Factor V (FV) present in platelet -granules has a significant but incompletely understood role in hemostasis. This report demonstrates that a fraction of platelets express very high levels of surface-bound, -granule FV on simultaneous activation with 2 agonists, thrombin and convulxin, an activator of the collagen receptor glycoprotein VI. This subpopulation of activated platelets represents 30.7% ± 4.7% of the total population and is referred to as convulxin and thrombin–induced-FV (COAT-FV) platelets. COAT-FV platelets are also observed on activation with thrombin plus collagen types I, V, or VI, but not with type III. No single agonist examined was able to produce COAT-FV platelets, although ionophore A23187 in conjunction with either thrombin or convulxin did generate this population. COAT-FV platelets bound annexin-V, indicating exposure of aminophospholipids and were enriched in young platelets as identified by the binding of thiazole orange. The functional significance of COAT-FV platelets was investigated by demonstrating that factor Xa preferentially bound to COAT-FV platelets, that COAT-FV platelets had more FV activity than either thrombin or A23187–activated platelets, and that COAT-FV platelets were capable of generating more prothrombinase activity than any other physiologic agonist examined. Microparticle production by dual stimulation with thrombin and convulxin was less than that observed with A23187, indicating that microparticles were not responsible for all the activities observed. These data demonstrate a new procoagulant component produced from dual stimulation of platelets with thrombin and collagen. COAT-FV platelets may explain the unique role of -granule FV and the hemostatic effectiveness of young platelets.
Platelets activated at sites of vascular injury play 2 key roles in normal hemostasis. By adhering to the exposed subendothelium and aggregating, they create a physical barrier that limits blood loss.1 In addition, platelets accelerate thrombin generation by providing a surface that promotes 2 procoagulant reactions, conversion of factor X to Xa and production of thrombin from prothrombin.2 These reactions are performed by homologous, membrane-bound, Ca++-dependent complexes: the tenase complex, consisting of the serine protease IXa and the nonenzymatic cofactor factor VIIIa,3 and the prothrombinase complex, composed of the serine protease factor Xa and the nonenzymatic cofactor factor Va.3 Furthermore, activated platelets also control at least 1 anticoagulant reaction, inactivation of factor Va by activated protein C (APC).4
Approximately 20% of factor V (FV) contained in whole blood is found in the α-granules of platelets5 and can be secreted after platelet activation.6 There is clinical and experimental evidence suggesting that platelet-derived FV plays a critical role in maintaining physiologic hemostasis. Factor V Quebec was originally described as an autosomal dominant bleeding disorder characterized by mild thrombocytopenia, fully functional plasma FV but defective platelet FV,7 suggesting that platelet-derived FV might be more important than plasma-derived FV. This concept is reinforced by the description of 2 patients with acquired inhibitors of FV. A patient with non-Hodgkin's lymphoma and gastrointestinal bleeding was found to have a FV inhibitor directed against both plasma and platelet FV,8 whereas a second patient with a neutralizing inhibitor active only against plasma-derived FV presented no bleeding tendency, despite surgical challenge.9 In addition, it has recently been shown that platelets can protect platelet-derived but not plasma-derived FV from proteolytic inactivation by APC.10Together, these observations indicate that platelet-derived, membrane-bound FV has a pivotal role in promoting and maintaining hemostasis at sites of vascular damage.
The ability of platelets to sustain assembly and activity of the tenase and prothrombinase complexes depends on the type of agonist used,11,12 and this correlates with the agonists' ability to induce expression of negatively charged membrane phospholipids.13 The most effective agonists are the Ca++ ionophore A23187, the complement membrane attack complex C5b-9, and the combined stimuli of collagen and thrombin.11,12 Therefore, the most important physiologic stimulus able to induce procoagulant activity at sites of endothelial damage would be the combined action of thrombin and collagen.11 In this report, we have investigated the ability of thrombin plus collagen to elicit platelet-FV surface expression and the functional competence of this platelet-derived FV.
Materials and methods
Materials
Collagen type I (calf skin), type III (human), type V (human), Sepharose CL-2B, FITC-goat-antimouse IgG (FITC-GAMG), and bovine serum albumin (BSA) were from Sigma Chemical Co, St Louis, MO. Collagen type VI (human) was obtained from Heyltex Corp, Houston, TX. Phycoerythrin-labeled streptavidin (PE-SA) and phycoerythrin-goat-antimouse IgG (PE-GAMG) were provided by Molecular Probes, Eugene, OR. Thiazole orange (TO) was from Becton Dickinson, San Jose, CA. Phycoerythrin-labeled annexin-V was from Pharmingen, San Diego, CA, and FITC-labeled annexin-V was provided by Boehringer-Mannheim Corp, Indianapolis, IN. Streptavidin TRI-COLOR was from Caltag Laboratories, Burlingame, CA. Chromogenic substrate S-2238 for thrombin determination was from Chromogenix, Mölndal, Sweden.
Convulxin was purified as published.14 Monoclonal antibodies (mAb) HFV-237, HVF-227, and HFV-271 against human factor V and HFXa-327 against human factor X were prepared as previously described.15 Prothrombin,16 FXa,17FV,18 and FV coagulant protein (VCP)19 were prepared as described. Monoclonal antibodies G5 recognizing P-selectin and TAB recognizing glycoprotein IIb/IIIa20 were provided by Dr R. P. McEver, Oklahoma University Health Sciences Center, Oklahoma City.
Buffers
The following buffers were used: acid citrate dextrose (ACD), 38.1 mmol/L citric acid, 74.8 mmol/L Na3 citrate, 136 mmol/L glucose. Buffered saline-glucose-citrate (BSGC), 129 mmol/L NaCl, 13.6 mmol/L Na3 citrate, 11.1 mmol/L glucose, 1.6 mmol/L KH2PO4, 8.6 mmol/L NaH2PO4, pH adjusted with NaOH to either 6.5 or 7.3. Phosphate-buffered saline (PBS), 150 mmol/L NaCl, 10 mmol/L NaH2PO4, pH 7.4. Saline, 150 mmol/L NaCl. TBS-gelatin, 100 mmol/L NaCl, 20 mmol/L Tris, pH 7.4 with 0.1% (w/v) gelatin.
Characterization of monoclonals against factor V
The anti-FV mAbs used for analysis of activated platelets were evaluated for their relative affinity toward FV and FVa. Polystyrene beads (6.4 μ) coated with phosphatidylcholine (PC), phosphatidylserine (PS), and phosphatidylethanolamine (PE) 40:20:40, as previously described,18 were provided by Dr Patricia Liaw, Oklahoma Medical Research Foundation, Oklahoma City. Beads representing 90 nmol/L total phospholipid were incubated in 10 mmol/L HEPES, pH 7.5 with 140 mmol/L NaCl, 2 mmol/L CaCl2, and 1 mmol/L MgCl2, 1 nmol/L FV or FVa, and 6 nmol/L of FITC-labeled antibody (HFV-237, 271, or 227) at room temperature for 20 minutes. Relative fluorescence associated with the beads was then determined by flow cytometry.
Human platelets
Informed consent was obtained in accordance with local Institution Review Board guidelines. Five milliliters of whole blood were drawn with a 19-gauge needle from the antecubital vein into a plastic syringe containing 0.5-mL ACD. Platelet-rich plasma (PRP) was prepared immediately by 1:2 dilution of whole blood with room temperature (RT) BSGC, pH 7.3, and centrifuged in 12 × 75 plastic tubes at 170g for 8 minutes at RT. Gel filtration of the platelets was performed by layering 2 mL of PRP onto a 25 × 60 mm (30 mL) column of Sepharose CL-2B equilibrated either with BSGC, pH 6.5 or 150 mmol/L NaCl. Gel-filtered platelets (GFP) were normalized to a concentration of 4 × 107 platelets/mL in BSGC, pH 7.3 for flow-cytometric studies or to 5 × 107platelets/mL in 10 mmol/L HEPES, pH 7.5, 140 mmol/L NaCl, 2 mmol/L CaCl2, 1 mmol/L MgCl2 for functional studies.
Collagen preparations
Collagens were dissolved at 1 mg/mL in 85 mmol/L acetic acid overnight at 4°C. Stock solutions were prepared with a 1:5 dilution in water to yield a final collagen concentration of 200 μg/mL in 17 mmol/L acetic acid and stored in glass tubes as previously described.21
Platelet activation for flow-cytometric studies
Reactions were performed in 12 × 75 mm polypropylene, round-bottom, culture tubes. For a final concentration of 20 μg/mL, 10 μL of collagen stock solution was diluted with 17 mmol/L acetic acid up to 40 μL and kept on ice until needed. Other agonists (convulxin, thrombin, TRAP, ionophore A23187) were diluted in 1 mg/mL BSA, 10 mmol/L HEPES pH 7.5, 140 mmol/L NaCl, up to 40 μL. Immediately before the assay was initiated, 50 μL of RT 100 mmol/L HEPES pH 7.5, 150 mmol/L NaCl (for collagen), or 10 mmol/L HEPES pH 7.5, 140 mmol/L NaCl (for other activators), each with 4 mmol/L CaCl2, 2 mmol/L MgCl2, and the relevant antibodies (see below). The reaction was initiated with 10 μL of GFP, allowed to proceed for 10 minutes at 37°C, and then stopped with 200 μL of ice-cold 1.5% formalin in PBS (or as described below for experiments avoiding platelet fixation). After 20 minutes fixation at RT, 3.5 mL of 1 mg/mL BSA in PBS (BSA/PBS) were added, the platelets pelleted at 1500g for 15 minutes at RT, and the pellet resuspended in 200 μL BSA/PBS with the appropriate detection system (see below). After 30 minutes of labeling and a further washing step when required, platelets were analyzed by flow cytometry.
Detection of surface expressed factor V
Anti-FV-mAb, either underivatized or biotin-conjugated, was present with platelets during the activation. After fixation and washing as described previously, underivatized mAb were detected with PE-GAMG, whereas biotin-conjugated mAb were labeled with 5 μg/mL PE-SA. In a separate set of experiments we verified that optimal binding of mAb to exposed FV was obtained within 30 seconds at 37°C. In experiments investigating the ability of reticulated platelets to express FV, biotin-conjugated anti-HFV-237 was used. After fixation and washing, the biotinylated antibody was labeled with PE-SA for 30 minutes. Platelets were then diluted into 600 μL TO for flow cytometric studies; compensation parameters were set to avoid cross-over fluorescence between FL1 (TO) and FL2 (PE-SA).
Analysis of intracellular factor V
Quiescent platelets at 4 × 106/mL in BSGC, pH 7.3, were fixed with 1% (final) formalin in PBS for 20 minutes at RT and washed with BSA/PBS as previously described. Platelets were permeabilized with 0.2% (w/v; final) saponin and incubated with 10 μg/mL of the relevant, biotinylated anti-FV monoclonal. After washing again, the biotinylated antibody was detected with 5 μg/mL PE-SA as above.
Detection of exposed negatively charged membrane phospholipids
Annexin-V was used as a probe for aminophospholipid exposure.13 GFP were activated in the presence of PE-labeled annexin-V as detailed previously. After a 10-minute incubation at 37°C, the 100 μL reaction mix was diluted with 600 μL of 10 mmol/L HEPES pH 7.5, 140 mmol/L NaCl, 2 mmol/L CaCl2, and promptly assayed by flow cytometry. In experiments investigating the ability of reticulated platelets to expose negatively charged aminophospholipids, PE-annexin-V and TO were used. After 10 minutes incubation at 37°C, the reaction mix was diluted in 600 μL of TO with 2 mmol/L CaCl2. Flow cytometer parameters were set to avoid cross-over fluorescence between FL1 (TO-staining) and FL2 (PE-annexin-V). In particular, the following controls were performed with each experiment: a sample of ionophore A23187–activated platelets labeled with PE-annexin-V but without TO confirmed that FL2 fluorescence did not mimic TO-positive events in the FL1 window, and a sample of unactivated platelets confirmed that TO-staining did not mimic positive events in the FL2 window.
Dual labeling experiments with annexin-V and HFV-237 were performed with slight modification of the parameters previously described. Platelets were activated with 5 nmol/L thrombin plus 500 ng/mL convulxin in the presence of PE-annexin-V, 0.5 μg/mL biotinylated HFV-237 and 4.5 μg/mL underivatized HFV-237 for 10 minutes at 37°C. Streptavidin-TRI-COLOR (5 μg/mL) was then added for 5 minutes at room temperature. Samples were diluted as above and analyzed for FL2 (PE) and FL3 (TRI-COLOR).
Detection of platelet-derived microparticles
Microparticles (MP) were distinguished according to size and ability to bind either FITC-annexin-V13 or FITC-TAB.20In experiments examining the time-dependent generation of MP on platelet activation, FITC-TAB was used, and the generation of MP was stopped by diluting the reaction mix in a buffer containing 5 mmol/L EDTA, 10 mmol/L HEPES pH 7.5, 140 mmol/L NaCl. EDTA stopped MP generation but did not affect TAB binding. MP were analyzed without formalin fixation or washing. For flow cytometric analysis of MP, forward scatter (FSC) was set on E01 (log scale), to allow a better visualization of the MP, which were defined as particles smaller (less FSC) than the parent platelet population. MP were expressed as percentage of total TAB-positive events.
Flow cytometric analysis
Flow cytometry was performed on a Becton Dickinson FACSCalibur, equipped with an argon-ion laser emitting at 488 nm (Becton Dickinson, Mountain View, CA). Parameters were set on a log scale.
Prothrombinase assay
GFP were normalized to 5 × 107 platelets/mL in 10 mmol/L HEPES pH 7.5, 140 mmol/L NaCl, 2 mmol/L CaCl2, 1 mmol/L MgCl2. Aliquots of 200 μL were activated with 20 μL of 1 mg/mL BSA in 10 mmol/L HEPES pH 7.5, 140 mmol/L NaCl, 2 mmol/L CaCl2, 1 mmol/L MgCl2 containing either no supplement (negative control) or the various agonists. After an incubation time of 10 minutes at 37°C, prothrombin was added to a final concentration of 1.4 μmol/L, and thrombin generation was started by addition of FXa (final 1 nmol/L). Every 30 seconds, aliquots of 20 μL were removed to 80 μL of ice cold buffer, containing 10 mmol/L EDTA, 10 mmol/L HEPES, 140 mmol/L NaCl, 0.5% (w/v) BSA, pH 7.5. Generated thrombin was assessed by adding the chromogenic substrate S-2238 (20 μL of 2 mmol/L) and measuring the rate of hydrolysis in a Vmax microplate reader at 405 nm (Molecular Devices). From the rate of change in absorbance, thrombin concentrations were calculated by comparison to a standard curve generated with purified thrombin.
In another set of experiments comparing the procoagulant activity of platelets versus platelet-derived MP, 660 μL of GFP were activated with various agonists as previously detailed. At different time points after activation (2, 9, and 19 minutes) 2 aliquots of 100 μL were removed. One was left untreated, the other was centrifuged at 13 800g for 1 minute.4 Prothrombin was then added to the first aliquot (platelets plus MP) and to the supernatant of the second aliquot (MP enriched), thrombin generation started by addition of 1 nmol/L FXa, and the reaction stopped every 30 seconds up to 2 minutes as previously detailed.
Factor V activity assay
FV coagulant activity was assayed in a 1-stage clotting assay using FV-deficient human plasma.22 Specifically, 200 μL of GFP normalized to 5 × 107 platelets/mL in 10 mmol/L HEPES pH 7.5, 140 mmol/L NaCl, 2 mmol/L CaCl2, and 1 mmol/L MgCl2 were activated with 20 μL of 1 mg/mL BSA in 10 mmol/L HEPES, 140 mmol/L NaCl, 2 mmol/L CaCl2, 1 mmol/L MgCl2, pH 7.5 containing the various agonists. After 2 minutes incubation at 37°C, a 50-μL aliquot was mixed with 50 μL TBS-gelatin buffer and 50 μL FV-deficient plasma; clot formation was initiated with 50 μL thromboplastin and monitored at 37°C using a coagulometer (Diagnostica Stago Model ST4). FV activity was determined on the basis of a standard curve constructed with normal plasma.22
Results
Characterization of monoclonals against factor V
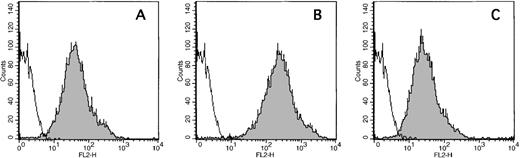
Three anti-FV mAbs were characterized for their relative affinities for FV versus FVa. Polystyrene beads coated with phospholipid (PC/PS/PE; 40:20:40) were prepared as previously described.18 Beads representing 90 nmol/L total phospholipid were incubated at room temperature with 1 nmol/L FV or FVa along with 6 nmol/L of the relevant, FITC-labeled antibody. Relative fluorescence associated with the beads was then determined by flow cytometry. The data presented in Figure 1indicate that anti-HFV-237, recognizing the FV light chain, bound both FV and FVa. Anti-HFV-271, recognizing the heavy chain, also reacted with both FV and FVa. Anti-HFV-227, recognizing the connecting region of FV, reacted with FV but not FVa as expected.
Characterization of anti-FV monoclonal antibodies.
Purified FV (solid line), FVa (shaded), and no protein (dotted) were added to phospholipid-coated beads as described in “Materials and methods.” FITC-labeled antibody against FV light chain (anti-HFV-237; Panel A), FV heavy chain (anti-HFV-271; Panel B), and FV connecting region (anti-HFV-227; Panel C) were then added and particle-bound fluorescence measured by flow cytometry.
Characterization of anti-FV monoclonal antibodies.
Purified FV (solid line), FVa (shaded), and no protein (dotted) were added to phospholipid-coated beads as described in “Materials and methods.” FITC-labeled antibody against FV light chain (anti-HFV-237; Panel A), FV heavy chain (anti-HFV-271; Panel B), and FV connecting region (anti-HFV-227; Panel C) were then added and particle-bound fluorescence measured by flow cytometry.
Platelet activation with thrombin and convulxin induces high levels of factor V surface expression in a discrete fraction of platelets.
We examined the potential for thrombin and convulxin, a specific agonist for the collagen receptor GPVI,14 to promote surface expression of α-granule FV. Figure2 shows the ability of thrombin (Figure2A), convulxin (Figure 2B), and ionophore A23187 (Figure 2C) as single agonists to induce expression of FV on the platelet surface. These single agonists were used at concentrations well above that required to induce maximal α-granule release as reported previously23and confirmed here (data not shown). Figure 2D shows that dual stimulation with both thrombin and convulxin results in a dramatically different pattern of FV distribution. A fraction of the platelets express very high levels of factor V (region M2), whereas the remainder still express factor V but at lower levels than that observed with convulxin alone (region M1 minus M2; referred to as low-level FV). We shall identify the high level of surface FV expression shown in region M2 as COAT-FV (convulxin andthrombin–induced FV). Costimulation of platelets with ionophore plus thrombin or ionophore plus convulxin also generates a COAT-FV population, although with these nonphysiologic agonist combinations essentially all platelets express high levels of surface bound FV. Duplication of the experiments in Figure 2 with antibody HFV-271 against FV heavy chain gives similar results for all agonists (data not shown). It is noteworthy that the majority of platelets stimulated with convulxin plus thrombin (Figure 2D) have a level of FV below that for platelets activated with convulxin alone (Figure 2B). The basis for this observation is not clear but may be explained as the mechanism for COAT-FV formation is elucidated. In addition, we recognize that reactions using thrombin may result in variable cleavage of platelet-derived FV to FVa or partially activated FV.24 Because the extent of FV cleavage in these experiments is uncertain, the term FV will be used here to describe all platelet-bound forms of FV, FVa, and partially activated FV that may be formed during these reactions.
Factor V binding to activated platelets.
In a representative experiment, gel-filtered human platelets were activated with 5 nmol/L thrombin (Panel A), 500 ng/mL convulxin (Panel B), 2 μmol/L A23187 (Panel C), thrombin plus convulxin (same concentrations; Panel D), thrombin plus A23187 (Panel E), or convulxin plus ionophore (Panel F) as described in “Materials and methods.” Surface-bound FV was detected with biotinylated monoclonal antibody HFV-237 against factor V and phycoerythrin-streptavidin (FL2). In each panel, control platelets are indicated by the line histogram, and stimulated cells are depicted with the shaded histogram. Region M1 represents all cells binding FV, and region M2 represents cells binding very high levels of FV. Cells in region M2 are referred to as COAT-FV (see text). Experiments performed with antibody HFV-271 gave similar results to those for HFV-237 (data not shown).
Factor V binding to activated platelets.
In a representative experiment, gel-filtered human platelets were activated with 5 nmol/L thrombin (Panel A), 500 ng/mL convulxin (Panel B), 2 μmol/L A23187 (Panel C), thrombin plus convulxin (same concentrations; Panel D), thrombin plus A23187 (Panel E), or convulxin plus ionophore (Panel F) as described in “Materials and methods.” Surface-bound FV was detected with biotinylated monoclonal antibody HFV-237 against factor V and phycoerythrin-streptavidin (FL2). In each panel, control platelets are indicated by the line histogram, and stimulated cells are depicted with the shaded histogram. Region M1 represents all cells binding FV, and region M2 represents cells binding very high levels of FV. Cells in region M2 are referred to as COAT-FV (see text). Experiments performed with antibody HFV-271 gave similar results to those for HFV-237 (data not shown).
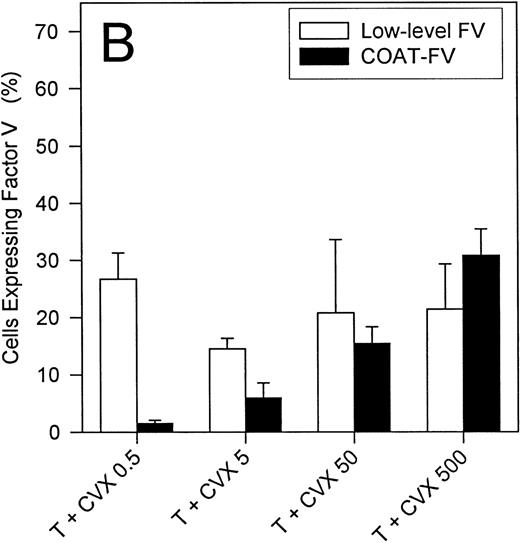
Figure 3A summarizes the ability of single agonists to express α-granule FV on the surface of activated platelets. Although these individual agonists are capable of stimulating FV expression on up to 80% of all platelets, none of them generate COAT-FV. On the other hand, the combined action of thrombin and convulxin results in a lower overall level of FV-positive events, even though this dual stimulation results in COAT-FV expression (Figure 3B). Furthermore, when thrombin is maintained at 5 nmol/L, there is a clear dose-response to convulxin for generation of COAT-FV (Figure 3B). When the convulxin concentration is fixed at 500 ng/mL, there is a dose-dependent increase in COAT-FV formation between 0.1 and 1 nmol/L thrombin, whereas with higher thrombin concentrations, the percentage of COAT-FV remains constant (data not shown).
Analysis of surface FV expression elicited by various agonists.
Platelets were stimulated with various agonists at the concentrations depicted on the abscissa. In Panel A single agonists were used similar to experiments in Figures 1A and 1B; the percentage of cells with surface FV is shown on the ordinate (mean ± 1 SD; n = 3-8). In Panel B, dual agonist stimulation was performed with thrombin held constant at 5 nmol/L and convulxin varied from 0.5 to 500 ng/mL. Two parameters are reported: COAT-FV and low-level FV corresponding to region M2 and region M1 minus M2, respectively, of Figure 2. Note that in Panel B the percentage of cells with low-level binding remains relatively constant, whereas the number of cells with COAT-FV increases with increasing convulxin concentration (n = 3-12).
Analysis of surface FV expression elicited by various agonists.
Platelets were stimulated with various agonists at the concentrations depicted on the abscissa. In Panel A single agonists were used similar to experiments in Figures 1A and 1B; the percentage of cells with surface FV is shown on the ordinate (mean ± 1 SD; n = 3-8). In Panel B, dual agonist stimulation was performed with thrombin held constant at 5 nmol/L and convulxin varied from 0.5 to 500 ng/mL. Two parameters are reported: COAT-FV and low-level FV corresponding to region M2 and region M1 minus M2, respectively, of Figure 2. Note that in Panel B the percentage of cells with low-level binding remains relatively constant, whereas the number of cells with COAT-FV increases with increasing convulxin concentration (n = 3-12).
We have also investigated whether COAT-FV is generated by the combined action of thrombin and collagen. Table 1summarizes the results obtained with collagen types I, III, V, and VI. By using all collagens at 20 μg/mL, a concentration that induces maximal α-granule degranulation,21 types I, V, and VI are able to promote COAT-FV to varying degrees, whereas collagen type III only induces low-level FV expression.
Effect of dual activation with thrombin and collagen on factor V expression on human platelets
| Collagen Type . | Low-Level Factor V . | COAT-FV . |
|---|---|---|
| I | 14.5 ± 5.6 | 36.4 ± 6.9 |
| III | 45.7 ± 13.8 | — |
| V | 19.4 ± 7.5 | 30.9 ± 9.6 |
| VI | 10.1 ± 3.9 | 61.6 ± 6.2 |
| Collagen Type . | Low-Level Factor V . | COAT-FV . |
|---|---|---|
| I | 14.5 ± 5.6 | 36.4 ± 6.9 |
| III | 45.7 ± 13.8 | — |
| V | 19.4 ± 7.5 | 30.9 ± 9.6 |
| VI | 10.1 ± 3.9 | 61.6 ± 6.2 |
To activate human platelets, 5 nmol/L thrombin and 20 μg/mL of collagen were used. Surface-bound FV was detected with monoclonal antibody HFV-237 (see “Materials and methods”), and the percentage of platelets expressing FV was then determined by flow cytometry. COAT-FV is defined in Figure 2 as events in region M2; low-level FV represents cells in region M1 minus those in region M2 of Figure 2. Data represent mean ± 1 SD, n = 4.
Factor V is detectable in all platelets and the complete molecule is expressed on the surface of activated platelets.
To investigate whether all the platelets contain FV, we permeabilized formalin-fixed platelets with saponin and incubated them with the 3 mAbs characterized in Figure 1. Figure 4demonstrates that all 3 mAbs recognize platelet FV and that all platelets contain FV. These 3 mAbs were also used to show that the entire factor V molecule is expressed on the surface of COAT-FV platelets, because each mAb resulted in the same percentage of COAT-FV cells on activation with thrombin plus convulxin: 30.7% ± 4.7% (mean ± 1 SD; n = 10) for anti-HFV-237; 29.6% ± 4.9% (n = 6) for anti-HFV-227; and 30.4% ± 8.9% (n = 6) for anti-HFV-271.
Detection of intracellular FV in quiescent platelets.
Control platelets were formalin-fixed and permeabilized with saponin as described in “Materials and methods.” Three different biotinylated, anti-FV monoclonal antibodies were then used to stain intracellular FV. Panel A is anti-HFV-237 that recognizes the FVa light chain; Panel B represents anti-HFV-227 that detects the FV connecting region (B domain); and Panel C is anti-HFV-271 that recognizes the heavy chain of FVa. The line histograms represent nonspecific antibody, and the shaded histograms represent the anti-FV monoclonals. All platelets bind the 3 monoclonals indicating that all platelets contain the entire FV molecule.
Detection of intracellular FV in quiescent platelets.
Control platelets were formalin-fixed and permeabilized with saponin as described in “Materials and methods.” Three different biotinylated, anti-FV monoclonal antibodies were then used to stain intracellular FV. Panel A is anti-HFV-237 that recognizes the FVa light chain; Panel B represents anti-HFV-227 that detects the FV connecting region (B domain); and Panel C is anti-HFV-271 that recognizes the heavy chain of FVa. The line histograms represent nonspecific antibody, and the shaded histograms represent the anti-FV monoclonals. All platelets bind the 3 monoclonals indicating that all platelets contain the entire FV molecule.
The exposure of negatively charged membrane phospholipids parallels the expression of COAT-FV but is not sufficient for its generation.
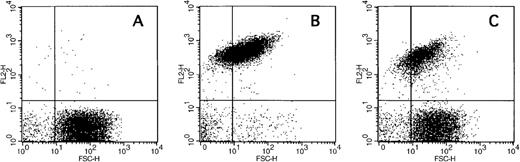
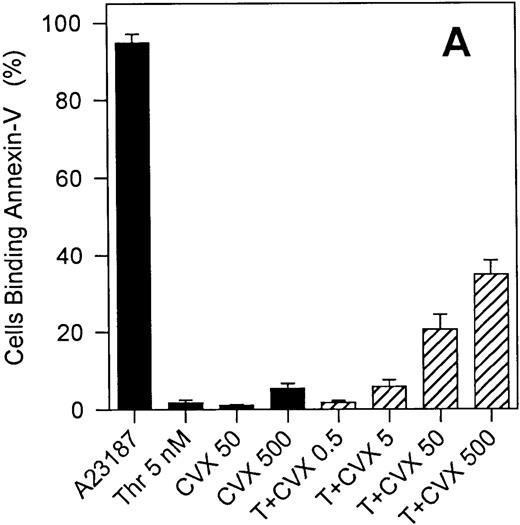
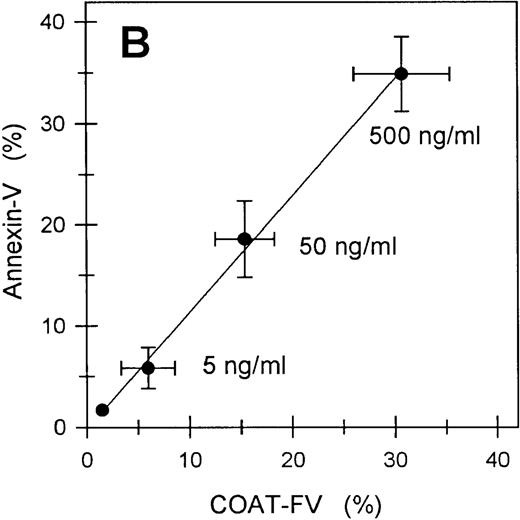
The generation of COAT-FV in response to thrombin and collagen (Table1) or thrombin and convulxin (Figure 3B) is restricted to a subpopulation of platelets similar to previous reports on the exposure of aminophospholipids by activated platelets.13 We therefore investigated whether these events might be associated. Exposure of negatively charged membrane phospholipids was monitored with fluorochrome conjugated annexin-V. Figure5 shows representative flow cytometric dot plots of annexin-V binding promoted by ionophore A23187 (Figure 5B) and the combined action of thrombin plus convulxin (Figure 5C). Ionophore resulted in essentially all platelets binding annexin-V, whereas thrombin plus collagen generated only a subpopulation of annexin-V-positive cells. Results obtained from several individuals are summarized in Figure 6. Except for A23187, single agonist stimulation of platelets resulted in very modest numbers of platelets binding annexin-V (Figure 6A); however, even though ionophore elicits a high level of annexin-V binding, it does not promote significant COAT-FV expression (Figure 2C). On the other hand, dual stimulation of platelets with 5 nmol/L thrombin and increasing concentrations of convulxin elicited annexin-V binding (Figure 6A). Interestingly, the percentage of platelets binding annexin-V in response to the combined stimulus of thrombin plus convulxin is very similar to the platelet fraction expressing COAT-FV (Figure 6B), suggesting that the same subpopulation of platelets is positive for surface FV and annexin-V. This is confirmed in Figure 6C in which dual staining of thrombin plus convulxin activated platelets indicates that the COAT-FV and annexin-V-positive populations are identical.
Annexin-V binding to stimulated platelets.
Platelets were stimulated with either 2 μmol/L A23187 (Panel B) or 5 nmol/L thrombin plus 500 ng/mL convulxin (Panel C) in the presence of phycoerythrin-labeled annexin-V (FL2). Panel A represents resting platelets. Note that thrombin plus convulxin results in only a fraction of the platelets binding annexin-V. Additional data are summarized in Figure 6.
Annexin-V binding to stimulated platelets.
Platelets were stimulated with either 2 μmol/L A23187 (Panel B) or 5 nmol/L thrombin plus 500 ng/mL convulxin (Panel C) in the presence of phycoerythrin-labeled annexin-V (FL2). Panel A represents resting platelets. Note that thrombin plus convulxin results in only a fraction of the platelets binding annexin-V. Additional data are summarized in Figure 6.
Annexin-V binding to platelets stimulated with single or dual agonists.
For Panel A, platelets were activated with the various agonists indicated on the abscissa, and the binding of phycoerythrin-labeled annexin-V was monitored. All convulxin concentrations represent ng/mL and T indicates thrombin at 5 nmol/L. Bars represent mean ± 1 SD, n = 6. Panel B demonstrates the percentage of platelets positive for COAT-FV (abscissa) and annexin-V (ordinate) on stimulation with 5 nmol/L thrombin and the convulxin concentration depicted in the plot. Data are extracted from Figures 3B and 6A. Panel C represents dual labeling of platelets stimulated with convulxin plus thrombin as described in “Materials and methods.” The abscissa (FL3) depicts biotin-HFV-237/streptavidin TRI-COLOR binding, and the ordinate (FL2) represents PE–annexin-V binding. Events in region R1 are positive for both annexin-V and FV.
Annexin-V binding to platelets stimulated with single or dual agonists.
For Panel A, platelets were activated with the various agonists indicated on the abscissa, and the binding of phycoerythrin-labeled annexin-V was monitored. All convulxin concentrations represent ng/mL and T indicates thrombin at 5 nmol/L. Bars represent mean ± 1 SD, n = 6. Panel B demonstrates the percentage of platelets positive for COAT-FV (abscissa) and annexin-V (ordinate) on stimulation with 5 nmol/L thrombin and the convulxin concentration depicted in the plot. Data are extracted from Figures 3B and 6A. Panel C represents dual labeling of platelets stimulated with convulxin plus thrombin as described in “Materials and methods.” The abscissa (FL3) depicts biotin-HFV-237/streptavidin TRI-COLOR binding, and the ordinate (FL2) represents PE–annexin-V binding. Events in region R1 are positive for both annexin-V and FV.
Both exposure of aminophospholipids and COAT-FV expression are increased among reticulated platelets.
Because only a portion of platelets express both COAT-FV and negatively charged membrane phospholipids in response to the combined action of thrombin and convulxin, we investigated whether this might be related to platelet age. To this purpose we used TO, a fluorescent dye that binds to the remnant RNA still contained in reticulated platelets, allowing identification of the youngest platelets in the circulation.25-27 Figure 7shows the exposure of negatively charged membrane phospholipids in response to 5 nmol/L thrombin and 500 ng/mL convulxin for a representative experiment. When TO-negative platelets are examined, 24.2% ± 7.0% (mean ± 1 SD; n = 6) of the cells bind annexin-V versus 73.1% ± 4.5% (P < .001) for the TO-positive platelets. Similarly, the percentage of COAT-FV expressing cells is enriched among the reticulated platelets: only 21.6% ± 3.1% (n = 5) of TO-negative platelets express COAT-FV in response to thrombin plus convulxin versus 65.6% ± 6.3% of the TO-positive platelets (P < .001).
Annexin-V binding to thiazole orange-positive and -negative platelets stimulated with thrombin plus convulxin.
Platelets were stimulated with 5 nmol/L thrombin plus 500 ng/mL convulxin and then stained with PE–annexin-V to label negatively charged surface phospholipids and thiazole orange (TO) to identify reticulated platelets. Panel A represents the TO+platelets, Panel B depicts the entire population, and Panel C represents the TO− platelets.
Annexin-V binding to thiazole orange-positive and -negative platelets stimulated with thrombin plus convulxin.
Platelets were stimulated with 5 nmol/L thrombin plus 500 ng/mL convulxin and then stained with PE–annexin-V to label negatively charged surface phospholipids and thiazole orange (TO) to identify reticulated platelets. Panel A represents the TO+platelets, Panel B depicts the entire population, and Panel C represents the TO− platelets.
Factor V peak surface expression is functionally relevant.
To assess the functional relevance of COAT-FV, we have used 3 different approaches. First, we investigated whether COAT-FV is able to bind plasma factor Xa (FXa). Exogenous FXa was added to platelets incubated with thrombin plus convulxin. Double labeling with biotin conjugated anti-HFV-237 and FITC-anti-HFX-327, a mAb directed against FX and FXa that does not inhibit FXa plasma clotting activity, demonstrated that all platelets expressing COAT-FV also maximally bind exogenous FXa (Table 2). Second, we examined the ability of variously activated platelets to affect the clotting time in FV-deficient plasma (Table 3). The combined activation by thrombin plus convulxin is a more potent inducer of platelet FV-activity than is any single agonist examined (P < .02); only when ionophore A23187 is potentiated by the addition of thrombin does the FV activity approximate that observed with thrombin plus convulxin. Third, we determined the ability of different platelet agonists to promote platelet dependent-prothrombin activation. Platelets were activated for 10 minutes with the agonists indicated in Figure 8, prothrombin and factor Xa were then added and the initial rate of prothrombin activation was determined. Prothrombin activation was nearly linear for the first 4 minutes (Figure 8A). Unstimulated platelets exhibited little ability to support prothrombin activation. Convulxin stimulated the platelet activity in a concentration-dependent fashion, and the combination of thrombin plus convulxin generated more prothrombinase activity than would be predicted for a simple summation of that for the individual agonists (Figure 8B).
Binding of FXa to activated platelets
| Platelets . | % Positive for FXa . | Mean Fluorescence . |
|---|---|---|
| Control | 0.8 ± 0.4 | 1.7 ± 0.3 |
| CVX | 19.5 ± 1.7 | 4.8 ± 1.1 |
| CVX + Thr (low-level FV) | 18.5 ± 1.0 | 4.4 ± 1.0 |
| CVX + Thr (COAT-FV) | 82.8 ± 5.9 | 20.1 ± 4.8 |
| Platelets . | % Positive for FXa . | Mean Fluorescence . |
|---|---|---|
| Control | 0.8 ± 0.4 | 1.7 ± 0.3 |
| CVX | 19.5 ± 1.7 | 4.8 ± 1.1 |
| CVX + Thr (low-level FV) | 18.5 ± 1.0 | 4.4 ± 1.0 |
| CVX + Thr (COAT-FV) | 82.8 ± 5.9 | 20.1 ± 4.8 |
Platelets were activated with 500 ng/mL convulxin (CVX) alone or in combination with 5 nmol/L thrombin (Thr) in the presence of 5 nmol/L FXa, 20 μg/mL FITC–anti-HFXa and 20 μg/mL biotin–anti-HFV237 as described in “Materials and methods.” Cells with surface-bound FV were identified as indicated in Figure 2. The percentage of cells positive for FXa as well as their mean FL1 fluorescence was then determined for each subpopulation of FV+ cells. Data are expressed as mean ± 1 SD; n = 3.
Factor V activity of activated platelets
| Agonist . | Factor V Activity (mU/mL) . |
|---|---|
| None | 3.4 ± 0.2 |
| Convulxin (500 ng/mL) | 25.6 ± 3.7 |
| Thrombin (5 nmol/L) | 91.9 ± 12.9 |
| TRAP (100 μmol/L) | 17.3 ± 1.6 |
| A23187 (2 μmol/L) | 12.9 ± 1.5 |
| VCP (2 nmol/L) | 8.3 ± 1.7 |
| Thrombin + convulxin | 156.3 ± 18.9 |
| Thrombin + A23187 | 107.7 ± 24.0 |
| Convulxin + A23187 | 16.9 ± 1.1 |
| TRAP + VCP | 48.3 ± 10.4 |
| A23187 + VCP | 55.6 ± 8.5 |
| Convulxin + VCP | 67.7 ± 11.6 |
| Agonist . | Factor V Activity (mU/mL) . |
|---|---|
| None | 3.4 ± 0.2 |
| Convulxin (500 ng/mL) | 25.6 ± 3.7 |
| Thrombin (5 nmol/L) | 91.9 ± 12.9 |
| TRAP (100 μmol/L) | 17.3 ± 1.6 |
| A23187 (2 μmol/L) | 12.9 ± 1.5 |
| VCP (2 nmol/L) | 8.3 ± 1.7 |
| Thrombin + convulxin | 156.3 ± 18.9 |
| Thrombin + A23187 | 107.7 ± 24.0 |
| Convulxin + A23187 | 16.9 ± 1.1 |
| TRAP + VCP | 48.3 ± 10.4 |
| A23187 + VCP | 55.6 ± 8.5 |
| Convulxin + VCP | 67.7 ± 11.6 |
Platelets activated with various agonists were added to FV-deficient plasma and 1-stage clotting times were measured as described in “Materials and methods.” Clotting times were converted to FV activity (mU/mL) by comparison with a normal plasma. TRAP, thrombin receptor agonist peptide; VCP, Factor V coagulant protein. Values are expressed as mean ± SE; n = 3-8.
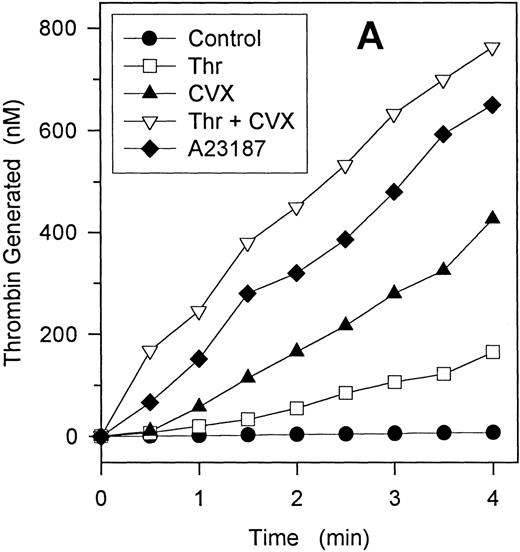
Prothrombinase activity generated by single and dual agonists.
Gel-filtered platelets were stimulated with various single- and dual-agonist combinations for 10 minutes as described in “Materials and methods.” Platelets were then added to exogenous Factor Xa and prothrombin, and the initial rate of prothrombin activation was determined. Panel A depicts a representative experiment and demonstrates that the initial rates of thrombin generation were linear for up to 4 minutes. Agonist concentrations were 5 nmol/L thrombin, 500 ng/mL convulxin, and 2 μmol/L A23187. Panel B represents the prothrombinase activity (nmol/L thrombin generated per minute) for various agonists. Ionophore (A) was 2 μmol/L; thrombin (T), 5 nmol/L; and convulxin (CVX) concentration was 500 ng/ml unless specifically designated (ng/mL) otherwise. Bars represent mean ± 1 SD; n = 3.
Prothrombinase activity generated by single and dual agonists.
Gel-filtered platelets were stimulated with various single- and dual-agonist combinations for 10 minutes as described in “Materials and methods.” Platelets were then added to exogenous Factor Xa and prothrombin, and the initial rate of prothrombin activation was determined. Panel A depicts a representative experiment and demonstrates that the initial rates of thrombin generation were linear for up to 4 minutes. Agonist concentrations were 5 nmol/L thrombin, 500 ng/mL convulxin, and 2 μmol/L A23187. Panel B represents the prothrombinase activity (nmol/L thrombin generated per minute) for various agonists. Ionophore (A) was 2 μmol/L; thrombin (T), 5 nmol/L; and convulxin (CVX) concentration was 500 ng/ml unless specifically designated (ng/mL) otherwise. Bars represent mean ± 1 SD; n = 3.
The role of microparticles (MP)
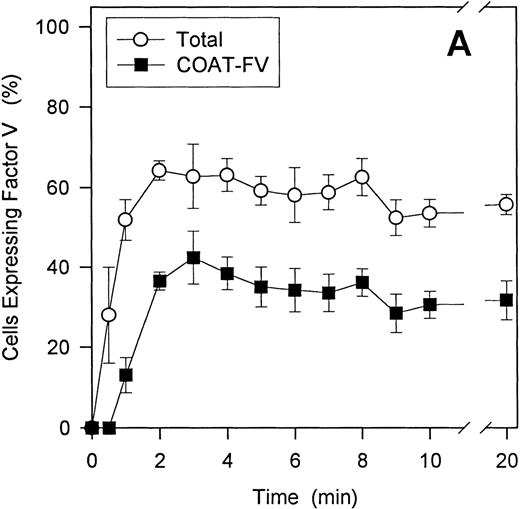
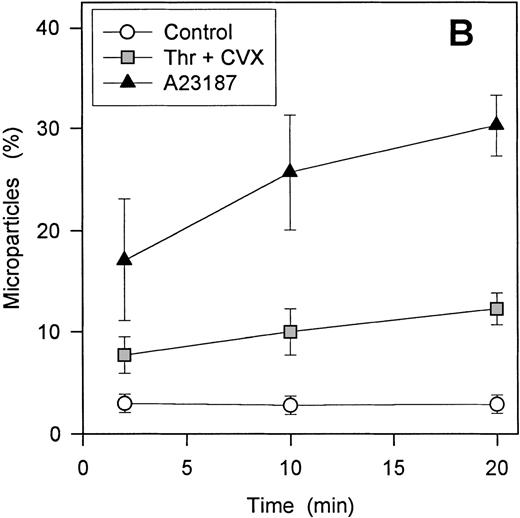
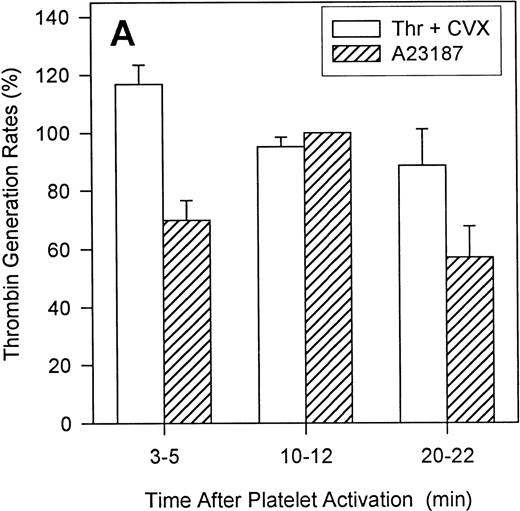
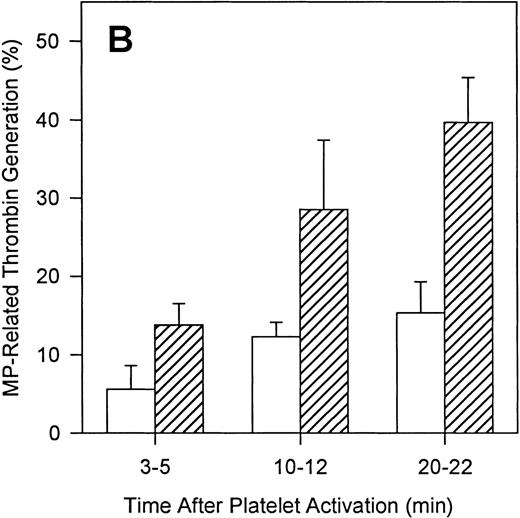
To define the relative contributions of platelets and platelet-derived MP to the observed procoagulant activity, we assessed the ability of thrombin and convulxin to promote MP-generation and compared this with the corresponding thrombin generation rates. Although surface expression of FV and the development of COAT-FV are essentially complete 2 minutes after platelet activation (Figure9A), the generation of MP is slower and is still increasing at 20 minutes (Figure 9B). Also, ionophore A23187 induces a higher percentage of MP than the combined stimulus of thrombin plus convulxin (Figure 9B). However, when prothrombinase activity is examined as a function of platelet activation time, thrombin plus convulxin is a more potent stimulus than ionophore A23187 shortly after platelet activation (3-5 minutes; Figure10A), a time when MP generation is still low (Figure 9B). After 10 to 12 minutes of platelet activation, both stimuli are equivalent in promoting thrombin generation, and after 20 to 22 minutes of activation, the thrombin/convulxin combination is again superior to that of A23187 alone (Figure 10A). These data suggest that the generation of microparticles is not tightly coupled to prothrombinase activity under these conditions. To further address this question, we assessed the level of residual procoagulant activity after separation of platelets and MP by centrifugation.4 At any given time the relative contribution of MP to the measured procoagulant activity is less than 20% for thrombin plus convulxin (Figure 10B); whereas the contribution of MP for A23187-induced prothrombinase activity reaches 40% after activation for 20 minutes (Figure 10B), a time point at which this agonist is less potent than the combined stimulus of thrombin plus convulxin in promoting the assembly of a functional prothrombinase complex (Figure 10A).
Time course of -granule FV surface expression and microparticle generation.
A time course for the generation of total FV-positive (region M1, Figure 2D) and COAT-FV (region M2, Figure 2D) cells after stimulation with 5 nmol/L thrombin plus 500 ng/mL convulxin is shown in Panel A. Both populations are essentially stable after 3 minutes of activation (mean ± 1 SD; n = 3). For Panel B, the percentage of MP after 2 to 20 minutes of stimulation was determined as detailed in “Materials and methods.” With 2 μmol/L A23187, there is a time-dependent increase in the percentage of MP, whereas the absolute number of MP and their time-dependent increase with 5 nmol/L thrombin plus 500 ng/mL convulxin stimulation is considerably less.
Time course of -granule FV surface expression and microparticle generation.
A time course for the generation of total FV-positive (region M1, Figure 2D) and COAT-FV (region M2, Figure 2D) cells after stimulation with 5 nmol/L thrombin plus 500 ng/mL convulxin is shown in Panel A. Both populations are essentially stable after 3 minutes of activation (mean ± 1 SD; n = 3). For Panel B, the percentage of MP after 2 to 20 minutes of stimulation was determined as detailed in “Materials and methods.” With 2 μmol/L A23187, there is a time-dependent increase in the percentage of MP, whereas the absolute number of MP and their time-dependent increase with 5 nmol/L thrombin plus 500 ng/mL convulxin stimulation is considerably less.
Contribution of microparticles to prothrombinase activity.
Platelets were activated with either 2 μmol/L A23187 or 5 nmol/L thrombin plus 500 ng/mL convulxin. At various times of platelet activation, samples were briefly centrifuged to pellet intact platelets and leave MP in the supernatant as described in “Materials and methods.” The prothrombinase activity of complete (Panel A) and microparticle-enriched supernatant (Panel B) samples was then determined. Prothrombinase rates for Panel A were normalized to the value for A23187 at 10 minutes (mean ± 1 SD; n = 3). For Panel B, the MP-related prothrombinase activity is presented as percentage of activity for the corresponding unfractionated sample.
Contribution of microparticles to prothrombinase activity.
Platelets were activated with either 2 μmol/L A23187 or 5 nmol/L thrombin plus 500 ng/mL convulxin. At various times of platelet activation, samples were briefly centrifuged to pellet intact platelets and leave MP in the supernatant as described in “Materials and methods.” The prothrombinase activity of complete (Panel A) and microparticle-enriched supernatant (Panel B) samples was then determined. Prothrombinase rates for Panel A were normalized to the value for A23187 at 10 minutes (mean ± 1 SD; n = 3). For Panel B, the MP-related prothrombinase activity is presented as percentage of activity for the corresponding unfractionated sample.
Discussion
In this report we demonstrate that the combined action of 2 physiologic agonists, thrombin and collagen, is able to promote high levels of α-granule factor V expression on the surface of a discrete fraction of platelets; we have referred to this population as COAT-FV platelets. Convulxin, a specific agonist for the collagen receptor GPVI,14 will substitute for collagen in this reaction. We also show that generation of COAT-FV parallels the exposure of negatively charged membrane phospholipids, although aminophospholipid exposure is not sufficient to generate COAT-FV platelets. Similarly, α-granule release is required but not sufficient for COAT-FV formation, since we observe a dose-dependent increase in COAT-FV formation (Figure 3B) with agonist concentrations well above that necessary to induce P-selectin expression in more than 95% of all platelets.23 In addition, platelets expressing FV, in particular COAT-FV, are functionally relevant and quantitatively more important under these conditions than platelet-derived MP in promoting procoagulant activity. Finally, our results demonstrate that COAT-FV formation is enriched in reticulated platelets, suggesting that young platelets are more likely than aged ones to undergo this transformation. Previous studies from our laboratory have demonstrated that aging platelets lose reactivity toward thrombin23 and collagen/convulxin (manuscript submitted). It is therefore conceivable that these age-related changes in reactivity toward single agonists are especially critical for an activation endpoint (COAT-FV formation), which relies on both of these agonists.
Two different mechanisms appear to control surface binding of FV released from α-granules. Low-level FV expression can be induced by all agonists examined and is independent from the exposure of negatively charged membrane phospholipids, confirming the existence of a FV binding site other than aminophospholipids.28 One candidate for a phospholipid-independent FV binding site on activated platelets is GPIa*/multimerin,29 a large disulfide-linked multimeric protein stored in α-granules30,31 which colocalizes with FV32 and remains associated with the platelet surface on activation.30,31 On the other side, COAT-FV expression is only induced by the combined stimulus of 2 agonists, requires the presence of extracellular calcium, results in the entire FV molecule being present on the cell surface, and parallels the exposure of aminophospholipids, although the latter is not sufficient for its generation. Moreover, only platelets expressing COAT-FV are able to maximally bind exogenous FXa. This is reminiscent of the model recently proposed by Bouchard et al33 for EPR-1 mediated binding of FXa. An FV-specific receptor could be expressed after maximal platelet stimulation, resulting in the generation of a highly functional procoagulant surface.
COAT-FV, coinciding with aminophospholipid exposure and the highest ability to bind FXa, theoretically represents the most efficient substrate for prothrombinase complex assembly. When 2 stimuli inducing similar amounts of negatively charged phospholipids are compared, the stimulus able to induce COAT-FV is more efficient in promoting thrombin generation. For instance, 500 ng/mL convulxin and the combined effect of 5 nmol/L thrombin plus 5 ng/mL convulxin both induce aminophospholipid exposure in about 5% of platelets (Figure 6), however, only the latter stimulus promotes COAT-FV (Figure 3) and this correlates with higher initial rates of prothrombin activation (Figure8B). A similar comparison can be drawn between thrombin alone and the combination 5 nmol/L thrombin plus 0.5 ng/mL convulxin. These observations demonstrate that COAT-FV, even though it is present in just a minority of platelets, is functionally more relevant than low-level FV. Moreover, ionophore A23187, inducing expression of negatively charged membrane phospholipids in more than 90% of the platelets (Figure 6) but no COAT-FV (Figure 3), results in prothrombinase activity approximating that of the combined stimulus of 5 nmol/L thrombin and 500 ng/mL convulxin (Figure 8B), a combination that promotes aminophospholipid exposure and COAT-FV in only 30% of the platelets (Figures 2 and 5). Ionophore is an even weaker promoter of procoagulant activity than the latter combination shortly after platelet activation (Figure 10A). Finally, we show that, under our conditions of dual stimulation with thrombin plus collagen, platelet-derived MP appear to contribute less than 20% of the prothrombinase activity in the absence of exogenous Va (Figure 10B). The difference between ours and previous studies12 in the relative contribution of platelets and platelet microparticles to prothrombinase activity may reflect that the latter study was performed in the presence of exogenous factor Va. Despite the fact that MP generated in vivo can stimulate coagulation34 and that MP-related procoagulant activity has been implicated in pathologic prothrombotic states,35 our results agree with previous observations4,36 37 and are consistent with the concept that under physiologic conditions an adequate hemostatic response must be rapid and localized to the site of vascular injury. This concept is supported by the observation that platelet FV appears to be uniquely important to hemostasis even in patients with near normal levels of plasma FV.
The current work presents a new model for vascular hemostasis in which the simultaneous engagement of the collagen receptor, GP VI, and thrombin activation generates a unique site on platelets that appears especially capable of supporting prothrombinase. This would provide for focal thrombin generation restricted to the site of vascular compromise. Furthermore, the age-dependence of COAT-FV generation may well explain the clinically observed hyperfunctionality of young platelets38 as well as the documented decrease in hemostatic competence of stored platelets.39
Supported in part by grants HL53 585 (G.L.D.) and P50 HL54 502 (C.T.E.) from the National Institutes of Health, the W. K. Warren Medical Research Institute, and the Swiss National Science Foundation (SSMBS grant, LA; 31-52 396.97, K.J.C.). C.T.E. is an investigator of the Howard Hughes Medical Institute.
Reprints:George L. Dale, PhD, Department Medicine, BSEB-302, Oklahoma University Health Sciences Center, PO Box 26901, Oklahoma City, OK 73190; e-mail: george-dale@ouhsc.edu.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.







This feature is available to Subscribers Only
Sign In or Create an Account Close Modal