Abstract
Opioid peptides affect different immune functions. We present evidence that these effects could be mediated by the modulation of TH1/TH2 cytokine production. BALB/cJ mice were immunized with 50 or 100 μg of the protein antigen keyhole-limpet hemocyanin (KLH), and treated acutely or chronically with the opioid antagonist naloxone. One and 2 weeks after immunization, the production of cytokines by splenocytes was evaluated by in vitro restimulation with KLH. The acute and chronic treatment with the opioid receptor antagonist naloxone decreased the production of interleukin (IL)–4 by splenocytes of BALB/cJ mice. In contrast, IL-2 and interferon-γ levels increased after naloxone treatment. Finally, the opioid antagonist diminished the serum immunoglobulin G anti–KLH antibody titers. These results suggest that naloxone increases TH1 and decreases TH2 cytokine production. The effect of naloxone could be ascribed to the removal of the regulatory effects exerted by endogenous opioid peptides, which could therefore activate TH2 and suppress TH1 cytokines.
Evidence for the involvement of the endogenous opioid peptides β-endorphin (BE) and met-enkephalin in the regulation of immune function is continuing to accumulate. Although in vitro experiments gave rise to contradictory results, showing both immunosuppressive and immunoenhancing effects of opioid peptides,1 most data indicate that, in vivo, particularly BE possesses immunosuppressive properties.1-4
We have shown that BE exerts a physiological inhibitory effect on cellular-mediated immune responses5 and that the administration in the rodent of the opiate receptor antagonist naloxone or of immunoglobulins, which neutralize the activity of BE, increases mitogen-induced splenocyte proliferation within minutes.5 6
Moreover, previous work from our group suggested a possible role of the endogenous opioids in the modulation of TH1/TH2 balance. In fact, the opioid receptor antagonist naloxone has been shown to affect TH1-mediated phenomena, such as skin graft rejection in mice7,8 and autoimmune encephalomyelitis in rats.9 10
The target of the effects of BE in the immune system could be the balance of the 2 types of mature T helper cells, TH1 and TH2. The TH1 and TH2 cells produce different patterns of cytokines: TH1 cells produce interleukin (IL)–2, interferon (IFN)–γ, and lymphotoxins, whereas TH2 cells produce IL-4, IL-5, IL-6, IL-10, and IL-13. TH1 cells are involved mostly in cell-mediated reactions, while the TH2 cytokines are commonly found in association with strong antibody and allergic responses.11 12
In the attempt to better define a possible physiological role for opioid peptides in the modulation of the balance between TH1 and TH2 cell types, in the present paper we analyze the effect of the in vivo treatment with the opioid receptor antagonist naloxone on splenocyte production of the TH1 cytokines IFN-γ and IL-2 and of the TH2 cytokine IL-4 in BALB/cJ mice immunized with the protein antigen keyhole-limpet hemocyanin (KLH). Serum IgM and IgG anti-KLH antibody titers were also evaluated.
Materials and methods
Animals
BALB/cJ male mice, 18 to 20 g body weight (Charles River, Calco, Italy), were used in the study. Animals were kept on a 12-hour light-dark cycle with water and food ad libitum and were housed 5 mice to a cage. Mice were allowed to acclimate to the laboratory conditions for a minimum of 10 days before experimental manipulations. Each experimental group consisted of 12 to 16 animals.
Immunization and naloxone treatment
Mice were injected intraperitoneally with 50 or 100 μg of the protein antigen KLH (Sigma, St Louis, MO) in a volume of 0.2 mL of saline and were killed for cytokine evaluation 7 or 14 days after immunization.
The opioid receptor antagonist naloxone HCl (S.A.L.A.R.S., Como, Italy) was injected subcutaneously at a dose of 5 mg/kg. The dose was chosen on the basis of our previous studies, which showed that this dose induces a relevant modulation of some immune parameters.5,6 16 One group of mice received only 1 acute injection of naloxone at the moment of immunization, while a second group of animals was chronically treated with subcutaneous naloxone once daily starting from the immunization day. The animals received the last naloxone injection 30 minutes before sacrifice. Control animals were immunized with KLH and were treated acutely or chronically with saline. Moreover, in order to rule out the possibility that the stress caused by handling and injections had an effect on cytokines, a group of animals were immunized with KLH and were not further handled or injected until the day of sacrifice.
Splenocyte cultures
One or 2 weeks after immunization, animals were killed by decapitation, and spleens were aseptically removed. Cells were teased from the spleens with the use of 20-gauge sterile needles through an incision made in the spleen capsule,5 centrifuged, and washed twice in Hanks balanced salt solution. Cells were suspended in RPMI supplemented with 10% fetal calf serum (FCS), 1% glutamine, 2% antibiotics, 10 mmol/L Hepes, and 50 mmol/L 2-ME (all from Sigma) and were plated at 7 × 106 cells in 24-well plates containing a final concentration of 80 μg/mL KLH in a total volume of 1 mL. Plates were incubated at 37°C in 5% CO2 and 95% air. Supernatants were collected after 48 and 72 hours in culture and stored frozen at −80°C for cytokine analysis. The concentration of 80 μg/mL used in vitro was chosen on the basis of previous pilot experiments. This concentration, in fact, induced an easily measurable, but not maximal, stimulation of cytokine production, in order to be able to detect a possible stimulation as well as any inhibition induced by naloxone treatment.
Measurement of interleukin-2, interleukin-4, and interferon-γ
The levels of IL-2 in 48- and 72-hour supernatants were determined by enzyme-linked immunosorbent assay (ELISA) protocol as standardized by Pharmingen (San Diego, CA). Briefly, the anti–IL-2 capture monoclonal antibody (mAb) 1(μg/mL) was absorbed on a polystyrene 96-well plate, and the IL-2 present in the sample was bound to the antibody-coated wells. The biotinylated anti–IL-2 detecting mAb (0.5 μg/mL) was added to bind the IL-2 captured by the first antibody. After washing, avidin-peroxidase (Sigma) was added to the wells to detect the biotinylated detecting antibody, and finally 2,2′-azino-bis (3ethylbenthiazoline-6-sulfonic acid) (ABTS, Sigma) substrate was added, and a colored product was formed in proportion to the amount of IL-2 present in the sample, which was measured at optical density 405 nm.
IL-4 production was measured in 48- and 72-hour supernatants with the use of the ELISA protocol outlined above with mAb anti–IL-4 at the same concentrations used for IL-2. IFN-γ was also evaluated with the same ELISA protocol except for the use of anti–IFN-γ capture and detecting antibody at 2 μg/mL and 1 μg/mL, respectively (all mAbs were from Pharmingen).
Anti–keyhole-limpet hemocyanin antibody enzyme-linked immunosorbent assay
Blood was collected at the time of sacrifice, and sera were stored at −20°C. Plates were coated overnight with 10 μg/mL KLH in a carbonate coating buffer, pH 9.6. Mice sera were diluted 1:25, 1:50, and 1:100 in phosphate-buffered saline (PBS)/Tween containing 1 mol/L NaCl and incubated for 3 hours at 37°C. Alkaline-phosphate–conjugated goat anti-mouse IgM (chain specific, Sigma), diluted 1:6000 in PBS/Tween, or anti-mouse IgG (chain specific, Sigma), diluted 1: 6000 in PBS/Tween, was then added, and plates were incubated overnight at 4°C. After washing, p-nitrophenyl-phosphate substrate at 1 mg/mL in carbonate buffer was added, and the colored product formed was measured at OD 405 nm. Serum from nonimmunized mice served as control.
Statistical analysis
Cytokine data were analyzed by means of 2-way analysis of variance (ANOVA), with treatments and hours of culture, doses of KLH, and time of immunization as factors, followed by a Tukey t test for multiple comparisons. Acute and chronic saline groups were treated as a single group. Serum antibody titers were analyzed with 2-way ANOVA, with treatments and serum dilution as factors.
Results
Keyhole-limpet hemocyanin–stimulated cytokine production
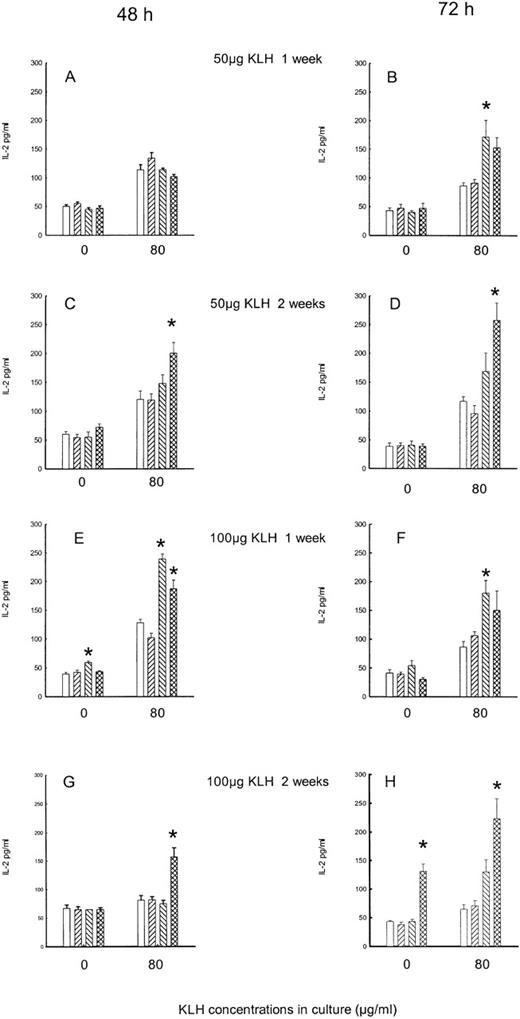
The stress linked to simple manipulation and handling of animals did not modify cytokine secretion, since levels of all cytokines did not differ in acutely or chronically saline-injected mice, in comparison with mice that were not further injected after KLH immunization (data not shown). Moreover, since no difference was observed in acute or chronically saline-treated animals, in the statistical analysis they were considered as a single group. Naloxone treatment induces significant changes. Figure 1 reports the effect of the acute and chronic treatment with naloxone on IL-2 production by splenocytes.
Effect of acute and chronic naloxone treatment on KLH-stimulated IL-2 production in vitro by splenocytes.
Animals were immunized with either 50 μg KLH (panels A-D) or 100 μg KLH (panels E-H) and killed after 1 week (panels A,B,E,F) or 2 weeks (panels C,D,G,H). Spleen cells were cultured with 0 or 80 μg/ml KLH for 48 hours (panels A,C,E,G) and 72 hours (panels B,D,F,H). Acute saline control is represented by white bars; chronic saline control is represented by right-slanted striped bars; acute naloxone treatment is represented by left-slanted striped bars; chronic naloxone treatment is represented by hatched bars. Results are expressed as mean ± SE. * = P < .01 versus saline controls.
Effect of acute and chronic naloxone treatment on KLH-stimulated IL-2 production in vitro by splenocytes.
Animals were immunized with either 50 μg KLH (panels A-D) or 100 μg KLH (panels E-H) and killed after 1 week (panels A,B,E,F) or 2 weeks (panels C,D,G,H). Spleen cells were cultured with 0 or 80 μg/ml KLH for 48 hours (panels A,C,E,G) and 72 hours (panels B,D,F,H). Acute saline control is represented by white bars; chronic saline control is represented by right-slanted striped bars; acute naloxone treatment is represented by left-slanted striped bars; chronic naloxone treatment is represented by hatched bars. Results are expressed as mean ± SE. * = P < .01 versus saline controls.
In the acute experiments, animals were treated with 1 injection of naloxone or saline at the moment of in vivo immunization with 50 or 100 μg KLH, and 7 or 14 days later, splenocytes were cultured in vitro with or without KLH for 48 and 72 hours. In the chronic experiments, animals were treated daily with naloxone or saline from the day of immunization to the day of sacrifice. With respect to the levels of IL-2 in all of the in vitro KLH-stimulated cultures, the 2-way ANOVA on data revealed a main effect of both acute and chronic treatment (F2,300 = 37.2, P < .001), with no significant difference between 48- and 72-hour cultures. Also, no significant difference was observed between 50 and 100 μg KLH (F1,300 = 0.6, P = .4) or between 1 and 2 weeks of immunization (F1,300 = 0.83,P = .36). The effect of naloxone treatments on spontaneous IL-2 production was still significant, although less evident (F2,300 = 4.54,P = .011). Moreover, in the saline group, the IL-2 levels were lower in 72-hour culture than in 48-hour culture (Tukey, q = 2.83, P < .05). In terms of the results in detail, when animals were immunized in vivo with 50 μg KLH, 1 week after immunization no effect of naloxone treatment was observed in the 48-hour cultures (Figure 1A); however, in the 72-hour supernatant, a significant increase in KLH-stimulated production was present after acute treatment with naloxone (Figure 1B). After 14 days of immunization with 50 μg KLH, a significant increase of IL-2 in KLH-stimulated cells was observed in 48-hour (Figure 1C) and 72-hour cultures (Figure 1D) after chronic naloxone treatment. The production of this cytokine showed an increase after naloxone treatment in the animals immunized with 100 μg KLH: 1 week after immunization, the enhancement of IL-2 production was evident in the 48-hour cultures after acute naloxone treatment in the unstimulated cultures and after both treatments in the stimulated ones (Figure 1E), while an increase was present after acute naloxone treatment in the 72-hour stimulated supernatant (Figure 1F). At 14 days after immunization, the increase was present after chronic naloxone treatment in KLH-stimulated cells in the 48-hour cultures, while in the 72-hour cultures the effect of chronic naloxone was also present in the unstimulated cell cultures (Figure 1G, 1H).
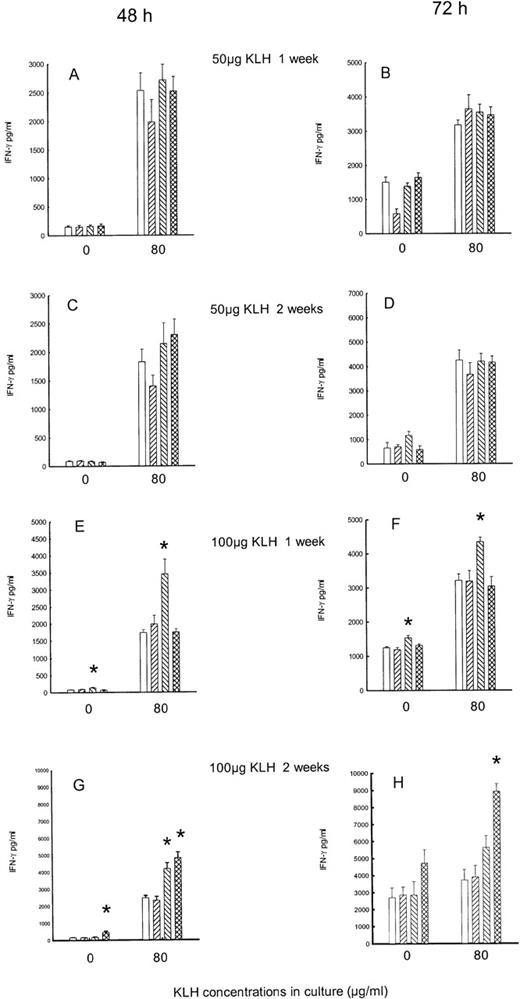
Independently of treatment, IFN-γ levels were higher in the 72-hour than in the 48-hour cultures (spontaneous secretion: F1,313 = 175.3, P < .001; KLH stimulated: F1,317 = 10.123, P < .001). Naloxone treatments induce a significant increase of IFN-γ levels in the in vitro KLH-stimulated cultures (F2,317 = 10.1,P < .001). Moreover, an interaction between the dose of KLH used for immunization and naloxone effect was evident, as the treatments were effective only in the animals immunized with 100 μg KLH (F2,317 = 11.26, P = .001). In fact, in the animals immunized with 50 μg KLH, the treatment with naloxone did not affect the production of IFN-γ (Figure2A-2D). On the contrary, in the 100 μg KLH–immunized animals, a significant increase of the cytokine was present. When IFN-γ was measured 7 days after immunization, a significant enhancement was evident in both the 48- and the 72-hour supernatant after acute treatment with naloxone for unstimulated as well as in vitro KLH-stimulated cultures (Figure 2E, 2F). At 2 weeks after immunization with 100 μg KLH, in 48-hour stimulated cultures a significant increase of IFN-γ was observed after chronic naloxone treatment in unstimulated cells, and after both acute and chronic naloxone treatment in KLH-stimulated cells (Figure 2G). In the 72-hour culture, an increase after chronic naloxone treatment in KLH-stimulated cells was evident (Figure 2H).
Effect of acute and chronic naloxone treatment on KLH-stimulated IFN-γ production by splenocytes.
Animals were immunized with either KLH (panels A,B,C,D) or 100 μg KLH (panels E,F,G,H) and killed after 1 week (panels A,B,E,F) or 2 weeks (panels C,D,G,H). Spleen cells were cultured with 0 or 80 μg/ml KLH for 48 h (panels A,C,E,G) and 72 h (panels B,D,F,H). Acute saline control is represented by white bars; chronic saline control is represented by right-slanted striped bars; acute naloxone treatment is represented by left-slanted striped bars; chronic naloxone treatment is represented by hatched bars. Results are expressed as mean ± SE. * = P < .01 versus corresponding saline controls.
Effect of acute and chronic naloxone treatment on KLH-stimulated IFN-γ production by splenocytes.
Animals were immunized with either KLH (panels A,B,C,D) or 100 μg KLH (panels E,F,G,H) and killed after 1 week (panels A,B,E,F) or 2 weeks (panels C,D,G,H). Spleen cells were cultured with 0 or 80 μg/ml KLH for 48 h (panels A,C,E,G) and 72 h (panels B,D,F,H). Acute saline control is represented by white bars; chronic saline control is represented by right-slanted striped bars; acute naloxone treatment is represented by left-slanted striped bars; chronic naloxone treatment is represented by hatched bars. Results are expressed as mean ± SE. * = P < .01 versus corresponding saline controls.
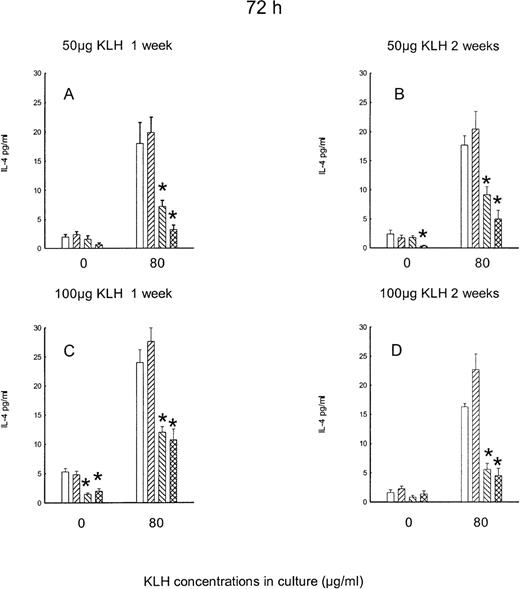
Independently of treatment, the production of IL-4 was barely detectable in the 48-hour cultures (data not shown). Figure3 reports the levels of IL-4 in the 72-hour cultures. The levels of IL-4 in the unstimulated cultures were also very low. The effect of naloxone treatment on KLH-stimulated IL-4 production is indeed opposite to the one observed on IL-2 and IFN-γ. A significant main effect of naloxone is in fact present (F2,151 = 38.29, P < .001), since both acute and chronic naloxone decrease IL-4 production. The effect is present with both doses of in vivo KLH and is present 1 week as well as 2 weeks after immunization.
Effect of acute and chronic naloxone treatment on KLH-stimulated IL-4 production in vitro by splenocytes.
Animals were immunized with either 50 μg KLH (panels A,B) or 100 μg KLH (panels C,D) and killed after 1 week (panels A,C) or 2 weeks (panels B,D). Spleen cells were cultured with 0 or 80 μg/ml KLH for 72 h. Acute saline control is represented by white bars; chronic saline control is represented by right-slanted striped bars; acute naloxone treatment is represented by left-slanted striped bars; chronic naloxone treatment is represented by hatched bars. Results are expressed as mean ± SE. * = P < .01 versus saline controls.
Effect of acute and chronic naloxone treatment on KLH-stimulated IL-4 production in vitro by splenocytes.
Animals were immunized with either 50 μg KLH (panels A,B) or 100 μg KLH (panels C,D) and killed after 1 week (panels A,C) or 2 weeks (panels B,D). Spleen cells were cultured with 0 or 80 μg/ml KLH for 72 h. Acute saline control is represented by white bars; chronic saline control is represented by right-slanted striped bars; acute naloxone treatment is represented by left-slanted striped bars; chronic naloxone treatment is represented by hatched bars. Results are expressed as mean ± SE. * = P < .01 versus saline controls.
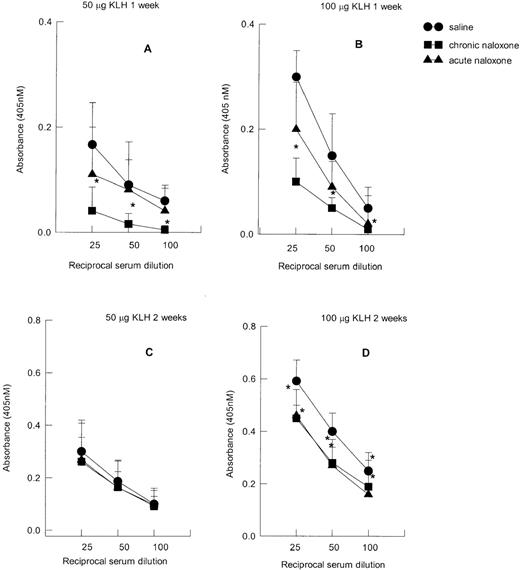
Keyhole-limpet hemocyanin antibody response
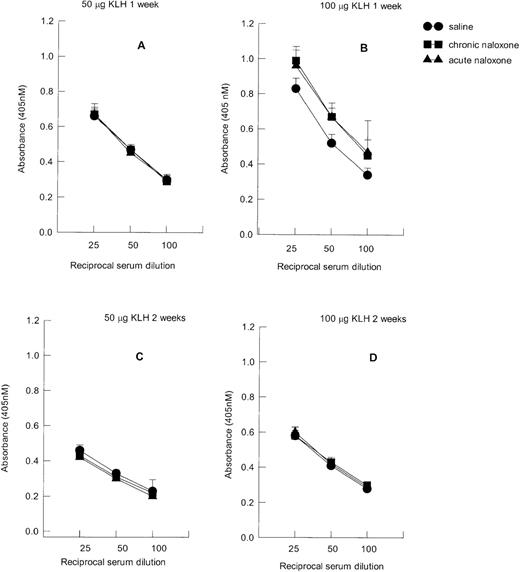
KLH IgM serum titers are reported in Figure4. The IgM titers were higher (F1,71 = 8.21, P = .006) in the groups of animals immunized with 100 μg (Figure 4B) in comparison with those immunized with 50 μg (Figure 4A). Moreover, higher titers were present 1 week after immunization (Figure 4A, B) than 2 weeks after immunization (Figure C, D) (F1,71 = 21.7,P < .001). Figure 4 also shows that neither acute nor chronic naloxone treatment modified IgM serum titers.
Effect of acute and chronic naloxone treatment on serum IgM anti-KLH antibody response.
Either animals were immunized with 50 μg KLH and killed after 1 week (A) or 2 weeks (C), or they were immunized with 100 μg KLH and killed after 1 week (B) or 2 weeks (D). Results are expressed as mean ± SE of 10 animals per group. * = P < .01 versus saline control.
Effect of acute and chronic naloxone treatment on serum IgM anti-KLH antibody response.
Either animals were immunized with 50 μg KLH and killed after 1 week (A) or 2 weeks (C), or they were immunized with 100 μg KLH and killed after 1 week (B) or 2 weeks (D). Results are expressed as mean ± SE of 10 animals per group. * = P < .01 versus saline control.
As reported in Figure 5, in the saline-treated animals immunized with 50 μg KLH (Figure 5A), the titers of anti-KLH IgG were lower than in the animals treated with 100 μg (Figure 5B) (F1,83 = 9.08, P = .003); moreover, higher titers were present 2 weeks after immunization (Figure 5C, D) in comparison with 1 week after (Figure 5A, B) (F1,83 = 30.17, P < .001).
Effect of acute and chronic naloxone treatment on serum IgG anti-KLH antibody response.
Animals were immunized with either 50 μg KLH and killed after 1 week (panel A) or 2 weeks (panel C), or with 100 μg KLH and killed after 1 week (panel B) or 2 weeks (panel D). Results are expressed as mean ± SE of 10 animals per group. * = P < .01 versus saline control.
Effect of acute and chronic naloxone treatment on serum IgG anti-KLH antibody response.
Animals were immunized with either 50 μg KLH and killed after 1 week (panel A) or 2 weeks (panel C), or with 100 μg KLH and killed after 1 week (panel B) or 2 weeks (panel D). Results are expressed as mean ± SE of 10 animals per group. * = P < .01 versus saline control.
A significant overall effect of naloxone treatment on IgG was present (F2,251 = 10.29, P < .001 (Figure 5).The post hoc comparison indicated that the antibody titers were in fact reduced in the animals chronically treated with naloxone 1 week after immunization with 50 μg KLH (P < .05) (Figure 5A, B), while in animals immunized with 100 μg KLH the IgG titers appeared to be reduced after both acute and chronic naloxone treatment (P < .05) (Figure 5D). No significant interactions (week × KLH dose × treatment) were present (F2,251 = .765, P = .466).
Discussion
The administration of the opioid antagonist naloxone appears to affect the pattern of cytokine production by splenocytes of BALB/cJ mice immunized with the protein antigen KLH. Acute as well as chronic naloxone treatments decrease IL-4 production in both unstimulated and in vitro KLH-stimulated splenocyte cultures. On the contrary, the production of the TH1 cytokines IL-2 and IFN-γ is increased by naloxone. Although slight differences are present depending on the dose of KLH used to immunize animals, the duration of immunization, and the duration of in vitro culture, no qualitative difference is observed: TH1 cytokines show a trend to increase and TH2 cytokines to decrease after naloxone.
Independently of treatment, although higher levels of IFN-γ and IL-4 are present after 72 hours of in vitro stimulation, the opposite is true for IL-2, since a decline of the levels of the cytokine is observed. IL-2 acts as an autocrine growth factor for T cells: IL-2, secreted by TH cells following stimulation by antigen, upregulates the expression of its own receptors, and the subsequent binding of IL-2 to this high-affinity receptor results in proliferation of the antigen-activated T cells. However, the half-life of cell-bound IL-2 is 25 to 30 minutes, with the IL-2/IL-2R complex being removed from the cell surface by internalization.17 The fact that lower amounts of free IL-2 are found at cells cultured for longer times could therefore be explained by massive utilization of the cytokine by the activated cells.
When TH1 cytokines are evaluated 7 days after immunization with the highest dose of KLH, acute treatment with naloxone is sufficient to increase TH1 cytokines, while chronic treatment is more efficacious in increasing TH1 cytokines 2 weeks after immunization.
We do not at the moment have a satisfactory explanation for this time course.
IL-4 production is greatly affected by naloxone treatment. A significant decrease of this cytokine is in fact present at both doses of antigen, at both 1 and 2 weeks after immunization, and after treatment with both acute and chronic naloxone. In BALB/cJ mice, one type of T helper population dominates over the other. BALB/cJ mice are susceptible to infection by intracellular pathogens, have a weak cell-mediated immune response, and consistently present a TH2 dominance.18,19 We cannot say, at the moment, whether naloxone could primarily act on the dominant TH2 population, affecting IL-4 secretion. Since IL-4 is inhibitory for the differentiation and effector function of the TH1 subset,11 12 the IL-4 decrease induced by naloxone could thereafter permit the production of TH1 cytokines. Alternatively, the effect on TH1 cytokines can precede and thereafter affect lL-4 production.Further studies are in progress in order to find a potential temporal dependence.
Consistent with the decrease of IL-4 production, naloxone also affects the primary antibody response, as shown by the low serum IgG levels that are present in the mice after naloxone treatment, confirming the link between IL-4 and antibody production.11 12 On the whole, the data obtained suggest that treatment with naloxone tends to skew the T-cell balance toward a TH1 pattern.
Given that naloxone is an almost pure antagonist at the μ-opioid receptor and is devoid of any intrinsic activity at the μ receptor, the effects of the drug are likely to be due to the removal of a regulatory tone exerted by endogenous opioid peptides. We previously showed that, in rat and human, naloxone increased T-lymphocyte proliferation, increased NK activity, and worsened the development of inflammatory responses.5,20 Similar effects were also shown by our laboratory with the use of a neutralizing antibody against the opioid peptide BE.6 20 It can therefore be hypothesized that the modulation of the TH1/TH2 cytokine pattern induced by naloxone could be due to the removal of BE effects, although the involvement of other opioid peptides (eg, met-enkephalin) could also be possible.
We and others have demonstrated that BE is produced and released by the cells of the immune system1,3,4,21,22 and that it can bind specific opioid receptors present on immunocytes.1 5Evidence therefore exists for an autocrine/paracrine activity of the opioid.
The data reported in this paper contribute a new insight on the role of opioid peptides in the modulation of the immune system. It becomes in fact questionable to claim a unique immunosuppressive or immunostimulatory role for opioid peptides and BE. It can rather be suggested that the opioid might exert an inhibitory control of TH1 cell populations, probably through the stimulation of TH2 cell types. In line with this hypothesis, the literature is often contradictory on the effects of opioid peptides on the immune system.1 3 Depending on the immune function evaluated (eg, cellular versus humoral) and on the preexisting TH1/TH2 balance (eg, after previous exposure of animals to different pathogens or by genetic predisposition), inhibition or stimulation of classical laboratory immune parameters has been reported.
The effect of chronic naloxone treatment on TH1 cytokines seems to be in contrast with what we previously reported in a different experimental model.5 In the earlier experiments, in fact, naloxone was given chronically to naive rats with a resting immune system, and lymphoproliferation, tested in vitro upon mitogen stimulation, was decreased. In the present experiments, the opioid antagonist is administered to immunized animals that present an in vivo stimulated immune response. Similarly, chronic naloxone treatment was able to increase TH1 cytokines in skin graft experiments. In this case, the drug was also given to an already activated immune system. These observations indicate that the status of the immune system of the host can be relevant in order to direct the opioid control of the immune responses.
The disruption of a correct TH1/TH2 balance is involved in the development of several immune diseases.23As a consequence, the effects that opioid peptides and naloxone exert on TH cell types can be relevant in immunopathology. Consistently, we demonstrated that the administration of naloxone, which, as shown in the present paper, increases the production of TH1 cytokines, worsened the development of experimental autoimmune encephalomyelitis9 where the TH1-type effector cell response is dominant.10Moreover, in a murine model of skin allograft rejection, which is driven by the development of a TH1 response,7we showed that naloxone significantly anticipated, while BE delayed, the onset of the rejection.8 We can hypothesize that the use of opioid peptides or their modulation and/or the use of opioid antagonists might be interesting new tools to achieve immune deviation.
In conclusion, our data indicate a role for opioid peptides in the modulation of TH1/TH2 balance in the complex network of immunoregulatory signals and offer a clue to the interpretation of conflicting results present in the literature.
Reprints:Paola Sacerdote, Dept. Pharmacology, via Vanvitelli 32, 20129 Milano, Italy; e-mail: paola.sacerdote@unimi.it.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal