was subtracted from each value. Each bar represents the mean of 6 determinations ± SD.
Factor XIIIa binds endothelial cells in solution
Immobilization of proteins on plastic can expose neoepitopes that are not exposed in the native proteins. Therefore, to determine whether soluble factor XIIIa binds to HMEC-1 cells, flow cytometric analysis of factor XIIIa binding was performed. Flow cytometry was conducted in the presence or absence of factor XIIIa, with or without primary antibody (goat antihuman factor XIIIa), and with or without secondary antibody (FITC-conjugated rabbit antigoat IgG F(ab')2). The addition of 15 μg/mL factor XIIIa (Figure5B) did not affect the mean fluorescence intensity compared with cells alone (Figure 5A). The addition of FITC-conjugated secondary antibody increased the mean fluorescence intensity to a similar extent when added alone (Figure 5C) or with primary antibody (Figure 5D) or with factor XIIIa (Figure 5E). However, there was a 4- to 5-fold increase in mean fluorescence intensity with cells incubated with 15 μg/mL factor XIIIa with both primary and secondary antibody (Figure 5F), indicating that factor XIIIa bound to the HMEC-1 cells in solution. Therefore, it seems unlikely that the adhesion of HMEC-1 cells to immobilized factor XIIIa was due to an artifact caused by the immobilization procedure or was mediated by a contaminating protein in the factor XIIIa preparation.
Soluble factor XIIIa binds to HMEC-1 cells.
Flow cytometry was conducted on HMEC-1 cells in the absence of added reagents (A), with 15 μg/mL factor XIIIa (B), with FITC-conjugated rabbit antigoat IgG secondary antibody (C), with goat antihuman factor XIIIa primary antibody plus secondary antibody (D), with factor XIIIa plus secondary antibody (E), or with factor XIIIa plus primary antibody and secondary antibody (F).
Soluble factor XIIIa binds to HMEC-1 cells.
Flow cytometry was conducted on HMEC-1 cells in the absence of added reagents (A), with 15 μg/mL factor XIIIa (B), with FITC-conjugated rabbit antigoat IgG secondary antibody (C), with goat antihuman factor XIIIa primary antibody plus secondary antibody (D), with factor XIIIa plus secondary antibody (E), or with factor XIIIa plus primary antibody and secondary antibody (F).
Factor XIIIa inhibits capillary tube formation in fibrin
To determine the effect of factor XIIIa on endothelial cell capillary tube formation, a 3-dimensional fibrin-based capillary tube formation assay was performed in the presence or absence of factor XIIIa. This assay has been shown to generate lumen-containing capillary-like structures40 with cell-cell junctions (Dallabrida and Farrell, manuscript submitted). Capillary tube formation in this assay is dependent on integrin αvβ3, and is inhibited both by antibodies against αvβ3 and by antisense RNA directed against the β3 subunit (Dallabrida and Farrell, manuscript submitted).
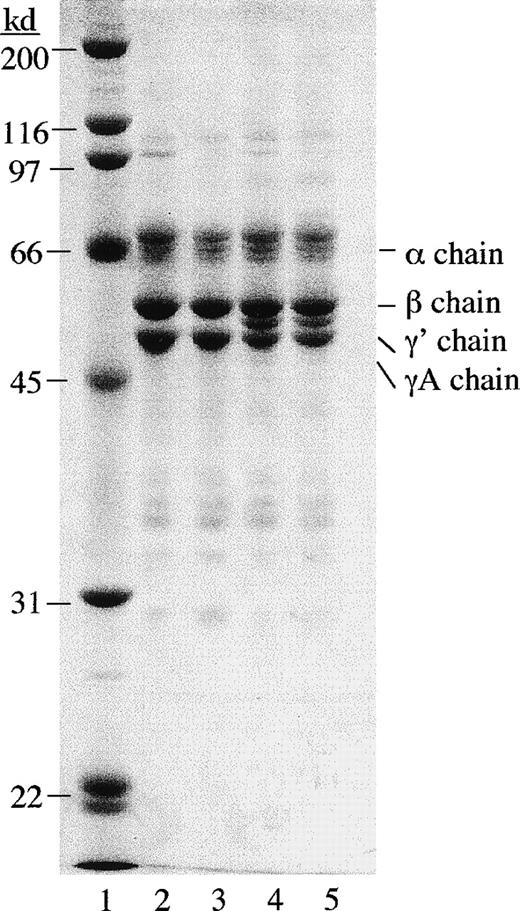
Contaminating factor XIIIa activity was first removed from γA/γA and γA/γ′ fibrinogen using mild urea treatment.34Factor XIIIa activity was also removed from FBS in the cell culture medium using a factor XIII antibody column. Urea-treated γA/γA and γA/γ′ fibrinogen appeared similar to untreated γA/γA and γA/γ′ fibrinogen on polyacrylamide gels under reducing conditions (Figure 6). Urea-treated fibrinogen was tested for clottability and rate of fibrinolysis and found to be indistinguishable from untreated fibrinogen (data not shown).
SDS polyacrylamide gel electrophoresis of urea-treated and untreated γA/γA and γA/γ′ fibrinogen.
Fibrinogen samples (5 μg) were run on a 10% polyacrylamide gel under reducing conditions and stained with Coomassie brilliant blue. Lane 1, molecular weight standards; lane 2, untreated γA/γA fibrinogen; lane 3, urea-treated γA/γA fibrinogen; lane 4, untreated γA/γ′ fibrinogen; and lane 5, urea-treated γA/γ′ fibrinogen.
SDS polyacrylamide gel electrophoresis of urea-treated and untreated γA/γA and γA/γ′ fibrinogen.
Fibrinogen samples (5 μg) were run on a 10% polyacrylamide gel under reducing conditions and stained with Coomassie brilliant blue. Lane 1, molecular weight standards; lane 2, untreated γA/γA fibrinogen; lane 3, urea-treated γA/γA fibrinogen; lane 4, untreated γA/γ′ fibrinogen; and lane 5, urea-treated γA/γ′ fibrinogen.
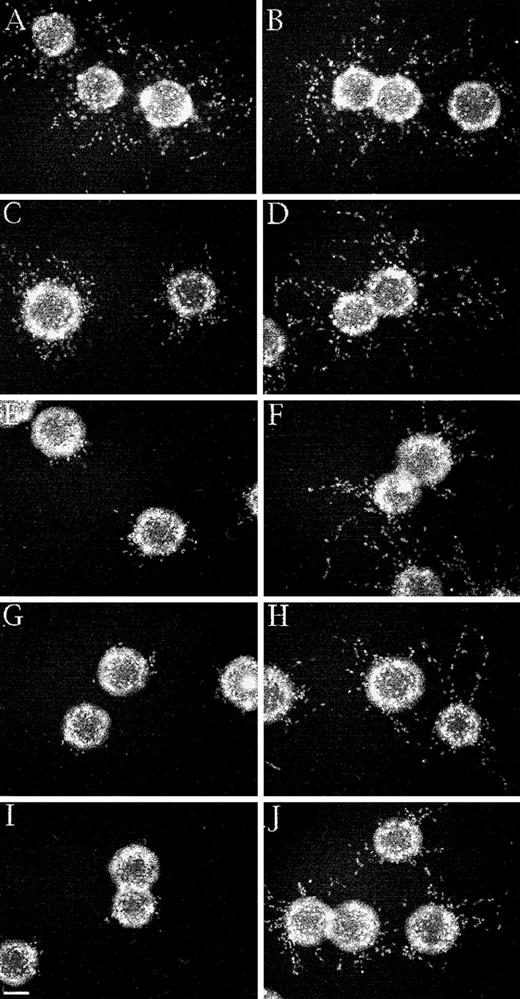
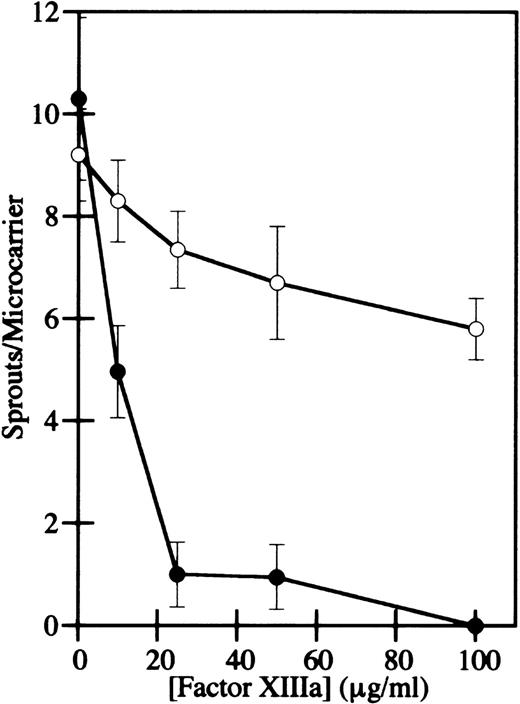
HMEC-1 cells were grown to confluence on microcarrier beads, the beads were added to γA/γA or γA/γ′ urea-treated fibrinogen, and α-thrombin was added to induce clot formation. After 2 to 4 days of culture in the presence of bFGF, HMEC-1 cell nuclei were stained with bis-benzimide and photographed. Capillary-like sprouts, defined as projections containing a minimum of 3 nuclei,40 were counted using fluorescence microscopy. In the absence of factor XIIIa activity, HMEC-1 cells migrated and formed sprouts in γA/γA and γA/γ′ fibrin similarly (Figure 7A and B), indicating that factor XIIIa is not required for capillary tube formation. When factor XIIIa was added to the cells after clotting, both the number and length of HMEC-1 cell sprouts decreased in a dose-dependent manner in both γA/γA (Figure 7C, E, G, and I) and γA/γ′ fibrin (Figure 7D, F, H, and J). However, the inhibition of sprout formation occurred at much lower factor XIIIa concentrations in γA/γA fibrin than in γA/γ′ fibrin. At 10 to 100 μg/mL factor XIIIa, sprouting was reduced 60% to 100% in γA/γA fibrin, but only 10% to 37% in γA/γ′ fibrin (Figure 8). Of particular note, physiological concentrations of factor XIIIa, 10 to 15 μg/mL, significantly inhibited sprout formation in γA/γA fibrin. The differential inhibition of capillary tube formation in γA/γA compared with γA/γ′ fibrin is unlikely to be due to increased factor XIIIa-mediated cross-linking in γA/γA fibrin, because greater cross-linking occurs in γA/γ′ fibrin.17In addition, the difference did not appear to be due to differences in adhesion of HMEC-1 cells to γA/γA fibrin compared with γA/γ′ fibrin, because HMEC-1 cells adhered similarly to each form of fibrin (data not shown). It should be noted that human umbilical vein endothelial cells also adhere similarly to recombinant γA/γA fibrinogen and γ′/γ′ fibrinogen.44 These results demonstrate that factor XIIIa inhibits endothelial cell capillary tube formation in fibrin, an effect that is more pronounced in γA/γA fibrin.
Differential inhibition of capillary tube formation by factor XIIIa in γA/γA and γA/γ′ fibrin.
A 3-dimensional fibrin-based capillary tube formation assay was performed using either γA/γA fibrin (A, C, E, G, and I) or γA/γ′ fibrin (B, D, F, H, and J). Nuclei were stained with bis-benzimide after 2 days and photographed. HMEC-1 cells in γA/γA (A) and γA/γ′ (B) fibrin without factor XIIIa showed numerous capillary-like sprouts. The addition of 10 μg/mL factor XIIIa (C, D), 25 μg/mL (E, F), 50 μg/mL (G, H), and 100 μg/mL (I, J) inhibited sprouting significantly in γA/γA fibrin (C, E, G, I), but only partially in γA/γ′ fibrin (D, F, H, J). Bar, 100 μm.
Differential inhibition of capillary tube formation by factor XIIIa in γA/γA and γA/γ′ fibrin.
A 3-dimensional fibrin-based capillary tube formation assay was performed using either γA/γA fibrin (A, C, E, G, and I) or γA/γ′ fibrin (B, D, F, H, and J). Nuclei were stained with bis-benzimide after 2 days and photographed. HMEC-1 cells in γA/γA (A) and γA/γ′ (B) fibrin without factor XIIIa showed numerous capillary-like sprouts. The addition of 10 μg/mL factor XIIIa (C, D), 25 μg/mL (E, F), 50 μg/mL (G, H), and 100 μg/mL (I, J) inhibited sprouting significantly in γA/γA fibrin (C, E, G, I), but only partially in γA/γ′ fibrin (D, F, H, J). Bar, 100 μm.
Concentration dependence of HMEC-1 capillary tube inhibition by factor XIIIa in γA/γA and γA/γ′ fibrin.
The number of sprouts per microcarrier for HMEC-1 cells in γA/γA (filled circles) or γA/γ′ fibrin (open circles) was determined. Cell sprouts were defined as projections containing a minimum of 3 nuclei. Each point represents the mean of triplicate determinations ± SD.
Concentration dependence of HMEC-1 capillary tube inhibition by factor XIIIa in γA/γA and γA/γ′ fibrin.
The number of sprouts per microcarrier for HMEC-1 cells in γA/γA (filled circles) or γA/γ′ fibrin (open circles) was determined. Cell sprouts were defined as projections containing a minimum of 3 nuclei. Each point represents the mean of triplicate determinations ± SD.
Discussion
The multiple activities of factor XIIIa as a mediator of cell adhesion and inhibitor of capillary tube formation extend the biologic role of factor XIIIa beyond its traditional role as a coagulation factor. The results show that endothelial cell adhesion is dependent on the a-subunit of factor XIII, but not the active site. These results are similar to those found for platelets, in which thrombin cleavage and Ca+2-induced conformational changes in factor XIIIa are required for adhesion,25 and in which the cell binding site is distinct from the catalytic active site of factor XIIIa.22 In contrast, these results differ from those found for fibroblasts, in which factor XIIIa transglutaminase activity is required for adhesion.24
However, 1 common feature of factor XIIIa adhesion to endothelial cells, fibroblasts, and platelets is the involvement of integrins in the adhesion process. Previous studies have also shown that factor XIIIa interacts with cells in an integrin-dependent manner.24,26,45 Integrin-mediated interactions between factor XIIIa and endothelial cells take on added significance because of the involvement of integrins in both normal and pathologic angiogenesis.27,46-48 The a-subunit does not contain a canonical integrin-binding RGD site, but does contain the sequence LDV near its carboxy terminus.49 This amino acid sequence mediates the binding of fibronectin to integrin α4β1,50 and may possibly play a role in cell adhesion to factor XIIIa. The LDV residues are solvent-exposed at opposite ends of the thrombin-activated factor XIII a2 dimer,51 raising the possibility that the LDV sequence may indeed be accessible to integrins.
The reason for the differential effect of factor XIIIa on capillary tube formation in γA/γA compared with γA/γ′ fibrin is unknown. γA/γ′ fibrinogen binds noncovalently to zymogen factor XIII,16 which leads to more rapid activation of factor XIII (Moaddel, Falls, and Farrell, manuscript in preparation), more rapid fibrin cross-linking by factor XIIIa, and increased resistance to fibrinolysis in γA/γ′ fibrin.17However, it is not clear if any of these effects are responsible for the inhibition of capillary tube formation by factor XIIIa. Paradoxically, γA/γ′ fibrin is more highly cross-linked than γA/γA fibrin, and has increased clot stability.17 Therefore, the decrease in endothelial cell capillary tube formation in γA/γA fibrin relative to γA/γ′ fibrin cannot be attributed to increased fibrin cross-linking. One possibility is that factor XIII is sequestered in γA/γ′ fibrin by binding to the γ′ chain site, making it unavailable for cell binding to integrins.
An implication of these findings is that high concentrations of factor XIIIa at a wound site may actually impede revascularization at that site. Physiologic concentrations of factor XIIIa significantly inhibited capillary tube formation in our assay using γA/γA fibrin, the predominant form of fibrin in human plasma. This effect may be even more pronounced in platelet-rich clots, where the release of platelet factor XIII results in high local concentrations of factor XIIIa.52 One possible physiologic consequence of this is that angiogenesis may be inhibited in a fibrin clot until fibrinolytic enzymes or passive diffusion clears the clot of coagulation factors. Such a mechanism may even contribute to the temporal orchestration of wound healing. Given the involvement of fibrinolytic factors53 and antithrombin III54 in angiogenesis, coagulation factors may also play a significant role in modulating angiogenesis.
Acknowledgments
We thank Dr Steven W. Levison (Pennsylvania State University, Hershey, PA) for the use of his fluorescence microscopy equipment. We gratefully acknowledge Dr Edwin W. Ades for providing the HMEC-1 cell line, Dr Walter Kisiel for providing α-thrombin, Dr Jun Mizuguchi for providing factor XIII, and Dr Edward F. Plow for providing the β2 integrin antibody TSI/18.
Supported in part by Student Awards from the American Heart Association, Pennsylvania Affiliate (S.M.D. and L.A.F.), Grant-in-Aid S98695P from the American Heart Association, Pennsylvania Affiliate (D.H.F.), and grant R29HL53997 from the National Institutes of Health (D.H.F.).
Reprints:David H. Farrell, Department of Oral Molecular Biology, School of Dentistry, Oregon Health Sciences University, 611 SW Campus Dr, Portland, OR 97201; e-mail: farrelld@ohsu.edu.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal