Factor VIII is tightly noncovalently linked to von Willebrand factor (vWF) in plasma with a stoichiometry of 1:50, and vWF deficiency results in secondary factor VIII deficiency, with accelerated clearance of factor VIII from the circulation. We used a murine model of severe von Willebrand disease (vWF knockout mice) to study the effect of a recombinant vWF/pro-vWF preparation (rpvWF) on factor VIII survival and to investigate whether low-density lipoprotein receptor-related protein (LRP) might be involved in the in vivo clearance of factor VIII in the absence of vWF. vWF-deficient mice received 70 U/kg rpvWF in the first series of experiments, and in a second series, 80 mg/kg receptor-associated protein (RAP) as a recombinant fusion protein to block the action of LRP. Factor VIII levels were measured at time 0, or 1 or 3 hours after administration of rpvWF or RAP. RAP induced a sustained rise in factor VIII levels comparable to that induced by rpvWF. In a third series, the preadministration of RAP resulted in a slower disappearance of factor VIII antigen (measured by an enzyme-linked immunosorbent assay specific for human factor VIII) after infusion of recombinant factor VIII. These findings suggest that the accelerated clearance of factor VIII seen in the absence of vWF may be a result of the involvement of LRP in factor VIII metabolism.
Factor VIII and von Willebrand factor (vWF) are functionally linked plasma glycoproteins that play a pivotal role in hemostasis. Deficiencies in these proteins result in the most common bleeding disorders in humans. A deficiency in factor VIII, a gene product of the X chromosome and the necessary cofactor for factor IX–mediated activation of factor X, results in hemophilia A, whereas a deficiency in vWF, which mediates platelet adhesion to the subendothelium and stabilizes factor VIII, results in von Willebrand disease (vWD).1 2
Each subunit of a vWF multimer contains a factor VIII binding site at its amino terminal end.3,4 Binding of the factor VIII heterodimer to vWF via the amino and carboxy terminal regions located within the factor VIII light chain is crucial for factor VIII survival in vivo.5,6 Although in vitro studies have yielded conflicting data for the factor VIII:vWF subunit ratio, factor VIII is tightly noncovalently linked to vWF in plasma with a stoichiometry of 1:50, suggesting that not every subunit of vWF is accessible for binding.7
Changes in the plasma concentration of vWF result in corresponding changes in factor VIII levels. The pathophysiologic significance of this interaction is best demonstrated in patients with vWD. Patients with type 1 vWD develop secondary factor VIII deficiency with, for example, a 50% decrease in vWF antigen (vWF:Ag) corresponding to reduction of 50% in circulating factor VIII. Such patients therefore not only have impaired primary hemostasis, but also a defect in the intrinsic coagulation pathway.8 The half-life of infused monoclonal antibody purified or recombinant factor VIII is significantly reduced (to less than 3 hours) in patients with vWD,9,10 and replacement therapy with vWF concentrate results in a rapid and sustained elevation of endogenous factor VIII with a half-life of up to 12 to 14 hours.11 Administration of human recombinant vWF into dogs with severe vWD also induces a rapid rise in canine factor VIII.12 The dependence of factor VIII survival on vWF is further reflected by vWD Normandy, a subtype of vWD in which patients have normal levels of vWF antigen and ristocetin cofactor activity as well as a normal multimeric pattern, but their factor VIII activity is decreased because of a mutation in the factor VIII binding region of vWF that leads to decreased or absent affinity of vWF for factor VIII and an accelerated clearance of factor VIII from the circulation.13 14
In vitro, vWF controls factor VIII activity through the prevention of factor VIII activation by factor Xa15 and the prevention of factor VIII binding to phospholipids and activated platelets.16,17 Factor VIII is very sensitive to enzymatic proteolysis, and vWF also protects factor VIII from proteolytic degradation by both activated protein C18 19 and factor Xa in vitro. However, it has never been demonstrated whether these effects explain the short half-life of factor VIII in the absence of vWF in vivo.
The low-density lipoprotein receptor-related protein (LRP) has been shown to mediate internalization of thrombin-activated factor VIII in vitro,20 suggesting a new aspect of the complexity of factor VIII metabolism. In addition, it was recently demonstrated that factor VIII binds to LRP in a reversible and dose-dependent fashion through the factor VIII light chain. vWF appeared to reduce LRP binding and to inhibit intracellular degradation of factor VIII.21LRP is a ubiquitously expressed, large, multifunctional endocytic receptor with structurally and functionally distinct sites.22 LRP binds a diverse group of ligands, including lipoproteins, lipoprotein lipase, protease inhibitors, and protease:inhibitor complexes, bacterial toxins, viruses, lactoferrin, and thrombospondin.23 Some of these molecules compete with each other for a common region on LRP, whereas others bind to independent sites. On the basis of this spectrum of unrelated ligands, it can be assumed that LRP is involved in a variety of pathophysiologic processes. It has also been demonstrated in rodents that LRP plays a role in the clearance of enzyme:inhibitor complexes involved in hemostasis such as t-PA,24 tissue factor pathway inhibitor (TFPI)25 and factor Xa.26
In this study, we took advantage of a murine model of severe vWD27 that mimics type 3 vWD to investigate whether LRP might play a role in factor VIII clearance in vivo. In the first series of experiments, factor VIII levels were observed in the vWF-deficient mice after administration of a recombinant human vWF pro-vWF preparation (rpvWF). In a second series, receptor-associated protein (RAP)22 24 was infused as a recombinant fusion protein to block the action of LRP. In a third series, a recombinant factor VIII preparation was administered after preinjection of RAP.
Materials and methods
Substances
Rat receptor-associated protein (RAP, 39 kD Protein) was made as a recombinant fusion protein with glutathione S-transferase (GST) as previously described28 with some modifications. The GST-tagged RAP protein was expressed in Escherichia coli BL 21 (Novagen, Madison, WI) transformed with a pGEX-GST-RAP construct.29 Bacteria were harvested by centrifugation at 4°C. The pellet was stored at −70°C until use. The expressed protein was extracted by freeze-thawing of the pellet and lysis of the bacteria by addition of 1 mg/mL Lysozyme (Boehringer Mannheim, Mannheim, Germany) in the presence of the protease inhibitors aprotinin (200 U/mL, Bayer, Leverkusen, Germany), phenylmethylsulfonyl fluoride (1 mmol/L, Sigma, St. Louis, MO), and benzamidine hydrocholoride (100 mmol/L, Sigma), as well as 0.5% Triton-X-100 (Sigma) at 4°C for lysis. The lysate was then homogenized with an Ultra-Turrax (IKA Labortechnik, Stauffen, Germany) and the supernatant was obtained by centrifugation at 39 000g for 15 minutes at 4°C. The GST-RAP was purified by affinity chromatography on glutathione (GSH) agarose (Pierce, Rockford, IL) by adding 10 mL of the lysate to 1 mL of the GSH agarose, followed by head-over-head rotation at 4°C overnight. Thereafter the gel was washed with phosphate-buffered saline. Specific bound protein was then eluted with 20 mmol/L GSH in 100 mmol/L Tris-hydrochloride, 120 mmol/L NaCl, pH = 8.0, dialyzed against several changes of 0.1 mol/L NH4HCO3 buffer, pH = 8.3, and concentrated by freeze-drying before use in the animal experiments.
The fusion protein thus obtained was formulated in a physiologically acceptable buffer containing 5 g/L glycine, 5 g/L lysine hydrochloride, 2.5 g/L trisodium citrate 2H2O, 0.6 g/L calcium chloride 2H2O, and 3 g/L sodium chloride. The same buffer was used without RAP as a control and for the formulation of recombinant factor VIII.
The recombinant human vWF pro-vWF preparation was produced by expression in a rodent cell line, Chinese hamster ovary (CHO) cells.30 The recombinant vWF was purified from the cell culture supernatant by ion-exchange chromatography, followed by immuno-affinity chromatography on an immobilized monoclonal antibody directed against mature vWF according to Mejan et al.31 As recently described,32 about 50% of the vWF in the resulting preparation (rpvWF) contained propeptide covalently linked to the mature vWF because the proteolytic system of the CHO cells was capable of only partially processing the expressed vWF. The preparation was essentially free of other proteins because of the specificity of the final purification step.
Recombinant human FVIII (rFVIII) was obtained as bulk material (Recombinate, Baxter Hyland Immuno, Thousand Oaks, CA).
Assays
Factor VIII activity was measured against a human reference plasma factor VIII standard containing 1 IU factor VIII/mL calibrated against the Third International Standard 91/666 using a 2-stage clotting method as well as a chromogenic method. The 2-stage clotting method was carried out as described by Austen and Rhymes33 using the reagents from a 2-stage factor VIII assay kit (Baxter Hyland Immuno, Vienna, Austria). The chromogenic assay was the Immunochrom Factor VIII:C kit (Baxter Hyland Immuno) as recently described.34Human factor VIII plasma-equivalent units were expressed in units per milliliter (U/mL) (100% equivalent to 1 IU/mL in humans).
Factor VIII antigen (FVIII:Ag) was determined by an enzyme-linked immunosorbent assay (ELISA) based on 2 different monoclonal antibodies directed against epitopes in the intact light chain of the FVIII molecule as recently described.35 The ELISA was specific for human FVIII and did not cross react with FVIII of murine origin.36
Total von Willebrand factor antigen (mature and pro-vWF) was measured by ELISA using a polyclonal rabbit antihuman vWF antibody from Diagnostica Stago (Asserachrom vWF, Boehringer-Mannheim, Mannheim, Germany) and was expressed in human plasma-equivalent units per milliliter (U/mL) using the standard preparation from the test kit.
Animals
Three strains of normal mice served as controls to establish physiologically normal murine FVIII levels using our assay systems: NMRI mice (Crl NMRI BR) and Balb/c mice (Balb/cAnNCrlBR) were obtained from Charles River, Sulzfeld, Germany, and had a body weight between 22 and 27 g. Mice from the C57 black strain originated from the Jackson Laboratories (C57Bl/6J) and were purchased from Charles River, Lyon, France.
A colony of mice with a targeted disruption of the vWF gene was established using a breeding pair from the original colony, which was generated by microinjecting targeted embryonic stem cells into the blastocoele cavity of the blastocysts of C57Bl mice.27 The vWF knockout mice completely lack the vWF protein and have been reported to have a secondary factor VIII deficiency with levels approximately 20% of those in normal mice.27
All experiments, treatment of the mice with various compounds or blood sampling, were performed under anesthesia. Ketamine hydrochloride (Ketavet, Parke Davis, Berlin, Germany), xylazine hydrochloride 2% solution (Rompun, Bayer, Leverkusen, Germany), and sodium pentobarbital (Nembutal, Sanofi, Libourne, France) were used as anesthetics.
The animals were housed in the animal care facilities of Baxter Hyland Immuno in Vienna and Orth/Donau. All animal studies were performed in accordance with Austrian federal law (Act BG 501/1989) regulating animal experimentation.
Administration of recombinant von Willebrand factor
Two groups of vWF-deficient mice (2 males and 2 females per group) received 70 ristocetin cofactor units (RCoF U) of rpvWF per kg body weight injected into a tail vein and were killed 1 or 3 hours after the injection for blood sampling by heart puncture. A third group (2 males and 2 females) without treatment served as the zero time point control.
Administration of receptor-associated protein
In another series of experiments, RAP was injected at a dose of 80 mg/kg body weight into a tail vein in vWF knockout mice. The animals were killed after 30 minutes (4 males, 4 females), 1 hour (5 males, 3 females), or 3 hours (2 females), and blood samples were taken by heart puncture. A fourth group (5 males and 5 females), killed 30 minutes after an injection of 20 mL of the formulation buffer per kg body weight, served as the zero time point control.
Priming with receptor-associated protein followed by recombinant factor VIII
In another experiment, vWF knockout mice were primed with RAP at a dose of 40 mg/kg body weight. An injection of 200 U/kg of human recombinant human factor VIII (rFVIII) was administered into a tail vein 15 minutes later. In the control group, mice were treated with 200 U/kg rFVIII alone. Three animals were used in each group, and plasma was obtained from every single animal at time points before and 15 minutes, and 1 and 3 hours after rFVIII administration. For drawing blood, a 2- to 3-mm piece of the tail was cut with a scalpel and the blood was collected with a lithium-heparinized capillary hematocrit tube (32 μL Li-heparin ring caps for Reflotron, Hirschmann Laborgeräte, Eberstadt, Germany) and sealed. The tail wounds were cauterized and another 2- to 3-mm tail piece was cut for the next time point. The capillaries were centrifuged with a hematocrit centrifuge (Type 2075, Hettich, Tuttlingen, Germany) and the hematocrit was evaluated. Subsequently, the weight of the capillary was measured and the mean weight of 10 sealed empty capillaries was subtracted to calculate the blood volume. For reasons of simplification, the specific gravity of blood was assumed as 1000g/L. The filled capillaries were cut with an ampoule cutter at the interface of the cellular and the liquid part of the capillary. The cell-free plasma was emptied by application of air pressure into an Eppendorf tube containing a known volume of 3.8% citrate solution as an anticoagulant. The diluted and anticoagulated plasma was subsequently centrifuged at 1200g for 10 minutes (Centrifuge 5415C, Eppendorf, Hamburg, Germany) at room temperature. The supernatant was subjected to FVIII determination. The dilution of the plasma through sample preparation was taken into account for calculations of the actual FVIII level.
Biometrical methods
In addition to group mean and standard deviation, the coefficient of variation was calculated to be able to compare variability, despite differences between groups in the magnitude of measurements. The results were statistically evaluated using the Student t test. The significance of the increase in endogenous murine factor VIII levels in the infusion experiments at the time points 1 and 3 hours after administration of the respective test substances was calculated in comparison to the values in the zero controls as previously defined.
In vivo recovery of human FVIII antigen was determined for the plasma samples taken 15 minutes after administration of human rFVIII according to the following formula: IVR (%) = [FVIII activity (U/mL)] × [plasma volume (mL)] / [FVIII infused (U)] × 100.
Results
Normal and baseline factor VIII levels
Because our assay systems were established for measuring human factor VIII, we determined the normal murine factor VIII levels for these systems by evaluating plasma samples from 3 typical laboratory mouse strains: NMRI, C57Bl, and Balb/c mice (Table1). In general, murine factor VIII did not exceed human levels, although the mean factor VIII level measured with the chromogenic assay in male Balb/c mice was 225% of normal human levels. Male mice had substantially higher factor VIII levels than females in all strains. Values measured with the chromogenic assay were at least twice as high as those measured with the 2-stage assay. The variability of the chromogenic assay was also greater than would be expected on the basis of screening of human plasma samples.
Plasma factor VIII levels determined in normal and von Willebrand factor-knockout mice with the 2-stage and chromogenic assays using a human standard plasma as reference
| Strain . | n . | FVIII Assay Mean U/mL ± SD (CV%) . | |
|---|---|---|---|
| 2-Stage . | Chromogenic . | ||
| NMRI | |||
| Male + female | 53 | 0.49 ± 0.39 (80%) | 1.03 ± 0.64 (62%) |
| Male | 33 | 0.57 ± 0.43 (75%) | 1.17 ± 0.75 (64%) |
| Female | 20 | 0.37 ± 0.27 (73%) | 0.80 ± 0.30 (38%) |
| C57B1/6J | |||
| Male + female | 20 | 0.28 ± 0.17 (61%) | 0.66 ± 0.39 (59%) |
| Male | 9 | 0.37 ± 0.19 (51%) | 0.80 ± 0.51 (64%) |
| Female | 11 | 0.20 ± 0.12 (57%) | 0.55 ± 0.21 (39%) |
| Balb/c | |||
| Male + female | 14 | 0.29 ± 0.19 (66%) | 1.60 ± 0.85 (53%) |
| Male | 8 | 0.34 ± 0.25 (72%) | 2.25 ± 0.26 (12%) |
| Female | 6 | 0.23 ± 0.05 (23%) | 0.74 ± 0.47 (63%) |
| vWD | |||
| Male + female | 4 | 0.07 ± 0.03 (40%) | 0.02 ± 0.01 (44%) |
| Male | 2 | 0.06 ± 0.02 (40%) | 0.02 ± 0.00 (16%) |
| Female | 2 | 0.09 ± 0.03 (34%) | 0.03 ± 0.01 (46%) |
| Strain . | n . | FVIII Assay Mean U/mL ± SD (CV%) . | |
|---|---|---|---|
| 2-Stage . | Chromogenic . | ||
| NMRI | |||
| Male + female | 53 | 0.49 ± 0.39 (80%) | 1.03 ± 0.64 (62%) |
| Male | 33 | 0.57 ± 0.43 (75%) | 1.17 ± 0.75 (64%) |
| Female | 20 | 0.37 ± 0.27 (73%) | 0.80 ± 0.30 (38%) |
| C57B1/6J | |||
| Male + female | 20 | 0.28 ± 0.17 (61%) | 0.66 ± 0.39 (59%) |
| Male | 9 | 0.37 ± 0.19 (51%) | 0.80 ± 0.51 (64%) |
| Female | 11 | 0.20 ± 0.12 (57%) | 0.55 ± 0.21 (39%) |
| Balb/c | |||
| Male + female | 14 | 0.29 ± 0.19 (66%) | 1.60 ± 0.85 (53%) |
| Male | 8 | 0.34 ± 0.25 (72%) | 2.25 ± 0.26 (12%) |
| Female | 6 | 0.23 ± 0.05 (23%) | 0.74 ± 0.47 (63%) |
| vWD | |||
| Male + female | 4 | 0.07 ± 0.03 (40%) | 0.02 ± 0.01 (44%) |
| Male | 2 | 0.06 ± 0.02 (40%) | 0.02 ± 0.00 (16%) |
| Female | 2 | 0.09 ± 0.03 (34%) | 0.03 ± 0.01 (46%) |
CV% = coefficient of variation.
Plasma factor VIII levels in the vWF knockout mice were approximately 20% of those in the C57 black mice, which were considered to be the most relevant control due to their genetic relationship with the vWF knockout mice. This finding was consistent with the plasma levels reported for the original vWF knockout colony.27
Effects of recombinant von Willebrand factor
Treatment of vWF knockout mice with the human rpvWF preparation resulted in an immediate increase in total vWF antigen from 0 to 1.6 U/mL, followed by a gradual decrease (0.8 U/mL at 3 hours).
Factor VIII plasma levels also increased for both assays after injection of rpvWF (Table 2), although with the 2-stage assay, an increase was not seen until the 3-hour measurement. As a consequence of the small numbers of animals and the large standard deviations, these results did not reach statistical significance.
Effect of recombinant von Willebrand factor on plasma factor VIII levels in von Willebrand factor knockout mice
| Measurement (hrs after injection) . | n . | FVIII Levels (U/mL) . | |||
|---|---|---|---|---|---|
| 2-Stage Assay . | Chromogenic Assay . | ||||
| Mean ± SD . | P . | Mean ± SD . | P . | ||
| Male + female | |||||
| 0 | 4 | 0.07 ± 0.01 | 0.02 ± 0.00 | ||
| 1 | 4 | 0.05 ± 0.06 | .2396 | 0.13 ± 0.15 | .1814 |
| 3 | 4 | 0.18 ± 0.14 | .1060 | 0.18 ± 0.13 | .0481 |
| Male | |||||
| 0 | 2 | 0.07 ± 0.01 | 0.02 ± 0.00 | ||
| 1 | 2 | 0.07 ± 0.09 | .4924 | 0.19 ± 0.24 | .2500 |
| 3 | 2 | 0.23 ± 0.17 | .2107 | 0.27 ± 0.12 | .1070 |
| Female | |||||
| 0 | 2 | 0.07 ± 0.02 | 0.02 ± 0.00 | ||
| 1 | 2 | 0.02 ± 0.02 | .0567 | 0.07 ± 0.01 | .0481 |
| 3 | 2 | 0.13 ± 0.12 | .3117 | 0.08 ± 0.02 | .0774 |
| Measurement (hrs after injection) . | n . | FVIII Levels (U/mL) . | |||
|---|---|---|---|---|---|
| 2-Stage Assay . | Chromogenic Assay . | ||||
| Mean ± SD . | P . | Mean ± SD . | P . | ||
| Male + female | |||||
| 0 | 4 | 0.07 ± 0.01 | 0.02 ± 0.00 | ||
| 1 | 4 | 0.05 ± 0.06 | .2396 | 0.13 ± 0.15 | .1814 |
| 3 | 4 | 0.18 ± 0.14 | .1060 | 0.18 ± 0.13 | .0481 |
| Male | |||||
| 0 | 2 | 0.07 ± 0.01 | 0.02 ± 0.00 | ||
| 1 | 2 | 0.07 ± 0.09 | .4924 | 0.19 ± 0.24 | .2500 |
| 3 | 2 | 0.23 ± 0.17 | .2107 | 0.27 ± 0.12 | .1070 |
| Female | |||||
| 0 | 2 | 0.07 ± 0.02 | 0.02 ± 0.00 | ||
| 1 | 2 | 0.02 ± 0.02 | .0567 | 0.07 ± 0.01 | .0481 |
| 3 | 2 | 0.13 ± 0.12 | .3117 | 0.08 ± 0.02 | .0774 |
P calculated for comparison to time point 0.
Effects of receptor-associated protein
The administration of RAP to the vWF knockout mice resulted in a statistically significant 2-fold increase in factor VIII activity in both assays within 30 minutes when compared with the control group, which received only buffer 30 minutes before blood sampling (= measurement 0 hours in Table 3). Elevated levels of factor VIII were maintained throughout the observation period (Table 3).
Effect of receptor-associated protein on plasma factor VIII levels in von Willebrand factor knockout mice
| Measurement (hrs after injection) . | n . | FVIII Levels (U/mL) . | |||
|---|---|---|---|---|---|
| 2-Stage Assay . | Chromogenic Assay . | ||||
| Mean ± SD (CV%) . | P . | Mean ± SD (CV%) . | P . | ||
| Male + female | |||||
| 0 | 10 | 0.05 ± 0.02 (34%) | 0.06 ± 0.03 (53%) | ||
| 0.5 | 8 | 0.10 ± 0.02 (25%) | .0002 | 0.11 ± 0.03 (30%) | .0013 |
| 1 | 8 | 0.09 ± 0.04 (44%) | .0108 | 0.10 ± 0.06 (61%) | .0421 |
| 3 | 2 | 0.07 ± 0.004 (6%) | .0052 | 0.11 ± 0.01 (11%) | .0060 |
| Male | |||||
| 0 | 5 | 0.04 ± 0.02 (51%) | 0.06 ± 0.04 (60%) | ||
| 0.5 | 4 | 0.11 ± 0.03 (24%) | .0043 | 0.11 ± 0.04 (40%) | .0742 |
| 1 | 5 | 0.10 ± 0.04 (40%) | .0144 | 0.11 ± 0.06 (53%) | .0867 |
| 3 | — | nd | nd | nd | nd |
| Female | |||||
| 0 | 5 | 0.05 ± 0.01 (14%) | 0.05 ± 0.02 (33%) | ||
| 0.5 | 4 | 0.08 ± 0.01 (17%) | .0052 | 0.11 ± 0.02 (20%) | .0024 |
| 1 | 3 | 0.06 ± 0.01 (14%) | .0702 | 0.08 ± 0.07 (88%) | .2450 |
| 3 | 2 | 0.07 ± 0.004 (6%) | .0118 | 0.11 ± 0.01 (11%) | .0091 |
| Measurement (hrs after injection) . | n . | FVIII Levels (U/mL) . | |||
|---|---|---|---|---|---|
| 2-Stage Assay . | Chromogenic Assay . | ||||
| Mean ± SD (CV%) . | P . | Mean ± SD (CV%) . | P . | ||
| Male + female | |||||
| 0 | 10 | 0.05 ± 0.02 (34%) | 0.06 ± 0.03 (53%) | ||
| 0.5 | 8 | 0.10 ± 0.02 (25%) | .0002 | 0.11 ± 0.03 (30%) | .0013 |
| 1 | 8 | 0.09 ± 0.04 (44%) | .0108 | 0.10 ± 0.06 (61%) | .0421 |
| 3 | 2 | 0.07 ± 0.004 (6%) | .0052 | 0.11 ± 0.01 (11%) | .0060 |
| Male | |||||
| 0 | 5 | 0.04 ± 0.02 (51%) | 0.06 ± 0.04 (60%) | ||
| 0.5 | 4 | 0.11 ± 0.03 (24%) | .0043 | 0.11 ± 0.04 (40%) | .0742 |
| 1 | 5 | 0.10 ± 0.04 (40%) | .0144 | 0.11 ± 0.06 (53%) | .0867 |
| 3 | — | nd | nd | nd | nd |
| Female | |||||
| 0 | 5 | 0.05 ± 0.01 (14%) | 0.05 ± 0.02 (33%) | ||
| 0.5 | 4 | 0.08 ± 0.01 (17%) | .0052 | 0.11 ± 0.02 (20%) | .0024 |
| 1 | 3 | 0.06 ± 0.01 (14%) | .0702 | 0.08 ± 0.07 (88%) | .2450 |
| 3 | 2 | 0.07 ± 0.004 (6%) | .0118 | 0.11 ± 0.01 (11%) | .0091 |
CV% = coefficient of variation; nd = not determined.
P calculated for comparison to time point 0.
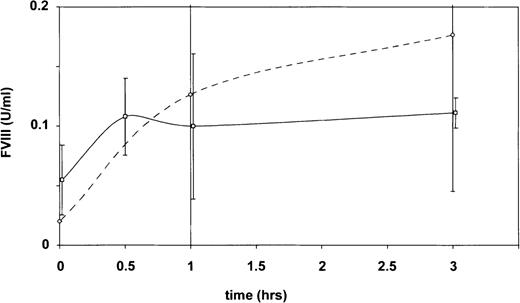
At the 3-hour time point, factor VIII had reached higher levels after administration of rpvWF than of RAP (Figure1).
Time course of plasma factor VIII activity in vWF knockout mice determined with the chromogenic assay after intravenous injection of either 80 mg/kg RAP (solid line, n = 2 to 10 animals per time point) or 70 RCoF U/kg rpvWF (broken line, n = 4 animals per time point).
Values are mean ± SD.
Time course of plasma factor VIII activity in vWF knockout mice determined with the chromogenic assay after intravenous injection of either 80 mg/kg RAP (solid line, n = 2 to 10 animals per time point) or 70 RCoF U/kg rpvWF (broken line, n = 4 animals per time point).
Values are mean ± SD.
Effects of priming with receptor-associated protein followed by recombinant factor VIII
On the basis of the measurement of human factor VIII antigen levels, the in vivo recovery of infused human rFVIII (200 U/kg) 15 minutes after infusion in vWF-deficient mice was only 6.2%. The infused rFVIII was cleared rapidly, and no antigen could be detected at 3 hours. When RAP (40 U/kg) was administered 15 minutes before the injection of rFVIII (200 U/kg), the rFVIII recovery at 15 minutes did not change (6.4%). However, after 1 hour, the circulating factor VIII antigen concentration after pretreatment with RAP was 2-fold that of rFVIII alone (Figure 2). Although this difference was not statistically significant, in the group of animals treated with rFVIII alone, factor VIII antigen had already disappeared completely in 2 of the 3 animals at 1 hour, whereas in the RAP group all 3 mice showed measurable human factor VIII antigen levels.
Factor VIII antigen levels measured by an ELISA specific for human factor VIII after administration of 200 IU/kg human rFVIII in vWF knockout mice with (solid line, n = 3) and without (broken line, n = 3) pretreatment with 40 mg/kg RAP.
Values are mean ± SD.
Factor VIII antigen levels measured by an ELISA specific for human factor VIII after administration of 200 IU/kg human rFVIII in vWF knockout mice with (solid line, n = 3) and without (broken line, n = 3) pretreatment with 40 mg/kg RAP.
Values are mean ± SD.
Discussion
Although it is known that factor VIII is rapidly cleared from the circulation8 in the absence of vWF, current knowledge about the mechanism of clearance is limited. Infusion studies with plasma-derived vWF concentrates have the disadvantage that factor VIII is always coinfused, thus complicating the interpretation of the factor VIII metabolism. The availability both of a human recombinant vWF preparation that contains no factor VIII and of a murine model of vWD resembling human severe type 3 vWD with undetectable vWF and low factor VIII levels allowed us to investigate the survival of factor VIII in the presence and absence of vWF in vivo.
We measured normal factor VIII plasma concentrations in 3 typical laboratory mouse strains (NMRI, C57Bl/6J, and Balb/c) to identify the maximum plasma level of factor VIII potentially obtainable by intervention in vWF knockout mice with secondary factor VIII deficiency. In all 3 strains, factor VIII levels measured with the 2-stage assay were well below normal human levels. With the exception of high levels in male Balb/C mice, factor VIII levels determined with the chromogenic assay were generally around 100% (equivalent to 1 U/mL in humans) or slightly below. This finding differs from the much higher normal levels reported for murine plasma factor VIII in a recent review of hemostasis in the mouse,37 which were based on a chromogenic assay in C57Bl mice of both sexes.38 We believe that our results are reliable because all assays were carried out in a single laboratory using identical reagents and equipment. Disagreement between reported murine factor VIII values may occur because of individual variability (shown by relatively high coefficients of variation in our data), sex differences, and differences between mouse strains as well as between the various factor VIII assays used.
Recombinant human vWF appears able to bind murine factor VIII to achieve a degree of stabilization of factor VIII in the circulation. We previously showed that a recombinant preparation containing only mature vWF induced a rise in endogenous factor VIII in vWF deficient pigs39 and dogs.12 Although the human rpvWF preparation used in this study consisted of 50% pro-vWF and of only 50% mature vWF capable of binding and thus stabilizing factor VIII, it induced a rise in endogenous factor VIII in vWF-deficient mice. In a previous study that also demonstrated that the pro-vWF contained in the preparation is rapidly processed to mature vWF in the circulation in vWF-deficient dogs, pigs, mice, and normal baboons,32 the factor VIII levels obtained with the same recombinant vWF preparation as in this study were similar to those found here.
The factor VIII response was not as pronounced as that often seen in patients with type 3 vWD after the administration of plasma-derived vWF concentrates, which could be due to poor binding of human vWF to mouse factor VIII. However, in contrast to the recombinant vWF preparation used here, which is free of factor VIII, all currently available vWF concentrates intended for human use contain factor VIII in varying amounts, contributing to the immediate rise in factor VIII. In fact, very highly purified vWF concentrates appear to result in similar levels of factor VIII after infusion in humans to those achieved by the recombinant rpvWF in mice.40-42
Although we were not able to calculate the half-life of rpvWF in this study in mice because of limitations on the number of time points we could evaluate, the aforementioned studies12,32 39 showed that the half-life of human mature vWF in plasma is long enough to support factor VIII stabilization even 24 hours after administration. In the mice described here, the factor VIII plasma levels also had continued to increase when measured 24 hours after administration of rpvWF (data not shown).
It is unlikely that the rise in factor VIII induced by recombinant vWF is a result of increased factor VIII synthesis in the liver, because it was recently shown in a porcine model of vWD that vWF elevates plasma factor VIII without induction of factor VIII-specific messenger RNA in the liver.43 Neither do the effects of vWF on factor VIII in these experiments appear to derive from an ability of vWF to protect factor VIII from APC-mediated degradation.19,44 Thrombin generation is impaired in humans and animals with factor VIII deficiency,45 and under stable conditions, it seems highly unlikely that thrombin is generated in sufficient amounts to allow protein C activation to a level that would inactivate factor VIII. The APC concentration necessary to inactivate factor VIII in vitro has been demonstrated to be in the nanomole range,18 whereas the circulating APC concentration in healthy volunteers is in the picomole range.46 Thus, the necessary concentrations for inactivation of factor VIII by APC would never be reached in vivo in vWF-deficient animals.
On the basis of reports that LRP mediates the in vitro cellular uptake and degradation of factor VIII,20 21 it seemed reasonable to investigate the hypothesis that LRP is involved in the clearance of factor VIII in vivo. The RAP blocks the effects of LRP by binding with high affinity to various sites that may be shared or overlap with ligand binding sites, thus antagonizing the binding of all known ligands to this receptor. If LRP were involved in the clearance of factor VIII, blocking it in vivo should result in changes in factor VIII levels. To investigate this effect in the absence of vWF, we administered recombinant RAP fusion protein as a single bolus injection into vWF knockout mice and followed the course of factor VIII levels over time.
The initial behavior of factor VIII after injection of RAP in vWF knockout mice was almost identical to that of rpvWF. Within a short period the endogenous factor VIII plasma levels doubled and stayed almost constant throughout the experiment. By establishing a control group of animals that received a bolus injection of buffer instead of RAP, we could exclude that the increase in factor VIII was a stress-related effect caused by the traumatic event of the injection. Thus, inhibition of LRP by a single bolus administration of RAP results in a prolonged survival of murine factor VIII in the circulation. This observation is consistent with the effect of RAP in vitro, where RAP inhibits the binding of factor VIII to LRP.21 On the basis of the short half-life of RAP, which is rapidly cleared from the circulation with a biphasic half-life of 30 seconds and 9 minutes,24 it is not surprising that its effect on factor VIII levels was not as sustained as that of rpvWF. An approach to overcoming the short half-life of RAP is the blocking of LRP by using adenoviral gene transfer techniques to induce overexpression of RAP in the liver of mice, which has been previously used to prolong the half-life of t-PA24 and FXa25 in vivo.
Consistent with the observations on endogenous factor VIII, the preadministration of RAP slowed the disappearance of human factor VIII antigen after infusion of recombinant human factor VIII. Thus, inhibition of LRP acts on both endogenous and exogenous factor VIII in the absence of vWF and this finding suggests that the accelerated clearance of administered factor VIII seen in the absence of vWF is a result of the involvement of LRP in factor VIII metabolism.
vWF binds factor VIII with a high affinity, with a dissociation constant of 0.2 to 0.4 nmol. In vitro, the formation of the factor VIII/vWF complex is a very rapid process with more than 95% binding in 12 seconds.47 This high-affinity, rapid complex formation might prevent factor VIII from binding to other ligands that bind with a lower affinity, such as FIXa, phospholipids48,49 or potentially LRP, to which factor VIII binds with moderate affinity (Kd approximately 50 nmol).21 Taking the natural limitations of animal models into consideration, this in vivo study suggests LRP-mediated endocytosis as a novel mechanism by which factor VIII is cleared from the circulation in the absence of vWF.
Acknowledgment
We thank Kathryn Nelson, ELS, for editorial assistance.
The establishment of the vWF knockout mice was supported by grant R01HL41002 from the National Institutes of Health.
Reprints:Hans Peter Schwarz, Baxter Hyland Immuno, Industriestr. 67, A-1221 Vienna, Austria; e-mail:schwarh@baxter.com.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal