Abstract
In a previous study, we reported that a single injection of cyclophosphamide (CTX) in tumor-bearing mice resulted in tumor eradication when the animals were subsequently injected with tumor-sensitized lymphocytes. Notably, CTX acted by inducing bystander effects on T cells, and the response to the combined CTX/adoptive immunotherapy regimen was inhibited in mice treated with antibodies to mouse interferon (IFN)–/β. In the present study, we have investigated whether CTX induced the expression of type I IFN, and we have characterized the CTX effects on the phenotype of T cells in normal mice. CTX injection resulted in an accumulation of type I IFN messenger RNA in the spleen of inoculated mice, at 24 to 48 hours, that was associated with IFN detection in the majority of the animals. CTX also enhanced the expression of the Ly-6C on spleen lymphocytes. This enhancement was inhibited in mice treated with anti–type I IFN antibodies. Moreover, CTX induced a long-lasting increase in in vivo lymphocyte proliferation and in the percentage of CD44hiCD4+ and CD44hiCD8+T lymphocytes. These results demonstrate that CTX is an inducer of type I IFN in vivo and enhances the number of T cells exhibiting the CD44hi memory phenotype. Since type I IFN has been recently recognized as the important cytokine for the in vivo expansion and long-term survival of memory T cells, we suggest that induction of this cytokine may explain at least part of the immunomodulatory effects observed after CTX treatment. Finally, these findings provide a new rationale for combined treatments with CTX and adoptive immunotherapy in cancer patients.
Cyclophosphamide (CTX) is a widely used chemotherapeutic agent in cancer therapy1 and in some autoimmune diseases.2 Combined regimens with CTX and immunotherapy are used in clinical trials with cancer patients.3-6 However, the mechanisms of CTX action are not fully understood. On the one hand, CTX can act as a conventional anticancer drug by affecting the in vivo proliferation of tumor cells.1 On the other hand, CTX can exhibit immunomodulatory effects, which may play an important role in the antitumor response.7-20 In this regard, many studies had reported that CTX can increase the efficacy of immunotherapeutic agents by removing tumor-induced suppressor T cells.21-25 However, the nature of these suppressor cells is still a matter of debate.26
By using transplantable mouse tumor models, we have recently reported that CTX can induce marked effects on T cells, which are important for a successful tumor eradication in response to adoptive immunotherapy.27 In particular, the results of an ensemble of experiments aimed at understanding the mechanisms underlying the synergistic antitumor response observed in tumor-bearing mice injected with CTX and tumor-sensitized lymphocytes have suggested that CTX acts by means of bystander effects (possibly through production of T-cell growth factors occurring during the rebound events after drug administration) that may sustain the proliferation, survival, and activity of the transferred lymphocytes.27 In considering which CTX-induced factor(s) can be important for explaining the effects on T cells and the antitumor response observed in tumor models, we focused our attention on type I interferon (IFN). In fact, the working hypothesis that this cytokine could be induced by CTX was suggested by 2 major considerations: (1) In our previous study,27 the synergistic antitumor response induced by the combined treatment with CTX and immune lymphocytes was abolished when mice were treated with a potent preparation of antibodies to mouse IFN-α/β,27 suggesting that this cytokine was somehow induced in our experimental system with tumor-bearing mice. (2) Recent reports have clearly indicated that type I IFN is the major factor responsible for the in vivo proliferation and long-term survival of certain subsets of T cells (especially CD44hiCD8 T lymphocytes) in response to viruses or other stimuli.28 29
All of this prompted us to investigate whether the injection of CTX in normal mice could result in any induction of type I IFN and to characterize the effects of CTX on T cells.
The results reported in this article demonstrate that CTX can induce type I IFN expression, which may represent an important mediator of the immunomodulatory effects of CTX, especially with regard to the expansion and persistence of memory T cells.
Materials and methods
Mice and in vivo treatments
Six- to 8-week-old male DBA/2 mice were obtained from Charles River Breeding Laboratories (Calco, Italy). All mice were treated in accordance with the European Community Guidelines. For bromodeoxyuridine (BrdU)-incorporation studies, mice were given drinking water containing BrdU (Sigma Chemical, St Louis, MO) at 0.8 mg/mL, which was made fresh and changed daily. CTX (Sigma) was dissolved in 0.15 mol/L NaCl (saline) and filter sterilized, and 0.5 mL of freshly prepared solution was injected intraperitoneally (CTX-treated mice). Polyinosinic-polycytidylic acid (poly [I:C]) (Sigma) was dissolved in saline at a concentration of 10 mg/mL. Frozen aliquots were thawed just before each experiment, and 0.15 mg of poly (I:C) was injected intraperitoneally in a volume of 0.15 mL of saline solution. Control preparations consisted of saline unless otherwise stated.
Antibody to mouse interferon /β
Sheep antibodies to mouse interferon α/β and normal sheep immunoglobulin were a generous gift from Dr Ion Gresser (Villejuif, France). The origin of sheep antibodies to mouse interferon α/β (sheep no. 1) and normal sheep immunoglobulin, their purification, and their assay have been previously described in detail.30 31Antibody titer was 1.6 × 106 neutralizing units against 8 interferon units; mice were injected with 0.2 mL of a 1:10 dilution on day −1, +2, and +4 with respect to CTX administration.
Preparation of spleen and lymph node cells suspensions
Mice were killed and spleen and lymph nodes were removed aseptically. Lymph node cells were pooled from cervical, axillary, inguinal, and mesenteric nodes. Tissues were gently disaggregated with a tissue homogenizer for 3 minutes at room temperature in lysis buffer (0.16 mol/L tromethamine [Tris]-buffered NH4Cl). After erythrocyte lysis, cells were washed in Rosewell Park Memorial Institute (RPMI) 1640 medium with 10% fetal calf serum (FCS), passed through a cell strainer (Falcon 2350, Becton Dickinson, Orlando, FL), and resuspended to approximately 1 × 107 viable cells/mL (determined by trypan blue exclusion) in RPMI 1640 medium with 2% FCS.
Flow cytometry
Monoclonal antibodies (mAbs) used to stain cell surface antigens were the following: biotinylated anti–Ly-6C (104-2.1, mouse immunoglobulin [Ig]–G) (PharMingen, San Diego, CA), biotinylated anti-CD44 (IM7, rat IgG) (PharMingen), phycoerythrin (PE)–conjugated anti-CD4 (GIBCO-BRL, Gaithersburg, MD), PE-conjugated anti-CD8 (GIBCO-BRL). Bound biotinilated L antibodies were detected with red 670-streptavidin (GIBCO-BRL). After surface staining by conventional techniques,32 cells were washed, resuspended in cold saline, and fixed by dropwise addition of cold 95% ethanol for 30 minutes on ice. For BrdU-incorporation studies, an intracellular staining method33 was used. Briefly, the cells were washed with PBS, then incubated with PBS containing 1% paraformaldehyde and 0.01% Tween 20 for 30 minutes. Cells were pelleted, then incubated with 50 Kunitz units DNAse I (Sigma) in 0.15 mol/L NaCl, 4.2 mmol/L MgCl2, pH 5.0, for 10 minutes. After washing, cells were incubated with fluorescein isothiocyanate–conjugated anti-BrdU mAb (Becton Dickinson, Mountain View, CA) and analyzed on FACsort flow cytometer (Becton Dickinson). A total of 10 000 events per sample were collected. Erythrocytes, dead cells, and tissue debris were excluded according to forward- and side-scatter properties in order to gate only live lymphocyte populations.
Interferon titration
Biological activity of serum IFN was determined as described elsewhere.34 One of the units, as expressed in the text, is the equivalent of 4 IFN reference units.
Detection of murine and β interferon messenger RNAs in the spleen by reverse transcriptase (RT)–polymerase chain reaction (PCR)
At different times after treatments, mice were killed and their spleens were immediately removed and directly homogenized with a tissue homogenizer in 2mL RNAzol B (Bioteck, Houston, TX) in an ice bath. Total RNA was then subjected to chloroform extraction and isopropanol precipitation. RNA was resuspended and treated with ribonuclease-free DNAse (Boehringer Mannheim, Germany), further purified, and then quantitated by UV absorbance at 260 nm. One microgram of total RNA was incubated for 5 minutes with oligo-(dt) 12-18 (Pharmacia, Uppsala, Sweden) at 75°C, cooled at room temperature, and reverse-transcribed by 200 U of Moloney murine leukemia virus reverse transcriptase (Bethesda Research Laboratories, Bethesda, MD) for 1 hour at 37°C in a final volume of 20 μL. We amplified 2 μL of complementary DNA in a final volume of 20 μL (10 mmol/L Tris-HCL, pH 8.3, 50 mmol/L KCl, 1.5 mmol/L MgCl2, 0.01% gelatin, 200 μmol/L deoxyribonucleoside triphosphate (dNTP), and 10 pmol of each primer) using a Perkin Elmer Thermal Cycler (Perkin Elmer, Norwalk, CT). Samples were heated at 94°C for 5 minutes, and each cycle was performed as follows: 40 seconds denaturation at 94°C, 40 seconds annealing at 62°C, and 1 minute extension at 72°C. At the end, samples were further incubated at 72°C for an additional 10 minutes. Table 1 reports the cytokine primer sequences, the number of amplification cycles, and the size of the fragment amplified in this study. For reaction product visualization, 10 μL of each PCR product was run on 1% agarose gel in 0.5 × tris borate dectrophoresis (TBE) buffer. As molecular weight markers, 1 μg of 132 bp DNA ladder (GIBCO-BRL) were run in parallel. As positive controls for IFN-α or IFN-β RT-PCR, we used messenger RNA (mRNA) extracted from IFN-α1–transduced Cl-11 cells35 and IFN β–transduced TSA/Cl-A4 cells,36 respectively. PCR products were visualized by means of ethidium bromide staining and UV transillumination. After electrophoresis, the relative density of mRNA bands stained with ethidium bromide was determined by LKB 2202 Ultrascan densitometer (Pharmacia, LKB, Uppsala, Sweden). Messenger RNA transcripts were expressed in absorbance units.
Primer sequences and PCR conditions for the evaluation of IFN mRNA expression
| Primer . | Nucleotide Sequence (5′-3′) . | Bp* . | No. Cycles . |
|---|---|---|---|
| β actin | 391 | 30 | |
| Sense | TGACGGGGTCACCCACACTGTGCCCATCTA | ||
| Antisense | CTAGAAGCATTGCGGTGGAGCATGGAGGG | ||
| Murine IFN-α1-2 | 162 | 40 | |
| Sense | TGTCTGATGCAGCAGGTGG | ||
| Antisense | AAGACAGGGCTCTCCAGAC | ||
| Murine IFN-β | 156 | 40 | |
| Sense | CCATCCAAGAGATGCTCCAG | ||
| Antisense | GTGGAGAGCAGTTGAGGACA |
| Primer . | Nucleotide Sequence (5′-3′) . | Bp* . | No. Cycles . |
|---|---|---|---|
| β actin | 391 | 30 | |
| Sense | TGACGGGGTCACCCACACTGTGCCCATCTA | ||
| Antisense | CTAGAAGCATTGCGGTGGAGCATGGAGGG | ||
| Murine IFN-α1-2 | 162 | 40 | |
| Sense | TGTCTGATGCAGCAGGTGG | ||
| Antisense | AAGACAGGGCTCTCCAGAC | ||
| Murine IFN-β | 156 | 40 | |
| Sense | CCATCCAAGAGATGCTCCAG | ||
| Antisense | GTGGAGAGCAGTTGAGGACA |
Number of base pairs of the amplified fragment.
Statistical analyses
Data were analyzed by Student t test.
Results
Detection of interferon activity in sera of mice treated with CTX
In a first set of experiments, mice were injected intraperitoneally with 2 doses of CTX (83 or 150 mg/kg body weight), with saline or poly (I:C) (positive control for IFN production). Sera were collected at different times after injection and tested for IFN activity by a standard biological assay on L929 cells. Table 2shows the IFN activity detected in sera from individual mice at 12, 24, and 48 hours after injection. No IFN activity was detected in sera from saline-treated control mice. In contrast, sera from poly (I:C)–treated mice exhibited high levels of IFN activity at 12 hours. Considerable levels of IFN (64 U/mL) were also found at 24 hours after poly (I:C) injection, while no IFN activity was detected at the subsequent time point. None of the 6 CTX-treated mice showed any presence of serum IFN at 12 hours after injection. However, at 24 hours, detectable IFN activity was found in 3 out of the 6 CTX-treated mice. At 48 hours, IFN activity (24 to 48 U/mL) was detected in the serum of 4 out of the 6 CTX-treated mice, with no significant difference with respect to the dose of CTX injected. At subsequent times, no IFN activity could be found in the sera of CTX-treated mice (data not shown).
Detection of IFN activity in the serum of mice injected with CTX
| Treatment . | Mouse No. . | Hours Posttreatment . | ||
|---|---|---|---|---|
| 12 . | 24 . | 48 . | ||
| Saline | 1 | < 4 | < 4 | < 4 |
| 2 | < 4 | < 4 | < 4 | |
| 3 | < 4 | < 4 | < 4 | |
| CTX 83 mg/kg | 1 | < 4 | < 4 | 32 |
| 2 | < 4 | < 4 | < 4 | |
| 3 | < 4 | 16 | 32 | |
| CTX 150 mg/kg | 1 | < 4 | < 4 | < 4 |
| 2 | < 4 | 16 | 24 | |
| 3 | < 4 | 32 | 48 | |
| Poly (I:C) | 1 | 256 | 64 | < 4 |
| 2 | 256 | 64 | < 4 | |
| 3 | 256 | 64 | < 4 | |
| Treatment . | Mouse No. . | Hours Posttreatment . | ||
|---|---|---|---|---|
| 12 . | 24 . | 48 . | ||
| Saline | 1 | < 4 | < 4 | < 4 |
| 2 | < 4 | < 4 | < 4 | |
| 3 | < 4 | < 4 | < 4 | |
| CTX 83 mg/kg | 1 | < 4 | < 4 | 32 |
| 2 | < 4 | < 4 | < 4 | |
| 3 | < 4 | 16 | 32 | |
| CTX 150 mg/kg | 1 | < 4 | < 4 | < 4 |
| 2 | < 4 | 16 | 24 | |
| 3 | < 4 | 32 | 48 | |
| Poly (I:C) | 1 | 256 | 64 | < 4 |
| 2 | 256 | 64 | < 4 | |
| 3 | 256 | 64 | < 4 | |
Six 7-week-old DBA/2 mice were injected intraperitoneally with 2 different doses of CTX (83 and 150 mg/kg body weight), poly (I:C) (0.15 mg/mouse), or saline. After different time intervals, mice were bled and serum samples collected and frozen. For IFN titration, sera were diluted 1:4 in 2% FCS RPMI 1640 to avoid nonspecific toxicity on cell substrates and processed for a standard IFN assay on L929 mouse cell monolayers, as described in “Materials and methods.” Data represent the IFN titer detected in the serum from each individual mouse, expressed in experimental units. One of these units is the equivalent of 4 international IFN units.
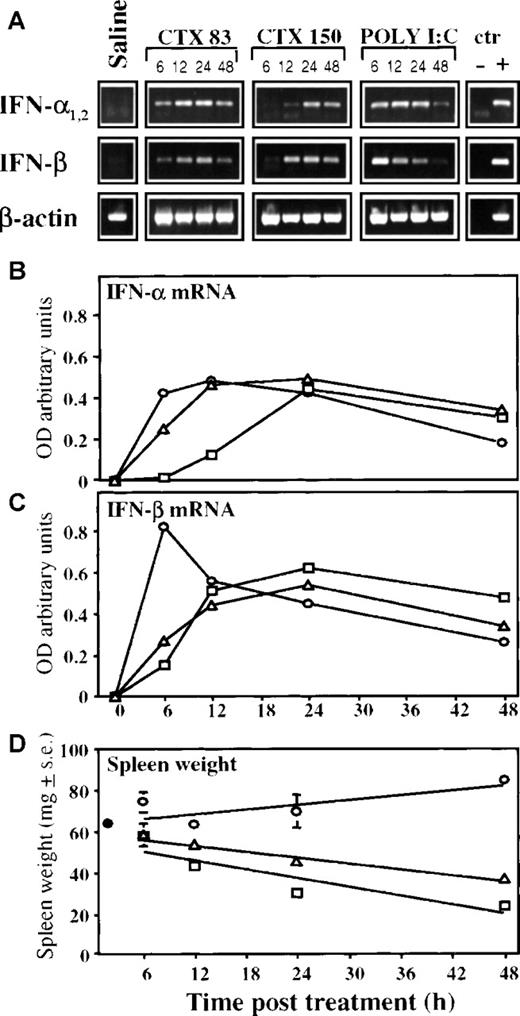
Kinetics of accumulation of IFN- and IFN-β messenger RNAs in the spleen of mice treated with cyclophosphamide
We then evaluated the levels of type I IFN mRNA expression in splenocytes harvested at different times after CTX injection. Thus, total RNA was extracted from spleen cells of mice treated with saline, CTX (83 or 150 mg/kg), or poly (I:C) and processed for RT-PCR by using 2 sets of primers specific for mouse IFN-α1-2 and IFN-β, and for β actin as a control (Figure 1). Injection of Poly (I:C) caused a transient but marked increase in the expression of both mRNAs for IFN-α and IFN-β, compared with untreated control mice (Figure 1A). In CTX-treated mice, the peak of mRNA expression for both IFN-α and IFN-β was almost comparable to that obtained with poly (I:C), but the kinetics was quite different. As shown by densitometric analyses (Figure 1B and 1C), IFN-α and IFN-β mRNA expression in spleen cells from mice injected with poly (I:C) reached its maximum level 6 to 12 hours after treatment. In CTX-treated mice, the maximal expression for both IFN mRNAs peaked between 12 and 24 hours. The apparent delay in the induction of IFN mRNA expression in mice injected with the higher dose of CTX (especially observed for IFN-α) may be somehow dependent on a CTX-induced toxicity. In this regard, there was a consistent decrease in the spleen weight in CTX-treated mice; this appeared more marked in animals treated with the higher dose of CTX. (Figure 1D).
RT-PCR analysis of IFN- and IFN-β mRNA levels in the spleen of mice treated with CTX or poly (I:C).
Seven-week-old DBA/2 mice were treated intraperitoneally with CTX (83 mg/kg, Δ, and 150 mg/kg, □); poly (I:C) (0.15 mg/mouse), ○; or saline, •. After 6, 12, 24, and 48 hours, mice were killed, and total spleen RNA was isolated and assayed for the presence of IFN-α and IFN-β mRNAs by RT-PCR as described in “Materials and methods.” Reaction products were run on 1% agarose gel in the presence of molecular markers (not shown). Values represent the mean ± SE of 3 mice per group. (A) Each band corresponds to a single mouse spleen. Densitometric values of ethidium bromide–stained bands, expressed as absorbance units (OD) and normalized to the control values, are reported, (B) for IFN-α mRNAs and (C) for IFN-β mRNAs. (D) Spleen weight of mice treated as above was measured 6, 12, 24, and 48 hours after treatment.
RT-PCR analysis of IFN- and IFN-β mRNA levels in the spleen of mice treated with CTX or poly (I:C).
Seven-week-old DBA/2 mice were treated intraperitoneally with CTX (83 mg/kg, Δ, and 150 mg/kg, □); poly (I:C) (0.15 mg/mouse), ○; or saline, •. After 6, 12, 24, and 48 hours, mice were killed, and total spleen RNA was isolated and assayed for the presence of IFN-α and IFN-β mRNAs by RT-PCR as described in “Materials and methods.” Reaction products were run on 1% agarose gel in the presence of molecular markers (not shown). Values represent the mean ± SE of 3 mice per group. (A) Each band corresponds to a single mouse spleen. Densitometric values of ethidium bromide–stained bands, expressed as absorbance units (OD) and normalized to the control values, are reported, (B) for IFN-α mRNAs and (C) for IFN-β mRNAs. (D) Spleen weight of mice treated as above was measured 6, 12, 24, and 48 hours after treatment.
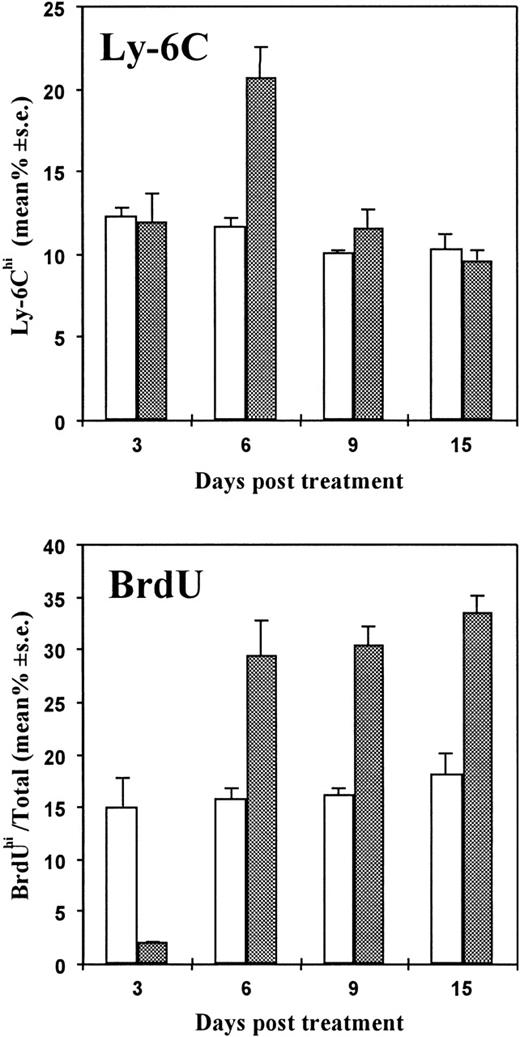
CTX injection results in the up-regulation of Ly-6C expression and bromodeoxyuridine incorporation in spleen lymphocytes
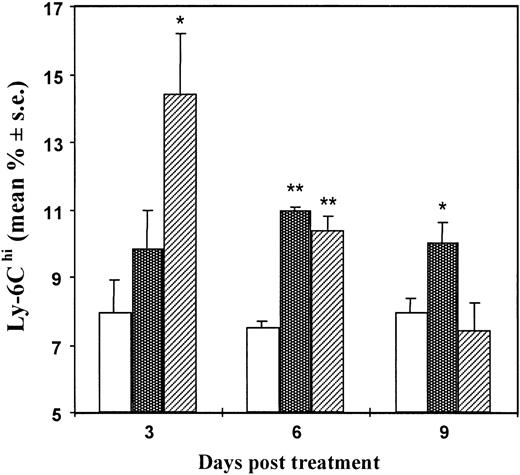
Ly-6C is a lymphocyte activation and differentiation antigen normally present on minor subsets of mature T cells, monocytes, macrophages, and endothelial cells. Previous studies have demonstrated that IFN-α/β is a cytokine capable of specifically enhancing Ly-6C expression.37 By cytofluorimetric analyses, we then evaluated the percentage of Ly-6C positive T cells with respect to the number of total T lymphocytes in the spleens of mice treated with a single injection of CTX (83 mg/kg) 3, 6, 9, and 15 days before sacrifice. In the same experiment, spleen lymphocytes were also analyzed for BrdU uptake. As shown in Figure2, there was a significant increase of Ly-6C positive cells in CTX-treated mice in comparison with controls at 6 days after CTX treatment. Cell proliferation data, obtained by the BrdU-labeling technique, showed a substantial decrease in the percentage of proliferating cells at day 3 after CTX administration, followed by a significant and long-lasting increase at the subsequent time points. In a subsequent experiment, illustrated in Figure3, we compared the Ly-6C expression in spleen lymphocytes at various times after treatment of mice with either poly (I:C) or CTX. Ly-6C expression was maximal in splenic lymphocytes 6 days after CTX treatment, whereas poly (I:C) induced a more intense but more transient Ly-6C antigen enhancement that peaked at day 3.
Effects of CTX injection on the expression of Ly-6C antigen and on BrdU incorporation in spleen lymphocytes at different times after treatment.
Seven- to 8-week-old DBA/2 mice were placed on BrdU water from day 0 to sacrifice. Mice were injected intraperitoneally with CTX (83 mg/kg) (dotted bars) or saline (white bars) at day 0. At different time intervals, spleens were taken and disaggregated. Cells were stained for Ly-6C; this was followed, after fixation, by nuclear staining for BrdU incorporation. Cell fluorescence was evaluated by fluorescence-activated cell sorter (FACS). There were 3 mice per group. The data show the mean (± SE) of the percentage of Ly-6Chi cells (upper panel) or BrdUhi cells (lower panel) with respect to the total number of spleen lymphocytes.
Effects of CTX injection on the expression of Ly-6C antigen and on BrdU incorporation in spleen lymphocytes at different times after treatment.
Seven- to 8-week-old DBA/2 mice were placed on BrdU water from day 0 to sacrifice. Mice were injected intraperitoneally with CTX (83 mg/kg) (dotted bars) or saline (white bars) at day 0. At different time intervals, spleens were taken and disaggregated. Cells were stained for Ly-6C; this was followed, after fixation, by nuclear staining for BrdU incorporation. Cell fluorescence was evaluated by fluorescence-activated cell sorter (FACS). There were 3 mice per group. The data show the mean (± SE) of the percentage of Ly-6Chi cells (upper panel) or BrdUhi cells (lower panel) with respect to the total number of spleen lymphocytes.
Effect of CTX versus poly (I:C) injection on Ly-6C expression in spleen lymphocytes at different times after treatment.
Seven- to 8-week-old DBA/2 mice were injected intraperitoneally with CTX (83 mg/kg) (dotted bars), poly (I:C) (0.15 mg/mouse) (striped bars), or saline (white bars). At different time intervals, spleens were taken and cells were stained for Ly-6C expression and processed for FACS analysis. There were 3 mice per group. The data show the mean (± SE) of the percentage of Ly-6Chi cells with respect to the number of total spleen lymphocytes. *P ≤ .05 versus controls; **P ≤ .001 versus controls.
Effect of CTX versus poly (I:C) injection on Ly-6C expression in spleen lymphocytes at different times after treatment.
Seven- to 8-week-old DBA/2 mice were injected intraperitoneally with CTX (83 mg/kg) (dotted bars), poly (I:C) (0.15 mg/mouse) (striped bars), or saline (white bars). At different time intervals, spleens were taken and cells were stained for Ly-6C expression and processed for FACS analysis. There were 3 mice per group. The data show the mean (± SE) of the percentage of Ly-6Chi cells with respect to the number of total spleen lymphocytes. *P ≤ .05 versus controls; **P ≤ .001 versus controls.
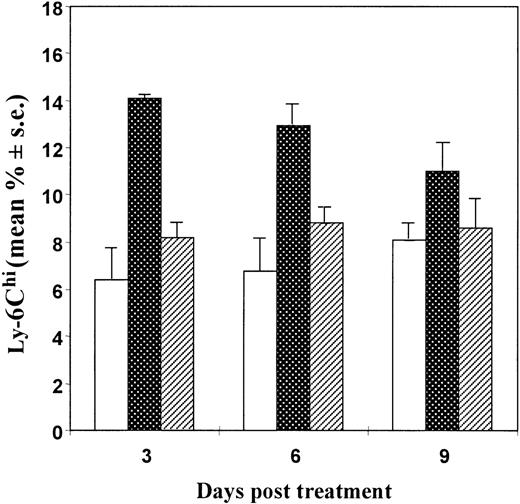
As shown in Figure 4, injection of mice with a potent preparation of anti–IFN-α/β antibodies resulted in a significant inhibition of the CTX-induced up-regulation of Ly-6C expression on spleen lymphocytes, suggesting that CTX-induced type I IFN was involved in enhancing the expression of this antigen.
Effects of injection of anti–IFN-/β antibodies on the CTX-induced up-regulation of Ly-6C expression in spleen lymphocytes from mice.
Seven- to 8-week-old DBA/2 mice were injected intraperitoneally with CTX (83 mg/kg) (dotted bars) or saline (white bars) at day 0. One day before and 2 and 4 days after CTX treatment, some mice were also injected intraperitoneally with 0.2 mL of an anti–murine IFN-α/β antibody preparation (160 000 IFN-neutralizing units/mouse/injection) (striped bars). At different time intervals, spleens were collected and cells were stained for Ly-6C expression and processed for FACS analysis. There were 3 mice per group. The data show the mean (± SE) of the percentage of Ly-6Chi cells with respect to the total number of spleen lymphocytes.
Effects of injection of anti–IFN-/β antibodies on the CTX-induced up-regulation of Ly-6C expression in spleen lymphocytes from mice.
Seven- to 8-week-old DBA/2 mice were injected intraperitoneally with CTX (83 mg/kg) (dotted bars) or saline (white bars) at day 0. One day before and 2 and 4 days after CTX treatment, some mice were also injected intraperitoneally with 0.2 mL of an anti–murine IFN-α/β antibody preparation (160 000 IFN-neutralizing units/mouse/injection) (striped bars). At different time intervals, spleens were collected and cells were stained for Ly-6C expression and processed for FACS analysis. There were 3 mice per group. The data show the mean (± SE) of the percentage of Ly-6Chi cells with respect to the total number of spleen lymphocytes.
Increase of the number of CD44hi T lymphocytes in the spleen and lymph nodes of CTX-treated mice
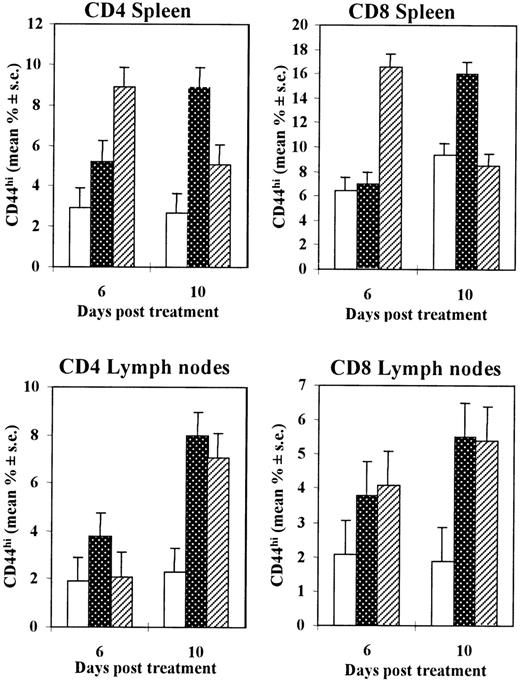
We then evaluated whether injection of CTX could affect the number of T cells in the spleen and lymph nodes at different times after injection. In general, no statistically significant differences were observed in the total number of CD4 or CD8 T lymphocytes recovered from the spleen or pooled lymph nodes of CTX-treated mice with respect to control injected mice on days 6 and 10, with the exception of a slight increase in the number of CD4 T cells detected in the spleen at 6 days after CTX injection (data not shown). Spleen or lymph node cells from mice treated with a single injection of CTX (83 mg/kg), poly (I:C), or saline were also labeled with biotinylated anti-CD44 and PE-conjugated anti-CD4 or anti-CD8 antibodies and assessed by cytofluorimetric analysis. As shown in Figure 5, there was a marked increase in the percentage of CD44hi T lymphocytes in both the spleen and the lymph nodes of CTX-injected mice, compared with saline-injected control animals. This increase reached its maximum level 10 days after treatment for both CD4+ and CD8+ T-cell subsets. In poly (I:C)–injected mice, there was also a marked increase in CD44hi cell percentage, but the kinetics of this increase appeared to be slightly different in spleen cells as compared with CTX-treated animals.
Increase in the percentage of CD44hi T lymphocytes in the CD4+ and CD8+ T cells from spleens and lymph nodes of mice treated with CTX or poly (I:C).
Seven- to 8-week-old DBA/2 mice were injected intraperitoneally with CTX (83 mg/kg) (dotted bars), poly (I:C) (0.15 mg/mouse) (striped bars), or saline (white bars). At different time intervals, spleens and a pool of lymph nodes were taken and disaggregated. Cells were stained for CD44 and for CD4 or CD8 surface antigen expression before being processed for cytofluorimetric analysis. There were 3 mice per group. The data show the mean (± SE) value of the percentage of CD44hi cells on CD4 (left panels) or CD8 (right panels) lymphocytes, in the spleen (upper panels) or in the pooled lymph nodes (lower panels).
Increase in the percentage of CD44hi T lymphocytes in the CD4+ and CD8+ T cells from spleens and lymph nodes of mice treated with CTX or poly (I:C).
Seven- to 8-week-old DBA/2 mice were injected intraperitoneally with CTX (83 mg/kg) (dotted bars), poly (I:C) (0.15 mg/mouse) (striped bars), or saline (white bars). At different time intervals, spleens and a pool of lymph nodes were taken and disaggregated. Cells were stained for CD44 and for CD4 or CD8 surface antigen expression before being processed for cytofluorimetric analysis. There were 3 mice per group. The data show the mean (± SE) value of the percentage of CD44hi cells on CD4 (left panels) or CD8 (right panels) lymphocytes, in the spleen (upper panels) or in the pooled lymph nodes (lower panels).
Discussion
In this study, we have provided the first evidence indicating that CTX is an inducer of type I IFN expression in mice. The induction of type I IFN by CTX was demonstrated not only by the presence of biologically active IFN in the serum of the injected mice at 24 to 48 hours, but also by a progressive accumulation of mRNAs for IFN-α and IFN-β in the spleens, starting at 6 hours and lasting for at least 48 hours after injection. The comparison of the kinetics of induction by CTX with that observed after injection of poly (I:C) (a typical strong inducer of type I IFN in mice) revealed that the peak of IFN-β mRNA induction by poly (I:C) occurred earlier (at 6 hours), but appeared to decrease more rapidly than that observed after CTX injection. As for IFN-α mRNA induction, the kinetics of accumulation of IFN-α mRNA in mice injected with poly (I:C) was similar to that observed in mice treated with the lower dose of CTX (83 mg/kg), whereas a certain delay in the induction kinetics was detected in mice injected with the higher dose of CTX (150 mg/kg). On the whole, these results indicate that CTX is an inducer of type I IFN at a time early after injection, before drug treatment might result in a strong myelosuppression27(Figure7A) or in a detectable decrease in the spleen weight (Figure 1D), substantially before the rebound phenomenon, which starts 5 to 6 days after CTX injection.27Thus, although the mechanisms of type I IFN induction by CTX remain unclear, our results suggest that the type I IFN induction is a direct effect of the chemotherapeutic agent on some mouse cells and does not represent a secondary response to drug-induced myelosuppression or to cell-stimulation events occurring during the rebound phase. This CTX-induced IFN production appears to be capable of directly affecting some lymphocyte functions. In fact, CTX induced an up-regulation in the expression of Ly-6C antigen (a marker known to be specifically induced by type I IFN), and this induction was inhibited by injecting the mice with a potent preparation of anti–IFN-α/β antibodies. Of interest, CTX was also capable of inducing an enhancement of the percentage of proliferating (ie, BrdU+) spleen lymphocytes as well as of modulating the phenotype of T cells, especially by enhancing the number of CD4+ and CD8+ T cells exhibiting a memory (CD44hi) phenotype in both the spleens and the lymph nodes of the injected mice.
The notion that CTX can induce release of cytokines and growth factors during the rebound events after drug-induced immunosuppression is well documented.38,39 Some studies have recently suggested that CTX can induce a pattern shift of cytokines from TH2 to TH1 in tumor models several days after injection.40 However, our finding that CTX can induce an early and long-lasting expression of type I IFN suggests that this event can be directly important in mediating some of the immunomodulatory effects of CTX observed in a number of experimental systems.
CTX is a currently used drug in cancer therapy. Although CTX is generally considered to be a typical chemotherapeutic agent capable of directly inhibiting tumor cell proliferation, many studies have shown that CTX can also induce multiple immunomodulatory effects.7-20 In mouse tumor models, some groups had suggested that CTX can act by inhibiting T suppressor cells.21-25 However, the nature of these suppressor cells assumed to be inhibited by CTX has remained elusive.26 In a recent study, in which we have investigated the mechanisms underlying the impressive antitumor response in tumor-bearing mice subjected to a single injection of CTX followed by adoptive immunotherapy,27 we provided evidence suggesting that CTX did not act by inhibiting T suppressor cells, but by rendering the mouse host capable of allowing survival and proliferation of the transferred immune lymphocytes, probably as a result of CTX-induced cytokines affecting T-cell functions.27,41 Notably, type I IFN was important in the response to the combined CTX/adoptive immunotherapy regimen, since the antitumor activity was abolished by injection of antibodies to IFN-α/β.27 Other groups have recently provided additional examples of CTX effects on T cells. For instance, Li and coworkers have recently reported that CTX given after active specific immunization augments antitumor immunity by modulation of TH1 commitment of CD4 T cells.42 Likewise, Apostolopoulos et al43 have reported a CTX-induced enhancement of cytotoxic lymphocyte (CTL) precursors' frequency in mice immunized with mucin 1–mannan fusion protein. In this regard, it is of interest to note that recent studies have revealed that type I IFN is an important cytokine in the regulation of T-cell response.44 Early studies had shown that type I IFN can inhibit T suppressor cells in mice.45 Type I IFN is involved in the polarization toward a TH1 type of immune response and has been shown to augment the generation and activity of CTL.46,47 Of interest, recent studies have shown that type I IFN can specifically induce in mice the proliferation and persistence of CD8+ T lymphocytes exhibiting the memory (CD44hi) phenotype.28,48 In light of all this, we may assume that the induction of type I IFN by CTX is important in inducing proliferation and persistence of T cells (especially CD8 T cells) and a TH1 type of immune response. There are some intriguing similarities in the effects induced by CTX and type I IFN, which may suggest, in light of the results reported in this article, that an induction of this cytokine may be involved in some of the responses observed after CTX treatment. CTX accelerates diabetes progression in non obese diabetic mice inducing a polarization toward a TH1 type of immune response49; notably, it has been postulated that type I IFN plays a role in diabetes as well as in the pathogenesis of other autoimmune diseases.50,51 In a different context—adoptive immunotherapy protocols in tumor models—it is worth mentioning that the antitumor efficacy of transferred lymphocytes has been shown to be markedly enhanced by CTX18,19 as well as by type I IFN.52 In the light of the collection of recent data showing the importance of type I IFN in the regulation of the T-cell turnover in mice,48,53,54 we suggest that, although CTX can induce several cytokines capable of affecting the antitumor activity of the transferred lymphocytes, type I IFN is the major factor that may allow the proliferation and long-term survival of at least some subsets of the injected immune cells (especially CD8+ T cells exhibiting the memory phenotype) when adoptive immunotherapy is performed after CTX injection. Finally, we believe that the finding that type I IFN, a cytokine endowed with well-recognized antitumor activity, is induced early after CTX injection may provide new insight for the definition of more selective strategies for the combined treatment with CTX and adoptive immunotherapy in cancer patients. In our previous work, we suggested that in order to attain the optimal effects of adoptive immunotherapy, it should be performed following chemotherapy at a time point when immune function is rebounding.27 Notably, we found that the antitumor response was optimal when immune cells were transferred in tumor-bearing mice approximately 6 hours after a single CTX injection, when an induction of type I IFN mRNA expression is observed in the spleen (Figure 1). We suggest, therefore, that adoptive immunotherapy should be performed shortly after chemotherapy (at a time when type I IFN is initially expressed in some lymphoid tissues), before the rebound overshoot is observed.
Acknowledgments
We are grateful to Dr Ion Gresser (Paris, France) for providing us with the sheep antibody to mouse IFN–α/β and for the helpful discussion and suggestions. We thank Ms C. Gasparrini for secretarial aid.
Supported in part by grants from the Associazione Italiana Ricerca sul Cancro (AIRC, Milan) and from the Italian Ministry of Health (Progetto di Ricerca sull'AIDS 1998, 30B/I).
Reprints:Enrico Proietti, Laboratory of Virology, Istituto Superiore di Sanità, Viale Regina Elena 299, 00161, Rome, Italy; e-mail: Proietti@virus1.net.iss.it.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal