Abstract
The role of granulocyte-macrophage colony-stimulating factor (GM-CSF) in the differentiation of dendritic cells (DCs) during pulmonary viral infection was investigated by using a mouse model of GM-CSF transgene expression established with an adenoviral vector (AdGM-CSF). GM-CSF gene transfer resulted in increased levels of GM-CSF in the lung, which peaked at day 4 and remained increased up to day 19. A striking cellular response composed predominantly of macrophage-like cells was observed in the lung receiving AdGM-CSF but not control vector. By FACS analysis, the majority of these cells were identified at an early time point as macrophages and later as mature/activated myeloid DCs characterized by CD11bbright, CD11cbright, MHC class IIbright, and B7.1bright. In contrast, GM-CSF had a weak effect on a small DC population that was found present in normal lung and was characterized by CD11cbright and CD11blow. By immunohistochemistry staining for MHC II, the majority of activated antigen-presenting cells were localized to the airway epithelium and peribronchial/perivascular areas in the lung. A concurrently enhanced Th1 immune response was observed under these conditions. The number of CD4 and CD8 T cells was markedly increased in the lung expressing GM-CSF, accompanied by increased release of interferon (IFN)γ in the lung. Furthermore, lymphocytes isolated from either lung parenchyma or local draining lymph nodes of these mice but not the control mice released large amounts of IFNγ on adenoviral antigen stimulation in vitro. These findings reveal that GM-CSF promotes the differentiation and activation of a myeloid DC population primarily by acting on macrophages during pulmonary immune responses.
The lung is constantly exposed to the external environment. Thus, the generation of pulmonary immune responses against invading pathogens, including bacteria and viruses, is critical for host defense. Dendritic cells (DCs) are the most potent antigen-presenting cells (APCs) essential for the development of primary immune responses against microbial pathogens.1Pulmonary DCs are normally distributed within the airway mucosa, alveolar septa, and perivascular parenchyma.2,3 Like DCs from other nonlymphoid tissues, pulmonary DCs are originated from either “myeloid” or “lymphoid” precursors and are believed to be functionally immature APCs in the tissue where they exert a sentinel function.1,4,5 On encounter with antigens, DCs move to the T-dependent areas of secondary lymphoid organs where they become mature DCs to activate antigen-specific lymphocytes. Granulocyte-macrophage colony-stimulating factor (GM-CSF), a well-known hematopoietic growth factor, is indispensable for DC differentiation and maturation from bone marrow progenitors or peripheral blood monocytes in a number of in vitro systems.6-11 It also has regulatory effects on purified pulmonary DCs.8-10,12However, relatively little is known about whether GM-CSF has such effects on DCs in vivo. GM-CSF-transduced tumor cells were shown to induce the most potent antitumor immunity compared with tumor cells transduced with other cytokines,13 and GM-CSF transgenic mice were found to have increased DCs in the peritoneal cavity and lymphoid organs.11,14 Furthermore, GM-CSF gene-deficient mice were more susceptible to lung infections by a range of opportunistic microorganisms.15,16 Indeed, GM-CSF has been found in increased amounts in a number of pulmonary immune and inflammatory conditions.17 Although these lines of evidence suggest a regulatory role of GM-CSF in host immune-inflammatory responses in the lung, the precise role of GM-CSF in the differentiation/activation of DCs in the lung during immune responses has remained to be elucidated. Because GM-CSF is induced primarily under immune-inflammatory conditions in the lung, its effect on APCs in the lung may be best studied in a transgenic model in which GM-CSF is expressed concurrently with an ongoing immune-inflammatory response.
In this study, we utilized a GM-CSF transgene model established by local lung delivery of a replication-deficient adenoviral GM-CSF gene transfer vector to examine the effect of GM-CSF on the differentiation and activation of pulmonary APCs, particularly DCs. This viral-mediated transgene approach targets GM-CSF transgene to the airway epithelial cells and gives rise to a prolonged raised GM-CSF level in the lung and thus allows us to study the effect of GM-CSF on APCs in the lung during host immune responses to viral infection.
Material and methods
Gene transfer vectors and antibodies
Construction and characterization of a replication-deficient adenoviral gene vector expressing murine GM-CSF (AdGM-CSF) have been previously described.18 An adenoviral vector Addl70-3 without transgene was used as a control.18 UV-inactivated wild-type adenovirus was used as adenoviral antigens for an in vitro antigen stimulation assay. Anti-mouse monoclonal antibodies, including FITC-labeled anti-CD8α (clone 53-6.7), PE-labeled anti-CD11c (clone HL3) and anti-CD4 (clone GK1.5), and biotinylated anti-CD3 (clone 145-2C11), anti-B7.1 (clone BB1), and anti-natural killer (NK) cell (clone DX5) antibodies, were purchased from PharMingen (Mississauga, ON, Canada). FITC-labeled anti-CD11b (anti-Mac1, clone M1/70.15) was purchased from Cedarlane Laboratories Limited (Hornby, ON, Canada). Anti-MHC II monoclonal antibody (mAb) (clone M5/114) was produced, purified on protein G, and labeled with biotin. The mAb 2.4G2 (anti-FcR IIb/III) was produced as ascites and purified on protein G. Binding of biotinylated antibodies was identified by streptavidin-conjugated peridinin chlorophyll protein (Becton Dickinson, San Jose, CA).
Mice and GM-CSF gene transfer in the lung
Female Balb/c and C3H mice of 10- to 14-week age were purchased from Harlan Laboratories (Indianapolis, IN) and housed under specific pathogen-free conditions before use at McMaster University Central Animal Facility. Mice were anesthetized and AdGM-CSF or Addl70-3 was intranasally (i.n.) delivered to mouse lung by a standardized procedure that we have previously described.19 Briefly, a dose of 0.6 × 109 plaque-forming units (pfu) of viral vector was diluted with phosphate-buffered saline (PBS) to a total volume of 30 μL and delivered into mouse lungs with a fine pipette tip in 2 aliquots (15 μL each). Mice were killed at days 2, 4, 7, 12, or 19 postgene transfer. We have previously shown that, following i.n. vector delivery, transgene is expressed primarily by bronchial epithelial cells and, to a certain extent, by alveolar macrophages.19
Bronchoalveolar lavage (BAL) and cytologic analysis
At each time point, BAL was performed as previously described.20 21 A total of 450 μL of PBS was used to lavage the lung, and usually 350 μL of BAL fluids were retrieved. BAL fluids were then spun in a microcentrifuge at 5000 rpm for 5 minutes, and supernatants were stored at −20°C until cytokine measurements. Cell pellets were resuspended in PBS and total cell numbers were counted on a hemocytometer. Cytospins were prepared by cytocentrifugation (Shandon Inc, Pittsburgh, PA). Differential cell counts were determined on Diff-Quik-stained (Baxter, McGaw Park, IL) cytospins by randomly counting 400-500 cells per slide.
Flow cytometric analysis of T-cell and NK-cell subsets in BAL and APCs in the lung
To phenotype immune cell subsets in the lung, BAL-derived cells were pooled from three AdGM-CSF-treated mice or five Addl70-3-treated mice 7 days after infection. About 0.3 × 106 cells were blocked by an anti-FcR antibody 2.4G2 for 15 minutes and then labeled with a combination of mAbs of biotinylated anti-CD3, PE-anti-CD4 and FITC-anti-CD8, or FITC-anti-CD3 and biotinylated anti-NK DX5. The basic staining procedure was carried out as previously described.22 A FACScan instrument was used (Becton Dickinson, Sunnyvale, CA) to collect list mode data (10 000 total events) for analysis. Analysis was carried out using Lysys II software (B-D) by first setting a forward- and side-scatter gate that included lymphocytes but excluded dead cells and debris.
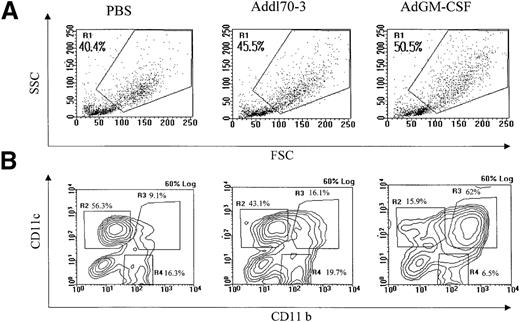
In separate experiments, mice were killed 5 or 12 days after delivery of AdGM-CSF, Addl70-3, or PBS. The lungs were removed from the chest with the heart and a portion of the trachea intact. Pulmonary vasculature was perfused with 5 mL of warm calcium and magnesium-free 1 X HBSS containing 5% fetal calf serum (FCS), 100 U/mL penicillin, and 100 g/mL of streptomycin via the right ventricle of the heart. Total lung mononuclear cells were isolated by collagenase digestion, followed by discontinuing gradient centrifugation as previously described.22 Approximately 0.3 × 106cells were labeled with mAbs in a combination of FITC-CD11b, PE-CD11c, and a biotinylated-antibody to a surface molecule of interest (MHC class II or B7.1) or biotinylated-isotype control antibody rat immunoglobulin (Ig)G2a. List mode data (20 000 total events) were collected on FACScan and analysis was performed on cells gated in high forward and scatter region (R1 region), distinct from regions defined for lymphocytes and debris (see Figure 1A). More than 90% of CD11c-positive cells were found within R1 region by back-gating analysis. The cells from R1 region were further divided into three subpopulations (R2-R4) on the basis of CD11b and CD11c expression (Figure 1B). The unstained cells gated on R1 region were used as negative controls, and the background was approximately 2% and 1.2% for PE-CD11c and FITC-CD11b, respectively. The absolute numbers of cell subsets based on CD11c and CD11b were calculated on the basis of the total number of cells recovered per mouse, multiplied by the fraction of cells in the high scatter region, and then multiplied by the fraction of cells with a given CD11c, CD11b phenotype.
Identification of pulmonary antigen-presenting cell (APC) populations under different conditions.
Total mononuclear cells were isolated from mouse lungs that received 0.6 × 109 plaque-forming units adenoviral gene vector expressing murine GM-CSF (AdGM-CSF) or Addl70-3, or 40 μL of sterile phosphate-buffered saline at day 12. Approximately 0.3 × 106 cells were immunostained with FITC-CD11b, PE-CD11c and biotinylated-MHC II, B7.1, or rat immunoglobulin (Ig)G2a antibodies. Data were collected from FACScan. (A) General cellular profile for each group was shown on forward-side scatter, macrophage/monocyte-like cell enriched population was gated as R1. (B) R1 region cells were further divided into three subpopulations in contour plot based on CD11b and CD11c expression. (C) The absolute cell numbers for each population derived from single mouse in different groups. Data are representative of three individual experiments.
Identification of pulmonary antigen-presenting cell (APC) populations under different conditions.
Total mononuclear cells were isolated from mouse lungs that received 0.6 × 109 plaque-forming units adenoviral gene vector expressing murine GM-CSF (AdGM-CSF) or Addl70-3, or 40 μL of sterile phosphate-buffered saline at day 12. Approximately 0.3 × 106 cells were immunostained with FITC-CD11b, PE-CD11c and biotinylated-MHC II, B7.1, or rat immunoglobulin (Ig)G2a antibodies. Data were collected from FACScan. (A) General cellular profile for each group was shown on forward-side scatter, macrophage/monocyte-like cell enriched population was gated as R1. (B) R1 region cells were further divided into three subpopulations in contour plot based on CD11b and CD11c expression. (C) The absolute cell numbers for each population derived from single mouse in different groups. Data are representative of three individual experiments.
T-cell enrichment from lung and draining lymph nodes and in vitro stimulation with adenoviral antigen
Mice were killed 12 days after delivery of AdGM-CSF or Addl70-3. Total lung mononuclear cells were isolated as above and were enriched for T cells by using a mouse T-cell-enrichment column (R&D System, Minneapolis, MN) according to manufacturer's instructions. The resultant population contained more than 90% T lymphocytes with 5% to 10% antigen-presenting cells. Cell suspensions were cultured in RPMI-10 medium (RPMI 1640, supplemented with 10% FCS, 100 U/mL penicillin, 100 g/mL streptomycin, and 2 mmol/L of L-Glutamine). From the same mice, mediastinal lymph nodes were collected and kept in HBSS, then gently ground between two sintered glass slides. The resultant lymphocyte suspension was filtered through one layer of nylon membrane (55 μm) and centrifuged at 1200 rpm for 10 minutes at 4°C. The cell pellets were washed once with PBS and resuspended in RPMI-10 medium.
Approximately 0.2 × 106 lung-derived or 0.3 × 106 lymph node-derived cells were seeded into 96-well plates and cultured with or without 1 × 109pfu/well of UV-inactivated adenovirus at 37°C for 72 hours. Other wells received 8 g/mL of an irrelevant mycobacterial antigen (PPD). The supernatants were taken at 72 hours and stored at −20°C until cytokine measurement.
Immunohistochemical staining for MHC II expression
Lung tissues were obtained at 12 days after delivery of AdGM-CSF or Addl70-3 and fixed in 30 mL of 4% formaldehyde in 75 mmol/L phosphate buffer (pH 7.4) for 24 hours. Paraffin sections were deparaffinized in xylene followed by 100% ethanol and then placed in freshly prepared methanol H2O2 solution for 30 minutes (200 mL methanol, 10 mL hydrogen peroxide, and 0.5 mL concentrated HCl) to block endogenous peroxidase activity. After rehydrolization, the slides were subjected to hot Hewlett's Epitope Recovery Buffer (10 mmol/L citrate/phosphate buffer, pH 6.85) for 10 minutes23 and then allowed to cool down to room temperature. The slides were blocked with 1% BSA/PBS for 15 minutes, followed by incubation with 1:100 rat anti-mouse biotinylated MHC II M5 mAb in 1% BSA/PBS overnight at room temperature. Following the incubation with 1:300 secondary biotinylated rabbit anti-rat antibody at room temperature for 1 hour and 1:1200 straptavidin/peroxidase conjugate for 90 minutes, the slides were developed by a conventional substrate/chromogen solution and counter-stained with 50% hematoxylin for 2 minutes.
Cytokine measurements
The level of cytokines in the BAL and culture supernatants was determined by using mouse specific enzyme-linked immunosorbent assay (ELISA) kits. Interferon (IFN)γ and GM-CSF ELISA kits were purchased from R&D Systems. The sensitivity of detection for these ELISA kits was 2 pg/mL. MIP-1 and MCP-1 ELISAs were developed as previously described.24
Results
GM-CSF transgene protein levels and cellular responses in the lung post-GM-CSF gene transfer
To determine the level of GM-CSF transgene protein in the lung, BAL fluids collected at various time points following lung GM-CSF gene transfer were measured for murine GM-CSF by ELISA. The level of GM-CSF markedly increased by day 2, peaked by day 4, still remained high at day 7, and significantly decreased by day 12 in the lung of mice after GM-CSF gene transfer (Table 1). In contrast, little GM-CSF was detected in the BAL from mice receiving the control vector Addl70-3. To evaluate cellular responses to GM-CSF in the lung, total and differential cell counts in the BAL were determined at various time points. The control vector induced only minimal cellular responses throughout the entire experiment (Table2). In contrast, GM-CSF induced an increase in total cell numbers that peaked at day 12, being 8 times as many as in mice receiving Addl70-3 (Table 2). Macrophages/monocytes represented a major cell type among increased leukocytes. The number of lymphocytes also markedly increased. For instance, at day 12, there were 50.4 × 104, 24 × 104, and 13.2 × 104 of macrophages/monocytes, lymphocytes, and neutrophils, respectively, in BAL from the lung of mice expressing GM-CSF versus 10.8 × 104, 0.64 × 104, and 0.17 × 104, respectively, in the lung of mice receiving Addl70-3 (Table 2). By day 19, although the cellular response almost completely resolved in the lung of control mice, the number of macrophages/monocytes in the lung of mice receiving AdGM-CSF still remained elevated. In addition to macrophages, lymphocytes, and neutrophils, there was also a small, but significant, increase in the number of eosinophils. The delayed peak cellular response, as compared with the earlier peak time of GM-CSF transgene product in the lung, suggests that such increased cell responses are not just the effect of GM-CSF on cell influx and that it is more likely a result of the enhancement by GM-CSF of cellular immune responses to viral infection that normally takes 7 to 10 days to peak. Consistent with the cellular profiles observed in the BAL, total mononuclear cells isolated from the lung tissue of mice expressing GM-CSF at day 12 were 10 times as high as that in control groups (data not shown).
The level of GM-CSF in BAL after intranasal delivery of AdGM-CSF or Addl70-3 (pg/ml)*
| . | Day 2 . | Day 4 . | Day 7 . | Day 12 . | Day 19 . |
|---|---|---|---|---|---|
| AdGM-CSF | 1734 ± 433 | 8501 ± 1838 | 3743 ± 1657 | 7.99 ± 0.28 | 12.7 ± 3.4 |
| Addl70-3 | 29.6 | 2.76 | 3.45 | 3.17 | 0 |
| . | Day 2 . | Day 4 . | Day 7 . | Day 12 . | Day 19 . |
|---|---|---|---|---|---|
| AdGM-CSF | 1734 ± 433 | 8501 ± 1838 | 3743 ± 1657 | 7.99 ± 0.28 | 12.7 ± 3.4 |
| Addl70-3 | 29.6 | 2.76 | 3.45 | 3.17 | 0 |
AdGM-CSF = adenoviral gene vector expressing murine GM-CSF; BAL = bronchoalveolar lavage; GM-CSF = granulocyte-macrophage colony-stimulating factor.
BAL samples were collected at various time points after intranasal delivery of AdGM-CSF or Addl70-3. The level of GM-CSF was measured by enzyme-linked immunosorbent assay. The results for AdGM-CSF group are expressed as mean ± SEM from three mice/time point, and mean from two mice/time point for control (Addl70-3) group.
Differential leukocytes in the lung after intranasal delivery of AdGM-CSF or Addl70-3 (×104/BAL)
| . | Total No. . | MΦ/MO . | PMN . | LC . | EOS . |
|---|---|---|---|---|---|
| Day 2 | 34.1 ± 4.9 (13.4) | 18.1 ± 0.6 (10.5) | 4.9 ± 1.9 (0.9) | 10.9 ± 2.6 (1.4) | 0.04 ± 0.04 (0) |
| Day 4 | 26.3 ± 0.7 (10.1) | 21.3 ± 0.7 (7.6) | 0.7 ± 0.1 (0.7) | 4.1 ± 0.3 (1.7) | 0.13 ± 0.04 (0) |
| Day 7 | 51.9 ± 7.1 (8.4) | 26.9 ± 3.2 (7.5) | 10.8 ± 2.1 (0.08) | 14.2 ± 2.3 (0.7) | 0.16 ± 0.16 (0.05) |
| Day 12 | 88.4 ± 11 (11.7) | 50.4 ± 7.8 (10.8) | 13.2 ± 2.8 (0.17) | 24.0 ± 3.8 (0.64) | 0.76 ± 0.24 (0.03) |
| Day 19 | 22.2 ± 3.3 (10.3) | 18.3 ± 3.1 | 0.38 ± 0.13 (9.6) | 1.94 ± 0.3 (0.2) | 0 (0.5) |
| . | Total No. . | MΦ/MO . | PMN . | LC . | EOS . |
|---|---|---|---|---|---|
| Day 2 | 34.1 ± 4.9 (13.4) | 18.1 ± 0.6 (10.5) | 4.9 ± 1.9 (0.9) | 10.9 ± 2.6 (1.4) | 0.04 ± 0.04 (0) |
| Day 4 | 26.3 ± 0.7 (10.1) | 21.3 ± 0.7 (7.6) | 0.7 ± 0.1 (0.7) | 4.1 ± 0.3 (1.7) | 0.13 ± 0.04 (0) |
| Day 7 | 51.9 ± 7.1 (8.4) | 26.9 ± 3.2 (7.5) | 10.8 ± 2.1 (0.08) | 14.2 ± 2.3 (0.7) | 0.16 ± 0.16 (0.05) |
| Day 12 | 88.4 ± 11 (11.7) | 50.4 ± 7.8 (10.8) | 13.2 ± 2.8 (0.17) | 24.0 ± 3.8 (0.64) | 0.76 ± 0.24 (0.03) |
| Day 19 | 22.2 ± 3.3 (10.3) | 18.3 ± 3.1 | 0.38 ± 0.13 (9.6) | 1.94 ± 0.3 (0.2) | 0 (0.5) |
AdGM-CSF = adenoviral gene vector expressing murine GM-CSF; BAL = bronchoalveolar lavage; GM-CSF = granulocyte-macrophage colony-stimulating factor; EOS = eosinophils; LC = lymphocytes; MΦ/Mo = macrophages/monocytes; PMN = polymorphonuclear neutrophils.
BAL samples were collected at various time points after intranasal delivery of 0.6 × 109 plaque-forming units of AdGM-CSF or Addl70-3. Differential cell types were determined on Diff-Quick stained cytospins. The results for AdGM-CSF treated group are shown and expressed as mean ± SEM from three mice per time point. The results from Addl70-3 control groups are placed in parenthesis and expressed as mean from two mice per time point.
Phenotypes of APCs induced by GM-CSF transgene expression in the lung
Having demonstrated that GM-CSF induced a marked cellular response of primarily monocytic nature in the lung, we analyzed the phenotype of this macrophage/monocyte population by FACS. To this end, total mononuclear cells were isolated from mouse lung tissue receiving AdGM-CSF, Addl70-3, or PBS at day 12 and stained with different combinations of mAbs to various leukocyte surface markers, including CD11b (Mac-1), CD11c, MHC II, and B7.1. A cell population (Figure 1A, R1 region) showing higher forward- and side-scatter properties was gated and subdivided into three populations, based on their relative CD11c and CD11b expression. These populations were cells expressing CD11blow and CD11cbright (R2 region), CD11bbright and CD11cbright (R3 region), or CD11bbright and CD11clow (R4 region), respectively (Figure 1B). CD11blow/CD11cbright cells were lung residential DCs, phenotypically similar to some DCs found in lymphoid organs.11,25-27CD11bbright/CD11cbright cells represented a myeloid DC phenotype also found in lymphoid organs11,26 and were a novel DC population induced by GM-CSF that we now identified in the lung. CD11bbright/CD11clow cells were macrophages.25 In PBS- or Addl70-3-treated animals, about 60%-80% of CD11c+ cells were CD11blow/CD11cbright. In contrast, GM-CSF expression resulted in a striking increase in the percentage of CD11b/CD11c double-positive cells, which accounted for about 90% of CD11c+ cells (Figure 1B). Thus, GM-CF induced an approximately 44-fold increase in the number of CD11bbright/CD11cbright DCs over that found in PBS- or Addl70-3-treated groups (Figure 1C). GM-CSF also induced a fourfold and threefold increase in the number of CD11bbright/CD11clow cells and CD11blow/CD11cbright cells, respectively (Figure 1C). These results indicate that GM-CSF plays a major inductive role in the differentiation of the CD11b/CD11c double-positive myeloid DC population.
Activation of DCs and macrophages by GM-CSF transgene expression in the lung
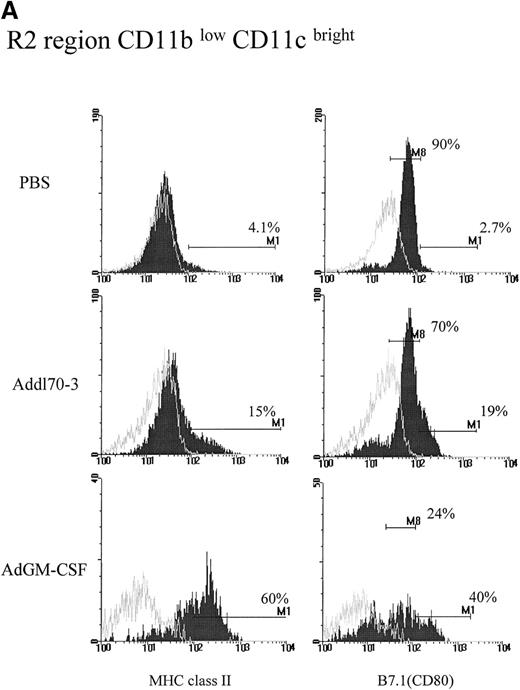
To examine the activating effect of GM-CSF on each APC population during viral infection, we also examined expression of phenotypic activation markers MHC class II and costimulatory molecule B7.1 that are expressed in high intensity by activated APC populations.28 29CD11blow/CD11cbright DCs in the PBS group expressed very low levels of MHC class II and moderate levels of B7.1 (Figure 2A). This type of DC in Addl70-3 control vector group expressed slightly enhanced expression of MHC class II and B7.1, which was likely a response to viral infection. In comparison, although there were 60% of CD11blow/CD11cbright cells in the GM-CSF group that expressed bright MHC II, about 40% of this type of cell translated from moderate to bright B7.1 expression, in contrast to the cells in control groups most of which only expressed moderate density of B7.1 (Figure 2A). In terms of absolute cell number, GM-CSF induced an approximately 14-fold and 7-fold increase in the number of DCs expressing bright MHC class II and B7.1, respectively, compared with control groups (Figure 2B).
Effect of granulocyte-macrophage colony-stimulating factor (GM-CSF) on activation of R2 region CD11blow/CD11cbright population during pulmonary adenoviral infection.
Following the procedure described in Figure 1, (A) MHC II and B7.1 expression were examined on R2 region for each group. Open histograms represent the background staining with the isotype control antibody, whereas solid histograms indicate staining with relevant monoclonal antibody against the indicated surface molecule. M1 markers represent the limit defining the expression at high levels of the corresponding marker. In the case of B7.1 expression, the majority of cells from R2 region in control groups expressed at dull level that labeled as M8. (B) The absolute cell numbers for double positive for CD11c and MHC II or B7.1 derived from single mouse in different groups.
Effect of granulocyte-macrophage colony-stimulating factor (GM-CSF) on activation of R2 region CD11blow/CD11cbright population during pulmonary adenoviral infection.
Following the procedure described in Figure 1, (A) MHC II and B7.1 expression were examined on R2 region for each group. Open histograms represent the background staining with the isotype control antibody, whereas solid histograms indicate staining with relevant monoclonal antibody against the indicated surface molecule. M1 markers represent the limit defining the expression at high levels of the corresponding marker. In the case of B7.1 expression, the majority of cells from R2 region in control groups expressed at dull level that labeled as M8. (B) The absolute cell numbers for double positive for CD11c and MHC II or B7.1 derived from single mouse in different groups.
The majority of CD11bbright/CD11cbright DCs in the PBS group did not express MHC II and B7.1 (Figure3A). There was only a moderately increased percentage of these cells from the Addl70-3 group expressing bright MHC II and B7.1, which likely represented a response to viral infection. In contrast, almost 100% of CD11b and CD11c double-positive cells from the GM-CSF group expressed high intensity of MHC II and B7.1 (Figure3A). Because a very small number of CD11b and CD11c double-positive cells was present in the lung of mice receiving PBS or Addl70-3, in contrast to that induced by GM-CSF (Figure 1C), the number of activated DCs coexpressing high density of CD11b, CD11c, MHC II, and B7.1 in the lung of the GM-CSF group was at least 60-fold greater than in the control groups (Figure 3B). These findings highlight a potent inductive effect of GM-CSF on this myeloid DC population in the lung during viral infection.
Effect of granulocyte-macrophage colony-stimulating factor (GM-CSF) on activation of R3 region CD11bbright/CD11cbright population during pulmonary adenoviral infection.
Following the procedure described in Figure 1, (A) MHC II and B7.1 expression on R3 region was examined for each group. Open histograms represent the background staining with the isotype control antibody, whereas solid histograms indicate staining with relevant monoclonal antibody against the indicated surface molecule. M1 markers represent the limit defining the expression at high levels of the corresponding marker. (B) The absolute cell numbers for triple positive for CD11b, CD11c, and MHC class II or B7.1 derived from single mouse in different groups.
Effect of granulocyte-macrophage colony-stimulating factor (GM-CSF) on activation of R3 region CD11bbright/CD11cbright population during pulmonary adenoviral infection.
Following the procedure described in Figure 1, (A) MHC II and B7.1 expression on R3 region was examined for each group. Open histograms represent the background staining with the isotype control antibody, whereas solid histograms indicate staining with relevant monoclonal antibody against the indicated surface molecule. M1 markers represent the limit defining the expression at high levels of the corresponding marker. (B) The absolute cell numbers for triple positive for CD11b, CD11c, and MHC class II or B7.1 derived from single mouse in different groups.
GM-CSF also had a significant effect on MHC class II and B7.1 expression on CD11bbright/CD11clow macrophages in R4 region (Figure 4A). In comparison, macrophages from the PBS group were not activated and few of them expressed MHC II and B7.1. There was a moderate increase in the percentage of these cells expressing MHC II and B7.1 in the Addl70-3 group, likely again as a result of viral infection (Figure 4A). Similar to the effect on lung residential CD11blow/CD11cbright DCs, GM-CSF induced about 9-fold and 7-fold increases in the number of macrophages expressing bright MHC class II and B7.1, respectively, compared with control groups (Figure 4B).
Effect of granulocyte-macrophage colony-stimulating factor (GM-CSF) on activation of R4 region CD11bbright/CD11clow population during pulmonary adenoviral infection.
Following the procedure described in Figure 1, (A) MHC II and B7.1 expression was examined on R4 region for each group. Open histograms represent the background staining with the isotype control antibody, whereas solid histograms indicate staining with relevant monoclonal antibody against the indicated surface molecule. M1 markers represent the limit defining the expression at high levels of the corresponding marker. (B) The absolute cell numbers for double positive for CD11b and MHC II or B7.1 derived from a single mouse in different groups.
Effect of granulocyte-macrophage colony-stimulating factor (GM-CSF) on activation of R4 region CD11bbright/CD11clow population during pulmonary adenoviral infection.
Following the procedure described in Figure 1, (A) MHC II and B7.1 expression was examined on R4 region for each group. Open histograms represent the background staining with the isotype control antibody, whereas solid histograms indicate staining with relevant monoclonal antibody against the indicated surface molecule. M1 markers represent the limit defining the expression at high levels of the corresponding marker. (B) The absolute cell numbers for double positive for CD11b and MHC II or B7.1 derived from a single mouse in different groups.
Differentiation of macrophages to myeloid DCs by GM-CSF in the lung
Having identified a potent effect of GM-CSF on the induction of a novel myeloid DC population characterized by CD11bbright, CD11cbright, MHC class IIbright, and B7.1bright in the lung, we investigated whether this DC population could have derived from a macrophage population expanded by GM-CSF at an earlier time. To this end, a group of mice were killed 5 days post-AdGM-CSF gene transfer. Mononuclear cells were isolated, immunostained, and analyzed in the way we did at day 12. A comparison was made between 5 days and 12 days post-GM-CSF gene transfer. GM-CSF markedly induced a CD11bbright/CD11clow macrophage population at day 5 after transgene expression (Figure5). These cells accounted for 46.3% of total analyzed cells as opposed to 16.3% found in PBS controls (Figure1B) and had increased MHC II expression (data not shown). In comparison, only 9% of analyzed cells were CD11bbright/CD11cbright.. At day 12, however, while macrophage population decreased dramatically to only 15.4% of total cells, 57.6% of cells were CD11bbright/CD11cbright. Of note, the percentage of CD11blow/CD11cbright cells remained similar to that found at day 5 (11.8% vs 9%). Thus, the phenotypic shift from macrophages to myeloid DCs during the course of GM-CSF transgene expression strongly suggests that CD11bbright/CD11cbright DCs induced by GM-CSF were primarily derived from a macrophage population activated earlier by GM-CSF.
Major effect of granulocyte-macrophage colony-stimulating factor (GM-CSF) on macrophages at earlier times in the lung.
In a separate experiment, mononuclear cells were isolated from mice receiving phosphate-buffered saline, Addl70-3, or adenoviral gene vector expressing murine GM-CSF (AdGM-CSF) at day 5 and immunostained in the exact same way as described in Figure 1. A parallel comparison analysis was carried out between the AdGM-CSF day 5 group and the AdGM-CSF day 12 group. R1 region cells as defined in Figure 1 were expressed in dot plot based on CD11b and CD11c expression. The number marked on each corner represents the percentage of a given phenotype out of total R1 region.
Major effect of granulocyte-macrophage colony-stimulating factor (GM-CSF) on macrophages at earlier times in the lung.
In a separate experiment, mononuclear cells were isolated from mice receiving phosphate-buffered saline, Addl70-3, or adenoviral gene vector expressing murine GM-CSF (AdGM-CSF) at day 5 and immunostained in the exact same way as described in Figure 1. A parallel comparison analysis was carried out between the AdGM-CSF day 5 group and the AdGM-CSF day 12 group. R1 region cells as defined in Figure 1 were expressed in dot plot based on CD11b and CD11c expression. The number marked on each corner represents the percentage of a given phenotype out of total R1 region.
Localization of APC in the lung by immunohistochemistry
We next examined the localization of APC in the lung by immunohistochemistry with an anti-MHC II mAb. Few MHC II-positive cells were found in the lung of mice receiving Addl70-3 (Figure6A). In contrast, many MHC II-bearing cells were localized to peribronchial and perivascular areas, many of which could represent activated GM-CSF-induced DCs although morphologically they appear to be macrophages (Figure 6B). In addition, some MHC II-positive cells were also localized to the airway epithelium, and because these cells possessed dendrites or projections, they were most likely intraepithelial dendritic cells (Figure 6B).
Distribution of MHC II positive dendritic cells (DCs) and macrophages in the lung.
Mice were killed 12 days after intrapulmonary injection of adenoviral gene vector expressing murine granulocyte-macrophage colony-stimulating factor (AdGM-CSF), Addl70-3, or phosphate-buffered saline. Lung tissues were isolated and fixed in 10% formalin and 3-cm sections were immunostained with monoclonal anti-MHC II M5 antibody and 50% hematoxylin counterstaining. (A) Addl70-3 control vector treatment; (B) AdGM-CSF treatment. Many MHC II-positive cells are seen in Figure 6B, some intraepithelial DC are marked with arrowheads. Bronchial lumen is marked by b. (Magnification for both panels is 450.)
Distribution of MHC II positive dendritic cells (DCs) and macrophages in the lung.
Mice were killed 12 days after intrapulmonary injection of adenoviral gene vector expressing murine granulocyte-macrophage colony-stimulating factor (AdGM-CSF), Addl70-3, or phosphate-buffered saline. Lung tissues were isolated and fixed in 10% formalin and 3-cm sections were immunostained with monoclonal anti-MHC II M5 antibody and 50% hematoxylin counterstaining. (A) Addl70-3 control vector treatment; (B) AdGM-CSF treatment. Many MHC II-positive cells are seen in Figure 6B, some intraepithelial DC are marked with arrowheads. Bronchial lumen is marked by b. (Magnification for both panels is 450.)
Enhanced immune responses in the lung by GM-CSF transgene expression
The primary function of DCs is to activate antigen-specific T cells, enhancing their proliferation and cytokine responses during primary immune responses.1,4,28,30 Having demonstrated that GM-CSF transgene expression markedly promoted the differentiation and activation of myeloid APC populations in the lung during immune responses to adenoviral infection, we further examined and compared the level of immune responses with pulmonary viral infection in the lung of mice receiving Addl70-3 or AdGM-CSF. To this end, we first examined by FACS analysis the number of immune subsets including NK, CD4, and CD8 T cells in the lymphocytic population present in BAL fluids recovered at day 7 postgene transfer. In our previous studies, we have found that the cellular profiles of lymphocytes, macrophages, and granulocytes in BAL fluids always mirror those seen at the histopathologic level.18-22 We have previously shown that T-cell responses are an important aspect of host anti-adenoviral immune response in the lung.31 The number of NK cells was found similar between control and GM-CSF groups (Figure 7). However, there were approximately 34-fold and 16-fold increases in the number of CD4 and CD8 T cells, respectively, in the lung of the GM-CSF group (Figure 7).
Determination of the number of subtypes of lymphocytes present in bronchoalveolar lavage (BAL) by FACS.
BALs were pooled out from three mice treated with adenoviral gene vector expressing murine granulocyte-macrophage colony-stimulating factor (AdGM-CSF) or Addl70-3, respectively, 7 days after gene transfer. Cells were immunostained with different combinations of monoclonal antibodies and examined by FACS analysis. Then, the absolute cell numbers of CD4, CD8, and natural killer cells were calculated, based on the total cell recovery and the percentage of each subtype.
Determination of the number of subtypes of lymphocytes present in bronchoalveolar lavage (BAL) by FACS.
BALs were pooled out from three mice treated with adenoviral gene vector expressing murine granulocyte-macrophage colony-stimulating factor (AdGM-CSF) or Addl70-3, respectively, 7 days after gene transfer. Cells were immunostained with different combinations of monoclonal antibodies and examined by FACS analysis. Then, the absolute cell numbers of CD4, CD8, and natural killer cells were calculated, based on the total cell recovery and the percentage of each subtype.
Further, we examined the level of an antiviral Th1 cytokine IFNγ in the lung. The level of this cytokine was only marginally increased in the lung of mice receiving the control viral vector. In sharp contrast, there was a significant induction of IFNγ in the lung of the GM-CSF group, which peaked at day 7 and remained high at day 12 and markedly declined by day 19 (Figure 8). In addition, we also observed increased levels of chemokines MIP-1α and MCP-1 in the BAL in the AdGM-CSF group, particularly at early time points (data not shown).
Interferon (IFN)γ content in the bronchoalveolar lavage (BAL) at various time points after delivery of adenoviral gene vector expressing murine granulocyte-macrophage colony-stimulating factor (AdGM-CSF) or Addl70-3.
BAL fluids were collected at days 4, 7, 12, and 19 postintranasal delivery of AdGM-CSF or Addl70-3, and IFNγ content was determined by enzyme-linked immunosorbent assay. The results are expressed as mean ± SEM for AdGM-CSF group (n = 3) and mean for Addl70-3 group (n = 2).
Interferon (IFN)γ content in the bronchoalveolar lavage (BAL) at various time points after delivery of adenoviral gene vector expressing murine granulocyte-macrophage colony-stimulating factor (AdGM-CSF) or Addl70-3.
BAL fluids were collected at days 4, 7, 12, and 19 postintranasal delivery of AdGM-CSF or Addl70-3, and IFNγ content was determined by enzyme-linked immunosorbent assay. The results are expressed as mean ± SEM for AdGM-CSF group (n = 3) and mean for Addl70-3 group (n = 2).
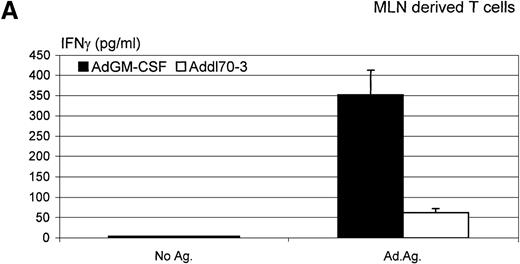
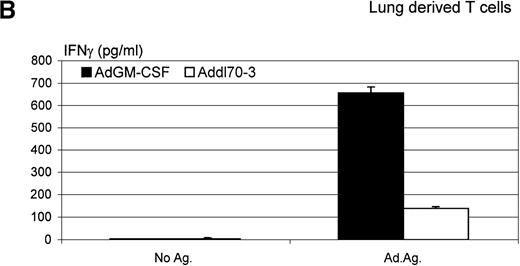
Furthermore, we isolated lymphocytes from both lung tissue and mediastinal lymph nodes 12 days after administration of AdGM-CSF or Addl70-3 and investigated the level of Th1-type lymphocyte response to adenoviral antigen stimulation in vitro by measuring the release of IFNγ. As shown in Figure 9, although there was an antigen-specific recall IFNγ response by lymphocytes isolated from the lung or mediastinal lymph nodes of mice infected with the control virus Addl70-3, such a response was enhanced many times in lung- or mediastinal lymph node-derived lymphocytes from mice infected with AdGM-CSF. These findings suggest that GM-CSF enhances a Th1-type host immune response to viral infection.
Interferon (IFN)γ level determined in ex vivo antigen-recall responses.
Mediastinal lymph nodes and lungs were collected from the mice receiving adenoviral gene vector expressing murine granulocyte-macrophage colony-stimulating factor (AdGM-CSF) or Addl70-3 at day 12. T lymphocytes were isolated and cultured at various conditions as described in “Material and methods.” Supernatants of (A) mediastinal lymph node-derived T-cell culture and (B) lung-derived T-cell culture were collected at 72 hours, and IFNγ level was measured by enzyme-linked immunosorbent assay. The difference in the level of IFNγ production between AdGM-CSF and control groups is statistically significant (t test,P ≤ .01). Data are representative of three individual experiments and expressed as mean ± SEM of triplicate samples.
Interferon (IFN)γ level determined in ex vivo antigen-recall responses.
Mediastinal lymph nodes and lungs were collected from the mice receiving adenoviral gene vector expressing murine granulocyte-macrophage colony-stimulating factor (AdGM-CSF) or Addl70-3 at day 12. T lymphocytes were isolated and cultured at various conditions as described in “Material and methods.” Supernatants of (A) mediastinal lymph node-derived T-cell culture and (B) lung-derived T-cell culture were collected at 72 hours, and IFNγ level was measured by enzyme-linked immunosorbent assay. The difference in the level of IFNγ production between AdGM-CSF and control groups is statistically significant (t test,P ≤ .01). Data are representative of three individual experiments and expressed as mean ± SEM of triplicate samples.
Discussion
In this study, we have used a unique mouse model of adenoviral-mediated GM-CSF transgene expression to investigate the role of GM-CSF in pulmonary DC differentiation and activation during an immune response to viral infection in the lung. We have shown an increased cellular response composed predominantly by macrophage/monocyte-like cells following GM-CSF gene transfer in the lung. By FACS analysis, two DC populations, CD11blow/CD11cbright and CD11bbright/CD11cbright, were identified with the latter being highly inducible by GM-CSF. The potent effect of GM-CSF on DC activation was demonstrated by up-regulation of both MHC class II and the costimulatory molecule B7.1 expression on CD11bbright/CD11cbright cells. Moreover, GM-CSF also had an effect on expansion and activation of lung residential DCs and macrophages characterized by surface expression of CD11blow, CD11cbright, MHC IIbright, and B7.1bright and of CD11bbright, CD11clow, MHC IIbright, and B7.1bright, respectively. These potent effects on APC by GM-CSF were found associated closely with enhanced Th1 immune responses, including increased CD4 and CD8 T cells, IFNγ release in the lung, and viral antigen-specific IFNγ response by lymphocytes from the lung or local draining lymph nodes.
GM-CSF is released by airway epithelial and endothelial cells, fibroblasts, macrophages, and carcinoma cells in response to a number of stimuli in vitro17,32-36 and has been found increased in the lungs during a number of pulmonary immune conditions.17However, the precise role of GM-CSF in the pathogenesis of these pulmonary conditions has remained poorly understood. Results from our current study have not only demonstrated a potent effect of GM-CSF on the differentiation and activation of DCs and macrophages but also revealed the nature of a novel DC phenotype driven by GM-CSF during an immune response to pulmonary viral infection. Our results suggest that GM-CSF has a weak effect on the differentiation of a DC population that is characterized by CD11blow and CD11cbright. However, GM-CSF dramatically induces the emergence of a DC population characterized by CD11bbright, CD11cbright, MHC IIbright, and B7.1bright, in addition to its activating effect on macrophages characterized by CD11bbright, CD11clow, MHC IIbright, and B7.1bright. Recently, Suda et al37 have reported that the number of DC precursors present in the pulmonary vascular compartment is 76% greater than that in the vena cava, and these precursors, on exposure to GM-CSF in vitro, have a strong ability to activate alloreactive T cells. It is possible that some of the GM-CSF-expanded DCs observed in our study derived from such precursors. However, Palucka et al38 have demonstrated that human macrophages could convert into DC in vitro in the presence of GM-CSF. Our current study has provided the first in vivo evidence to support such conversion. We found that macrophage population was induced 5 days after GM-CSF transgene delivery and dramatically decreased at the time when a myeloid DC population emerged at day 12. Thus, these findings, together with the fact that GM-CSF is a well-known stimulator of macrophage proliferation,17,18,39strongly suggest that GM-CSF-induced DCs derived primarily from expanded macrophages. In further support of our findings, such CD11b-expressing myeloid DCs have recently been identified in lymphoid organs.11,25 Compared with lymphoid-derived DCs, myeloid-derived DCs are believed to play a differential immune-stimulatory role in host defense.30,40-42 This selective effect on myeloid DCs by GM-CSF suggests that GM-CSF is a proimmune cytokine in the lung. Indeed, we observed a markedly enhanced immune response at both cellular and cytokine levels during adenoviral infection in the lung. Our recent demonstration that airway allergic sensitization to repeated airway ovalbumin challenges only occurred in mice that expressed GM-CSF transgene has lent further support to a key proimmune role of GM-CSF in the lung.43
In contrast to a pronounced effect of GM-CSF on macrophage-derived DCs, the effect of GM-CSF on CD11blow/CD11cbright DC population was minimal. Although GM-CSF transgene expression did not markedly expand this cell population in the lung, it enhanced the level of MHC II expression. Of note, GM-CSF did not markedly enhance B7.1 expression on this cell population. It is thus possible that enhanced MHC II expression on these cells was an indirect effect from GM-CSF via its effect on IFNγ release. This notion is supported by an in vitro observation by Larsen et al44 that, different from GM-CSF, IFNγ selectively induces MHC II but not B7.1 expression on murine DC or even inhibits B7.1 expression on Langerhans cells.45GM-CSF has been shown to be unable to induce the differentiation of DCs of lymphoid origin.11,27 We also found that these lung residential DCs expressed little CD11b but bright CD11c and a moderate level of B7.1 and that GM-CSF had little effect on their differentiation. Together, these findings suggest that these cells are likely of lymphoid origin. However, consistent with the other study,46 we have failed to detect any CD8α expression, a lymphoid-lineage marker, on any of the DCs in the lung (data not shown), suggesting a lack of such DCs in the lung. Nevertheless, it is worthwhile to bear in mind that not all lymphoid DCs express CD8α.27
In summary, we have shown a potent effect of GM-CSF on induction of two important APC populations, DCs and macrophages, during pulmonary viral infection. We have also revealed that GM-CSF exerts its effect on a myeloid-derived but not “lymphoid”-derived DC population. Our findings not only suggest that GM-CSF is a potent immune enhancer during pulmonary immune responses but also provide the rationale for using GM-CSF as an immune adjuvant to prevent or treat pulmonary infectious or malignant diseases, in particular, those occurring in immune-compromised hosts.
Acknowledgments
The authors wish to thank Anna Zganiacz and Mary Jo Smith for their excellent technical assistance.
Supported by grants from the Medical Research Council (MRC) of Canada, McMaster University, Hamilton Health Sciences Corporation, and St Joseph's Hospital.
J.W. is an MRC-CLA (Canadian Lung Association) fellow. Z.X. is an MRC scholar and holder of Ontario Premier's Research Excellence Award.
Reprints:Zhou Xing, Health Sciences Centre, Rm 4H19, Department of Pathology & Molecular Medicine, McMaster University, Hamilton, Ontario, Canada L8N 3Z5; e-mail: xingz@fhs.csu.mcmaster.ca.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.











This feature is available to Subscribers Only
Sign In or Create an Account Close Modal