We investigated the molecular basis of hypofibrinogenemia in a man with a normal thrombin clotting time. Protein analysis indicated equal plasma expression of 2 different Bβ alleles, and DNA sequencing confirmed heterozygosity for a new Bβ235 P→L mutation. Protein analysis also revealed a novel γD chain, present at a ratio of 1:2 relative to the γA chain. Mass spectrometry indicated a 14 d decrease in the γD-chain mass, and DNA sequencing showed this was caused by a novel γ82 A→G substitution. DNA sequencing established heterozygosity for 2 further mutations: T→C in intron 4 of the A gene and A→C in the 3′ noncoding region of the Bβ gene. Studies on the man's daughter, together with plasma expression levels, discounted both the A and Bβ mutations as the cause of the low fibrinogen, suggesting that the γ82 mutation caused the hypofibrinogenemia. This was supported by analysis of 31 normal controls in whom the Bβ mutations were found at polymorphic levels, with an allelic frequency of 5% for the Bβ235 mutation and 42% for the Bβ 3′ untranslated mutation. The γ82 mutation was, however, unique to the propositus. Residue γ82 is located in the triple helix that separates the E and D domains, and aberrant packing of the helices may explain the decreased fibrinogen concentration.
Fibrinogen is the focal point of the coagulation cascade, which results in the conversion of fibrinogen to fibrin monomer and the eventual covalent cross-linking of the fibrin polymer. Fibrinogen, a 340-kd glycoprotein, is synthesized in the liver as a dimer with each half composed of 3 different polypeptide chains (Aα, Bβ, and γ). These chains, and each half molecule, are linked by a network of 29 disulfide bonds to form the circulating protein.1 The central E domain of the trinodal structure is connected to 2 globular D domains by a triple helix of Aα, Bβ, and γ chains. These peripheral D domains are composed of the independently folding homologous C-terminal regions of the Bβ and γ chains,2 while the C-terminal half of the Aα chain extends freely in solution, but appears to fold back and form a globular structure that interacts with the central E domain.3
Some 40 different mutations have been identified as causing dysfibrinogenemia,4 and a few of these mutations also result in decreased plasma expression. Notable among these are fibrinogens Marburg and Otago, where in homozygotes a 25% and 56% C-terminal truncation of the Aα chain results in a polymerization defect and hypofibrinogenemia, with circulatory levels of 0.6 and 0.1 mg/mL respectively.5,6 Surprisingly, a heterozygote for the severe Otago truncation showed no plasma expression of the variant and had a normal fibrinogen concentration (1.6 mg/mL). Similarly, while Marburg heterozygotes expressed the variant chain at only 10%, they too had normal fibrinogen concentrations (average, 1.9 mg/mL). This suggests that the synthesis of Aα chains is not limiting, and indeed other studies have shown that the expression of fibrinogen is regulated largely by sequences within the Bβ gene.7 Here we report the investigation of an individual with moderate hypofibrinogenemia and identify a novel γ-chain mutation as the apparent cause. This is the first case of hypofibrinogenemia (as distinct from dysfibrinogenemia) for which the molecular basis has been identified, and it is the first report of a compound heterozygote for 2 fibrinogen mutations.
Materials and methods
Citrate-anticoagulated blood was collected, and standard coagulation assays were used to measure thrombin and reptilase times. Functional fibrinogen levels were determined by means of the Clauss method, and gravimetric levels by quantification of fibrinopeptide release from whole plasma.8
Fibrinogen was purified by precipitation with 20% saturated ammonium sulfate, and the pellet was washed 3 times with 25% saturated ammonium sulfate.8 Sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) (7.5% reducing gels) was carried out as described by Laemmli,9and relative band intensities were determined by densitometric scanning of Coomassie stained gels. For chain separation, fibrinogen (5 mg/mL) was dissociated by reduction in 8 mol/L urea, 0.1 mol/L Tris/HCl (pH 8.0), and 15 mmol/L dithiothreitol for 4 hours at 37°C, and individual chains were isolated by high-performance liquid chromatography (HPLC) on a Phenomenex (Torrence, CA) C-4 column (25 × 0.45 cm). After injections of 1 mg of protein, the column was developed with a 0.05% trifluoroacetic acid/acetonitrile gradient at a flow rate of 0.75 mL/min.10
HPLC peak crests spanning a volume of 200 μL were collected and analyzed directly by electrospray ionisation (ESI) mass spectrometry (MS) on a VG Platform quadrupole analyzer.11 We injected 10 to 30 μL of each peak into the ion source at 5 μL/min. The probe was charged at + 3500 V and the source maintained at 60°C. The mass range 700 of 1600 m/z was scanned every 3 seconds, and a cone voltage ramp of 30 to 60 V was applied over this range. Up to 100 scans were averaged in acquiring the raw data. Calibration was made over this same m/z range by use of the charge series generated by human α globin, and data were acquired and processed by means of MassLynx software (Micromass, Manchester, UK) and transformed onto a true molecular mass scale with the use of maximum entropy (MaxEnt) software (Micromass) as described previously.10
Tryptic digests were prepared from approximately 0.25 mg of HPLC-isolated Bβ and γ chains. The individual chains were dried under N2 and redissolved in 50 μL of 25 mmol/L NH4HCO3 in 10% acetonitrile. Trypsin (3 μg) was added and the reaction incubated for 16 hours at 37°C. After being dried under vacuum with P2O5, digests were redissolved in 200 μL of 0.1% HCOOH, 50% acetonitrile, and 20 μL was analyzed by ESI MS. The m/z range of 300 to 1600 was scanned every 3.5 seconds.
Genomic DNA was isolated from buffy coat by a standard procedure.12 The entire coding region and flanking intronic sequences of all 3 fibrinogen genes were amplified by polymerase chain reaction (PCR),13 and PCR products were purified with the use of HiPure PCR purification cartridges (Boehringer, Mannheim, Germany). Cycle sequencing was performed with either an amplification primer or an internal sequencing primer with the use of 33P-radiolabeled dideoxy-terminators and Thermosequenase (Amersham, Amersham, UK) according to the manufacturer's instructions.
The frequency of the Bβ235 P→L and γ82 A→G mutations (single-letter amino acid code) were determined by screening 31 unrelated normal individuals using an allele-specific PCR assay.14 The allele-specific primer γ82G (5′-GATCCTATATTACAGATATGATAGACGCTCG-3′) and the primer Fn959γ (5′-TATAAATGGGGAAAACACAT-3′) were used to amplify a 202-bp product from the γ82 G allele while, in a separate reaction, the allele-specific primer β235L (5′-TCTCATTCAACCTGACAGTTCTGTCAAAGT-3′) and the primer Fn6050β (5′-GTGCTGGAATTACAGGTATG-3′) were used to amplify a 174-bp product from the β235 L allele. These primers were used at a final concentration of 0.2 μmol/L while β-globin primers at 0.025 μmol/L (β globin 5′: 5′-GCCGTGCCAGAGAGCCAA-3′; β globlin 3′: 5′-TTAGGGTTGCCCATAACAGC-3′) provided a 514-bp internal control. Each 25 μL PCR reaction also contained 200 μmol/L deoxynucleotide triphosphates, 50 mmol/L KCl, 10 mmol/L Tris/HCl (pH 8.3), 1.5 mmol/L MgCl2, 0.1% (wt/vol) gelatin, and 10 to 20 ng template DNA. Reactions were hot-started by the addition of 1 U of Taq DNA polymerase after the first denaturation. PCR was performed for 35 cycles of denaturation at 94°C for 30 seconds, annealing at 58°C for 30 seconds, and extension at 72°C for 1 minute, and products were electrophoresed in 2% (wt/vol) agarose and visualized by ethidium bromide staining.
The frequency of the Bβ 3′-untranslated sequence variation was determined by HinfI restriction digestion of a 262-bp PCR product amplified from the same 31 unrelated controls. Each 50 μL reaction contained 0.5 μmol/L Fn8349β (5′-CAGTTCTAGTTGATTGCGAG-3′), and 0.5 μmol/L Fn8610β (5′-GCTTCTCCTTCCTTACAAGT-3′), and amplification was as above. Products were analyzed on 3% (wt/vol) Nusieve/agarose (3:1) (FMC, Rockland, MN) after the addition of 10 UHinfI and a further 3 hours' incubation at 37°C.
Results
Case report
Hypofibrinogenemia was first diagnosed at age 79 years when the patient developed a large wound hematoma following inguinal hernia repair. During the procedure, some bleeding was noted; however, this appeared to be easily controlled. Reexploration of the wound hematoma disclosed surgical bleeding points in the spermatic cord and external iliac vein. The patient admitted to only a mild tendency to increased bleeding from cuts in the past. A retropubic prostatectomy was performed at age 59 years and required a urethral catheter for 2 weeks.
Surgical resection of a pharyngoesophageal pouch was carried out after infusing a single preoperative dose of cryoprecipitate, which increased fibrinogen levels from 1.0 mg/mL to 3.7 mg/mL (kinetic assays). Hemostasis was clinically normal during the procedure and convalescence. At age 88 years, he developed transient ischemic attacks, angina, and mild left ventricular failure. Treatment included aspirin and persantin, but aspirin was withdrawn after development of rectal bleeding. At this time, he also had a mild thrombocytopenia (102 × 109/L), which may be an early indication of myelodysplasia.
There is no history of hepatitis, and coagulation tests have consistently shown low functional fibrinogen of 0.7 to 1.1 mg/mL (normal range 1.5 to 4.0) and gravimetric levels of 0.8 mg/mL together with normal thrombin (18 seconds) and reptilase times (18 seconds). Fibrin degradation products were negative; the activated partial thromboplastin time has given high normal values, 30 to 34 seconds (22 to 33 seconds), prothrombin time 12.6 (range, 10.0 to 15.0), von Willebrand factor antigen 85% (range, 50% to 150%), ristocetin cofactor 90% (range, 50% to 150%), factor VIII 55% (range, 50% to 150%), factor IX 47% (range, 50% to 150%), factor XI 91% (range, 50% to 150%), and factor XII 57% (range, 50% to 150%).
It is concluded that the patient has a mild tendency toward increased bleeding that is clinically apparent only in the presence of moderate or severe tissue injury, but that may be enhanced by antiplatelet agents, such as aspirin. In the absence of clinical laboratory assessment for qualitative platelet defects, factor XIII, and fibrinolytic parameters, the origin of this patient's mild bleeding tendency remains an open question. His daughter, the only other family member available for study, appeared normal with a functional fibrinogen concentration of 3.5 mg/mL and a thrombin clotting time of 18 seconds.
Protein and DNA analysis
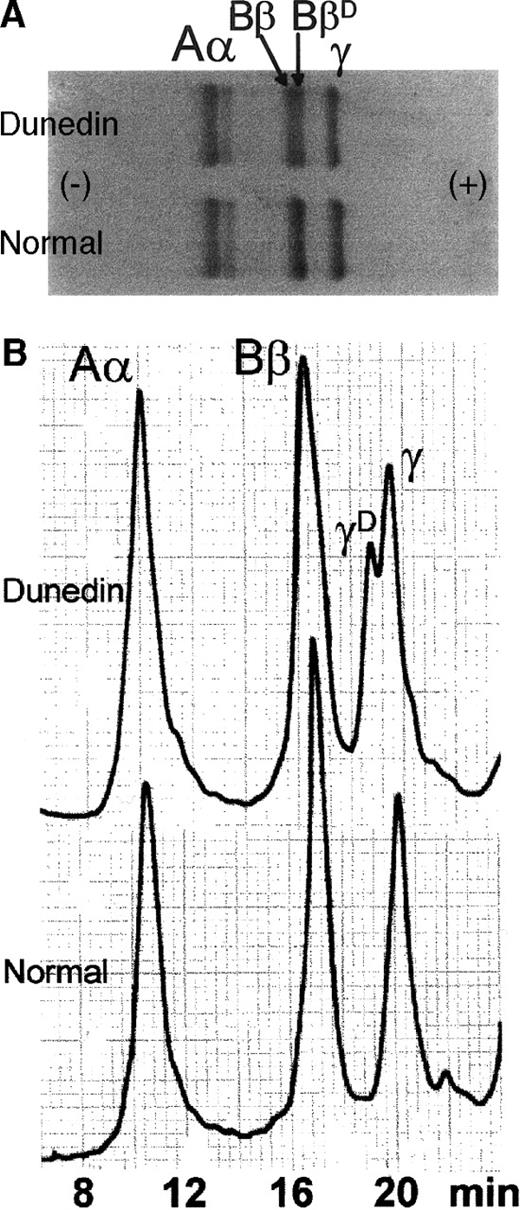
Analysis of purified fibrinogen from the propositus by SDS-PAGE showed a doublet Bβ band together with a normal pattern of Aα and γ chains (Figure 1A). The doublet Bβ band was consistently present in several different plasma samples, and the 2 components were in a 1:1 ratio in the different samples. Western blots with an anti–Aα chain antiserum showed reactivity only against the Aα doublet and not against the Bβ doublet. This suggested a Bβ mutation, but the equal expression of the 2 alleles negated the possibility that the mutation might be the cause of the hypofibrinogenemia. Further analysis of the reduced fibrinogen chains by reverse-phase HPLC also indicated an abnormal pattern, but here (Figure 1B) the Aα and Bβ peaks appeared normal, and a more hydrophilic γD chain was present in addition to the normal γA chain. Again, this unusual pattern was consistently reproduced with fibrinogen purified from several different plasma samples, and the ratio of γD to γAwas found to be 1:2. SDS-PAGE of the purified HPLC peaks showed that the Bβ peak consisted of 2 components of similar intensity and that the separated γD and γA peaks comigrated on electrophoresis (not shown).
Analysis of purified fibrinogen.
Fibrinogen from Dunedin plasma is shown in comparison with normal fibrinogen. (A) Analysis by SDS-PAGE (7.5% reducing gel). Note additional BβD band. (B) Analysis by reverse phase HPLC. Note new γD peak.
Analysis of purified fibrinogen.
Fibrinogen from Dunedin plasma is shown in comparison with normal fibrinogen. (A) Analysis by SDS-PAGE (7.5% reducing gel). Note additional BβD band. (B) Analysis by reverse phase HPLC. Note new γD peak.
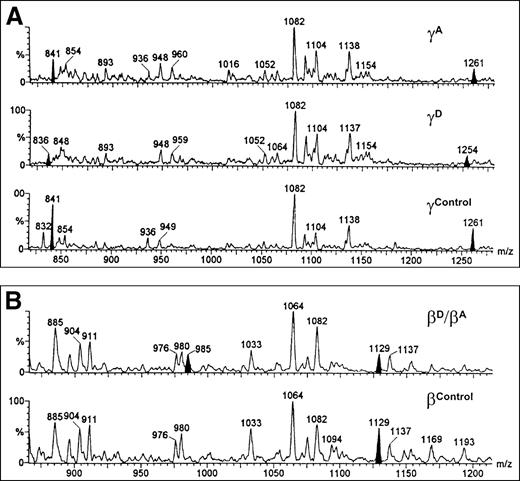
Individual HPLC peaks were also examined after direct injection into an ESI mass spectrometer. This indicated molecular weights of 66 156 d for the Aα chains and 54 200 d for the major (monosialo) isoform of the Bβ-chain mixture. However, these values were not significantly different from the control values (66 150 and 54 193 d, respectively). The monosialo isoform of the γA and γD chains, purified from the propositus, had masses of 48 369 and 48 356 d, respectively. The difference between these values seemed significant, and a mean decrease of 14 d (SD=3 d) was observed for the γD chain in 5 separate experiments.
Tryptic peptide mapping was used to locate the site of this putative genetic lesion. ESI MS maps of purified γA and γD chains showed that both the M+2H and M+3H ions of peptide T8 (residues γ63-68) were missing from the γDdigests. These ions have theoretical m/z values of 1261.4 and 841.2, respectively, and they can be clearly seen in the γA- and control γ-chain digests (Figure 2A). In the γD mass spectrum, they are replaced by signals at 1254.0 and 836.2 m/z, indicating a mass of 2506 d for peptide T8D, compared with 2520 d for T8A. PCR amplification and direct sequencing of DNA encoding the T8 peptide (exons 3 and 4) indicated heterozygosity for a γ82 A (GCT)→G (GGT) substitution (Figure3D). This is the first report of this substitution, which we have named the fibrinogen Dunedin mutation.
ESI MS tryptic peptide maps.
(A) γ chains showing (from top) purified γA from the propositus, purified γD chains from the propositus, and γA chains from a control. Note the disappearance of 1261 and 841 m/z ions and their replacement by ions at 1254 and 836 m/z. (B) Mixture of BβD/BβA from propositus and BβA chains from a control. Note the 50% decrease of the 1129 m/z ion and the appearance of a new ion at 985 m/z.
ESI MS tryptic peptide maps.
(A) γ chains showing (from top) purified γA from the propositus, purified γD chains from the propositus, and γA chains from a control. Note the disappearance of 1261 and 841 m/z ions and their replacement by ions at 1254 and 836 m/z. (B) Mixture of BβD/BβA from propositus and BβA chains from a control. Note the 50% decrease of the 1129 m/z ion and the appearance of a new ion at 985 m/z.
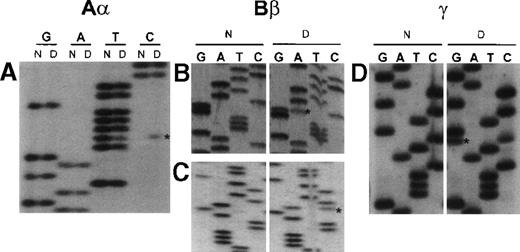
Unique fibrinogen gene sequence variations detected in subject from Dunedin.
Abnormal bases are denoted by an asterisk. Lanes labeled N correspond to normal control sequence, while those labeled D are from propositus. Nucleotide positions are based on the numbering in the respective Genbank entries M64 982 (Aα), M64 983 (Bβ) and M10 014 (γ). (A) Sequence surrounding nucleotide 3237 in intron 4 of the Aα-chain gene obtained with the forward primer Fn3003γ (5′-TTACAGACAAATCACTCAGCAGCT-3′). Dunedin subject is heterozygous for a T→C transition. (B) Sequence surrounding nucleotide 5906 in exon 5 of the Bβ-chain gene obtained with the reverse primer Fn6050β (5′-GTATGGACATTAAGGTCGTG-3′). Dunedin subject is heterozygous for a G→A (coding strand C→T) transition, which predicts the substitution Bβ235 P→L. (C) Sequence surrounding nucleotide 8525 in the 3′ untranslated region of the Bβ-chain gene obtained with the reverse primer Fn8610β (5′-TGAACATTCCTTCCTCTTCG-3′). Dunedin subject is heterozygous for an A→C transversion. (D) Sequence surrounding nucleotide 2525 in exon 4 of the γ-chain gene obtained with forward primer Fn2479γ (5′-GGATTTTTATGTCTCTGATC-3′). Dunedin subject is heterozygous for a C→G transversion, which predicts the substitution γ82 A→G.
Unique fibrinogen gene sequence variations detected in subject from Dunedin.
Abnormal bases are denoted by an asterisk. Lanes labeled N correspond to normal control sequence, while those labeled D are from propositus. Nucleotide positions are based on the numbering in the respective Genbank entries M64 982 (Aα), M64 983 (Bβ) and M10 014 (γ). (A) Sequence surrounding nucleotide 3237 in intron 4 of the Aα-chain gene obtained with the forward primer Fn3003γ (5′-TTACAGACAAATCACTCAGCAGCT-3′). Dunedin subject is heterozygous for a T→C transition. (B) Sequence surrounding nucleotide 5906 in exon 5 of the Bβ-chain gene obtained with the reverse primer Fn6050β (5′-GTATGGACATTAAGGTCGTG-3′). Dunedin subject is heterozygous for a G→A (coding strand C→T) transition, which predicts the substitution Bβ235 P→L. (C) Sequence surrounding nucleotide 8525 in the 3′ untranslated region of the Bβ-chain gene obtained with the reverse primer Fn8610β (5′-TGAACATTCCTTCCTCTTCG-3′). Dunedin subject is heterozygous for an A→C transversion. (D) Sequence surrounding nucleotide 2525 in exon 4 of the γ-chain gene obtained with forward primer Fn2479γ (5′-GGATTTTTATGTCTCTGATC-3′). Dunedin subject is heterozygous for a C→G transversion, which predicts the substitution γ82 A→G.
When mass spectra of tryptic digests of the Bβ-chain mixture were compared with control digests, 2 features were of particular relevance. The signal at 1129.3 m/z was approximately half the intensity seen in the controls, and there was a new ion present at 985.0 m/z (Figure 2B). Since the 1129.3 m/z signal corresponds to the predicted M+2H ion (1129.7) of peptide T27, this suggested that the propositus was heterozygous for a mutation occurring between residues Bβ218 and Bβ237. This is also consistent with the initial SDS gels, which showed 2 different Bβ components of similar intensity.
Direct sequencing of amplified DNA confirmed heterozygosity for a novel Bβ variant when a C→T mutation was identified in exon 5 (Figure 3B). As expected, this Bβ235 P→L substitution is located in tryptic peptide T27 (GGETSEMYLIQPDSSVKPYR237). Trypsin is unable to cleave the -K-P- bond in this peptide; however, the P→L substitution renders the K susceptible to cleavage. Predictably, this would result in the appearance of a new peptide (GGETSEMYLIQPDSSVK), and since this sequence is preceded by an RK sequence, another new peptide (KGGETSEMYLIQPDSSVK) would also be expected. This latter peptide would have an expected M+2H ion at 985.1 m/z, and this was indeed seen in the patient's spectrum at 985.0 m/z (Figure 2B).
While it was possible that the γ82 A→G mutation (rather than the coexpressed Bβ235 P→L mutation) might explain the hypofibrinogenemia, it was also possible that an additional mutation might contribute to the low circulatory fibrinogen. With this in mind, we sequenced the entire coding region and the intron/exon boundaries of the Aα, Bβ, and γ genes. We detected 2 additional new heterozygous mutations during this analysis: 1 in intron 4 of the Aα gene (Figure 3A) and the other in the 3′ untranslated region of the Bβ gene (Figure 3C).
Since the Aα-, Bβ-, and γ-chain genes are present as a cluster on chromosome 4, it was important to establish which of the 4 mutations cosegregated and which was associated with the hypofibrinogenemia. Unfortunately, only 1 daughter was available for study. Examination of her DNA and purified fibrinogen showed that she had inherited the Aα intron 4 and the Bβ untranslated mutations, but that she had neither the Bβ235 P→L nor the γ82 A→G amino acid substitutions. Thus, the γ82 A→G and Bβ235 P→L mutations must be on the same chromosome in the father, and since the daughter has a normal fibrinogen concentration (3.5 mg/mL), 1 of these substitutions must be responsible for the hypofibrinogenemia. However, the equivalent expression of the 2 Bβ variants in the father suggests that the γ defect is the actual cause of his hypofibrinogenemia.
The finding of multiple mutations implied that some might be at polymorphic frequency in the population, so we examined DNA from 31 normal individuals. PCR amplification followed by HinfI digestion established an allelic frequency of 42% for the Bβ 3′ untranslated A→C mutation, while allele-specific amplification followed by agarose gel electrophoresis indicated a frequency of 5% for the Bβ235 P→L mutation. SDS-PAGE of fibrinogen from the latter individuals confirmed the presence of a Bβ doublet band. The mean plasma fibrinogen for individuals heterozygous for these 2 mutations were 3.0 and 3.1 mg/mL respectively. The γ82 A→G substitution was, however, unique to the propositus.
We also examined the father and daughter for 4 other fibrinogen gene-cluster polymorphisms; both were homozygous for the common AαTaqI, Bβ BclI, Bβ MnlI, and BβHindIII alleles (the latter being associated with higher fibrinogen levels).
Discussion
Plasma fibrinogen levels are genetically determined15and several studies have shown an association between polymorphisms in the fibrinogen gene cluster and elevated fibrinogen. In turn, these high levels have been correlated with an increased risk of arterial thrombosis and coronary artery disease.16-19Hypofibrinogenemia may occur as an acquired condition associated with acute liver disease, but few genetic determinants of hypofibrinogenemia have been characterized. Very low levels can result in prolonged bleeding and miscarriage,6 and surprisingly, some hypofibrinogenemic individuals may also suffer from thrombotic tendencies.20
Here we have identified 4 novel mutations (1 Aα, 2 Bβ, and 1 γ) in an individual with hypofibrinogenemia. This raised the question: which mutation was actually responsible for the decreased plasma expression of the fibrinogen? This was resolved by 3 different approaches: family studies, measurement of the relative plasma levels of the different allelic products, and gene frequency analysis in normal subjects.
The Aα intron 4 mutation was excluded on the basis that it was also identified in the patient's normal daughter. This woman also had the Bβ 3′ untranslated A→C mutation, but not the Bβ235 P→L substitution. However, both of the β mutations were excluded as possible causes of the hypofibrinogenemia because unrelated individuals with these mutations did not have hypofibrinogenemia and because there was equivalent expression of the 2 Bβ alleles in the propositus. This coexpression was evidenced by, the 2 Bβ bands on SDS-PAGE, and the 50% reduction in intensity of the Bβ T8 peptide on ESI MS peptide mapping.
The evidence that the γ82 A→G mutation was the actual cause of the hypofibrinogenemia comes from (1) the decreased plasma level of this variant chain, (2) its absence in the unaffected daughter, and (3) its absence in the normal group that was examined.
Chain separation by HPLC established that the γD/γA chain ratio was 1:2 in plasma fibrinogen. It may seem implausible that this small decrease in the relative plasma expression could satisfactorily explain the hypofibrinogenemia. However, after DNA sequencing of all 3 fibrinogen genes, and elimination of 3 other candidate mutations, we are left with the conclusion that the γ82 A→G substitution is most probably responsible for the low fibrinogen concentration.
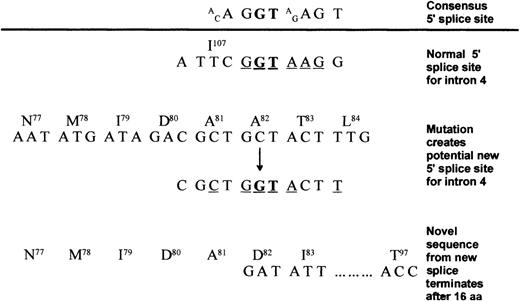
At least 2 mechanisms may be postulated. If the 2 γ genes are expressed at equal levels and incorporated randomly into the 340-kd dimer, then 75% of the molecules (25% Aα2Bβ2 γD2 and 50% Aα2 Bβ2 γDγA) will contain a γD chain. In this case. any destabilization resulting from the γD aberration would affect the stability of molecules containing γA chains as well, thereby maintaining a relatively high γD/γA ratio, but having a major negative impact on fibrinogen concentration. Alternatively, it is possible that the GCT→GGT mutation at codon 82 might activate a cryptic 5′ splice site in exon 4 (Figure 4). This putative new site appears as attractive as the normal 5′ site, and if both are used, normal splicing would produce the mutant γD chain, while aberrant splicing would produce an abnormal chain truncated after residue 97. This chain would be nonviable, and the product of the γD gene would be decreased by a value reflected in the observed 1:2 γD/γA ratio. However, aberrant splicing appears insufficient to account for the hypofibrinogenemia, since the supply of γ chain messenger RNA is not limiting.7 Also heterozygotes for variants with no, or very low, plasma expression have normal fibrinogen levels.6 Although we favor the former protein instability mechanism for explaining the hypofibrinogenemia, mutations within the promoter have not been excluded.
Possible intron 4 splicing events as a result of the γ82 A→G substitution.
Conserved bases are underlined, and the invariant GT is in bold. The consensus 5′ splice site21 is shown above the line with the normal 5′ splice site for intron 4 shown below. γ82 A→G introduces a potential 5′ splice site by creating a new GT with 4 of the surrounding 7 bases adhering to the consensus sequence. Translation of this message would result in a truncated polypeptide ending in the novel sequence82DICRKYIIQIIKRLLT.97
Possible intron 4 splicing events as a result of the γ82 A→G substitution.
Conserved bases are underlined, and the invariant GT is in bold. The consensus 5′ splice site21 is shown above the line with the normal 5′ splice site for intron 4 shown below. γ82 A→G introduces a potential 5′ splice site by creating a new GT with 4 of the surrounding 7 bases adhering to the consensus sequence. Translation of this message would result in a truncated polypeptide ending in the novel sequence82DICRKYIIQIIKRLLT.97
Neither the γ82 nor the Bβ235 mutation appears to have any effect on polymerization as judged by thrombin time assay. This is not surprising considering their respective locations, in the center of the triple helix and in the β-chain D domain close to its junction with the triple helix. The triple helix contains no known functional sites and is essentially a spacer between the functional E and D domains. Although the γ-chain D domain contains a number of critical polymerization sites, the crystal structure shows that Bβ235 is well away from homologous sites in the Bβ chain. Bβ235 P occurs immediately before the third β sheet of the D domain. This position is quite tolerant of different side chains, being occupied by Q in the γ chain and I in the alternate αEtranscript.22 Predictably, therefore, the P-to-L mutation would be quite benign, and this prediction was confirmed by finding it at a polymorphic frequency in a normal population.
Residue γ82 is located in the protease-sensitive region in the middle of the triple helix that connects the E domain to the outer D domains and is not included in the available crystal structures. Sequence in this region is not highly conserved, but large hydrophobic residues of Y and V occupy the corresponding position in the Aα and Bβ strands. Glycine is underrepresented in this region, with only 3 occurrences in the 337 residues that make up the triple helix. The absence of a side chain on the new G could destabilize the helix packing and impair molecular assembly or render the mature molecule more susceptible to proteolytic degradation.
Our finding that γ82 is an important determinant of plasma fibrinogen levels highlights the role of mutation analysis in defining novel functional sites. This case suggests that the triple helix structure can affect the assembly, secretion, or half-life of fibrinogen. Future studies should examine these concepts and the detailed structure of this domain. The finding of 3 new polymorphisms suggests that the fibrinogen genes are more variable than presently recognized and that care needs to be taken when assigning a phenotype to a particular DNA mutation.
Acknowledgment
We thank Silvia Parkin for performing the coagulation studies.
Supported by the Canterbury Medical Research Foundation, Lottery Health, and the Health Research Council of New Zealand.
Reprints: S. O. Brennan, Molecular Pathology Laboratory, Canterbury Health Laboratories, Christchurch Hospital, Christchurch, New Zealand; e-mail: steve.brennan@chmeds.ac.nz.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal