The endothelial cell protein C receptor (EPCR) facilitates protein C activation by the thrombin-thrombomodulin complex. Protein C activation has been shown to be critical to the host defense against septic shock. In cell culture, tumor necrosis factor- (TNF-) down-regulates EPCR expression, raising the possibility that EPCR might be down-regulated in septic shock. We examined EPCR mRNA and soluble EPCR levels in mice and rats challenged with lethal dose 95 levels of endotoxin. Toxic doses of TNF- failed to alter EPCR mRNA levels in mice. Rather than EPCR mRNA levels falling in response to endotoxin, as predicted from cell-culture experiments, they rose approximately 3-fold 6 hours after exposure to endotoxin before returning toward baseline levels at 24 hours after exposure. Soluble EPCR levels rose approximately 4-fold. Infusion of hirudin, a specific thrombin inhibitor, before endotoxin exposure almost completely blocked the increase in EPCR mRNA and soluble EPCR. Consistent with the idea that the responses were mediated by thrombin, thrombin infusion (5 U/kg of body weight for 3 hours) resulted in an approximately 2-fold increase in EPCR mRNA and soluble EPCR. Incubation of rat endothelial cells with thrombin or murine protease-activated receptor 1 agonist peptide resulted in a 2-fold increase in EPCR mRNA. These results indicate that thrombin plays a major role in up-regulating EPCR mRNA and shedding in vivo.
The protein C anticoagulant pathway provides a critical, on-demand mechanism for regulation of blood coagulation (reviewed by Esmon1). The pathway is initiated when thrombin binds to thrombomodulin on the surface of the endothelium, and this complex catalyzes protein C activation. Activated protein C (APC) functions as an anticoagulant by proteolytic inactivation of the coagulation cofactors, factor Va and factor VIIIa. Patients with protein C deficiency usually have life-threatening thrombotic complications in infancy2 that can be corrected by administration of protein C.3 In addition to modulating the coagulation response, the protein C anticoagulant pathway also appears to modulate the inflammatory response. In vivo, APC administration prevented the lethal effects of Escherichia coli infusion in a baboon model of gram-negative sepsis,4 and preliminary clinical results suggest that protein C is effective in treating certain forms of septic shock.5-7 In vitro, APC has been reported to inhibit endotoxin-induced tumor necrosis factor-α (TNF-α) elaboration by monocytes8 and to inhibit leukocyte adhesion to selectins.9 The exact mechanism by which the protein C pathway modulates inflammatory responses remains unknown.
In an effort to gain further insights into the mechanisms by which APC might modulate inflammation, we sought to identify candidate protein C/APC receptors. In pursuit of this goal, we10 and others11 identified high-affinity binding sites for protein C and APC on vascular endothelium. The responsible glycoprotein, named the endothelial cell protein C receptor (EPCR), was identified by expression cloning and was suggested to be a member of the CD1-major histocompatibility complex class I family of molecules on the basis of sequence homology.10 Protein C binding to EPCR augments protein C activation by the thrombin-thrombomodulin complex on the cell surface.12 Binding of APC to soluble forms of EPCR blocks the APC anticoagulant activity and the ability of APC to inactivate factor Va without altering sensitivity to inhibition by protein C inhibitor and α1-antitrypsin.13 Presumably, this inhibition of APC anticoagulant activity reflects a change in enzyme specificity toward a new, unidentified substrate.
Immunohistochemical analysis of human and baboon organs indicated that EPCR expression is quite specific to endothelial cells and that it is expressed primarily on the surface of large vessels.14 In endothelial cell cultures of human, bovine, or murine origin, EPCR expression was down-regulated by the inflammatory mediator TNF-α.10,15 Because protein C activation and function were shown to play a critical role in the host defense against bacterial challenge,4,16,17 we thought it was important to understand the regulation of EPCR in vivo during challenges with endotoxin. During our analysis of the 5′ flanking region of the murine and human EPCR genes, we identified a thrombin response element, CCCACCCC, that in the context of the rest of the EPCR promoter, was necessary for the induction of murine EPCR mRNA by thrombin in cell culture.18 The presence of a positive regulatory element in the gene raised the question of whether thrombin might overcome down-regulation by TNF-α in vivo.
In this study, we found that EPCR mRNA and soluble EPCR levels rose soon after challenge with endotoxin in vivo and that this rise could be diminished by the specific thrombin inhibitor, hirudin. These results suggest that the dominant regulation of EPCR expression in gram-negative sepsis is mediated by thrombin and not TNF-α.
Materials and methods
Materials
The endotoxin, lipopolysaccharide B from E coli O127:B8 (LPS) (Difco Laboratories, Detroit, MI), was dissolved in saline. Owren's buffer, standard human plasma for fibrinogen assays, and leech hirudin (440 U/mg) were from Sigma (St Louis, MO). One hirudin unit was defined as the amount required to inhibit 1 NIH U of thrombin. Bovine thrombin was prepared by activation of purified bovine prothrombin with factor Xa, factor Va, phospholipid, and calcium as previously described19 and stored at −80°C. Murine protease-activated receptor (mPAR) 1 agonist peptide (SFFLRNPSE) and mPAR2 agonist peptide (SLIGRL) were provided by Shaun Coughlin (Department of Medicine, University of California San Francisco). Hybond-N nylon membrane and αphosphorus 32 (32P)-cytidine triphosphate (11 000 GBq/mmol) were from Amersham (Arlington Heights, IL).
In vivo experiments
The study protocol was approved by the Institutional Animal Care and Use Committees of the Oklahoma Medical Research Foundation. Male Sprague-Dawley rats (Charles River, Wilmington, MA) aged 7 to 10 weeks, body weight 235 to 380 g, were maintained for at least 1 week in the Oklahoma Medical Research Foundation animal facility before the experiments. For the study, rats were anesthetized with urethane (Sigma, 1 g/kg of body weight administered intraperitoneally). Polyethylene tubing was inserted into the femoral vein for LPS, hirudin, and thrombin infusions and into the femoral artery for blood sampling. In experiments with LPS, bolus injections of LPS (50 mg/kg) were used. Control animals were given injections of saline. When hirudin was used, it was dissolved in saline and infused intravenously (100 U/kg per hour for 6.2 hours at a rate of 0.9 mL per hour). Thirty minutes after the start of the hirudin infusion, LPS (50 mg/kg) was injected as a bolus. In some control experiments, hirudin was infused as described above, but a bolus injection of saline was given instead of the LPS. Six hours after the injection of LPS or saline, the hearts and lungs were perfused transcardially with 50 mL of saline before removal and storage at −80°C until RNA extraction. Plasma or serum was collected at selected times after LPS injection for measurement of soluble EPCR and fibrinogen.
For the experiments that used TNF-α infusion, BALB/c mice were infused with either endotoxin (20 mg/kg intraperitoneally) or murine TNF-α (3.6 mg/kg intravenously). At the selected times, organs were removed and the RNA was prepared as described above for the rat specimens.
In thrombin-infusion experiments, thrombin was infused into the femoral vein at rates of 5 U/kg per hour. Three hours after the initiation of thrombin infusion, lungs were removed for mRNA assessment, and blood was collected for measurement of soluble EPCR.
Assays of fibrinogen
Fibrinogen consumption was determined by plasma thrombin time. Plasma was diluted to 1:10 by using Owren's buffer and incubated at 37°C for 2 minutes; 50 μL of 100 U/mL of bovine thrombin was then added to initiate clotting. The clotting time was measured with an ST4 coagulometer. Fibrinogen content was expressed as a relative percentage compared with fibrinogen content of the rat plasma before LPS injection.
Cell culture
Rat aortic endothelial cells were isolated as previously described20 and cultured in Dulbecco's minimum essential medium with 10% fetal bovine serum. Cells were used before the 18th passage. The cells were cultured in serum-free medium (Opti-minimum essential medium plus insulin-like growth factor) for 24 hours before stimulation with bovine thrombin, mPAR1, or mPAR2.
Northern blot analysis
Total RNA was isolated from different rat organs or from cells as previously described.21 RNA (15 μg) was electrophoresed in 1% agarose gels with formaldehyde and blotted onto Hybond-N membranes. The membranes were UV-cross-linked for 4 minutes, baked for 2 hours at 80°C, and then prehybridized for 6 hours at 42°C in hybridization buffer (5% dextran sulfate, 5 × SSC, 5 × Denhardt's solution, 50% formamide, and 200 μg/mL sonicated and denatured salmon-sperm DNA). 32P-labeled murine EPCR cDNA probes prepared by using a random-primer labeling method (Rediprime, Amersham) were suspended in hybridization buffer and incubated with the membrane overnight at 42°C. The membranes were then washed for 15 minutes at each of the following steps: 2 × SSC, 0.1% sodium dodecyl sulfate, 1 time at room temperature; twice at 55°C; and twice at 65°C. The radioactivity on the membrane was quantified with a PhosphorImager (425S; Molecular Dynamics, Sunnyvale, CA). A BamHI linearized plasmid containing the entire CHO-B cDNA (provided by Rodger P. McEver's laboratory), a housekeeping gene that did not change when endothelial cells were treated with mediators,22 was used to normalize the data from the PhosphorImager.
Assays of soluble EPCR in rat serum
A soluble form of recombinant murine EPCR was prepared by truncating the sequence just before the transmembrane domain and adding an HPC4 dodecapeptide for affinity purification, followed by a stop codon, analogous to methods used to prepare human soluble EPCR.23The soluble receptor was purified from conditioned culture supernatants of stably transfected 293T cells as previously described.23The recombinant soluble murine EPCR has all the known properties of its human counterpart, including binding of human protein C/activated protein C and inhibition of human activated protein C anticoagulant activity (data not shown). Goat polyclonal antibody to the recombinant soluble murine EPCR was prepared and the IgG was purified as previously described23 before use in the enzyme-linked immunosorbent assay. Goat antimurine soluble EPCR polyclonal antibody was biotinylated with biotinamidocaproate N-hydroxysuccinimide ester by using standard procedures.
Microtiter plates (96 wells) were left coated overnight at 4°C with 50 μL of 10 μg/mL of goat polyclonal antibody against recombinant soluble murine EPCR in 0.1 mol/L of sodium carbonate (pH 9.6). The following steps were then performed at room temperature. The wells were washed and then blocked for 1 hour with 0.1% (wt/vol) gelatin in Tris-buffered saline (TBS; 0.1 mol/L of sodium chloride and 0.02 mol/L of Tris-hydrochloric acid (HCl) [pH 7.5]). The wells were washed with TBS containing 0.1% Tween and incubated for 1 hour with 50 μL of the diluted rat serum (from 1/10 to 1/50). Dilutions of recombinant EPCR (50 μL of 1 to 1000 ng/mL) were added to the wells and used to prepare a standard curve for calculating rat soluble EPCR levels. The wells were washed 3 times and incubated for 1 hour with 50 μL of 2 μg/mL of biotin-goat-antimurine EPCR polyclonal antibody. The plates were washed 3 times, and 50 μL of 0.25 μg/mL of streptavidin-alkaline phosphatase conjugate (Gibco, Grand Island, NY) was added to each well and incubated for an additional hour. The color was developed by adding Blue-Phos substrate, and the optical density at 650 nm was measured on a Vmax microplate reader (Molecular Devices). The standard curve was linear (r = 0.99) from 1 to 300 ng/mL, and samples were diluted with the same buffer to fall within the linear range.
For analysis of tissue EPCR levels, hearts and lungs were removed from saline-treated controls or animals treated with LPS (50 mg/kg) for 6 hours or thrombin (5 U/kg per hour) for 3 hours before organ removal. The reason for organ removal in the LPS-treated animals at 6 hours and the thrombin-treated animals at 3 hours is that the onset of peak thrombin formation after endotoxin administration occurs 2 to 4 hours after endotoxin infusion. The rats were perfused transcardially as described for the mRNA isolation, and the tissue was soaked in physiologic saline to remove external blood before organ removal. The lungs and hearts were removed, weighed, and suspended in 2 mL/g of 10 mmol/L of HEPES (pH 7.5), 1% Triton × 100, 0.25 mol/L of sucrose, 50 mmol/L of benzamidine HCl, 0.02% sodium azide, 2 mmol/L of EDTA, 1 mmol/L of phenylmethanesulfonyl fluoride, 1 μg/mL of leupeptin, and 1μg/mL of aprotinin. The tissue was then homogenized and centrifuged at 12 000 rpm in an IEC microcentrifuge (IEC, Neeham Heights, MA) for 10 minutes at 4°C. The supernatants were stored at −80°C until they were analyzed for antigen content. The analysis was performed as described above for soluble EPCR.
Statistical analysis
Comparisons among the 3 groups (control, LPS, and LPS plus hirudin) of the results of the fibrinogen assessment were made by using the Dunnett test. The Student t test was used to compare changes in EPCR expression between 2 groups (control and LPS, and LPS and LPS plus hirudin).
Results
Tissue distribution of rat EPCR mRNA in vivo
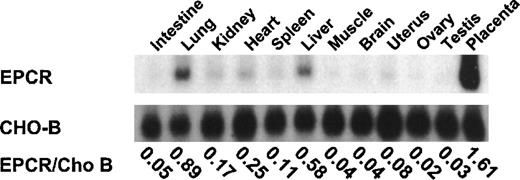
To determine the organ specificity of EPCR expression and to identify organs suitable for analysis of mRNA levels in response to inflammatory mediators, we performed Northern blot analysis in different rat organs. EPCR mRNA levels were highest in the placenta, lung, liver, and heart but relatively low in other tissues. Northern blot studies with CHO-B, a housekeeping gene, indicated that the level of CHO-B was similar among the different organs (Figure1). Because the heart and lung had relatively high levels of expression and both organs were impaired functionally by inhibition of protein C-EPCR binding in animals treated with E coli,24 these organs were chosen for examining the influence of inflammatory mediators on EPCR expression. A similar organ distribution of EPCR expression was found in mice.
Tissue distribution of rat endothelial cell protein C receptor.
Different organs from normal rats were homogenized and total RNA was extracted. Fifteen micrograms of RNA was electrophoresed, transferred to a Hybond-N membrane, and hybridized with murine endothelial cell protein C receptor (EPCR) complementary DNA (cDNA) and CHO-B cDNA probes. The ratios of EPCR to CHO-B are indicated below the blots for each organ.
Tissue distribution of rat endothelial cell protein C receptor.
Different organs from normal rats were homogenized and total RNA was extracted. Fifteen micrograms of RNA was electrophoresed, transferred to a Hybond-N membrane, and hybridized with murine endothelial cell protein C receptor (EPCR) complementary DNA (cDNA) and CHO-B cDNA probes. The ratios of EPCR to CHO-B are indicated below the blots for each organ.
Regulation of EPCR mRNA levels by LPS
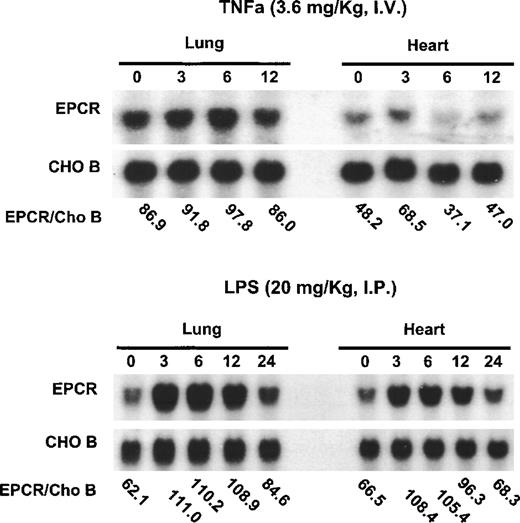
Previous experiments demonstrated that EPCR mRNA is down-regulated by TNF-α in cultured endothelial cells.10,15 Because TNF-α is a major mediator of endotoxin shock,25 these observations led to the hypothesis that EPCR would be down-regulated by endotoxin in vivo, possibly contributing to the inability of this anticoagulant pathway to prevent the disseminated intravascular coagulation associated with endotoxin shock. To test this hypothesis directly, we administered toxic doses of TNF-α or endotoxin to mice (Figure 2). TNF-α did not alter EPCR mRNA levels (Figure 2A). In contrast, endotoxin increased mRNA levels, with maximum levels occurring between 3 hours and 6 hours after endotoxin infusion before levels returned toward baseline values at 24 hours after the administration of endotoxin or TNF-α (Figure 2B). To control for gel loading, the CHO-B levels were also examined.
Up-regulation of EPCR mRNA levels in mice by lipopolysaccharide B from Escherichia coli O127:B8 but not by TNF-.
Mice were injected intravenously with TNF-α (3.6 mg/kg of body weight) or intraperitoneally with lipopolysaccharide B from E coli O127:B8 (LPS) endotoxin (20 mg/kg). RNA was obtained from the heart and lungs at the times indicated (hours after administration of LPS or TNF-α) and analyzed by Northern blotting. The ratios of EPCR to CHO-B are indicated below the blots for each time point. The blots at the top represent the experiments with TNF-α; those at the bottom represent the experiments with endotoxin.
Up-regulation of EPCR mRNA levels in mice by lipopolysaccharide B from Escherichia coli O127:B8 but not by TNF-.
Mice were injected intravenously with TNF-α (3.6 mg/kg of body weight) or intraperitoneally with lipopolysaccharide B from E coli O127:B8 (LPS) endotoxin (20 mg/kg). RNA was obtained from the heart and lungs at the times indicated (hours after administration of LPS or TNF-α) and analyzed by Northern blotting. The ratios of EPCR to CHO-B are indicated below the blots for each time point. The blots at the top represent the experiments with TNF-α; those at the bottom represent the experiments with endotoxin.
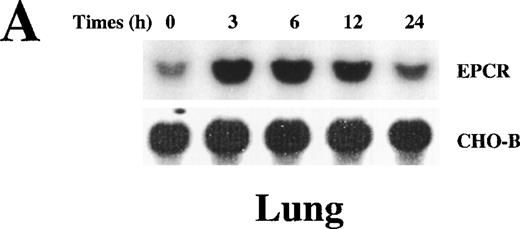
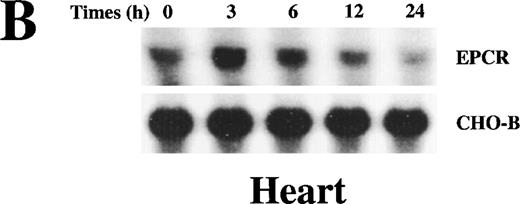
Because we wanted to analyze the relation between the levels of soluble EPCR and EPCR mRNA, we performed the remainder of the experiments in rats. When lethal dose 95 (LD95) levels of endotoxin were infused into rats, EPCR mRNA levels in the heart and lung rose as a function of time after endotoxin challenge. After endotoxin injection, EPCR mRNA levels increased, reaching a peak between 3 hours and 6 hours, and then declined toward baseline values by 24 hours (Figure3). The response in the lung and heart was similar. Immunohistochemical analysis of the tissues before and after endotoxin exposure indicated that EPCR expression was still restricted to the large vessels and was low to absent in the capillaries (data not shown), as was found previously in tissue from a patient who died of bronchopulmonary dysplasia.14 The increase in EPCR was temporally linked to fibrinogen consumption, which is indicative of thrombin generation. Therefore, we considered the possibility that thrombin might be responsible for up-regulation of the EPCR gene.
Up-regulation of EPCR mRNA levels in rat lung and heart tissue by LPS administration.
Total RNA was extracted from rat lungs (A) and hearts (B) at 0, 3, 6, 12, and 24 hours after LPS injection, and Northern blot analysis was performed.
Up-regulation of EPCR mRNA levels in rat lung and heart tissue by LPS administration.
Total RNA was extracted from rat lungs (A) and hearts (B) at 0, 3, 6, 12, and 24 hours after LPS injection, and Northern blot analysis was performed.
Thrombin generation contributes to induction of rat EPCR mRNA
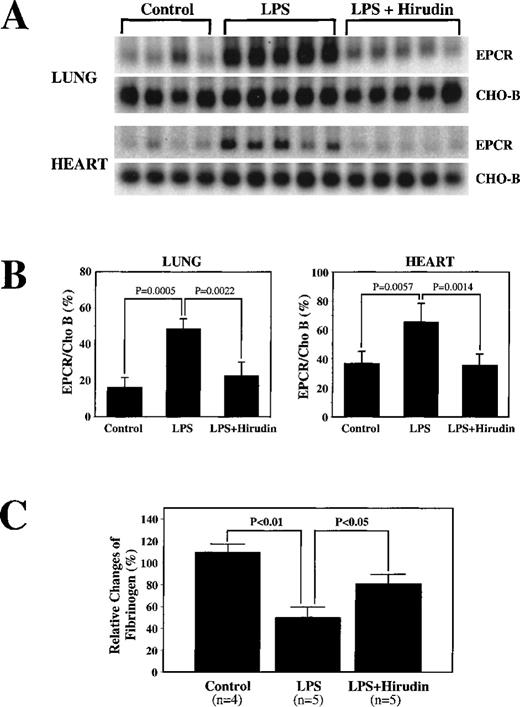
To examine the possible role of thrombin in endotoxin-mediated up-regulation of EPCR mRNA, we infused hirudin, a specific thrombin inhibitor, before administration of LPS. As expected, control experiments in which hirudin alone was infused did not result in changes in either EPCR mRNA or fibrinogen levels (data not shown). Hirudin diminished the LPS-mediated EPCR mRNA up-regulation in lung tissue (Figure 4A), decreasing the response from a 2.9-fold increase to a 1.4-fold increase (P = .0022) (Figure 4B). Similar results were obtained in heart tissue. The fibrinogen level decreased less in the presence of hirudin than in its absence, but there was still a decrease (Figure 4C), indicating either that the hirudin had not totally inhibited thrombin or that other factors contributed to the decreases in fibrinogen. These results implicate thrombin as a major mediator of endotoxin-induced up-regulation of EPCR mRNA.
Decrease by hirudin administration of LPS-mediated up-regulation of EPCR mRNA levels.
(A) Rats were surgically prepared and injected with saline (N) or LPS alone (LPS) or infused with hirudin before and after LPS injection (LPS + hirudin). Total RNA was prepared from rat lungs and hearts isolated 6 hours after LPS injection and used for Northern blot analysis. (B) Below the Northern blot is the quantitation of the change based on the ratio of the intensity of EPCR to CHO-B mRNA on the PhosphorImager. (C) Changes in fibrinogen levels in the above animals treated with LPS or LPS plus hirudin.
Decrease by hirudin administration of LPS-mediated up-regulation of EPCR mRNA levels.
(A) Rats were surgically prepared and injected with saline (N) or LPS alone (LPS) or infused with hirudin before and after LPS injection (LPS + hirudin). Total RNA was prepared from rat lungs and hearts isolated 6 hours after LPS injection and used for Northern blot analysis. (B) Below the Northern blot is the quantitation of the change based on the ratio of the intensity of EPCR to CHO-B mRNA on the PhosphorImager. (C) Changes in fibrinogen levels in the above animals treated with LPS or LPS plus hirudin.
Thrombin alone can up-regulate EPCR expression in vivo
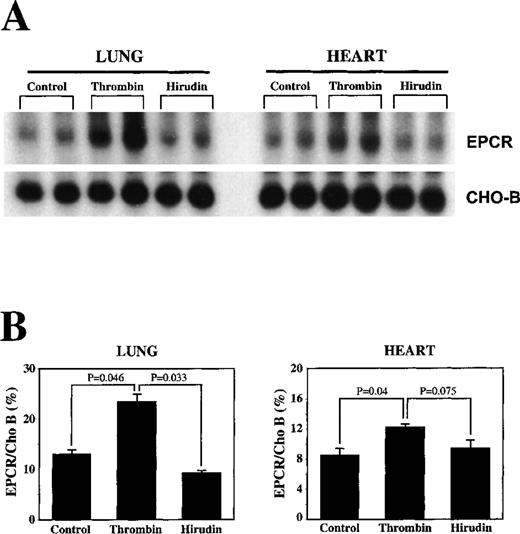
To test directly the possibility that thrombin can up-regulate EPCR mRNA, thrombin was infused into rats for 3 hours at the rate of 5 U/kg per hour. EPCR mRNA levels had risen approximately 2-fold by the end of this infusion (Figure 5). Hirudin was used as a control protein in the infusion; there was no change in EPCR mRNA levels in response to hirudin infusion alone.
Increases in EPCR mRNA levels in rat lungs with infusion of thrombin.
Bovine thrombin (5 U/kg infused per hour) and hirudin (100 U/kg infused per hour) were infused into the femoral vein of rats, and the lungs and hearts were removed 3 hours later for the extraction of total RNA. (A) Northern blot analysis. (B) Below the Northern blot is the quantitation of the change based on normalization of the signal of EPCR mRNA to that of CHO-B mRNA on the PhosphorImager.
Increases in EPCR mRNA levels in rat lungs with infusion of thrombin.
Bovine thrombin (5 U/kg infused per hour) and hirudin (100 U/kg infused per hour) were infused into the femoral vein of rats, and the lungs and hearts were removed 3 hours later for the extraction of total RNA. (A) Northern blot analysis. (B) Below the Northern blot is the quantitation of the change based on normalization of the signal of EPCR mRNA to that of CHO-B mRNA on the PhosphorImager.
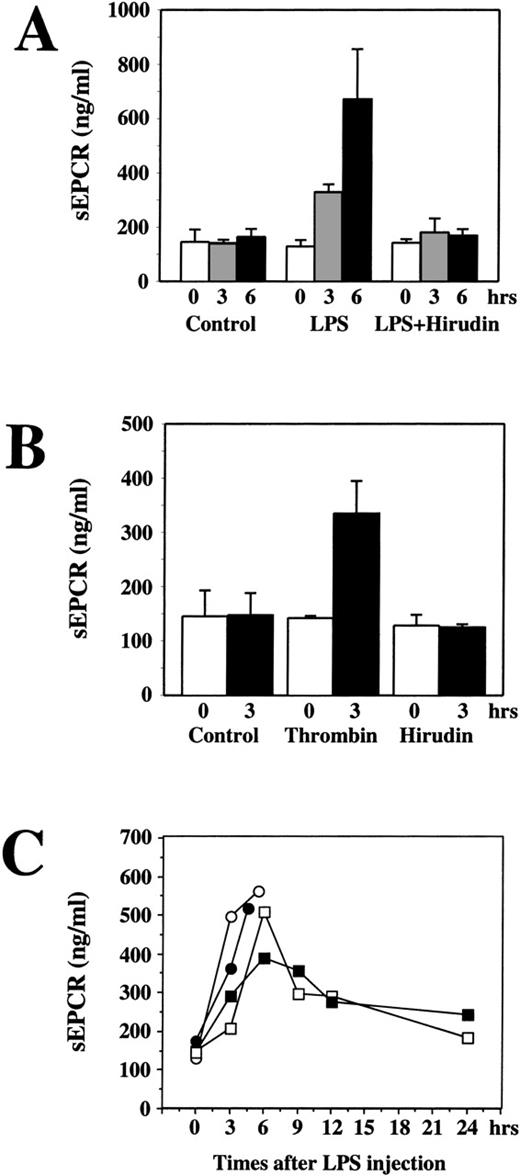
Endotoxin-induced increases in soluble EPCR levels in rats can be blocked by hirudin
In addition to increasing the endotoxin up-regulation of EPCR mRNA, endotoxin injection increased soluble EPCR antigen levels in serum (approximately 4-fold after 6 hours) (Figure6A). Like the mRNA elevation, this rise in serum EPCR levels was also blocked by hirudin (Figure 6A). These results implicate thrombin as a major mediator of endotoxin-induced shedding of soluble EPCR. To further test this hypothesis, thrombin was infused for 3 hours at the rate of 5 U/kg per hour. At the end of this infusion, the serum EPCR levels had risen approximately 2-fold (Figure6B) compared with the control and hirudin-only groups.
Increases by LPS in the soluble EPCR levels in rats that can be blocked by hirudin.
(A) Serum from normal controls (n = 6), rats given LPS (n = 4), and rats given LPS plus hirudin (n = 6) at 0, 3, and 6 hours after administration of the agents were assayed for soluble EPCR levels by enzyme-linked immunosorbent assay (ELISA). (B) Changes in soluble EPCR levels in rats infused with bovine thrombin or hirudin alone. (C) Rats were injected with LPS (20 mg/kg) through the tail vein. Serum samples were collected at the indicated times through the tail vein and were assayed for the soluble EPCR by ELISA. In the 2 animals that died, the last data point corresponds to the serum sample obtained at the time of death.
Increases by LPS in the soluble EPCR levels in rats that can be blocked by hirudin.
(A) Serum from normal controls (n = 6), rats given LPS (n = 4), and rats given LPS plus hirudin (n = 6) at 0, 3, and 6 hours after administration of the agents were assayed for soluble EPCR levels by enzyme-linked immunosorbent assay (ELISA). (B) Changes in soluble EPCR levels in rats infused with bovine thrombin or hirudin alone. (C) Rats were injected with LPS (20 mg/kg) through the tail vein. Serum samples were collected at the indicated times through the tail vein and were assayed for the soluble EPCR by ELISA. In the 2 animals that died, the last data point corresponds to the serum sample obtained at the time of death.
We next examined the time course of soluble EPCR release. Because most rats die within 24 hours when injected with an LD95 dose (50 mg/kg) of endotoxin, we reduced the dose to 20 mg/kg. Serum was collected through the tail vein at different times. Even this lower dose caused 2 of the 4 rats to die within 24 hours. In Figure 6C, therefore, each animal is represented individually, with a different symbol, in the time course. The last point represents serum collected at the time of death except for the 2 animals that survived for 24 hours. The soluble EPCR levels rose with time. In the surviving animals, the levels returned toward baseline values by 24 hours.
Thrombin and mPAR1 can up-regulate EPCR mRNA levels in cell culture
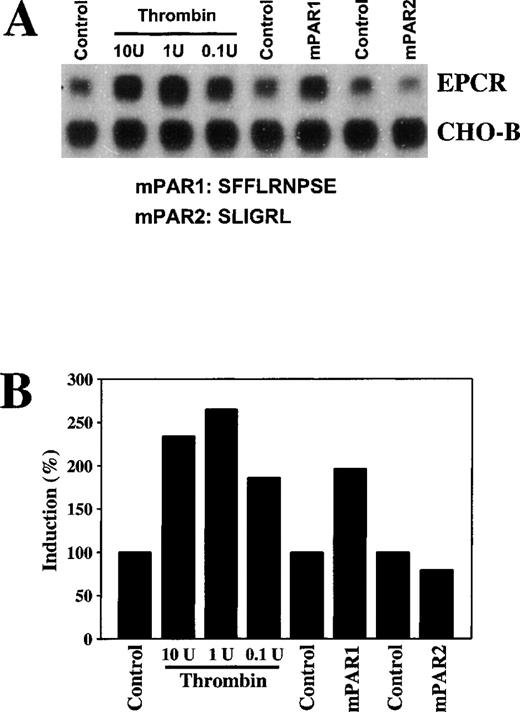
The in vivo thrombin response could be indirect. To determine whether thrombin affected expression of EPCR in rat aortic endothelial cells directly in culture, these cells were treated with thrombin. After a 6-hour incubation with even relatively low concentrations of thrombin (0.1 U/mL), EPCR mRNA levels were increased (Figure 6A). Thrombin increased EPCR mRNA in a time-dependent manner, with peak levels reached at 12 hours of incubation (data not shown).
To test whether thrombin-mediated up-regulation of EPCR is mediated by a protease-activated receptor, we stimulated the cells with the agonist peptide SFFLRNPSE for mPAR126 or SLIGRL for mPAR2.27 After a 6-hour incubation, the mPAR1 agonist peptide increased EPCR mRNA levels approximately 2-fold (Figure7), a response similar to that observed with thrombin. Incubation with mPAR2 had no effect on EPCR mRNA levels. These results indicate that thrombin mediates up-regulation of EPCR mRNA in endothelial cells through mPAR1.
Increases in EPCR mRNA levels in endothelial cells in culture by thrombin and murine protease-activated receptor 1.
Rat aortic endothelial cells were maintained in serum-free media for 24 hours and were then treated with 0.1, 1, or 10 U/mL of bovine thrombin, 10 μmol/L of murine protease-activated receptor (mPAR) 1 peptide, and 10 μmol/L of mPAR2 peptide for 6 hours. (A) Total RNA was extracted and 15 μg of the RNA was analyzed by Northern blotting and compared with CHO-B mRNA levels. (B) The changes in the mRNA levels were quantitated with a PhosphorImager on the basis of the increase in the ratio of the EPCR intensity to the CHO-B intensity.
Increases in EPCR mRNA levels in endothelial cells in culture by thrombin and murine protease-activated receptor 1.
Rat aortic endothelial cells were maintained in serum-free media for 24 hours and were then treated with 0.1, 1, or 10 U/mL of bovine thrombin, 10 μmol/L of murine protease-activated receptor (mPAR) 1 peptide, and 10 μmol/L of mPAR2 peptide for 6 hours. (A) Total RNA was extracted and 15 μg of the RNA was analyzed by Northern blotting and compared with CHO-B mRNA levels. (B) The changes in the mRNA levels were quantitated with a PhosphorImager on the basis of the increase in the ratio of the EPCR intensity to the CHO-B intensity.
Endotoxin does not change tissue levels of EPCR
Given that endotoxin elicits both an increase in EPCR mRNA and soluble EPCR levels, the question of whether there is a net change in tissue EPCR expression arises. We found that EPCR levels in the heart and lung changed very little in response to either endotoxin or thrombin infusion (Table 1). These results suggest that the increase in mRNA may prevent the shedding of the receptor from diminishing EPCR levels in the endothelium.
Endothelial cell protein C receptor (EPCR) levels in tissue from control rats and from rats given lipopolysaccharide B from E coli O127:B8 (LPS) or thrombin
| Tissue . | Control (n = 5) . | LPS (n = 5) . | Thrombin (n = 2) . |
|---|---|---|---|
| Lung | 904.1 ± 284.5 | 675.2 ± 255.4 | 984.7 ± 263.1 |
| Heart | 1324.7 ± 278.1 | 1593.6 ± 645.2 | 1290.6 ± 312.9 |
| Tissue . | Control (n = 5) . | LPS (n = 5) . | Thrombin (n = 2) . |
|---|---|---|---|
| Lung | 904.1 ± 284.5 | 675.2 ± 255.4 | 984.7 ± 263.1 |
| Heart | 1324.7 ± 278.1 | 1593.6 ± 645.2 | 1290.6 ± 312.9 |
Rats were either injected with endotoxin (50 mg/kg) or infused with thrombin (5 U/kg per hour for 3 hours). Six hours after endotoxin injection and 3 hours after thrombin infusion, they were perfused transcardially and lung and heart tissue were extracted with use of Triton X-100. EPCR antigen in the tissues was measured by an enzyme-linked immunosorbent assay. In controls, the results are expressed as the mean ± SE. In rats given LPS or the thrombin infusion, the results are expressed as the mean ± SD.
EPCR levels are given in mg/g.
Vascular distribution after endotoxin challenge
One possible cause of EPCR mRNA up-regulation is that EPCR expression is selectively increased in the microvasculature. Immunohistochemical staining, performed essentially as described previously,14 failed to detect increases in EPCR expression in the microvasculature. In addition, the EPCR expression levels in the kidney and brain did not appear to change significantly, which constitutes strong evidence against the possibility of organ-specific differences in regulation (data not shown).
Discussion
These studies demonstrate that, contrary to predictions derived from in vitro cell-culture studies, the in vivo response to LPS is to up-regulate EPCR mRNA levels. Presumably, this is due to increases in transcription, since a putative thrombin response element is present in the 5′ region of the EPCR gene, expression of luciferase reporter constructs containing this element can be enhanced by thrombin treatment of the transfected cells,18 and transgenic animals with this region of the EPCR promoter have increased expression of the transgene only if the thrombin response element is not mutated (Gu and Esmon, unpublished observations). Our findings with hirudin indicate that the endotoxin-mediated up-regulation has a strong requirement for thrombin generation. This could result from either thrombin effects on the endothelium or the generation of thrombin-dependent products, which could include, for instance, platelet-release products. On the basis of the cell-culture data, which showed that thrombin could increase EPCR mRNA directly, it appears that at least some of the stimulation is due to direct thrombin effects on endothelium. This is at least partly mediated by mPAR1 because mPAR1 agonist peptide and thrombin induced a similar enhancement of EPCR mRNA. Thus, it is likely that the thrombin-induced increase in EPCR mRNA serves to up-regulate EPCR protein synthesis. This increased synthesis may be offset by increased shedding of the receptor, as reflected by the substantial increases in serum EPCR levels observed in response to endotoxin.
Studies have shown that thrombin or the PAR1 agonist peptide can enhance EPCR shedding from human umbilical vein endothelial cells.28 This is apparently mediated by the activation of a metalloproteinase. It is unlikely that the soluble EPCR that appeared after endotoxin treatment resulted from endothelial cell death, since hirudin, which did not protect the animals from the lethal effects of endotoxin, blocked the endotoxin-induced soluble EPCR formation. It is also unlikely that the soluble EPCR was caused by alternative splicing of the mRNA. Although we have detected alternatively spliced mRNA that would code for soluble EPCR, the levels of the alternatively spliced transcript were low both before and after endotoxin treatment (unpublished observations).
Other agonists can also elicit EPCR shedding, raising the question of which agonists are most important in vivo. Our current data indicate that thrombin appears to be critical for shedding, at least in the context of endotoxin. We previously found, however, that other agonists can work synergistically with thrombin to augment shedding from endothelium in culture.28 The possibility that thrombin works synergistically with other agonists to increase EPCR shedding further cannot be addressed readily by in vivo studies.
Our original goal in searching for EPCR was to attempt to explain the ability of APC to modulate the response to lethal levels of E coli in baboons4 and to protect against meningococcemia in humans.5-7 When EPCR mRNA and function were found to be down-regulated in response to endotoxin and TNF-α in cell culture, it appeared that EPCR might not be a candidate for the in vivo response, at least in meningococcemia. Patients with meningococcemia are often treated relatively late in the disease course— many hours after the onset of the infection, when the cell-culture data would predict that EPCR levels would be extremely low. The observations presented here suggest that EPCR would be relatively well expressed under these conditions. Consistent with this possibility, immunohistochemical studies of vessels from a patient who died of respiratory distress syndrome revealed high levels of EPCR expression.14 On the basis of these findings and the observation that the macromolecular specificity of APC is altered when bound to soluble EPCR,13the possibility that this receptor is involved in modulating the inflammatory responses in gram-negative sepsis remains viable. Initial experiments in a primate model of E coli septic shock have provided preliminary support for this idea.24 In these experiments, blocking protein C binding to EPCR with a monoclonal antibody dramatically increased leukocyte migration into the tissues and induced a capillary leak syndrome in response to low-level E coli infusion.
In studies with results possibly related to the current findings, we identified moderately high levels of EPCR in the plasma of healthy human donors.29 The levels of plasma EPCR were elevated in inflammatory disease states such as sepsis and lupus erythematosus.30 It is possible that the induction of mRNA observed here is a mechanism to help compensate for the loss of EPCR from the membrane surface that occurs in disease states associated with coagulation and inflammation. The observation that the shedding of EPCR and the increase in mRNA are temporally similar and both mediated by thrombin suggests that the release of soluble EPCR may play an important, unidentified role in the physiologic response to endotoxin. In support of this conclusion, we observed specific binding sites for soluble EPCR on activated leukocytes31 and found that the binding interaction is mediated largely by proteinase 3, the autoantigen found in Wegener's granulomatosis. Alternatively, although the levels of soluble EPCR achieved in this study were too low to impair APC anticoagulant function, most of the soluble EPCR would be in complex with the protein C-APC, since the concentrations of these components are higher than the Kd for EPCR-protein C interaction (≈30-50 nmol/L). Therefore, if the complex does have altered specificities, increases in soluble EPCR would allow expression of these activities throughout the blood stream.
Acknowledgments
We thank Jeff Box for assistance with the figures, Jeff Mollica for performing the plasma APC assays, Zoltan Laszik for performing the immunohistochemical studies, and Nici Barnard for preparing the final manuscript.
Supported by a grant awarded by the National Heart, Lung and Blood Institute of the National Institutes of Health (grant PO1 HL 54804) to C.T.E.
Reprints:Charles T. Esmon, Cardiovascular Biology Research, Oklahoma Medical Research Foundation, 825 NE 13th Street, Oklahoma City, OK 73104; e-mail: charles-esmon@omrf.ouhsc.edu.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal