Factor VIIIa is a trimer of A1, A2, and A3-C1-C2 subunits. Inactivation of the cofactor by human activated protein C (APC) results from preferential cleavage at Arg336 within the A1 subunit, followed by cleavage at Arg562 bisecting the A2 subunit. In the presence of human protein S, the rate of APC-dependent factor VIIIa inactivation increased several-fold and correlated with an increased rate of cleavage at Arg562. (Active site-modified) factor IXa, blocked cleavage at the A2 site. However, APC-catalyzed inactivation of factor VIIIa proceeded at a similar rate independent of factor IXa, consistent with the location of the preferential cleavage site within the A1 subunit. Addition of protein S failed to increase the rate of cleavage at the A2 site when factor IXa was present. In the presence of factor X, cofactor inactivation was inhibited, due to a reduced rate of cleavage at Arg336. However, inclusion of protein S restored near original rates of factor VIIIa inactivation and cleavage at the A1 site, thus overcoming the factor X-dependent protective effect. These results suggest that in the human system, protein S stimulates APC-catalyzed factor VIIIa inactivation by facilitating cleavage of A2 subunit (an effect retarded in the presence of factor IXa), as well as abrogating protective interactions of the cofactor with factor X.
Factor VIII, an essential blood coagulation protein deficient or defective in individuals with hemophilia A, is synthesized as a 300 kd precursor protein1,2 with domain structure A1-A2-B-A3-C1-C2.3 Factor VIII is processed to a series of divalent metal ion-dependent heterodimers,4-6 produced by cleavage at the B-A3 junction generating a heavy chain (A1-A2-B domains) and a light chain (A3-C1-C2 domains). Thrombin converts factor VIII to the active cofactor, factor VIIIa, by limited proteolysis.7 Thrombin cleaves factor VIII heavy chain at Arg740, which liberates the B domain, and at Arg372, which bisects the contiguous A1-A2 domains into the A1 and the A2 subunits. (Factor VIIIa subunits are designated relative to the domain sequence A1-A2-B-A3-C1-C23 and are as follows: A1, residues 1-372; A2, residues 373-740; A3-C1-C2, residues 1690-2332. Noncovalent subunit associations are denoted by [/] and covalent associations are denoted by [-].) Cleavage of the light chain at Arg1689 liberates an acidic rich region and creates a new NH2-terminus. Thus, factor VIIIa is a heterotrimer of subunits designated as A1, A2, and A3-C1-C2.8,9 The A1 and A3-C1-C2 subunits retain the stable divalent metal ion-dependent linkage, whereas the A2 subunit is weakly associated with the dimer through electrostatic interactions.9,10 Factor VIIIa functions as a cofactor for factor IXa in the surface-dependent conversion of factor X to factor Xa, increasing the kcat of the reaction by several orders of magnitude.11
Human activated protein C (APC) is a potent anticoagulant and its importance as a regulator of blood coagulation is apparent from a tendency for thrombosis in individuals with protein C deficiency (see Dahlback12 for a recent review). The anticoagulant effect is phospholipid and Ca++ dependent and results from the selective inactivation of factor Va and factor VIIIa. Previous studies have identified 2 sites in factor VIIIa that are cleaved by APC. The site identified at Arg3367 is located near the C-terminal end of the A1 subunit, whereas the site at Arg56213 bisects the A2 subunit. The latter site is cleaved rapidly by bovine APC and this event correlated with loss of factor VIIIa activity.13The rate of cleavage within the A2 subunit was further accelerated by the presence of protein S,14 a cofactor for APC.15 In the presence of active site-modified factor IXa, cleavage at the A2 site was protected by a mechanism consistent with steric blocking.14 This protection was eliminated in the presence of protein S. Recent results suggest that, although equivalent sites in factor VIII/factor VIIIa are cleaved by human APC, the initial cleavage occurs at Arg336 within the A1 subunit and correlates with the APC-dependent loss of cofactor activity.16
In this study, we investigate the cleavage and inactivation of factor VIIIa by human APC in the absence and presence of protein S. We further examine the influence of factor IXa and factor X (the enzyme and substrate, respectively, of the factor X activating complex), on cofactor inactivation because association of factor VIIIa with these proteins represent conditions under which attack by APC would likely occur. This rationale is further supported by the observations that APC cleavage sites are within and are likely influenced by these macromolecular interactive sites. Arg562 is located within a factor IXa interactive site,17 whereas Arg336 is adjacent to residues 337-372 shown to represent an interactive site for factor X.18 Results of this study show that rate of bond cleavage and site specificity of APC are altered by these macromolecules.
Materials and methods
Reagents
The reagents Glu-Gly-Arg chloromethyl ketone (Calbiochem), phospotidylserine (PS), phosphotidylcholine (PC), phosphotidylethanolamine (PE) (Sigma, St Louis, MO), α-thrombin, factor IXa, factor X, factor Xa (Enzyme Research Labs, South Bend, IN), human APC, and human protein S (Hematologic Technologies, Burlington, VT) were purchased from the indicated vendors. Phospholipid vesicles composed of 20% PS, 40% PC, and 40% PE were prepared using octyl glucoside as previously described.19 Tick anticoagulant peptide (TAP) was a gift from S. Krishnaswamy. Anti-factor VIII monoclonal antibodies R8B129 and 58.12 (a gift from Bayer Corporation, Berkeley, CA) recognize epitopes at the C-terminal end of A2 and the N-terminal end of A1 domains, respectively.
Proteins
Recombinant factor VIII preparations were gifts from Bayer Corporation and The Genetics Institute. Factor VIII (1.8 μmol/L) in 20 mmol/L HEPES, pH 7.2, 100 mmol/L NaCl, 5 mmol/L CaCl2, 0.01% Tween was activated with thrombin (60 nmol/L) for 10 minutes at room temperature and resultant factor VIIIa was purified using CM-sepharose chromatography.20 Peak factor VIIIa fractions were 1.5 to 2 μmol/L and were stored in aliquots at −80°C. Factor VIIIa activity was monitored using a 1-stage clotting assay. The active site of factor IXa was modified with EGR-CK to yield EGR-factor IXa.21 This modified form of factor IXa was used to eliminate the contribution of factor IXa-catalyzed cleavage at Arg336 from the analysis.
Inactivation of factor VIIIa by human activated protein C
The rate of factor VIIIa inactivation was monitored using a 1-stage clotting assay. Factor VIIIa (200 nmol/L) was reacted at 37°C with APC (40 nmol/L) in buffer containing 20 mmol/L HEPES, pH 7.2, 100 mmol/L NaCl, 5 mmol/L CaCl2, 0.01% Tween, and 100 μmol/L PSPCPE vesicles. Reactions containing factor X were supplemented with TAP (1 μmol/L) to eliminate proteolysis by any traces of contaminating factor Xa. Control reactions showed that the presence of TAP had no effect on the rate of APC-catalyzed inactivation of factor VIIIa in the absence of factor X. Other components are as indicated in the figure legends. Time course reactions were initiated with the addition of APC. Aliquots were removed at indicated times and assayed immediately for residual clotting activity and factor VIIIa subunit composition.
Electrophoresis and Western blotting
SDS-PAGE was performed using the method of Laemmli,22with a Bio-Rad minigel system. Electrophoresis was performed at 150V for 1 hour. The proteins were transferred to polyvinylidene difluoride membrane using a Bio-Rad mini-transblot apparatus at 0.5 A for 30 minutes in a buffer containing 10 mmol/L CAPS, pH 11, and 10% (vol/vol) methanol. Western blotting used the indicated primary antibodies, followed by goat antimouse horseradish peroxidase-conjugated secondary antibody. The secondary antibody signal was detected using the ECL system (Amersham, Arlington Heights, IL) with luminol as substrate, and the blots were exposed to film for various times. Films were scanned and band densities (obtained from a linear exposure range) were quantitated by using Scan Analysis software (BioSoft, Ferguson, MD).
Data analysis
Results of time course studies are plotted as percentage of initial activity or concentration. Rates of APC-catalyzed inactivation of factor VIIIa activity and proteolysis of A1 and A2 subunits were calculated from the linear portion (initial time points) of the plotted data. In most cases, initial rates were estimated using best fit lines through points where less than 30% of the substrate had been converted to product. The percentage of intact subunit remaining was calculated from band densities using the formula: (density of intact subunit/[density of intact subunit + density of derived fragment]) × 100 and converted to a concentration value based on the initial substrate concentration. Rates were determined as nmol/L factor VIIIa (subunit)/min/nmol/L APC and are expressed as minute−1 in the text.
Results
Inactivation of factor VIIIa by human activated protein C and protein S
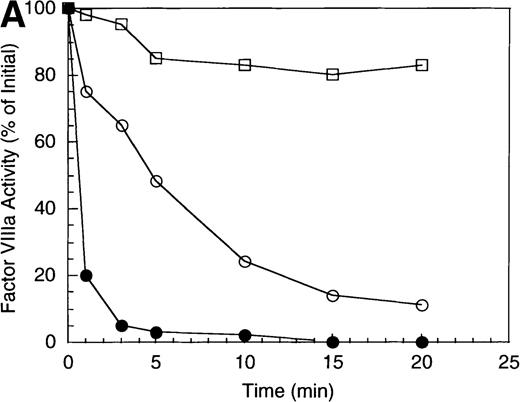
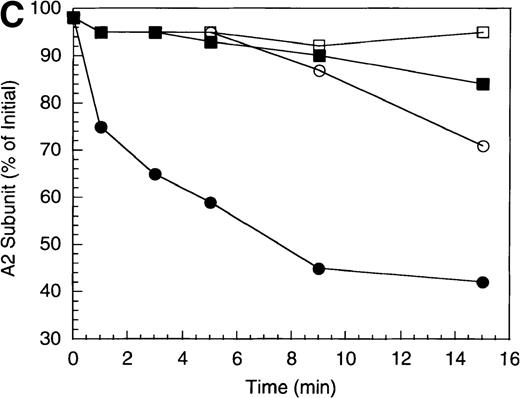
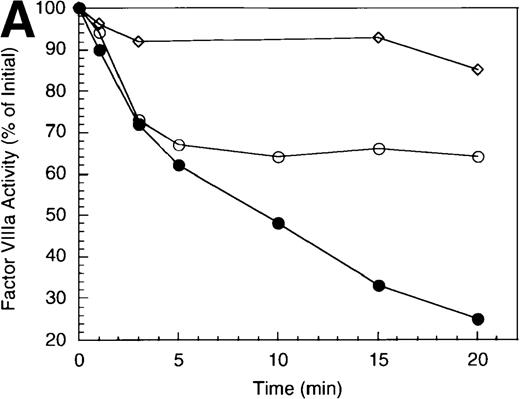
The inactivation of factor VIIIa (200 nmol/L) by APC (40 nmol/L) was examined in the absence and presence of a saturating concentration of protein S (250 nmol/L). The high factor VIIIa concentration was used to minimize spontaneous decay of the cofactor, due to dissociation of the A2 subunit.23 In the absence of protein S, APC-catalyzed inactivation of factor VIIIa proceeded at a rate of approximately 1.2 minute−1 (Figure 1A), based on factor VIIIa activity lost at the early time points and assuming negligible inactivation because of nonproteolytic decay of the cofactor. In the presence of protein S, the rate of factor VIIIa inactivation by APC increased approximately 3-fold (approximately 4 minutes−1). However, this value may underestimate the inactivation rate given the conversion of a significant fraction of substrate (approximately 80%) by the 1 minute time point.
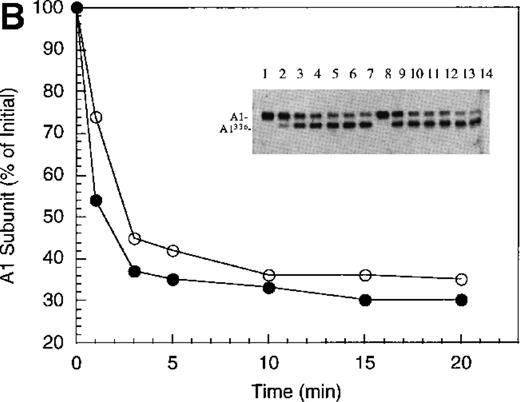
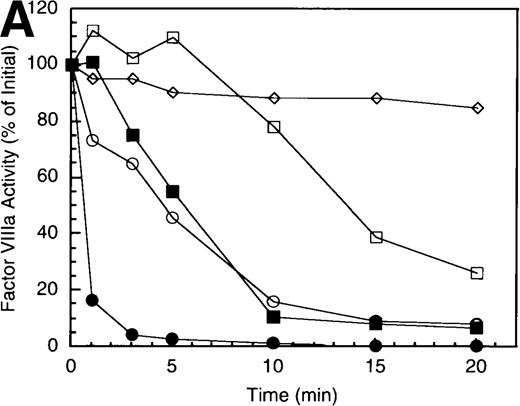
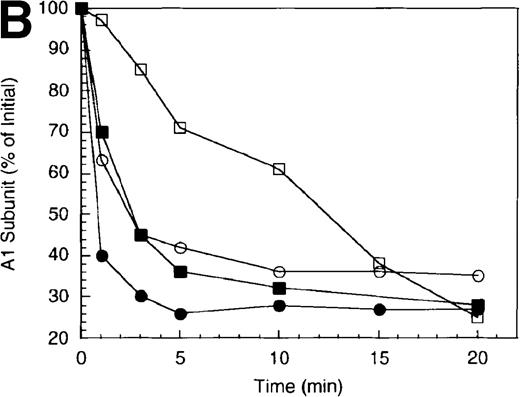
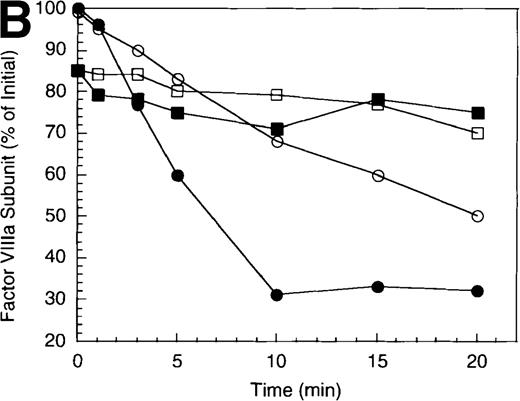
Inactivation of factor VIIIa by human APC and protein S.
(A) Factor VIIIa activity was determined for reactions containing 200 nmol/L factor VIIIa alone (open squares), factor VIIIa plus 40 nmol/L APC (open circles) and factor VIIIa plus APC plus 250 nmol/L protein S (closed circles). (B) Residual A1 subunit was determined for the above reactions run in the absence (open circles) and presence (closed circles) of protein S. Curves were derived from scans of the Western blots obtained using the 58.12 anti-A1 subunit antibody. These data are shown in the inset, where lanes 1-7 and 8-14 represent time points at 0, 1, 3, 5, 10, 15, and 20 minutes after addition of APC and APC plus protein S, respectively. (C) Residual A2 subunit was determined for the above reactions run in the absence (open circles) and presence (closed circles) of protein S. Curves were derived from scans of the Western blots obtained using the R8B12 anti-A2 subunit antibody, which is shown in the inset. Lane designations are as in B. Low levels of the C-terminal derived fragment (A2c; approximately 10% total A2) were identified in the control (time = 0) lanes before addition of APC. The origin of this material is not known.
Inactivation of factor VIIIa by human APC and protein S.
(A) Factor VIIIa activity was determined for reactions containing 200 nmol/L factor VIIIa alone (open squares), factor VIIIa plus 40 nmol/L APC (open circles) and factor VIIIa plus APC plus 250 nmol/L protein S (closed circles). (B) Residual A1 subunit was determined for the above reactions run in the absence (open circles) and presence (closed circles) of protein S. Curves were derived from scans of the Western blots obtained using the 58.12 anti-A1 subunit antibody. These data are shown in the inset, where lanes 1-7 and 8-14 represent time points at 0, 1, 3, 5, 10, 15, and 20 minutes after addition of APC and APC plus protein S, respectively. (C) Residual A2 subunit was determined for the above reactions run in the absence (open circles) and presence (closed circles) of protein S. Curves were derived from scans of the Western blots obtained using the R8B12 anti-A2 subunit antibody, which is shown in the inset. Lane designations are as in B. Low levels of the C-terminal derived fragment (A2c; approximately 10% total A2) were identified in the control (time = 0) lanes before addition of APC. The origin of this material is not known.
To examine the rates of proteolytic cleavage at each of the cleavage sites and correlate these events with cofactor inactivation, Western blot analysis was performed after separation of subunits by SDS-PAGE and transfer to polyvinylidene difluoride membranes. Results show that A1 subunit was cleaved (at Arg336) at a rate of approximately 1.3 minute−1 (Figure 1B), which essentially accounts for the observed rate of factor VIIIa inactivation. In the presence of protein S, the rate of cleavage of A1 subunit was modestly increased (approximately 2.3 minute−1). In contrast, cleavage of the A2 subunit (at Arg562) was negligible in the absence of protein S (approximately 0.15 minute−1 Figure 1C), whereas cleavage of A2 subunit was increased approximately 5-fold (approximately 0.8 minute−1) in the presence of protein S. These results suggest that the enhanced proteolysis of A2 likely contributes to the rapid rate of APC-dependent cofactor inactivation in the presence of protein S. In the absence of APC, addition of protein S had no effect on factor VIIIa activity (data not shown).
In general, a disparity appears to exist notably in those reactions including protein S (shown in Figure 1 and below) in that extended time points show the near complete loss of activity, whereas residual intact A1 and A2 subunits persist. The reason for this likely reflects nonproteolytic decay of factor VIIIa accelerated by the reduced concentrations on intact, functional subunits. For example, extended time points show that approximately 70% of A1 subunit (Figure 1B) and approximately 45% of A2 subunit (Figure 1C) are cleaved by APC plus protein S, whereas the factor VIIIa activity essentially approaches zero (Figure 1A). Because these reactions contained 200 nmol/L factor VIIIa, cleavage of 70% of A1 subunit and 45% of A2 subunits would reduce the concentration of intact A1 in the A1/A3-C1-C2 dimer and intact A2 to approximately 60 nmol/L and 110 nmol/L, respectively. Given a dissociation constant for the interaction of A1/A3-C1-C2 dimer with A2 of approximately 260 nmol/L,23 these concentrations predict approximately 15 nmol/L factor VIIIa at equilibrium (based on a quadratic equation) and represents approximately 7% of the initial factor VIIIa present at the start of the reaction. In the absence of protein S, a similar rate and extent of cleavage of A1 occurs; however, the rate of cleavage of A2 subunit is markedly reduced. Because cleavage of A1 facilitates dissociation of A2 subunit and free A2 subunit is a poor substrate for APC,13 more A2 is present in the intact form. Thus, for the same given amount of A1/A3-C1-C2, this increase in intact A2 concentration yields a greater amount of active factor VIIIa at equilibrium. Therefore, the increased rate and extent of cleavage of A2 in the presence of protein S (Figure 1C) is compatible with both the increased rate and extent of cofactor inactivation (Figure 1A). Another possible contributing factor to the disparity of residual activity versus subunit concentration may reflect functionally inactive protein that is resistant to cleavage because of partial denaturation, giving the appearance of persistence of intact subunit in the absence of activity.
Factor IXa limits the protein S-dependent stimulation of APC-catalyzed factor VIIIa inactivation.
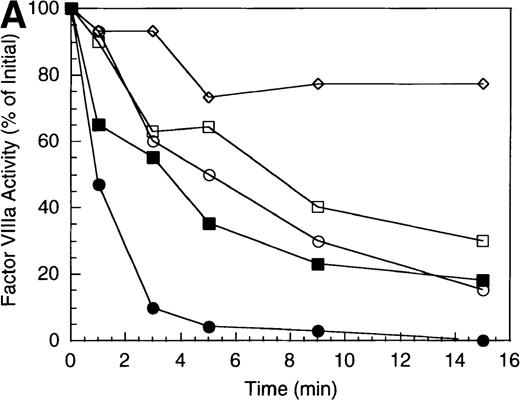
Previous work from our laboratory has shown that active site-modified factor IXa (EGR-factor IXa) protected factor VIIIa from inactivation by bovine APC.14 Protection appeared to be achieved by blocking the primary site of cleavage (Arg562) by the bovine enzyme. This protection was overcome by addition of protein S. An experiment was performed to determine whether the presence of EGR-IXa might influence inactivation of factor VIIIa by human APC in both the presence and absence of protein S. As shown in Figure2A (open symbols), the presence of a 2-fold molar excess of EGR-factor IXa relative to factor VIIIa showed essentially no effect on the rate of cofactor inactivation by human APC (0.5 minute−1), compared with the absence of factor IXa (0.5 minute−1). This result was consistent with the location of the primary inactivating cleavage site for the human enzyme residing within the A1 subunit. When reactions were supplemented with protein S (Figure 2A, closed symbols), the rate of inactivation was approximately 2-fold greater in the absence of factor IXa (approximately 2.9 minute−1 versus 1.6 minute−1 in the absence and presence of factor IXa, respectively). Furthermore, cofactor inactivation was incomplete in the presence of EGR-factor IXa (approximately 20% residual activity), compared with near zero activity in its absence, indicating that complex formation between factor VIIIa and factor IXa limited the effect of protein S.
Effect of EGR-factor IXa on the inactivation of factor VIIIa by human APC and protein S.
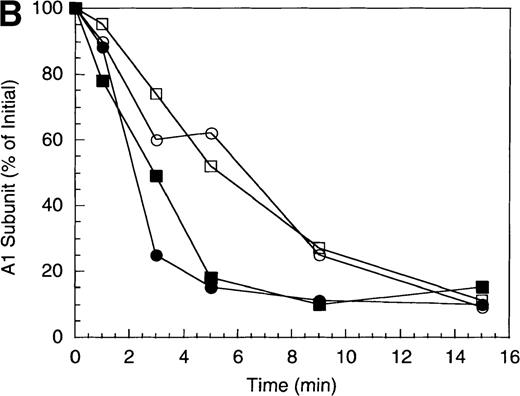
(A) Factor VIIIa activity was determined for reactions containing 200 nmol/L factor VIIIa alone (open diamonds), factor VIIIa plus 40 nmol/L APC (circles), and factor VIIIa plus APC plus 400 nmol/L EGR-factor IXa (squares). Open symbols were in the absence and closed symbols were in the presence of 250 nmol/L protein S. (B) Residual A1 subunit was determined for the above reactions run in the presence of APC (circles) and APC plus EGR-factor IXa (squares). Open and closed symbols represent the absence and presence of protein S, respectively. Curves were derived from scans of the Western blots obtained using the 58.12 anti-A1 subunit antibody. (C) Residual A2 subunit was determined for the above reactions run in the presence of APC (circles) and APC plus EGR-factor IXa (squares). Open and closed symbols represent the absence and presence of protein S, respectively. Curves were derived from scans of the Western blots obtained using the R8B12 anti-A2 subunit antibody.
Effect of EGR-factor IXa on the inactivation of factor VIIIa by human APC and protein S.
(A) Factor VIIIa activity was determined for reactions containing 200 nmol/L factor VIIIa alone (open diamonds), factor VIIIa plus 40 nmol/L APC (circles), and factor VIIIa plus APC plus 400 nmol/L EGR-factor IXa (squares). Open symbols were in the absence and closed symbols were in the presence of 250 nmol/L protein S. (B) Residual A1 subunit was determined for the above reactions run in the presence of APC (circles) and APC plus EGR-factor IXa (squares). Open and closed symbols represent the absence and presence of protein S, respectively. Curves were derived from scans of the Western blots obtained using the 58.12 anti-A1 subunit antibody. (C) Residual A2 subunit was determined for the above reactions run in the presence of APC (circles) and APC plus EGR-factor IXa (squares). Open and closed symbols represent the absence and presence of protein S, respectively. Curves were derived from scans of the Western blots obtained using the R8B12 anti-A2 subunit antibody.
Western blot analysis revealed that the rate of cleavage of the A1 subunit was essentially unchanged in the absence (0.5 minute−1) and presence (0.4 minute−1) of EGR-factor IXa (Figure 2B, open symbols). Similarly, inclusion of factor IXa did not alter the rate of A1 cleavage in the presence of protein S (approximately 1.2 minutes−1 for both the absence and presence of EGR-FIXa, closed symbols). However, cleavage at the A2 site was virtually eliminated in the presence of EGR-IXa independent of protein S (Figure 2C). This failure to cleave A2 subunit in the protein S-containing reaction when factor IXa is present appeared to limit the stimulation typically observed with protein S. In the absence of factor IXa, protein S stimulated the rate of A2 cleavage by APC (approximately 1.2 minute−1) to a value that was equivalent to the rate of cleavage of A1 subunit. Because cleavage of either factor VIIIa subunit results in inactive cofactor,24 25 the summation of these rates of A1 and A2 subunit cleavage was in good agreement with the overall rate of cofactor inactivation (approximately 2.9 minute−1). These results suggest that, similar to those observed with bovine APC, the presence of factor IXa protects factor VIIIa from cleavage within the A2 subunit. However, in the human system, a saturating level of protein S relative to APC appears unable to overcome this protective effect.
Factor X protects from APC by modulating cleavage at the A1 site.
Factor X serves as substrate for the intrinsic factor Xase. Recent work has identified a primary factor X interactive site for factor VIIIa within residues 337-372 of the A1 subunit.18 Because the presence of factor X protects factor VIIIa from factor IXa-catalyzed cleavage at Arg336,26 we wished to determine whether association of factor X with factor VIIIa would influence inactivation by APC. Inclusion of factor X dramatically reduced the rate of factor VIIIa inactivation by APC (Figure 3A) suggesting that association with factor X might block accessibility to the primary cleavage site. In a reaction containing APC but lacking protein S (open symbols), inclusion of factor X resulted in initially stable cofactor activity that subsequently decayed at a rate about 20% that of the reaction lacking factor X (0.2 minute−1versus 0.95 minute−1 in the presence and absence of factor X, respectively). Inclusion of protein S (closed symbols) eliminated the initial stabilization of cofactor activity by factor X and yielded an inactivation profile essentially indistinguishable from the reaction run in the absence of factor X and protein S. This result suggested that protein S could overcome the factor X-dependent protective effect.
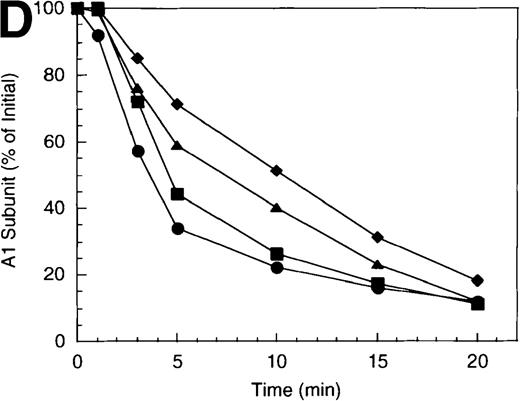
Effect of factor X on the inactivation of factor VIIIa by human APC and protein S.
(A) Factor VIIIa activity was determined for reactions containing 200 nmol/L factor VIIIa alone (open diamonds), factor VIIIa plus 40 nmol/L APC (circles), and factor VIIIa plus APC plus 400 nmol/L factor X (squares). Open symbols were in the absence and closed symbols were in the presence of 250 nmol/L protein S. (B) Residual A1 subunit was determined for the above reactions run in the presence of APC (circles) and APC plus factor X (squares). Open and closed symbols represent the absence and presence of protein S, respectively. Curves were derived from scans of the Western blots obtained using the 58.12 anti-A1 subunit antibody. (C) Residual A2 subunit was determined for the above reactions run in the presence of APC (circles) and APC plus factor X (squares). Open and closed symbols represent the absence and presence of protein S, respectively. Curves were derived from scans of the Western blots obtained using the R8B12 anti-A2 subunit antibody. (D) Residual A1 subunit was determined after addition of 40 nmol/L APC to reactions containing 0 (circles), 200 (squares), 400 (triangles), and 800 nmol/L (diamonds) factor X.
Effect of factor X on the inactivation of factor VIIIa by human APC and protein S.
(A) Factor VIIIa activity was determined for reactions containing 200 nmol/L factor VIIIa alone (open diamonds), factor VIIIa plus 40 nmol/L APC (circles), and factor VIIIa plus APC plus 400 nmol/L factor X (squares). Open symbols were in the absence and closed symbols were in the presence of 250 nmol/L protein S. (B) Residual A1 subunit was determined for the above reactions run in the presence of APC (circles) and APC plus factor X (squares). Open and closed symbols represent the absence and presence of protein S, respectively. Curves were derived from scans of the Western blots obtained using the 58.12 anti-A1 subunit antibody. (C) Residual A2 subunit was determined for the above reactions run in the presence of APC (circles) and APC plus factor X (squares). Open and closed symbols represent the absence and presence of protein S, respectively. Curves were derived from scans of the Western blots obtained using the R8B12 anti-A2 subunit antibody. (D) Residual A1 subunit was determined after addition of 40 nmol/L APC to reactions containing 0 (circles), 200 (squares), 400 (triangles), and 800 nmol/L (diamonds) factor X.
Western blotting revealed that the A1 site but not the A2 site was protected in the presence of factor X. The rate of A1 cleavage was reduced nearly 7-fold in the presence of factor X (approximately 0.2 minute−1), compared with the absence of factor X (1.4 minute−1) (Figure 3B, open symbols). In the presence of protein S (closed symbols), this differential was only a factor of 2 (1.5 minute−1 versus 3.0 minute−1in the presence and absence of factor X, respectively). Conversely, rates of cleavage of A2 were independent of the presence of factor X with little if any cleavage observed in the absence of protein S (Figure 3C, open symbols) and similar rates of cleavage (approximately 0.2 minute−1) in its presence (closed symbols).
Comparison of cleavage rates at the A1 site as a function of factor X concentration is illustrated in Figure 3D. For this experiment, factor X concentration was varied from 0 to 800 nmol/L. Increasing factor X concentration resulted in incremental decreases in the rate of A1 subunit cleavage (over approximately 5-fold range of rates). Taken together, these results suggest factor X selectively protects from APC-catalyzed at the A1 site by a mechanism consistent with competition between APC and factor X.
APC-catalyzed inactivation of factor VIIIa in the presence of EGR-factor IXa and factor X.
Attack of factor VIIIa by APC likely occurs while the cofactor is associated with factor IXa in the factor Xase complex which can bind (and activate) factor X. To examine the influence of these simultaneous macromolecular interactions, we examined APC-catalyzed inactivation of factor VIIIa and proteolysis of subunits in the presence of both EGR-IXa and factor X (Figure 4). Under these reaction conditions, the rate and extent of factor VIIIa inactivation catalyzed by APC was dramatically reduced and the latter appeared to plateau at approximately 60% of the original activity (Figure 4A). Addition of protein S primarily enhanced the extent of cofactor inactivation, which approached approximately 20% of initial activity at the 20 minutes time point. Analysis of the A1 and A2 subunits (Figure 4B) showed results consistent with the sum of the individual contributions. The A2 subunit was essentially uncleaved independent of protein S (squares), likely resulting from its protection by EGR-factor IXa. Alternatively, the relatively slow cleavage of the A1 subunit (circles) (approximately 0.18 minute−1), likely reflecting interaction with factor X, was somewhat accelerated (0.4 minute−1) by the presence of protein S. However, significantly lower levels of A1 subunit were cleaved in the presence of both EGR-factor IXa plus factor X and this result was consistent with the high residual levels of factor VIIIa activity observed. These results suggest that protein S is required to manifest APC-dependent inactivation of factor VIIIa when complexed in the intrinsic factor Xase.
Effect of EGR-factor IXa and factor X on the inactivation of factor VIIIa by human APC and protein S.
(A) Factor VIIIa activity was determined for reactions containing 200 nmol/L factor VIIIa alone (open diamonds), factor VIIIa plus 40 nmol/L APC, and 400 nmol/L each EGR-factor IXa and factor X in the absence (open circles) and presence (closed circles) of 250 nmol/L protein S. (B) Residual A1 (circles) and A2 (squares) subunits were determined for the above reactions. Open and closed symbols represent the absence and presence of protein S, respectively.
Effect of EGR-factor IXa and factor X on the inactivation of factor VIIIa by human APC and protein S.
(A) Factor VIIIa activity was determined for reactions containing 200 nmol/L factor VIIIa alone (open diamonds), factor VIIIa plus 40 nmol/L APC, and 400 nmol/L each EGR-factor IXa and factor X in the absence (open circles) and presence (closed circles) of 250 nmol/L protein S. (B) Residual A1 (circles) and A2 (squares) subunits were determined for the above reactions. Open and closed symbols represent the absence and presence of protein S, respectively.
Discussion
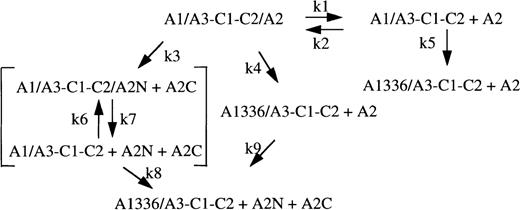
Previous studies have demonstrated that factor VIII inactivation by human APC correlates with rapid cleavage at Arg336 within the A1 domain16 while inactivation by bovine APC results from initial attack at Arg562 within the A2 domain (subunit) of factor VIII (factor VIIIa).13 Consistent with these results, we find inactivation of the factor VIIIa by human APC results from preferential cleavage at the A1 site. These results support a parallel scheme for factor VIIIa inactivation wherein cleavage at either site (Arg336 or Arg562) yields loss of activity (Regan et al,24 Amano et al,25 and Figure 5). This scheme also incorporates the nonproteolytic decay of factor VIIIa resulting from dissociation of A2 subunit. With the use of reaction conditions described in this report, the initial rate of APC-catalyzed inactivation of factor VIIIa (1.2 minute−1) reflects cleavage at Arg336 (1.3 minute−1). Both cofactor inactivation and A1 subunit cleavage are primarily defined by k4 because, at initial time points, k5 is negligible as a result of little spontaneous decay of factor VIIIa and because little product has been generated by the k3/k8 pathway (k3 approximately 0.15 minute−1).
APC-catalyzed and nonproteolytic schemes for factor VIIIa decay.
Heterotrimeric factor VIIIa is represented as A1/A3-C1-C2/A2. A1336 and A2N + A2C represent fragments derived from cleavage of A1 and A2 subunits, respectively. Fragment A2N possesses an A1-interactive site.36 Constants k1 and k2 represent the dissociation and association rate constants, respectively, for reversible interaction of factor VIIIa and A1/A3-C1-C2 dimer plus A2 subunit. Constants k3 and k4 represent initial rate constants for cleavage of factor VIIIa at Arg562 and Arg336, respectively. Constants k5 and k8 represent rate constants for cleavage of A1/A3-C1-C2 to A1336/A3-C1-C2 and are likely equivalent to k4. Constants k6 and k7 represent the association and dissociation rate constants for interaction of fragment A2N with A1/A3-C1-C2 and may be similar to k2 and k1, respectively. Constant k9, the rate constant for cleavage of free A2 by APC, is negligible.13
APC-catalyzed and nonproteolytic schemes for factor VIIIa decay.
Heterotrimeric factor VIIIa is represented as A1/A3-C1-C2/A2. A1336 and A2N + A2C represent fragments derived from cleavage of A1 and A2 subunits, respectively. Fragment A2N possesses an A1-interactive site.36 Constants k1 and k2 represent the dissociation and association rate constants, respectively, for reversible interaction of factor VIIIa and A1/A3-C1-C2 dimer plus A2 subunit. Constants k3 and k4 represent initial rate constants for cleavage of factor VIIIa at Arg562 and Arg336, respectively. Constants k5 and k8 represent rate constants for cleavage of A1/A3-C1-C2 to A1336/A3-C1-C2 and are likely equivalent to k4. Constants k6 and k7 represent the association and dissociation rate constants for interaction of fragment A2N with A1/A3-C1-C2 and may be similar to k2 and k1, respectively. Constant k9, the rate constant for cleavage of free A2 by APC, is negligible.13
However, results described in this report indicate that the tendency for cleavage site selectivity is governed by the presence of other protein factors. Addition of human protein S to the reaction increases the initial rate of factor VIIIa inactivation at least 3-fold and correlates with an approximate 5-fold increase in the rate of cleavage at Arg562 within the A2 subunit. This increase in the rate of A2 subunit cleavage likely reflects direct acceleration of k3 because (1) cleavage at Arg336 reduces the affinity of the A1/A3-C1-C2 dimer for A2 subunit13,27 and (2) free A2 subunit is a poor substrate for APC.13 Thus cleavage of A2 subunit via k9 is negligible. Interestingly, protein S stimulated the rate of A1 subunit cleavage by approximately 2-fold. This effect may derive in part from the rate increase in k3 and the supposition that k8 is likely equivalent to k4. This observation suggests that protein S may alter the orientation of the enzyme relative to this substrate. Indeed, recent fluorescence analyses have indicated that protein S alters the distance of closest approach of the APC active site with membrane surface, as well as the environment of the active site of APC.28
Factor VIIIa functions as a cofactor for the serine protease, factor IXa, in the conversion of factor X to factor Xa. Thus factor VIIIa as part of the intrinsic factor Xase complex is likely the true substrate for APC. Interestingly, both factor IXa and factor X influence cleavage rate and site selectivity, and these parameters are further modulated by the presence of protein S. Previous work in our laboratory has shown that factor IXa protects factor VIIIa from proteolytic inactivation by bovine APC by selectively blocking cleavage at the A2 site.14 Addition of bovine protein S to the reaction resulted in an increase in cleavage rate at the A2 site thus overcoming this protection. These observations led to the identification of a factor IXa-interactive site comprised of residues 558-565.17 We have now examined the ability of active site-modified factor IXa to protect factor VIIIa from inactivation by human APC. The modified form of the enzyme was used to eliminate the contribution of factor IXa-catalyzed cleavage at the A1 site. In contrast to results obtained with bovine APC, cofactor inactivation by human APC proceeds at a similar rate independent of EGR-IXa. This result is consistent with the location of the preferential cleavage site within the A1 subunit and inactivation largely following the k4 pathway. However, addition of a saturating level of human protein S relative to APC failed to significantly increase the rate of cleavage at the A2 site, suggesting that this site remains protected by its interaction with EGR-IXa and k3 remains negligible. These results indicate that bovine and human APC interact differentially with substrate factor VIIIa alone or when factor VIIIa is complexed with factor IXa.
A2 subunit is essential for cofactor activity.9,10 Thus, cleavage at Arg336 promoting dissociation of A2 subunit thereby limits factor Xase activity. This effect has been demonstrated in the homologous cofactor, factor Va, where cleavage at Arg306 results in dissociation of the A2 domain.29 Interestingly, the factor IXa-dependent protection of A2 subunit from APC offers the potential for persistence of residual factor VIIIa activity. Factor IXa has been shown to stabilize the inter-factor VIIIa subunit interaction30,31 by a mechanism that may involve tethering the A2 subunit and A3 domain.31 The effect of this interaction is to reduce the dissociation rate constant for A2 subunit by as much as 10-fold.26 Thus, in the presence of factor IXa, cleavage of the A1 subunit may not necessarily result in complete release of A2 subunit. This effect, coupled with resistance to cleavage of A2 independent of protein S, may allow for significant levels of cofactor activity in the presence of APC provided the factor Xase complex is maintained. Indeed, we observed persistence of factor VIIIa activity (approximately 20% of initial), following extended time points in reactions containing factor IXa that were run in the presence of APC plus protein S, compared with near zero activity for reactions lacking factor IXa. This capacity for factor IXa to stabilize the factor VIIIa trimer by apparently reducing the dissociation rate constant (k1; 26) becomes more pronounced as factor VIIIa subunits are cleaved, thereby reducing the concentration of functional subunits and limiting the extent of factor VIIIa reassociation.
Factor VIII residues 337-372 represent a factor X interactive site in the cofactor.18 The affinity of factor X for this site is fairly weak (Kd approximately 1-3 μmol/L); however, this value was determined using a solid phase assay in the absence of a phospholipid surface. In the presence of factor X, inactivation of factor VIIIa by human APC is inhibited and correlates with a reduced rate of cleavage at Arg336 in the A1 subunit. This result is consistent with occupancy of the factor X site sterically blocking the protease and/or overlapping with an interactive site for APC. However, inclusion of human protein S restores near original rates of factor VIIIa inactivation and cleavage at the A1 site, thereby overcoming the factor X-dependent protective effect. These results suggest a competition between APC and factor X for binding factor VIII that is weighted in favor of the former in the presence of protein S. This observation may reflect the capacity of protein S to enhance the affinity of APC for surfaces,26 thereby facilitating APC-catalyzed factor VIIIa inactivation by abrogating protective interactions of the cofactor with other macromolecules.
Recombinant factor VIII substrates in which Arg residues at 562 and 336 were individually altered to preclude cleavage have been recently used to characterize inactivation by APC.25 Although the information obtained from this analysis was limited because of reagent quantity, results showed that a single mutation inhibited cleavage at only that mutated site and that there is not a required order for cleavage at the 2 sites in factor VIIIa. Furthermore, rates of inactivation observed for the individual mutations were markedly less than that observed for wild-type factor VIII, suggesting that while either cleavage is inactivating, cleavages at both sites maximize the overall rate of inactivation.
A more strictly ordered pathway for bond cleavage by APC appears to exist with the substrate, factor Va. Sequential cleavage of Arg506, followed by Arg306 in the factor Va heavy chain is the primary pathway for inactivation of this cofactor by APC.32 In a comprehensive study, Rosing and coworkers33 evaluated the effects of protein S and factor Xa on bond cleavages after inactivation of factor Va. Interestingly, these investigators found that protein S accelerated factor Va inactivation by selectively promoting the cleavage at Arg306 by approximately 20-fold. Furthermore, it was shown that factor Xa, the enzyme that associates with factor Va to form prothrombinase, protects factor Va by selectively blocking cleavage at Arg506. Thus, it appears that some continuity is maintained between the substrates factor Va and factor VIIIa in that protein S accelerates the cleavage rate at the slower reacting site and that complexing of the cofactor in Xase or prothrombinase protects the A2 site from cleavage.
Little functional information is available on the molecular basis for factor VIIIa inactivation following bond cleavage. Cleavage at Arg562 bisects the A2 subunit, whereas cleavage at Arg336 precedes the C-terminal acidic region of the A1 subunit. The former cleavage occurs within a factor IXa interactive site and affects the factor VIIIa-dependent modulation of the factor IXa active site, as judged by fluorescence anisotropy.24 The A1/A3-C1-C2 dimer plus A2 subunit reconstitutes cofactor activity and produces a factor VIIIa-like effect on the anisotropy of Fl-FFR-FIXa. However, when A2 was replaced by A2 subunit that had been cleaved by APC to the A2N/A2C fragments, no activity was regenerated and the resulting fluorescence signal was equivalent to that observed with the dimer alone.24 Cleavage at Arg336, in addition to promoting dissociation of A2 subunit, directly affects cofactor interactions within the factor Xase. Although intact A1/A3-C1-C2 dimer increased Fl-FFR-FIXa anisotropy and bound factor X in a solid phase assay, these activities were absent in the APC-cleaved, A1336/A3-C1-C2 dimer.24
APC-catalyzed inactivation of factor Va is a primary mechanism to dampen prothrombinase, as illustrated by thrombophilia that results from APC resistance due to the Arg506Gln missense mutation in factor VLeiden (see Dahlback34 for review). The APC-catalyzed inactivation of factor VIIIa represents an alternate mechanism for dampening of the intrinsic factor Xase, which may also occur by factor VIIIa subunit dissociation as well as factor IXa-catalyzed inactivation of the cofactor. Although the relative contribution of these components to factor Xase regulation remains poorly understood, recent inferences35 derived from study of model systems suggest the APC pathway may serve a minor role in limiting factor Xase. This conclusion was primarily based on rates of cleavage of factor VIIIa subunits relative to cleavages in factor Va using reconstituted reactions containing approximate plasma levels of clotting factors, including factors IX, X, and prothrombin. In factor VIIIa, the A2 appeared stable in digests and this persistence of A2 correlates well with our results showing its relative unreactivity under these reaction conditions, even when protein S is present. Further, A1 cleavage significantly lagged behind APC-catalyzed cleavages in factor Va. Taken together, these results are consistent with the inactivation of factor VIIIa by APC as a secondary mechanism for down-regulation of intrinsic factor Xase.
Acknowledgments
We thank James Brown of Bayer Corporation and Debra Pittman of the Genetics Institute for the gifts of recombinant factor VIII. We also thank S. Krishnaswamy for the gift of TAP and James Brown for the 58.12 anti-factor VIII monoclonal antibody.
Supported by grants HL 30616 and HL 38199 from the National Institutes of Health.
Reprints:Philip J. Fay, Vascular Medicine Unit, PO Box 610, University of Rochester Medical Center, 601 Elmwood Ave, Rochester, NY, 14642; e-mail: philip_fay@urmc.rochester.edu.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal