Abstract
Perforin is known to display a membranolytic activity on tumor cells. Nevertheless, perforin release during natural killer (NK)–cell activation is not sufficient to induce membrane target-cell damage. On the basis of the ability of perforin to interact with phospholipids containing a choline phosphate headgroup, we identify the platelet-activating factor (PAF) and its membrane receptor as crucial components in tumor cell killing activity of human resting NK cells. We demonstrate for the first time that upon activation, naive NK cells release the choline phosphate–containing lysolipid PAF, which binds to perforin and acts as an agonist on perforin-induced membrane damage. PAF is known to incorporate cell membranes using a specific receptor. Here we show that interferon-γ (IFN–γ) secreted from activated NK cells ends in PAF-receptor expression on perforin-sensitive K562 cells but not on perforin-resistant Daudi cells. In order to prove the capacity of PAF to interact simultaneously with its membrane PAF receptor and with perforin, we successfully co-purified the 3 components in the presence of bridging PAF molecules. The functional activity of this complex was further examined. The aim was to determine whether membrane PAF-receptor expression on tumor cells, driven to express this receptor, could render them sensitive to the perforin lytic pathway. The results confirmed that transfection of the PAF-receptor complementary DNA into major histocompatibility complex class I and Fas-receptor negative tumor cells restored susceptibility to naive NK cells and perforin attack. Failure of IFN-γ to induce membrane PAF receptor constitutes the first described mechanism for tumor cells to resist the perforin lytic pathway.
Natural killer (NK) cells are a distinct subpopulation of lymphocytes that play an important role in natural immunity to tumor cells. Resting or naive NK cells are capable of killing target cells without requiring prior activation or sensitization. On the basis of the ability of NK cells to kill tumors lacking major histocompatibility complex (MHC) class I, the identification of killer cell inhibitory receptors CD94-NKG2 of the C-type lectin-like family and those belonging to the immunoglobulin superfamily and of a recently identified novel set of phosphorylated polypeptides termed killer-activating-receptor–associated proteins have considerably clarified the regulation of NK-cell activation.1-4 Upon interaction with their targets, NK effector cells release their granule protein content, essentially perforin and granzymes, into the intercellular space. The perforin-dependent granule exocytosis pathway proved to represent the main lytic pathway in tests that compared perforin knocked-out mice with wild-type littermates.5-10In addition, studies performed in perforin-deficient mice emphasized the role of perforin in control of tumor growth through NK and T-cell cytotoxicity.11,12 Perforin exerts its homeostatic role either by induced target-cell necrosis13-16 or by relocalization of granzyme B to its cytoplasmic and nuclear substrates,17-21 a phenomenon responsible for induced target-cell apoptosis.22,23
Perforin is a phospholipid-binding protein that can disrupt the membrane of mammalian cells. Perforin-induced membrane damage is related to successful binding and insertion into the lipid bilayer, ending in pore formation. Uellner and coworkers24 showed that the removal of carbohydrates from the C-terminus of perforin allowed its C2 phospholipid-binding domain to bind Ca++ and to initiate interactions with the negatively charged target-cell surface. Since perforin is primarily hydrophilic in nature, increasing hydrophobic interactions between perforin and the membrane phospholipid bilayer is required for protein insertion.25-27 Several studies on the lipid dependence of perforin interaction with target-cell membranes have been reported in the literature.27-31 It appears that both the lipid sidechain composition, which is predicted to alter the membrane fluidity,30,31 and the phospholipid headgroup identity could influence perforin lytic activity.27-29 In the presence of Ca++, perforin was indeed shown to be capable of binding directly to various lipid molecules, provided a phosphorylcholine headgroup was present.29 These results were in agreement with 2 other studies,27,28 which demonstrated that choline phosphate–containing lipids represented powerful ligands for perforin. In contrast, cephaline-containing phospholipids (phosphatidylethanolamine and phosphatidylserine) were virtually ineffective in perforin binding.28
Here we show that, upon activation, resting human NK cells release not only perforin but also choline phosphate–containing lysolipids, among which is the platelet-activating factor (PAF). The significance of this finding remained unknown. We suspected that PAF could affect NK-cell activity as a direct ligand for the lytic protein perforin. Moreover, it is well established that PAF molecules act through a specific cell membrane choline receptor, the PAF receptor, which has been cloned.32-34 In this study, we explored whether PAF and its specific receptor, when expressed on target-cell membranes, could enhance NK-cell perforin-mediated cytotoxicity. The results clearly demonstrated that, in the presence of extracellular Ca++ and PAF, perforin was able to initiate tumor-cell membrane damage, provided that target PAF-receptor membrane expression could be induced via IFN-γ, an essential cytokine released after NK-cell activation.35 Our present results indicate that the lipid mediator PAF and its receptor are crucially involved in the cytolytic function of resting NK cells. They further suggest that target failure to express membrane PAF receptor could constitute a mechanism for tumor cells to resist the perforin lytic pathway.
Materials and methods
Assay for perforin membranolytic activity
Perforin cytotoxicity was followed by means of a 4-hour51Cr-release assay. The targets we used, K562 and Daudi cells, were negative for Fas receptor (CD95) expression by fluorescence-activated cell sorting (FACS) analysis (data not shown). These cells have previously been shown to undergo perforin-induced acute necrosis with the use of NK cells as effectors.36 As already described, 51Cr-release assays were performed.37 The effector-target–cell suspensions were incubated at 37°C in 5% C02 in the presence or absence of defined concentrations of PAF (1-0-octadecyl-2-0-acetyl-sn-glycero-3-phosphocholine) C16:0 alkyl moieties (10−10 to 10−5 mol/L), supplied by France Biochem. The PAF-receptor antagonist WEB 2086 or SR 27417 was added into the assays at a concentration of 2 μmol/L.38-40 In experiments using transfected PAF-receptor–expressing Daudi cells as targets, the cell suspensions were incubated with and without the PAF-receptor antagonist WEB 2086 (2 μmol/L).
Preparation of human natural killer effector cells
Human NK cells were purified as shown previously.37Adult peripheral blood mononuclear cells were prepared by standard Ficoll-Hypaque procedures. After 1 hour of adherence to plastic at 37°C in 5% CO2, nonadherent cells were loaded on a discontinuous Percoll gradient (Pharmacia Fine Chemicals, Uppsala, Sweden) and centrifuged for 30 minutes at 500g. Cells were recovered from the low-density fraction and purified for NK cells by lysis in the presence of anti-CD3 and anti-CD19 hybridomas at 1:100 dilution of an ascite fluid and rabbit complement (Fillorga Laboratories, Paris, France). On purification, the cell population was analyzed by flow cytometry using anti-CD16 (fluorescein isothiocyanate [FITC]–labeled), anti-CD56 (FITC-labeled), and anti-CD3ε (FITC-labeled) monoclonal antibodies (mAbs) from Becton Dickinson Immunocytometry Systems, Inc (San José, CA). NK cells were isolated to greater than 93% purity. Freshly isolated, purified human NK cells were tested for cytolytic activity in 51Cr-release assays in cell culture medium in which fetal calf serum (FCS) was replaced by bovine serum albumin (BSA) (2 mg/mL). These cells were referred to as resting or naive NK cells.
Release by natural killer cells of platelet-activating factor, perforin, and interferon-γ
Naive NK cells were activated with the use of 2 × 106 K562 and Daudi hematopoietic tumoral cells, in an E-to-T ratio of 50:1, in duplicate. PAF assay and characterization were performed as already described.41Briefly, PAF released from NK cells were ethanol-extracted from supernatants at different points of NK/target-cell incubation. After centrifugation (1500g, 20 minutes), the ethanolic supernatants were dried, recovered in 50 μL of 60% ethanol, and stored at −20°C until assayed. PAF activity was measured by platelet aggregation of washed rabbit platelets. Aspirin-treated washed rabbit platelets were stirred in 300 μL Tyrode buffer containing 0.25% gelatin, 1 mmol/L creatine phosphate, and 10 U/mL creatine phosphokinase (pH 7.4). Aggregating activity of the samples was measured by means of a calibration curve obtained with 2.5-20 pg of synthetic C18:0 PAF. Results were expressed in picograms of PAF as the mean of duplicate samples. The NK-cell–secreted lipid material was further characterized as PAF on the basis of studies on the aggregating activity in the presence of 0.1 mmol/L BN 52021 or CV 3988, 2 specific PAF-receptor antagonists, and on the retention time during thin-layer chromatography analysis as shown.42
The capacity of the target K562 and Daudi cells to induce from freshly isolated human NK cells the release of granule protein content into the intercellular space was examined as already described.43 At determined times of NK/target-cell incubation, the supernatants were harvested (50 μL), and the secreted BLT-esterase activity was measured. Each sample (20 μL) was incubated in enzyme-linked immunosorbent assay (ELISA) plates with 200 μL of BLT-esterase substrate: 1.5 mg BLT (N-carbobenzoxy L-lysine-thiobenzyl ester) (Sigma) and 1.5 mg of 5.5′-dithio-bis-(2-nitrobenzoic acid)/20 mL (Tris)–HCl 0.2 mol/L, pH 8 (Sigma). After 1 hour of incubation at 37°C, absorption was measured at 450 nm. The spontaneous BLT release was determined by incubating NK cells in medium alone. The maximum release was determined by adding 50 μL of 10% NP-40 to 100 μL of NK-cell suspension. The percentage of specific BLT-esterase activity was calculated as follows: ([experimental − spontaneous]/[maximum − spontaneous]) × 100, where experimental is experimental BLT-esterase release (A450); spontaneous is spontaneous BLT-esterase release (A450); and maximum is maximum BLT-esterase release (A450).
At relevant times of NK/target-cell incubation, IFN-γ release from NK cells into the supernatants (50 μL) was also determined. The high sensitive IFN-γ human ELISA system (Biotrak, Amersham) was used for the IFN-γ assays. Control tests received either targets or effectors only.
Target-cell interferon-γ activation
Target K562 and Daudi cells were first prepared for flow cytometry analysis of membrane PAF-receptor expression before and after 10 and 30 minutes of NK-cell contact into the NK assays. K562 cells expressing glycophorin A and Daudi cells expressing the CD19 antigen were selected in a flow cytometry analysis (FASCan, Becton Dickinson) with the use of the appropriate mAbs (FITC-labeled anti–glycophorin A [Immunotech, Marseilles, France]; FITC-labeled anti-CD19 [Becton Dickinson Immunocytometry Systems, Inc) and were then analyzed for PAF-receptor expression. On the other hand, 1 × 106 K562 and Daudi cells were incubated with different concentrations of IFN-γ (Protech Inc, Rocky Hill, NJ) (50 to 500 U/mL) for various times (30 minutes and 1, 2, and 4 hours), washed, and analyzed for PAF-receptor membrane expression by flow cytometry with the use of an anti–human PAF-receptor mAb (Cayman Chemical Co, MI). The myelomonocytic cell line U937 was used as a positive control of PAF-receptor expression. The neutralizing anti–IFN-γ antibody named MAS 290 was used for blocking at a concentration of 25 ng/mL and was purchased from Valbiotech (Paris, France). Mouse normal pooled sera were used in blocking experiments as isotype control.
Binding assays of [3H]platelet-activating factor to perforin
Antiperforin 6.4 (immunoglobulin [Ig]M, antiperforin) mAb (PharmaCell, Paris, France) coupling to Affi-Gel 10 gel (Affi-Gel 10 gel, Bio-Rad Laboratories) was first performed. The gel was washed with 20 vol of cold 10 mmol/L sodium acetate, pH 4.5. Then, 1 mL of the gel was mixed with 0.5 mL of antiperforin mAb (0.1 mol/L MOPS, pH 7.5, containing 6.3 mg antiperforin) and was sufficiently agitated to give a uniform suspension. The gel was gently shaken for 12 hours at 4°C. The coupling reaction was halted by adding 0.1 mol/L ethanolamine-HCl (pH 8) for 4 hours at 4°C. Perforin was then purified by specific immunoprecipitation from the human NK-cell line YT2C2. These cells (3.6 × 106) were lysed with 1 mL cold lysis buffer (10 mmol/L Hepes-NaOH, pH 7.4, containing 25 mmol/L KCl, 5 mmol/L EDTA, 1 mmol/L DTT, and 0.5% NP-40) at 4°C for 1 minute. The cytoplasmic cell lysate was mixed with the antiperforin coupling gel in a coupling buffer: 10 mmol/L Tris-HCl, pH 8.0, containing 0.5 mol/L NaCl, 5 mmol/L EDTA, 0.2 mmol/L ABESF (4-2-aminoethyl-benzene sulfonyl fluoride), 0.5% NP-40, and 0.02% NaN3 which was gently agitated for 4 hours at 4°C. The gel was washed with phosphate-buffered saline (PBS) (20 mmol/L NaH2PO4, pH 7.0, containing 150 mmol/L NaCl, 5 mmol/L KCl, 5 mmol/L MgCl2, 1 mmol/L CaCl2, 6 mmol/L glucose, and 0.25% BSA). The experiments were further carried out by the specific binding of [3H] octadecyl-9,10-PAF C18:0 (141.6 Ci/mmol) (purchased from Dupont de Nemours Division NEN) to 50 μL perforin-antibody gel in a total volume of 300 μL PBS containing 20 nmol/L [3H]PAF. The mixture was incubated at 20°C for 30 minutes. The binding gel was then washed 5 times with the cold buffer mentioned above, and the radioactivity was measured by liquid scintillation counting after gel incubation with 0.1 mol/L NaOH. Specific binding was determined as the total radioactivity bound minus the radioactivity bound in the presence of unlabeled PAF C18:0 from France Biochem. The perforin-negative Jurkat cell line was used as control under the same conditions.
Coimmunoprecipitation of the platelet-activating factor receptor, platelet-activating factor, and perforin as a ternary complex
Biotinylation of membrane cell surface proteins of IFN-γ–induced PAF-receptor–positive K562 cells was first carried out. Cells were washed 3 times with ice-cold PBS (pH 8.0), resuspended at a concentration of 25 × 106 cells/mL in PBS in which 0.5 mg of Sulfo-NHS-LC-Biotin (Pierce, Rockford, IL) was added per mL of reaction volume, incubated during 2 hours and washed 3 times with ice-cold PBS. In order to purify cell membranes, the biotinylated cells were resuspended at 107 cells/mL in ice-cold PBS containing protease inhibitors; they were equilibrated at 0°C in a nitrogen cavitation bomb for 1 minute; nuclei were removed by centrifugation at 800 rpm at 4°C for 8 minutes; and the membrane fraction was obtained by centrifugation at 14 × 103rpm at 4°C for 1 hour. The anti–PAF-receptor mAb (Cayman Chemical) was covalently cross-linked with Protein G Sepharose (Pharmacia), as previously shown.44 Membrane proteins were lysed at 4°C for 45 minutes with the following buffer: 20 mmol/L Hepes, pH 7.5, containing 150 mmol/L NaCl, 5 mmol/L MgCl2, 1 mmol/L CaCl2, 1 mmol/L DTT, 1 mmol/L AEBSF, and 0.5% NP-40. The anti–PAF-receptor sepharose was mixed with biotinylated K562 membrane proteins and incubated at 4°C for 12 hours. The beads were washed 3 times with the same ice-cold buffer containing 1 mol/L NaCl. PAF-receptor immunoprecipitated beads were then washed 2 times in binding buffer (Hepes 10 mmol/L, pH 7.4, containing 137 mmol/L NaCl, 2.6 mmol/L KCl, 6 mmol/L glucose, 1.3 mmol/L CaCl2, 1 mmol/L MgCl2, 0.02% NaN3 and 0.25% BSA).
Immunopurified PAF-receptor microspheres (100 μL) were incubated with 20 nmol/L [3H]PAF (1.4 mL) at 20°C for 1 hour. After this binding step, beads were washed 3 times with the same ice-cold buffer. They were then incubated with the perforin-positive NK-cell YT2C2 cytoplasmic fraction in the same binding buffer at 20°C for 1 hour and then washed 3 times with the washing buffer (Hepes 10 mmol/L, pH 7.4, containing 137 mmol/L NaCl, 2.6 mmol/L KCl, 6 mmol/L glucose, 1.3 mmol/L CaCl2, 1 mmol/L MgCl2, and 0.02% NaN3). Proteins were eluted with the use of a buffer of 20 mmol/L Tris, pH 6.8, containing 4% sodium dodecyl sulfate (SDS) and 20% glycerol, at 95°C for 5 minutes. Then 30 μL of elution proteins were incubated with 10 μL of Laemmli electrophoresis buffer at 95°C for 5 minutes to Western blot analysis. Western blotting was performed by loading the proteins on 10% SDS–polyacrylamide gel electrophoresis. The human antiperforin (antiperforin, perforin A2 2d4.2.a8.E11, kindly provided by G. M. Griffiths, Sir William Dunn School of Pathology, South Parks Rd, Oxford, UK) was added at 1/50 final dilution for 1 hour, and then washed and detected with a sheep antimouse IgG peroxydase conjugate (1/2000 final dilution for 1 hour; Amersham, Arlington Heights, IL), as previously described.45 PAF-receptor proteins were tested by streptavidin immunoblotting. The binding of [3H]PAF was analyzed by radioactive controls. U937 PAF-receptor positive membrane biotinylated fractions were used as positive controls whereas PAF-receptor negative Jurkat cell line membrane biotinylated fractions and perforin-negative cell lysates from the same cell line were used as negative controls under the same conditions.
Daudi cell transfections
The EcoR1 insert of the plasmid pBluescript vector32containing the total coding region of the human PAF-receptor complementary DNA (cDNA) (1.8 Kb) was subcloned into the EcoR1 site of the pCI-neo vector (Promega), in both sense (pCI-neo PAF-receptor sense) and antisense (pCI-neo PAF-receptor antisense) orientations. Wild-type Daudi cells, purchased from ATCC, were maintained in RPMI 1640 with 10% FCS, 2 mmol/L glutamine, penicillin (100 U/mL), and streptomycin (100 μg/mL). Daudi cells (1 × 107) were electroporated (Gene Pulser, Bio-Rad) at 250 V and a capacitance of 960 microfarads in the presence of the above-mentioned expression vectors (10 μg) and with a plasmid expressing no gene (pCI-neo). Membrane PAF-receptor expression was analyzed by flow cytometry (FASCan, Becton Dickinson) with the use of an anti–PAF-receptor mAb (Cayman Chemical). At 4 days after electroporation, membrane PAF-receptor expression was maximal at the surface of transfected Daudi cells (data not shown). Wild-type and transfected Daudi cells were used as targets in short-term51Cr-release assays with naive NK cells used as effectors.
Results
Natural-killer–cell lytic activity is enhanced by the phospholipid platelet-activating factor
Addressing the question of tumor cell susceptibility to perforin-mediated lysis in humans, we took advantage of a model in which K562 and Daudi cells, both of which are MHC class I and Fas-receptor (FasR) negative, were shown to induce activation of resting human NK cells, degranulation, and perforin release.43 The kinetic of degranulation was analyzed and shown to take place 5 minutes after incubation of freshly isolated NK effectors with K562 targets (Table 1). A maximum release (90%), as measured by the percentage of BLT-esterase activity, occurred at that time. With the use of Daudi cells as targets, a similar ability to induce a granule protein content release from NK cells was clearly observed, as shown in Table 1. However, if NK-cell activation and degranulation ended up in 51Cr release from K562 cells, it did not result in Daudi cell lysis (Table1).
Release by NK cells of PAF, perforin, and IFN-γ compared with PAF receptor expression on targets and 51Cr release
| Time . | 0 Min . | 5 Min . | 10 Min . | 30 Min . | 60 Min . | 120 Min . | 180 Min . | 240 Min . |
|---|---|---|---|---|---|---|---|---|
| PAF release (pg) | ||||||||
| NK + K562 | 0 | 0 | 0 | 175 | 87.5 | 0 | — | 0 |
| NK + Daudi | 0 | 0 | 62.5 | 250 | 0 | 0 | — | 0 |
| Percentage of BLT esterase | ||||||||
| NK + K562 | 0 | 98 | 54 | 50 | 47 | 45 | 45 | 49 |
| NK + Daudi | 0 | 78 | 44 | 42 | 42 | 38 | 41 | 42 |
| IFN-γ release (pg) | ||||||||
| NK + K562 | 0 | 715 | 645 | 708 | 733 | 730 | — | 780 |
| NK + Daudi | 0 | 716 | 707 | 721 | 727 | 729 | — | 714 |
| Percentage of target PAF-receptor expression | ||||||||
| NK + K562 | 0 | — | 33 | 23 | — | — | — | — |
| NK + Daudi | 0 | — | 0 | 1 | — | — | — | — |
| Percentage of 51Cr release | ||||||||
| NK + K562 | — | — | 5 | 5 | 11 | 21 | 30 | 29 |
| NK + Daudi | — | — | 0 | 0 | 0 | 4 | 6 | 4 |
| Time . | 0 Min . | 5 Min . | 10 Min . | 30 Min . | 60 Min . | 120 Min . | 180 Min . | 240 Min . |
|---|---|---|---|---|---|---|---|---|
| PAF release (pg) | ||||||||
| NK + K562 | 0 | 0 | 0 | 175 | 87.5 | 0 | — | 0 |
| NK + Daudi | 0 | 0 | 62.5 | 250 | 0 | 0 | — | 0 |
| Percentage of BLT esterase | ||||||||
| NK + K562 | 0 | 98 | 54 | 50 | 47 | 45 | 45 | 49 |
| NK + Daudi | 0 | 78 | 44 | 42 | 42 | 38 | 41 | 42 |
| IFN-γ release (pg) | ||||||||
| NK + K562 | 0 | 715 | 645 | 708 | 733 | 730 | — | 780 |
| NK + Daudi | 0 | 716 | 707 | 721 | 727 | 729 | — | 714 |
| Percentage of target PAF-receptor expression | ||||||||
| NK + K562 | 0 | — | 33 | 23 | — | — | — | — |
| NK + Daudi | 0 | — | 0 | 1 | — | — | — | — |
| Percentage of 51Cr release | ||||||||
| NK + K562 | — | — | 5 | 5 | 11 | 21 | 30 | 29 |
| NK + Daudi | — | — | 0 | 0 | 0 | 4 | 6 | 4 |
The capacity of naive NK cells to secrete the phospholipid PAF during the NK/target-cell contact was analyzed. NK effector cells released the significant amount of 62.5 pg of PAF after 10 minutes of interaction with Daudi cells, whereas a maximal value of 250 pg of PAF was found at 30 minutes (Table 1). Then the lipid mediator became undetectable. We observed that the kinetic of PAF release from NK cells when incubated with K562 targets was slightly delayed, compared with the use of Daudi cells as activators, with a maximal value of 175 pg obtained at 30 minutes but with the same general kinetic profile of PAF metabolism (Table 1).
We subsequently explored whether PAF addition could overcome Daudi cell resistance to NK-cell–dependent lysis. To answer this question, we used resting NK cells and targets in 51Cr-release assays to which exogenous PAF (C16:0 alkyl moieties) was added. The concomitant increase in extracellular concentration of C16:0 PAF completely failed to affect Daudi cell lysis (Figure 1a). In contrast, K562 cells displayed, after addition of C16:0 PAF, a significantly higher susceptibility to membrane damage, as demonstrated by an increase of 22 ± 2% of 51Cr target-cell release (Figure 1A). These results demonstrated an agonistic effect of PAF in the lytic pathway on NK perforin-sensitive target cells.
PAF and perforin-mediated NK target-cell lysis.
(A) The phospholipid PAF enhances perforin-mediated NK target-cell lysis. Resting NK cells were used in standard cytotoxic assays against the MHC class I and FasR-negative K562 and Daudi cells, both of which were shown to be capable of inducing NK-cell activation and degranulation. Perforin-induced cell lysis was measured by target-cell release of sodium-51Cr–labeled cytoplasmic proteins via membrane damage and perforin pores. Resting human NK cells were mixed with labeled target cells at the various E-to-T ratios of 50:1, 25:1, 12:1, and 6:1, and target-cell lysis was measured at 4 hours. Naive NK cells were very efficient in provoking 51Cr release from K562 targets. Following addition of PAF to targets (PAF C16:0 alkyl moieties, [1 μmol/L]), K562 cells displayed a higher susceptibility to the lytic activity of perforin, as reflected by an increase in the amount of 51Cr target-cell release. In contrast, increasing extracellular concentrations of C16:0 PAF turned out to be inefficient in overcoming the failure of naive NK cells to induce Daudi cell lysis. (B) Inhibition of the PAF receptor. PAF receptor activity on K562 target cells was inhibited either by blocking its IFN-γ–induced expression using a neutralizing anti–IFN-γ antibody or by adding specific PAF receptor antagonists, such as WEB 2086 and SR 27417. Resting NK cells were used as effectors. These processes led to a potent inhibitory activity on NK-cell lysis, as shown by a significant decrease in the amount of NK/target-cell51Cr release.
PAF and perforin-mediated NK target-cell lysis.
(A) The phospholipid PAF enhances perforin-mediated NK target-cell lysis. Resting NK cells were used in standard cytotoxic assays against the MHC class I and FasR-negative K562 and Daudi cells, both of which were shown to be capable of inducing NK-cell activation and degranulation. Perforin-induced cell lysis was measured by target-cell release of sodium-51Cr–labeled cytoplasmic proteins via membrane damage and perforin pores. Resting human NK cells were mixed with labeled target cells at the various E-to-T ratios of 50:1, 25:1, 12:1, and 6:1, and target-cell lysis was measured at 4 hours. Naive NK cells were very efficient in provoking 51Cr release from K562 targets. Following addition of PAF to targets (PAF C16:0 alkyl moieties, [1 μmol/L]), K562 cells displayed a higher susceptibility to the lytic activity of perforin, as reflected by an increase in the amount of 51Cr target-cell release. In contrast, increasing extracellular concentrations of C16:0 PAF turned out to be inefficient in overcoming the failure of naive NK cells to induce Daudi cell lysis. (B) Inhibition of the PAF receptor. PAF receptor activity on K562 target cells was inhibited either by blocking its IFN-γ–induced expression using a neutralizing anti–IFN-γ antibody or by adding specific PAF receptor antagonists, such as WEB 2086 and SR 27417. Resting NK cells were used as effectors. These processes led to a potent inhibitory activity on NK-cell lysis, as shown by a significant decrease in the amount of NK/target-cell51Cr release.
Perforin purified by immunoprecipitation efficiently binds to the phospholipid platelet-activating factor
These results led us to examine the capacity of human purified perforin to bind to the phospholipid mediator PAF. [3H]PAF C18:0 was exposed to immunopurified perforin, as shown in “Materials and methods,” and the protein was then counted for [3H] incorporation. As shown in Figure2, perforin efficiently bound to PAF in the presence of Ca++ and Mg2+, as demonstrated by the detection of a 20 × 103disintegration-per-minute radioactivity from perforin after [3H]PAF coincubation (n = 3). Unlabeled PAF competitively inhibited the binding of [3H]PAF, with 50% inhibition observed by mixing 1 vol C18:0 PAF with 1 vol C18:0 [3H]PAF at 20°C (data not shown). There was no [3H]PAF binding in the control tests (the perforin-negative Jurkat cell line cytoplasmic extracts, the antiperforin mAb alone, the gel itself, and BSA) (Figure 2).
Purified human perforin efficiently binds to PAF.
Perforin was immunopurified from the perforin-positive YT2C2 NK-cell line using an appropriate binding gel (antiperforin 6.4-IgM, antiperforin mAb, coupled with Affi-Gel 10 gel). (A) Successful purification of perforin was controlled by a Western blot using the specific antiperforin antibody 2d4-perf. In lane C, for YT cell line extracts as positive control, the procedure revealed 2 bands at the molecular weight of 66 and 30-kd as recently reported by Uellner et al.24 Perforin was further purified by ultracentrifugation to obtain the active form of 66-kd (Uellner et al24), expressed in lane 1. No other band was detected in lanes 2, 3, 4, and 5 using, respectively, the perforin-negative Jurkat cell line lysate, the gel cross-linked to the mAb, the gel alone, or the gel with an irrelevant protein BSA instead of perforin. Active perforin was then exposed to [3H]PAF C18:0 during 30 minutes at 20°C and washed 5 times with the cold buffer as shown in “Materials and methods.” The resulting radioactivity was then measured for perforin. (B) In this assay, lane 1 shows the formation of perforin-[3H]PAF complexes expressed in disintegrations per minute. In contrast, no significant radioactivity was detected in lanes 2, 3, 4, and 5 using, respectively, the perforin-negative Jurkat cell line lysate, the gel cross-linked to the mAb, the gel alone, or the gel with an irrelevant protein BSA instead of perforin as already mentioned for Western blot analysis.
Purified human perforin efficiently binds to PAF.
Perforin was immunopurified from the perforin-positive YT2C2 NK-cell line using an appropriate binding gel (antiperforin 6.4-IgM, antiperforin mAb, coupled with Affi-Gel 10 gel). (A) Successful purification of perforin was controlled by a Western blot using the specific antiperforin antibody 2d4-perf. In lane C, for YT cell line extracts as positive control, the procedure revealed 2 bands at the molecular weight of 66 and 30-kd as recently reported by Uellner et al.24 Perforin was further purified by ultracentrifugation to obtain the active form of 66-kd (Uellner et al24), expressed in lane 1. No other band was detected in lanes 2, 3, 4, and 5 using, respectively, the perforin-negative Jurkat cell line lysate, the gel cross-linked to the mAb, the gel alone, or the gel with an irrelevant protein BSA instead of perforin. Active perforin was then exposed to [3H]PAF C18:0 during 30 minutes at 20°C and washed 5 times with the cold buffer as shown in “Materials and methods.” The resulting radioactivity was then measured for perforin. (B) In this assay, lane 1 shows the formation of perforin-[3H]PAF complexes expressed in disintegrations per minute. In contrast, no significant radioactivity was detected in lanes 2, 3, 4, and 5 using, respectively, the perforin-negative Jurkat cell line lysate, the gel cross-linked to the mAb, the gel alone, or the gel with an irrelevant protein BSA instead of perforin as already mentioned for Western blot analysis.
Interferon-γ induced membrane platelet-activating factor–receptor expression on K562 cells but not on Daudi cells
PAF molecules bind and incorporate cell membranes using a specific receptor that has been cloned.33 34 We determined whether PAF could enhance NK-cell lytic activity through efficient membrane binding of perforin/PAF complexes to the PAF receptor. We analyzed PAF-receptor expression on K562 and Daudi targets, but neither group of cells had a detectable level of membrane PAF receptor (Figure3).
IFN-γ induces K562 cells to rapidly express membrane PAF receptors.
K562 and Daudi cells were stimulated with 300 U/mL of human IFN-γ for 30 minutes and for 1, 2, and 4 hours and were analyzed by flow cytometry for PAF receptor membrane expression. U937 cells were used as positive controls. The background signal is shown (dotted lines). Inset numbers indicate the percentage of cells expressing the protein and the mean fluoresence intensity at the indicated times. Regarding the capacity of tumor cells to express membrane PAF receptor, K562 cells were IFN-γ sensitive; Daudi cells, IFN-γ resistant.
IFN-γ induces K562 cells to rapidly express membrane PAF receptors.
K562 and Daudi cells were stimulated with 300 U/mL of human IFN-γ for 30 minutes and for 1, 2, and 4 hours and were analyzed by flow cytometry for PAF receptor membrane expression. U937 cells were used as positive controls. The background signal is shown (dotted lines). Inset numbers indicate the percentage of cells expressing the protein and the mean fluoresence intensity at the indicated times. Regarding the capacity of tumor cells to express membrane PAF receptor, K562 cells were IFN-γ sensitive; Daudi cells, IFN-γ resistant.
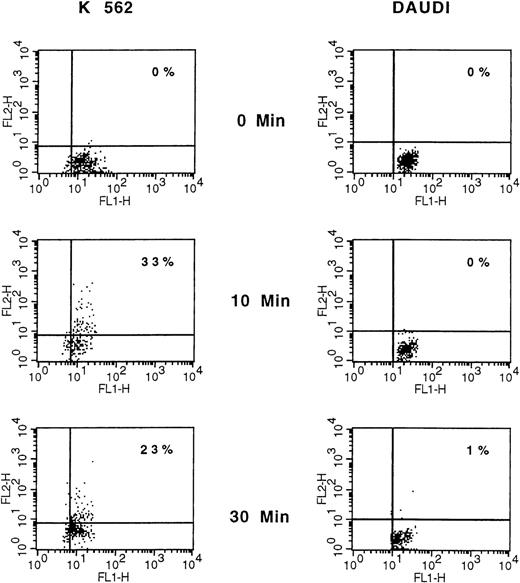
We subsequently explored whether membrane PAF-receptor expression could be induced on targets by coincubated NK cells. The expression of PAF receptors on K562 and Daudi target cells was measured at 3 times (0, 10, and 30 minutes), using flow cytometry analysis and appropriate double-fluorescence labeling (PAF receptor and glycophorin A for K562 cells; CD19 for Daudi cells). However, owing to a rapid and expected NK-mediated induced–target-cell lysis, only a limited number of double-labeled and viable target cells could be analyzed (ie, 5.102). In such conditions, 33% of K562 target cells were clearly found to express membrane PAF receptors at 10 minutes (Table 1 and Figure 4). In contrast, there were no Daudi PAF-receptor–positive cells in the NK assays at the same time and thereafter (Table 1, Figure4).
Comparative analysis of membrane PAF-receptor expression on K562 and Daudi cells.
A representative experiment is shown. K562 and Daudi cells were analyzed before and after 10 and 30 minutes of NK cell attack, stained with anti–PAF-receptor mAb detected with phosphatidylethanolamine (PE)–goat antimouse antibody and with FITC-conjugated antiglycophorin (K562) or anti-CD19 (Daudi). Data are displayed as dot plots, and PAF-receptor expression was analyzed only on glycophorin (K562) and CD19 (Daudi) positive cells. Only a limited number of double-labeled and viable target cells could be analyzed (ie, 5.102), owing to the expected rapid NK-mediated induced–target-cell lysis. Cells that are double-stained with corresponding PE- and FITC-conjugated antibodies are represented in the upper right quadrant.
Comparative analysis of membrane PAF-receptor expression on K562 and Daudi cells.
A representative experiment is shown. K562 and Daudi cells were analyzed before and after 10 and 30 minutes of NK cell attack, stained with anti–PAF-receptor mAb detected with phosphatidylethanolamine (PE)–goat antimouse antibody and with FITC-conjugated antiglycophorin (K562) or anti-CD19 (Daudi). Data are displayed as dot plots, and PAF-receptor expression was analyzed only on glycophorin (K562) and CD19 (Daudi) positive cells. Only a limited number of double-labeled and viable target cells could be analyzed (ie, 5.102), owing to the expected rapid NK-mediated induced–target-cell lysis. Cells that are double-stained with corresponding PE- and FITC-conjugated antibodies are represented in the upper right quadrant.
Among the cytokines produced by activated NK cells, we hypothesized that IFN-γ could be a reliable candidate for the induction of membrane PAF-receptor expression on targets. Following a 2-hour treatment by IFN-γ (300 U/mL), we observed a large increase in the percentage of membrane positive PAF-receptor K562 cells (73.3 ± 7%) (Figure 3). This increase persisted after 4 hours. In contrast, IFN-γ turned out to be ineffective in inducing Daudi cells to express the receptor (Figure 3), even when the cells were further exposed to the cytokine for 16 hours (data not shown). The next step was to further confirm the secretion of IFN-γ by NK cells during their interaction with targets. After a 5-minute interval of NK interaction with both target-cell lines, 715 pg of IFN-γ were indeed found. The amount of released IFN-γ remained virtually constant during the 4 hours of NK/target incubation, with a slight decrease at 10 minutes (Table 1).
In order to provide further insights into the role of PAF receptor on NK-cell lytic activity, we studied the effect of a neutralizing anti–IFN-γ antibody (25 ng/mL) and of different PAF-receptor antagonists (2 μmol/L) on lysis of K562 targets by resting NK cells. Inhibition of the IFN-γ pathway led to a 50 ± 5% reduction of NK-cell killing activity. This effect was not seen when an irrelevant antibody was used (Figure 1B). We also observed that addition in the K562 assays of the PAF-receptor antagonists, WEB 2086 and SR 27417, was followed by a potent inhibitory activity on NK-cell lysis as shown by a decrease of, respectively, 50 ± 7% and 72 ± 5% in the amount of NK target-cell 51Cr release (Figure 1B). Such a reduced, but not totally abolished, NK-cell cytotoxicity could be explained by either an incomplete membrane PAF-receptor saturation or ligation. Through the induction of PAF-receptor expression on target cells, IFN-γ is therefore involved in the successful perforin lytic pathway induced by naive NK cells.
Perforin, platelet-activating factor, and platelet-activating factor receptor form a ternary complex
We immunopurified the PAF receptor from biotinylated membranes of IFN-γ–treated K562 cells. This receptor was then exposed, in the presence of Ca++ and Mg2+, to [3H]PAF C18:0 and to perforin present in a YT2C2 NK-cell–line lysate. Radioactivity and perforin detection within the ternary complex were then analyzed as appropriate, as shown in “Materials and methods.” The successful in vitro constitution of a ternary complex was demonstrated by the presence of the 3 components, PAF receptor, [3H]PAF, and perforin, in the same elution fraction. The PAF-receptor protein was detected in the complex as a 42-kd band using stavidin immunoblotting (Figure5A). Western blot analysis also revealed the presence of a 66-kd band equivalent to active perforin, as reported by Uellner et al,24 using the same specific antiperforin antibody 2d4-perf (Figure 5B). Finally, the presence of [3H]PAF in the complex was demonstrated by the concomitant detection of a 6 × 103 cpm radioactivity, as shown in column 2 of Figure 5. In contrast, purified PAF receptors failed to bind to perforin in the absence of bridging PAF molecules (Figure 5). These data proved the capacity of PAF to interact simultaneously, in these experimental conditions, with its receptor and the perforin protein.
Perforin, PAF, and PAF receptor form a ternary complex.
PAF receptors were immunopurified from the biotinylated membrane of IFN-γ–treated K562 cells with the use of the specific anti–PAF-receptor mAb. Purified receptors were incubated with or without C18:0 [3H]PAF in the presence of Ca++and Mg2+ and then exposed to YT2C2 cell cytoplasmic extracts for perforin-binding analysis. To confirm the presence of the 3 components (PAF receptor, PAF, perforin) in the same elution fraction, Western blot analysis using, respectively, anti-PAF receptor (A1), antiperforin (A2) mAbs, and [3H]PAF radioactivity detection (B) were performed. This procedure allowed simultaneous detection of PAF receptor at the molecular weight (mw) of 42-kd (A1, lane 2); perforin at the mw of 66 kd (A2, lane 2); and radioactivity due to the presence of [3H]PAF in the complex (B, column 2). In the absence of [3H]PAF (B, column 3), PAF receptor was still detected (A1, lane 3) but perforin failed to bind to the receptor (A2, lane 3). In the absence of PAF receptors (Jurkat cell membrane extracts, A1, lane 4), [3H]PAF (B, column 4) and perforin (A2, lane 4) failed to form the ternary complex. The YT2C2 cell line was used as negative control for PAF receptor expression (A1, lane 1), as positive control for perforin expression (A2, lane1), and as negative control for [3H]PAF binding (B, column 1).
Perforin, PAF, and PAF receptor form a ternary complex.
PAF receptors were immunopurified from the biotinylated membrane of IFN-γ–treated K562 cells with the use of the specific anti–PAF-receptor mAb. Purified receptors were incubated with or without C18:0 [3H]PAF in the presence of Ca++and Mg2+ and then exposed to YT2C2 cell cytoplasmic extracts for perforin-binding analysis. To confirm the presence of the 3 components (PAF receptor, PAF, perforin) in the same elution fraction, Western blot analysis using, respectively, anti-PAF receptor (A1), antiperforin (A2) mAbs, and [3H]PAF radioactivity detection (B) were performed. This procedure allowed simultaneous detection of PAF receptor at the molecular weight (mw) of 42-kd (A1, lane 2); perforin at the mw of 66 kd (A2, lane 2); and radioactivity due to the presence of [3H]PAF in the complex (B, column 2). In the absence of [3H]PAF (B, column 3), PAF receptor was still detected (A1, lane 3) but perforin failed to bind to the receptor (A2, lane 3). In the absence of PAF receptors (Jurkat cell membrane extracts, A1, lane 4), [3H]PAF (B, column 4) and perforin (A2, lane 4) failed to form the ternary complex. The YT2C2 cell line was used as negative control for PAF receptor expression (A1, lane 1), as positive control for perforin expression (A2, lane1), and as negative control for [3H]PAF binding (B, column 1).
Platelet-activating-factor–receptor complementary DNA transfection into Daudi cells restores susceptibility to naive natural-killer-cell–induced lysis
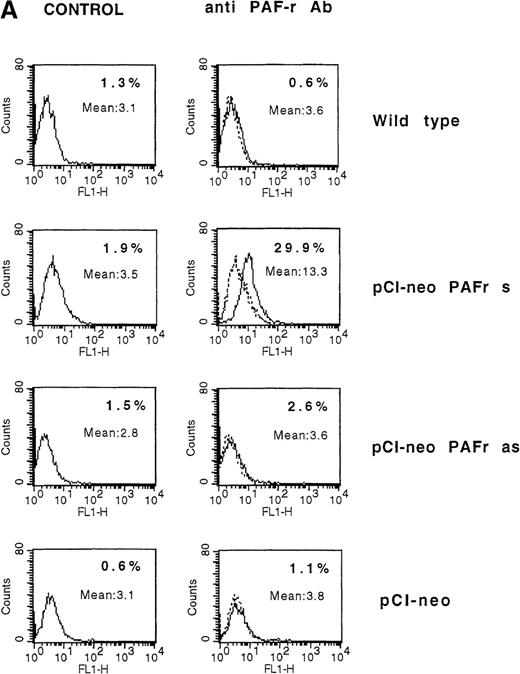
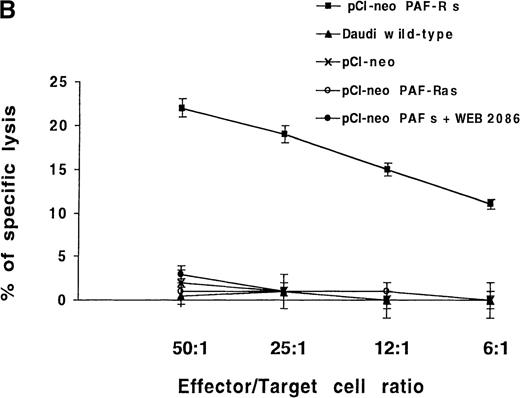
The observed failure of NK cells to end in membrane PAF-receptor expression on Daudi targets, via IFN-γ secretion, could explain the tumor-cell resistance to the perforin lytic pathway. To establish that the absence of PAF receptor on Daudi cells was responsible for their resistance to the NK-cell perforin lytic activity, we transiently transfected Daudi cells with the human PAF-receptor cDNA. This led to PAF-receptor membrane expression on 30 ± 5% of cells, as measured by flow cytometry analysis 4 days after transfection (Figure6A). The transfected cells were used as targets in 4-hour 51Cr-release assays using resting, purified human NK cells as effectors. Five successive experiments showed that an efficient NK-induced target-cell 51Cr release (20 ± 3%) was repeatedly observed when PAF-receptor–expressing Daudi cells were used as targets (Figure 6B). Moreover, the ability of NK cells to induce a cytolytic activity in PAF-receptor–positive Daudi cells was totally reversed by the addition of the PAF-receptor antagonist WEB 2086 into the lytic assays (Figure6B). Such an observed complete inhibition of perforin-mediated lysis via WEB addition was likely due to the low level of PAF-receptor expression obtained on transfected Daudi cells. We conclude that PAF-receptor expression on Daudi cells was necessary and sufficient to restore NK-cell susceptibility to the perforin-dependent lytic pathway.
(A) Daudi cells transfected with the PAF-receptor cDNA express membrane PAF receptors. Wild-type Daudi cells were transfected by the plasmid pCI-neo vectors expressing the sense PAF receptor cDNA gene (pCI-neo.PAF-R s), the PAF receptor antisense cDNA gene (pCI-neo. PAF-R as), and by a plasmid expressing no gene (pCI-neo) control vector. At 4 days later, PAF receptor membrane expression was quantified by flow cytometry using an mAb anti-PAF receptor on nonpermeabilized Daudi transfected cells (solid lines). Cells were stained with propidium iodide to facilitate discrimination among live and dead cells. The background signal is shown (dotted lines). Inset numbers indicate the percentage of cells expressing the PAF receptor on untransfected Daudi cells (wild-type), Daudi cells transfected with pCI-neo PAF receptor sense gene (pCI-neo PAF-R s), Daudi cells transfected with the pCI-neo PAF receptor antisense gene (pCI-neo PAF-R as), and Daudi cells transfected with pCI-neo. (B) PAF-receptor cDNA transfection in the MHC class I and FasR-negative NK-resistant Daudi cells restores susceptibility to NK perforin-dependent lysis. Wild-type Daudi cells and PAF-receptor–positive (pCI-neo PAF-R s), PAF-receptor–negative (pCI-neo PAF-R as and pCI-neo plasmids) transfected Daudi cells were used independently as targets in short-term 51Cr-release assays using naive NK cells as effectors. PAF-receptor–positive Daudi cell susceptibility to NK cells was tested with and without a PAF-receptor antagonist, WEB 2086, added at 2 μmol/L. Resting NK cells were mixed with labeled target cells at the E-to-T ratios of 50:1, 25:1, 12:1, and 6:1, and perforin-induced Daudi cell lysis was measured at 4 hours. Naive NK cells were totally unable to lyse PAF-receptor–negative Daudi cells (wild-type and PAF-receptor–negative transfectants) but were efficient in killing PAF-receptor–positive Daudi cells. The susceptibility of PAF-receptor–expressing Daudi cells to NK lysis was totally reversed in the presence of the PAF receptor antagonist (pCI-neo PAF-R s + WEB).
(A) Daudi cells transfected with the PAF-receptor cDNA express membrane PAF receptors. Wild-type Daudi cells were transfected by the plasmid pCI-neo vectors expressing the sense PAF receptor cDNA gene (pCI-neo.PAF-R s), the PAF receptor antisense cDNA gene (pCI-neo. PAF-R as), and by a plasmid expressing no gene (pCI-neo) control vector. At 4 days later, PAF receptor membrane expression was quantified by flow cytometry using an mAb anti-PAF receptor on nonpermeabilized Daudi transfected cells (solid lines). Cells were stained with propidium iodide to facilitate discrimination among live and dead cells. The background signal is shown (dotted lines). Inset numbers indicate the percentage of cells expressing the PAF receptor on untransfected Daudi cells (wild-type), Daudi cells transfected with pCI-neo PAF receptor sense gene (pCI-neo PAF-R s), Daudi cells transfected with the pCI-neo PAF receptor antisense gene (pCI-neo PAF-R as), and Daudi cells transfected with pCI-neo. (B) PAF-receptor cDNA transfection in the MHC class I and FasR-negative NK-resistant Daudi cells restores susceptibility to NK perforin-dependent lysis. Wild-type Daudi cells and PAF-receptor–positive (pCI-neo PAF-R s), PAF-receptor–negative (pCI-neo PAF-R as and pCI-neo plasmids) transfected Daudi cells were used independently as targets in short-term 51Cr-release assays using naive NK cells as effectors. PAF-receptor–positive Daudi cell susceptibility to NK cells was tested with and without a PAF-receptor antagonist, WEB 2086, added at 2 μmol/L. Resting NK cells were mixed with labeled target cells at the E-to-T ratios of 50:1, 25:1, 12:1, and 6:1, and perforin-induced Daudi cell lysis was measured at 4 hours. Naive NK cells were totally unable to lyse PAF-receptor–negative Daudi cells (wild-type and PAF-receptor–negative transfectants) but were efficient in killing PAF-receptor–positive Daudi cells. The susceptibility of PAF-receptor–expressing Daudi cells to NK lysis was totally reversed in the presence of the PAF receptor antagonist (pCI-neo PAF-R s + WEB).
Discussion
Perforin and granzymes, which belong to the granule exocytosis lytic pathway, are used by cytotoxic T lymphocytes (CTLs) and NK cells in acquired and innate immunity.14 However, CTLs as well as NK cells can use Fas ligand–induced cytotoxicity, provided that targets express the membrane Fas receptor.36,46 Granzyme B is involved in target-cell death apoptosis, while perforin is responsible for target-cell membrane damage as measured by 51Cr release. Even if the mechanisms by which both proteins might interact are still under investigation,17,18,20 the production of perforin-deficient mice has established beyond any doubt the powerful role of the protein perforin in tumor growth control.11,12Moreover, a recent study performed in double granzyme A and B knocked-out mice has clearly indicated that target-cell membrane damage leading to cell death was independent of granzymes and related solely to perforin cytolytic activity.10
Naive NK cells primarily use Fas-independent and perforin-based cytotoxicity.47 In a previous study, using naive human NK cells as effectors and Fas-receptor negative targets, we showed that NK-cell lytic activity was totally abolished in the presence of 0.75 mmol/L EGTA, further confirming a perforin-induced Ca++-dependent cytolysis.43 In addition, dependent on the hematopoietic tumor target cell, NK-cell activation and perforin release were insufficient to regularly determine successful target-cell lysis.43 This observation suggested that a structure present at the surface of some but not all tumoral targets could be critical in facilitating perforin-mediated target-cell lysis induced by resting NK cells.
Perforin lytic activity has been shown to be dependent on the presence of phosphorylcholines acting as specific Ca++-dependent receptors for the protein.29 We here demonstrated a concomitant release by NK cells, upon activation by tumor cells, of lytic proteins and of PAF choline phosphate–containing molecules. This observation raised the question of the potential facilitating effect of the NK-cell–secreted phospholipid mediator on perforin lytic activity. Purified human perforin was indeed shown to efficiently bind to the phospholipid PAF. Furthermore, our present results identified PAF and its specific membrane receptor as a critical component in the lytic activity of naive NK cells. The major argument comes from the observation that transfection of the PAF-receptor cDNA into NK-resistant MHC class I and FasR negative Daudi cells confers susceptibility to NK-cell–induced perforin-mediated lysis.
It has been demonstrated that a larger amount of perforin binds perforin-resistant cell membranes following treatment with tunicamycin, an inhibitor for N-glycosylation.48 This observation suggests that the protective cell surface layer, which contains a large carbohydrate content, could prevent perforin from efficient binding to membrane targets. On the other hand, hydrophobic interactions between perforin and phospholipids in target-cell membranes are known to represent essential requirements for protein insertion and pore formation.25-27 In this context, phosphorylcholines represent suitable ligands for the protein.27-29 As perforin is primarily a hydrophilic protein, the role of the phosphocholine PAF as a facilitating partner in inducing a perforin conformational switch from a hydrophilic state to a membrane-inserted hydrophobic form remains to be demonstrated. Whatever the role of PAF on perforin function, our present study favors the use by tumor cells of PAF receptors to increase the concentration of phosphorylcholines at membrane-binding sites, through PAF recruitment. This process seems to enhance perforin-induced target-cell pore formation. The successful constitution we obtained in vitro of a tri-molecular complex, consisting of perforin, PAF, and its receptor, strongly supports such a facilitating role of the bridging PAF molecules in perforin function. We are aware that perforin can determine membrane damage without the presence of PAF, as shown by the capacity of the protein to induce lysis of sheep red blood cells. However, the matrix composition of erythrocytes, which contain in their membranes 23% of phosphorylcholines31 and lack a protective cell surface layer,49 is likely to allow direct access and facile pore formation by perforin. Finally, lack of PAF-receptor detection on erythrocyte membranes could explain the observed inhibition of perforin-induced hemolysis when choline-phosphate lysolipids are added into the red blood cell assays.28
In addition to perforin and PAF, IFN-γ is also released by activated NK cells. This cytokine has been implicated in tumor-cell cytotoxicity, but the mechanism(s) of such a target-cell activity remained undetermined. Here we showed that IFN-γ was capable of inducing membrane PAF-receptor expression in NK-sensitive K562 cells, but not in NK-resistant Daudi cells. The observed failure of NK cells to achieve PAF-receptor membrane expression on Daudi targets, through IFN-γ secretion, could explain tumor-cell resistance to the perforin lytic pathway. Whatever the strategy used by Daudi cells to down-regulate PAF-receptor membrane expression, it allowed tumor cells to escape the immune response. PAF-receptor expression variability has already been reported among hematopoietic tumoral cells,50,51 suggesting that this process could constitute a more general phenomenon of NK-cell resistance. A role of both IFN-γ and perforin in the suppression of tumor growth has been described in mice52; this is in agreement with our present in vitro data using human cells.
In conclusion, we showed that a tumor cell could escape perforin attack by preventing efficient perforin insertion into its cell membrane. We propose PAF and its receptor as facilitating partners in the perforin lytic pathway as these elements confer target-cell susceptibility to cytolytic activity induced by naive NK cells.
Acknowledgments
We are very grateful to Claude Gazin for constructing the expression vectors with the PAF-receptor cDNA. We thank Eva Ninio for helpful suggestions concerning the ligation of perforin to [3H]PAF and the use of PAF-receptor antagonists (WEB 2086 and SR 27417) that she kindly provided.
Supported by grants from ARC, La Ligue Nationale contre le Cancer (Laboratoire associe n°10, Comite de Paris).
Reprints:Marilyne Sasportes, INSERM U462, Hôpital Saint-Louis, 1 Avenue Claude Vellefaux, 75475 Paris Cedex 10, France; e-mail: msasportes@chu-stlouis.fr.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

![Fig. 1. PAF and perforin-mediated NK target-cell lysis. / (A) The phospholipid PAF enhances perforin-mediated NK target-cell lysis. Resting NK cells were used in standard cytotoxic assays against the MHC class I and FasR-negative K562 and Daudi cells, both of which were shown to be capable of inducing NK-cell activation and degranulation. Perforin-induced cell lysis was measured by target-cell release of sodium-51Cr–labeled cytoplasmic proteins via membrane damage and perforin pores. Resting human NK cells were mixed with labeled target cells at the various E-to-T ratios of 50:1, 25:1, 12:1, and 6:1, and target-cell lysis was measured at 4 hours. Naive NK cells were very efficient in provoking 51Cr release from K562 targets. Following addition of PAF to targets (PAF C16:0 alkyl moieties, [1 μmol/L]), K562 cells displayed a higher susceptibility to the lytic activity of perforin, as reflected by an increase in the amount of 51Cr target-cell release. In contrast, increasing extracellular concentrations of C16:0 PAF turned out to be inefficient in overcoming the failure of naive NK cells to induce Daudi cell lysis. (B) Inhibition of the PAF receptor. PAF receptor activity on K562 target cells was inhibited either by blocking its IFN-γ–induced expression using a neutralizing anti–IFN-γ antibody or by adding specific PAF receptor antagonists, such as WEB 2086 and SR 27417. Resting NK cells were used as effectors. These processes led to a potent inhibitory activity on NK-cell lysis, as shown by a significant decrease in the amount of NK/target-cell51Cr release.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/95/7/10.1182_blood.v95.7.2329/5/m_bloo00734001ax.jpeg?Expires=1765943338&Signature=LY51cXfIV9hVWc~SiEj7ZE7cBCnR3VolqWOf67lRn3r1bpB4rE1Dg3GyW4exHzl5ri-fU5Zxesei~VV4FBX6AE9eZi6GBbwj5fjmvbYK4FgPg7C2ptX52i0bKKUfL5hrTip-PREvgW-oe~voFYRS1sTYMEueagQ9WJ6KIL0aIQgYXdCAH0PZ0XprIKpanUP7EqGsMO2Q1r92uoudq-mxPpauocoHX5XIfTkJHxy1~qxyio0uOM9Sv8QiOb5Ia9sxZ9XNYQdmF2tpgDj3zUU2y9sMJjSYz4VZ4pYVUOgTHVGFKslZcOkDzOwYE0EWYXdgt4ioj-yX3cnukBhMZZ75~A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 1. PAF and perforin-mediated NK target-cell lysis. / (A) The phospholipid PAF enhances perforin-mediated NK target-cell lysis. Resting NK cells were used in standard cytotoxic assays against the MHC class I and FasR-negative K562 and Daudi cells, both of which were shown to be capable of inducing NK-cell activation and degranulation. Perforin-induced cell lysis was measured by target-cell release of sodium-51Cr–labeled cytoplasmic proteins via membrane damage and perforin pores. Resting human NK cells were mixed with labeled target cells at the various E-to-T ratios of 50:1, 25:1, 12:1, and 6:1, and target-cell lysis was measured at 4 hours. Naive NK cells were very efficient in provoking 51Cr release from K562 targets. Following addition of PAF to targets (PAF C16:0 alkyl moieties, [1 μmol/L]), K562 cells displayed a higher susceptibility to the lytic activity of perforin, as reflected by an increase in the amount of 51Cr target-cell release. In contrast, increasing extracellular concentrations of C16:0 PAF turned out to be inefficient in overcoming the failure of naive NK cells to induce Daudi cell lysis. (B) Inhibition of the PAF receptor. PAF receptor activity on K562 target cells was inhibited either by blocking its IFN-γ–induced expression using a neutralizing anti–IFN-γ antibody or by adding specific PAF receptor antagonists, such as WEB 2086 and SR 27417. Resting NK cells were used as effectors. These processes led to a potent inhibitory activity on NK-cell lysis, as shown by a significant decrease in the amount of NK/target-cell51Cr release.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/95/7/10.1182_blood.v95.7.2329/5/m_bloo00734001bx.jpeg?Expires=1765943338&Signature=OBLizSYQqGFc1~iiF0xvgy7~0K5GDY1jDfNg0O0k7LdT~DTCWFAFHCvi16jVuMwvq9GCttJgelFaDNWjB7Q9yLPIAzoMLDwUcyE9D9230kEdxy-fR~NXvzjpQM0xUtVywLYZEm3IvLVxYi661ZUv2QmJwcSAfDhFrdC55DAAi9vaohe9vrgbmTN~d7N1JvP7lPGmLXi9pY2YSrA1CtK-OMOzU9t3APE5y5MtLOHxZ4In-24VUqK8ekH-2HQOt0MeL65yIN2cXAi-SmguHgohL3TSKc2wlGpeQuqbekYofGgI-GnKhTpk3zJRDITYSdqOoKkEkhNM8G035YUy0N1hjg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 2. Purified human perforin efficiently binds to PAF. / Perforin was immunopurified from the perforin-positive YT2C2 NK-cell line using an appropriate binding gel (antiperforin 6.4-IgM, antiperforin mAb, coupled with Affi-Gel 10 gel). (A) Successful purification of perforin was controlled by a Western blot using the specific antiperforin antibody 2d4-perf. In lane C, for YT cell line extracts as positive control, the procedure revealed 2 bands at the molecular weight of 66 and 30-kd as recently reported by Uellner et al.24 Perforin was further purified by ultracentrifugation to obtain the active form of 66-kd (Uellner et al24), expressed in lane 1. No other band was detected in lanes 2, 3, 4, and 5 using, respectively, the perforin-negative Jurkat cell line lysate, the gel cross-linked to the mAb, the gel alone, or the gel with an irrelevant protein BSA instead of perforin. Active perforin was then exposed to [3H]PAF C18:0 during 30 minutes at 20°C and washed 5 times with the cold buffer as shown in “Materials and methods.” The resulting radioactivity was then measured for perforin. (B) In this assay, lane 1 shows the formation of perforin-[3H]PAF complexes expressed in disintegrations per minute. In contrast, no significant radioactivity was detected in lanes 2, 3, 4, and 5 using, respectively, the perforin-negative Jurkat cell line lysate, the gel cross-linked to the mAb, the gel alone, or the gel with an irrelevant protein BSA instead of perforin as already mentioned for Western blot analysis.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/95/7/10.1182_blood.v95.7.2329/5/m_bloo00734002aw.jpeg?Expires=1765943338&Signature=KhPjM5RrqbWYb8r5omaZeDAItbWTZExvv0oKlGR9UWLAePplq~kwohgATdlEZFTEN1hYIB8YopwRRjHDXrCPZnQFx-IKKy9AByrtBDinC8628u1P1Mfq95BW3oKkVOXK~ClumGG~Bm2zlYt7M-n0YtW24nHa3Rq~cOV~9JmIXea-IgH2T1aWwQyC7d0wDOQnSKfOC5UC3Hr2qqEXpYgly1QiYo28mP1ne~75jTLdy0Bco~gUb5uEGhR74p8amzDKnkT6X0l1nVggM71QE9PmFeoGMYJQ0M5hHar4wgLs3EM5Hs1LNjxs0aVcm0oQh9Bxrbtm1zKP-18kIfZK3Gd9Eg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 2. Purified human perforin efficiently binds to PAF. / Perforin was immunopurified from the perforin-positive YT2C2 NK-cell line using an appropriate binding gel (antiperforin 6.4-IgM, antiperforin mAb, coupled with Affi-Gel 10 gel). (A) Successful purification of perforin was controlled by a Western blot using the specific antiperforin antibody 2d4-perf. In lane C, for YT cell line extracts as positive control, the procedure revealed 2 bands at the molecular weight of 66 and 30-kd as recently reported by Uellner et al.24 Perforin was further purified by ultracentrifugation to obtain the active form of 66-kd (Uellner et al24), expressed in lane 1. No other band was detected in lanes 2, 3, 4, and 5 using, respectively, the perforin-negative Jurkat cell line lysate, the gel cross-linked to the mAb, the gel alone, or the gel with an irrelevant protein BSA instead of perforin. Active perforin was then exposed to [3H]PAF C18:0 during 30 minutes at 20°C and washed 5 times with the cold buffer as shown in “Materials and methods.” The resulting radioactivity was then measured for perforin. (B) In this assay, lane 1 shows the formation of perforin-[3H]PAF complexes expressed in disintegrations per minute. In contrast, no significant radioactivity was detected in lanes 2, 3, 4, and 5 using, respectively, the perforin-negative Jurkat cell line lysate, the gel cross-linked to the mAb, the gel alone, or the gel with an irrelevant protein BSA instead of perforin as already mentioned for Western blot analysis.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/95/7/10.1182_blood.v95.7.2329/5/m_bloo00734002bx.jpeg?Expires=1765943338&Signature=jKS1j8iZAwMl5FwmbklzpH~yV3~xgIjDIfVi5RbjQM05RRvC9yJ6eYueM~NbAqjBPjvUFwzqe2EVBda4zRBWioXkUBk93VBfTxztfiQGzGjW-XoQY5-6rbx-LUKwVloFO-Sl67mhCWVZE2r18CxbCw0-rWk3YUCbkM0JG9iHwiSRpO4VeVdcZwGfj3HtMsK5NoCdyqIMbcgu8hgWnCunUmdTwPUFJPeu-bY~9IHKkwmA~f0EIxx2N4kJ0U1cEW2cibDULIBIC8pucexjRrF4drgtbrFAi0y5FfdXK-~LuLyLyigSNeEuqa7gnCam8IA5-uhPrBCQKevJzf-ZfFyv9Q__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Fig. 5. Perforin, PAF, and PAF receptor form a ternary complex. / PAF receptors were immunopurified from the biotinylated membrane of IFN-γ–treated K562 cells with the use of the specific anti–PAF-receptor mAb. Purified receptors were incubated with or without C18:0 [3H]PAF in the presence of Ca++and Mg2+ and then exposed to YT2C2 cell cytoplasmic extracts for perforin-binding analysis. To confirm the presence of the 3 components (PAF receptor, PAF, perforin) in the same elution fraction, Western blot analysis using, respectively, anti-PAF receptor (A1), antiperforin (A2) mAbs, and [3H]PAF radioactivity detection (B) were performed. This procedure allowed simultaneous detection of PAF receptor at the molecular weight (mw) of 42-kd (A1, lane 2); perforin at the mw of 66 kd (A2, lane 2); and radioactivity due to the presence of [3H]PAF in the complex (B, column 2). In the absence of [3H]PAF (B, column 3), PAF receptor was still detected (A1, lane 3) but perforin failed to bind to the receptor (A2, lane 3). In the absence of PAF receptors (Jurkat cell membrane extracts, A1, lane 4), [3H]PAF (B, column 4) and perforin (A2, lane 4) failed to form the ternary complex. The YT2C2 cell line was used as negative control for PAF receptor expression (A1, lane 1), as positive control for perforin expression (A2, lane1), and as negative control for [3H]PAF binding (B, column 1).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/95/7/10.1182_blood.v95.7.2329/5/m_bloo007340051w.jpeg?Expires=1765943338&Signature=3PhYwjny5I67HhzccB84oOcsqbOsHDT-UZ3mYghKFupgZSac5C6oyAJHHEpEbyOonR-oP~Rwk9J0Nk0C5aPR3ZUVMJOfgMu9wghh8D0vuvsFSCkTFKo0I7H3kMCXJKdZ49~BXokpwk-sTBtwvCIjZXQOmFirpopXE3mWeq1NjRaQ81eMjM9ECtkSVXv4AKdMg8kfRO4qmIzyWnyboy-2pQuFLhl8sNQmmee40TUO5ab1dnhqbRy75cQhSEKKu0FwkS4CD1eHfV9d170FEx-md~p9EM-hJFWR1Pl5ApVbYyYvwUyJ8gC8XjEgBBVYpevsOYRmr-EHRA5HS1547YMrQQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 5. Perforin, PAF, and PAF receptor form a ternary complex. / PAF receptors were immunopurified from the biotinylated membrane of IFN-γ–treated K562 cells with the use of the specific anti–PAF-receptor mAb. Purified receptors were incubated with or without C18:0 [3H]PAF in the presence of Ca++and Mg2+ and then exposed to YT2C2 cell cytoplasmic extracts for perforin-binding analysis. To confirm the presence of the 3 components (PAF receptor, PAF, perforin) in the same elution fraction, Western blot analysis using, respectively, anti-PAF receptor (A1), antiperforin (A2) mAbs, and [3H]PAF radioactivity detection (B) were performed. This procedure allowed simultaneous detection of PAF receptor at the molecular weight (mw) of 42-kd (A1, lane 2); perforin at the mw of 66 kd (A2, lane 2); and radioactivity due to the presence of [3H]PAF in the complex (B, column 2). In the absence of [3H]PAF (B, column 3), PAF receptor was still detected (A1, lane 3) but perforin failed to bind to the receptor (A2, lane 3). In the absence of PAF receptors (Jurkat cell membrane extracts, A1, lane 4), [3H]PAF (B, column 4) and perforin (A2, lane 4) failed to form the ternary complex. The YT2C2 cell line was used as negative control for PAF receptor expression (A1, lane 1), as positive control for perforin expression (A2, lane1), and as negative control for [3H]PAF binding (B, column 1).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/95/7/10.1182_blood.v95.7.2329/5/m_bloo007340052x.jpeg?Expires=1765943338&Signature=E-f1qWYeM1VGnl0CXzlH4oZX3X015E09fANd0w6GObTUS3HjbD3H2tRJRy9nt0sGiCZ52pzVuh3bnoLWFCmCuHjzwzScgPwMzJJQu-kJnvM9p1vHh4varL5fgaTOis51c7HSc6wlSn7EWf6uYHsi-AOI1lKX7VmioHByFdJ~X1yMTP5q1QiSBKXKovImrOVO45~zHRt5xsENQ47s54k~EhHLX1IOqL1w8NEhYfzcr4PlBTZDOhfzbLpAAfnQr-ZYNaQVAvyQMC8SGRO0t5ubDL1DGfH97VS5Gtt2abbUWCXdfaFX467d9wqD2ovuU6ctdSIcywxw5YF19jOyW0TVeQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal