The chemotactic potencies of ELR+-CXC chemokines during acute inflammation are regulated by their binding affinities and by their ability to activate, desensitize, and internalize their specific receptors, CXCR1 and CXCR2. To gain insight into the fine mechanisms that control acute inflammatory processes, we have focused in this study on the highly potent ELR+-CXC chemokine Granulocyte Chemotactic Protein 2 (GCP-2), and on its ability to control the cell surface expression of CXCR1 and CXCR2. Although GCP-2 has been considered an effective ligand for both CXCR1 and CXCR2, our findings demonstrated that it was a potent inducer of CXCR2 internalization only. A functional hierarchy was shown to exist between GCP-2 and 2 other ELR+-CXC chemokines, IL-8 and NAP-2, in their abilities to induce CXCR1 and CXCR2 internalization, according to the following: IL-8 > GCP-2 > NAP-2. By the use of pertussis toxin (PTx), it was demonstrated that the actual events of Gi-coupling to CXCR2 do not have a major role in the regulation of its internalization. Rather, CXCR2 internalization was shown to be negatively controlled by induction of signaling events, as indicated by the promotion of CXCR2 internalization following exposure to wortmannin, a potent inhibitor of phosphatidylinositol (PI) 3 kinases and PI4 kinases. Furthermore, our results suggest that rab11+-endosomes participate in the trafficking of CXCR2 through the endocytic pathway, to eventually allow its recycling back to the plasma membrane. To conclude, our findings shed light on the interrelationships between GCP-2 and other ELR+-CXC chemokines, and determine the mechanisms involved in the regulation of GCP-2–induced internalization and recycling of CXCR2.
Human Granulocyte Chemotactic Protein 2 (GCP-2) is a member of the ELR-expressing CXC subfamily of chemokines (ELR+-CXC) and acts as a potent chemoattractant of neutrophils in the course of acute inflammation.1-5 The high chemotactic potency of GCP-2 is well illustrated in mice, where it is the predominant neutrophil chemoattractant.4,6,7Moreover, GCP-2 is highly produced by MG-63 osteosarcoma cells and induces neovascularization,4,5 7-11 suggesting that it may be involved in tumor development and metastasis formation.
As a member of the ELR+-CXC subfamily of chemokines, human GCP-2 is one of several CXC chemokines that differ in their ability to attract and activate neutrophils.4,5,10-18 The differential functional capabilities of these chemokines are in part the result of their divergent abilities to bind and activate 2 receptors, CXCR1 and CXCR2, which are expressed on neutrophils.19-26 Interleukin 8 (IL-8), the most potent of all human ELR+-CXC chemokines, binds to both receptors with high affinity, whereas most other ELR+-CXC chemokines (such as Neutrophil Activating Protein 2 [NAP-2]), bind with high affinity to CXCR2 only.21-26In that respect, GCP-2 is of major interest, being the only ELR+-CXC chemokine, except for IL-8, that is an effective ligand for CXCR1, in addition to CXCR2.24-26 These characteristics of GCP-2 may allow it to serve as a “backup chemokine” in case inappropriate down-regulation of IL-8 expression has occurred.
The redundancy in ELR+-CXC chemokines and in their receptors may provide multiple levels of regulation that allow for chemokine- and receptor-specific control of inflammatory processes. Fine tuning of ELR+-CXC chemokine-induced responses could also be achieved by the different abilities of these chemokines to regulate the induction of intracellular events, which follow the binding of each of them to its specific receptors, CXCR1 and/or CXCR2.
CXCR1 and CXCR2 belong to a superfamily of G protein-coupled receptors (GPCR), whose signaling is mediated by their coupling to heterotrimeric G proteins, resulting in the exchange of GDP for GTP on the α subunit of the G protein.27-29 Studies of CXCR1 and CXCR2 have demonstrated that the cascade of events that follows leads to specific cellular responses, which are strictly regulated by homologous desensitization. The process of homologous desensitization is induced by high concentrations of ligands and is mediated primarily by receptor phosphorylation.30-34 In addition, one of the major determinants that may control the functionality of CXCR1 and CXCR2 is the level of their expression, determined by rapid dynamics of internalization and recycling back to the plasma membrane.31,32 34-38
Since the fine control of neutrophil responses results from the coordinated activity of several ELR+-CXC chemokines, it is of major importance to elucidate the regulation of CXCR1 and CXCR2 by members of this subfamily. In that respect, the aim of our study is to gain insight into the regulation of these receptors by the highly potent neutrophil chemoattractant, GCP-2. Because receptor expression is a principal regulator of the functionality of CXCR1 and CXCR2, we have focused on the hierarchical relationships between GCP-2 and other ELR+-CXC chemokines and their abilities to induce receptor internalization, and on the intracellular events that are involved in GCP-2–induced CXCR2 internalization and recycling. The results of our study shed light on the coordinated interaction of ELR+-CXC chemokines with their receptors and on the tight regulation of their activities.
Materials and methods
DNAs for human CXCR1 and human CXCR2
Cell cultures, transfections, and characterization of receptor expression by transfected cells
Human embryonal kidney 293 cells (HEK 293 cells) were grown and stably transfected as previously described.35,39 Scatchard analysis showed that CXCR1 and CXCR2 bound IL-8 with similarly high affinity. A higher binding affinity of IL-8 to CXCR1 as compared to NAP-2 was observed, whereas both ligands showed a similarly high affinity of binding to CXCR2.23 The binding characteristics of GCP-2 to CXCR1 and CXCR2 were determined as previously described,24,25 indicating the moderately higher binding capability of GCP-2 to CXCR2 than to CXCR1. To further increase the expression level of transfected receptors, stable cell lines expressing CXCR1 or CXCR2 were subjected to cytofluorometric sorting, using monoclonal antibodies to the amino terminus of CXCR1 or CXCR2.35,39 Fluorescence-activated cell sorting (FACS) analyses showed that a high percentage (over 90%) of the transfected cells expressed the receptor on the cell surface with high mean fluorescence value. Control transfections were performed with the vector (pRc/CMV) alone, and the resulting cells did not specifically bind IL-8, NAP-2, and GCP-2, or antibodies specific for human CXCR1 or CXCR2.23,24,35 39
Analysis of receptor downmodulation by FACS analysis
This analysis was performed as previously described.35Briefly, aliquots of stable HEK 293 transfectants were removed and supplemented with GCP-2, IL-8, or NAP-2 (GCP-2, NAP-2: PeproTech, Inc, Rocky Hill, NJ; IL-8: Dainippon, Japan), while no chemokines were added to control tubes. The cells were incubated at 37°C for the indicated time periods. The cells were washed in CSB (phosphate-buffered saline [PBS] containing 1% fetal calf serum, 0.02% NaN3, and 25 mmol/L Hepes) and incubated with monoclonal mouse anti-CXCR1 or anti-CXCR2 antibodies (R&D Systems, Minneapolis, MN; IgG2a, 1 μg/5 × 105 cells). Baseline staining was obtained by adding CSB to the cells instead of anti-CXCR1 or anti-CXCR2 antibodies. Following incubation and washings, the cells were incubated with fluorescein isothiocyanate (FITC)-conjugated goat anti-mouse IgG antibodies (Jackson ImmunoResearch Laboratories, West Grove, PA), washed, and resuspended. The effects of PTx and of wortmannin on internalization levels were determined by pre-incubating the cells with the compounds for 2 hours and 1 hour, respectively, at 37°C. FACSort® (Becton Dickinson, San Jose, CA), was used to analyze 5000 live cell events. Percent receptor downmodulation was calculated from the mean channel fluorescence values of cells treated with ligand at 37°C vs the mean channel fluorescence of cells not treated with ligand, under similar conditions. P values were calculated by Student t test.
Determination of receptor re-expression on the plasma membrane
The procedure used to determine receptor re-expression was similar to that used to evaluate receptor downmodulation, but matched samples were allowed to undergo a receptor recovery process that was performed as previously described.35 To determine the contribution of de novo protein synthesis to receptor reappearance on the cell membrane, cell samples matching those undergoing receptor recovery were incubated with medium containing 10 μg/mL cycloheximide (during the recovery phase) (Sigma, St Louis, MO). Analysis of the cells following cycloheximide treatment indicated that cell viability was not affected by this treatment. Mean fluorescence values of cells that were not exposed to GCP-2, and of cells exposed to GCP-2 and allowed to undergo recovery, were used to determine the level of receptor re-expression. P values were calculated by Student t test.
Chemotaxis assays
The migration of CXCR2-expressing HEK 293 cells was assessed by a 48-well microchemotaxis chamber technique as previously described.39 Briefly, the lower compartment of the chamber was loaded with aliquots of medium, or 100 ng/mL GCP-2 diluted in medium, while the upper compartment of the chamber was loaded with cells (resuspended in a similar medium). The 2 compartments were separated by a 10 μm pore-sized polycarbonate PVPF coated with 50 μg/mL rat collagen type I (Collaborative Biomedical Products, Bedford, MA). Following 5 to 6 hours incubation at 37°C, the filter was removed, fixed, and stained. The effects of pertussis toxin (PTx) and of wortmannin on the migration of the cells were determined by pre-incubating the cells with the compounds for 2 hours and 1 hour, respectively, at 37°C, followed by washings. The migrated cells in 3 high-power fields (in each of the triplicates performed) were counted by light microscopy. The statistical significance of the number of cells migrating in response to stimuli vs to BSA medium was evaluated using Student t test.
Confocal analyses of receptor downmodulation
Aliquots of stable CXCR2-expressing HEK 293 cells were supplemented with 1000 ng/mL GCP-2, while no chemokines were added to control tubes. The cells were incubated at 37°C for the indicated time points, fixed in 4% paraformaldehyde, and centrifuged onto 0.5% gelatin-coated slides. From this stage on, the entire procedure was performed at room temperature. Cells were permeabilized in 0.2% triton X-100 for 30 minutes, and blocking was performed for 1 hour in blocking buffer (PBS supplemented with 0.25% gelatin, 0.15% saponin, and 3% goat serum). Primary antibodies were added for 1.5 hours, after diluting them in washing buffer (PBS supplemented with 0.25% gelatin, 0.15% saponin, and 0.1% goat serum). The primary antibodies included polyclonal rabbit antibodies against human CXCR2 (0.5 μg/60 μL; Santa Cruz Biotechnology, Santa Cruz, CA) and monoclonal mouse antibodies against human rab11 (2 μg/60 μL; Transduction Laboratories, Lexington, KY). After rinsing the cells in washing buffer, incubations were performed for 1 hour with secondary antibodies: rhodamine-conjugated goat anti-rabbit IgG (3.5 μg/60 μL) and FITC-conjugated goat anti-mouse IgG (8 μg/60 μL) (both antibodies were purchased from Jackson ImmunoResearch Laboratories). No cross-reactivity of the rhodamine-conjugated goat antibodies with mouse anti-rab11 antibodies, or of the FITC-labeled goat antibodies with rabbit anti-CXCR2 antibodies, was distinguished. Following additional washings, stained cells were analyzed using a Zeiss confocal laser scanning microscope.
Results
Characteristics of GCP-2 induced downmodulation of CXCR1 and CXCR2
To determine the effect of GCP-2 on cell surface expression of CXCR1 and CXCR2, we first exposed CXCR1- and CXCR2-expressing HEK 293 cells to high concentrations of this chemokine for 2 hours at 37°C (high concentrations of ligands were previously shown to be required for optimal induction of receptor internalization31,32 35). The expression of CXCR1 and CXCR2 was determined by anti-CXCR1 or anti-CXCR2 specific antibodies, using FACS analysis. Similar experiments that were performed at 4°C indicated that the pre-incubation of the cells with chemokines did not prevent the antibodies from binding to cell surface–expressed CXCR1 and CXCR2 (data not shown).
As shown in Figure 1, 3000 ng/mL GCP-2 were required to potently downmodulate CXCR1 expression, at the level of 33.7 ± 0.9% (Figure 1A) (This value is the mean ± SD of 4 independent experiments). One thousand ng/mL GCP-2 did not induce a significant downmodulation of CXCR1 (data not shown). These results indicate that GCP-2 differs from IL-8 in its ability to induce reduction in cell surface expression of CXCR1, since IL-8 was previously shown to elicit 71.8 ± 5.3% downmodulation of CXCR1 expression in concentrations of 1100 ng/mL.35 (To rule out the possibility that GCP-2–induced low levels of CXCR1 internalization were due to an artifact, experiments with IL-8 were performed in parallel and on the same batch of cells, and indicated that IL-8 induced high levels of CXCR1 downmodulation.) However, GCP-2 proved to be a more potent inducer of CXCR1 internalization when compared to NAP-2, since high concentrations of NAP-2 (2000 ng/mL) did not induce any significant reduction in CXCR1 expression.35
GCP-2–induced downmodulation of CXCR1 and CXCR2 expression.
CXCR1- or CXCR2-transfected HEK 293 cells incubated with 3000 and 1000 ng/mL GCP-2, respectively, for 2 hours at 37°C. The cells were washed and stained with anti-CXCR1 or anti-CXCR2 specific antibodies, and subjected to fluorescence-activated cell sorting (FACS) analysis as described in “Materials and methods.” (A) CXCR1-expressing cells. (B) CXCR2-expressing cells. Counts indicate relative cell number; baseline, cells stained with cell sorter buffer (CSB) instead of antibodies to CXCR1 or CXCR2. A representative experiment of 4 to 5 performed is shown.
GCP-2–induced downmodulation of CXCR1 and CXCR2 expression.
CXCR1- or CXCR2-transfected HEK 293 cells incubated with 3000 and 1000 ng/mL GCP-2, respectively, for 2 hours at 37°C. The cells were washed and stained with anti-CXCR1 or anti-CXCR2 specific antibodies, and subjected to fluorescence-activated cell sorting (FACS) analysis as described in “Materials and methods.” (A) CXCR1-expressing cells. (B) CXCR2-expressing cells. Counts indicate relative cell number; baseline, cells stained with cell sorter buffer (CSB) instead of antibodies to CXCR1 or CXCR2. A representative experiment of 4 to 5 performed is shown.
Exposure of CXCR2-expressing cells to 1000 ng/mL GCP-2 resulted in 65 ± 2.3% downmodulation of CXCR2 cell surface expression (Figure 1B). (This value is the mean ± SD of 5 independent experiments.) In that respect, the downmodulation-inducing potencies of GCP-2 and IL-8 were similar since 1000 ng/mL of IL-8 induced 78.9 ± 2.4% downmodulation of CXCR2 expression.35 On the other hand, GCP-2 proved to be more potent than NAP-2 in induction of CXCR2 downmodulation, because the latter chemokine induced only 37.4 ± 6.6% reduction in receptor expression on exposure to 2000 ng/mL.35
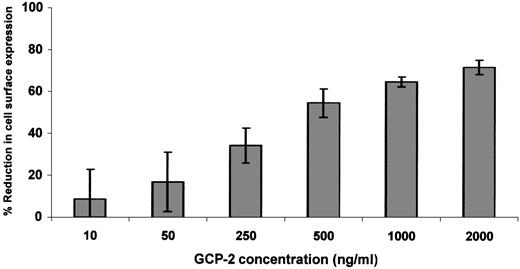
Since relatively high levels of receptor downmodulation were observed only in GCP-2–treated CXCR2-expressing cells, dose response and kinetics experiments were performed on GCP-2–induced downmodulation of CXCR2 only. Exposure of CXCR2-expressing cells to 50 ng/mL GCP-2 did not induce significant reduction in receptor expression (Figure2). At 250 ng/mL of GCP-2, moderate levels of CXCR2 downmodulation (34.5 ± 8.3%) were observed, and plateau levels were reached on exposure to 1000 to 2000 ng/mL of this chemokine (65 ± 2.3% and 72 ± 3.5%, respectively) (Figure 2). Further analysis of GCP-2–induced reduction in cell surface expression of CXCR2 indicated that on exposure to 1000 ng/mL GCP-2 for 5 minutes, 27 ± 6.1% downmodulation was observed, increasing to 55.6 ± 1.8% following 30 minutes' exposure, and reaching a plateau level following 90 minutes' exposure to GCP-2 (65.8 ± 4.4%).
Dose response of GCP-2–induced downmodulation of CXCR2.
CXCR2-expressing HEK 293 cells were incubated with various concentrations of GCP-2 for 2 hours at 37°C. The cells were washed and stained with anti-CXCR2–specific antibodies, and subjected to fluorescence-activated cell sorting (FACS) analysis as described in “Materials and methods.” Each value represents the mean ± SD of 5 independent experiments.
Dose response of GCP-2–induced downmodulation of CXCR2.
CXCR2-expressing HEK 293 cells were incubated with various concentrations of GCP-2 for 2 hours at 37°C. The cells were washed and stained with anti-CXCR2–specific antibodies, and subjected to fluorescence-activated cell sorting (FACS) analysis as described in “Materials and methods.” Each value represents the mean ± SD of 5 independent experiments.
Following determination of the ability of GCP-2 to induce CXCR1 and CXCR2 downmodulation, confocal analyses were performed to determine whether receptor downmodulation resulted from GCP-2–induced internalization of both receptors. These analyses indicated that exposure to high concentrations of GCP-2 induced internalization of CXCR2 (Figure 3C, D). As shown in Figure3A, the majority of the receptors in CXCR2-expressing cells that were not exposed to GCP-2 were localized on the cell surface. However, on exposure to 1000 ng/mL GCP-2 for 1 hour, the distribution of CXCR2 was different, as indicated by the fact that the majority of the receptor-specific staining was associated with a cytoplasmic granular localization (Figure 3C, D). Although GCP-2 induced only 33.7% reduction in CXCR1 cell surface expression (Figure 1), the process could be observed by confocal analysis in a moderate number of cells, and was characterized as receptor internalization (data not shown).
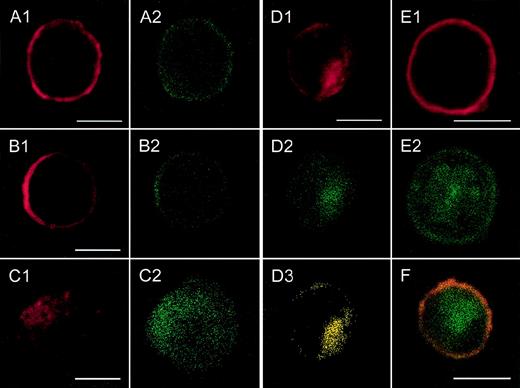
Localization of CXCR2 expression and of the expression of rab11+ endosomes by confocal analysis.
CXCR2-expressing HEK 293 cells were incubated with or without 1000 ng/mL GCP-2, at 37°C for 1, 5, and 60 minutes. The GCP-2–exposed cells were subdivided into 2 groups. One group of cells was washed and stained immediately after exposure to GCP-2 with anti-CXCR2 and anti-rab11–specific antibodies, as described in “Materials and methods.” The cells of the other group were washed and allowed to recover at 37°C for 90 minutes, in the presence or absence of 10 μg/mL cycloheximide. The cells were washed and stained with anti-CXCR2 and anti-rab11–specific antibodies, and subjected to confocal analysis as described in “Materials and methods.” In all the pictures shown, the red color represents the expression of CXCR2, as distinguished by the staining with rabbit antibodies against CXCR2, followed by rhodamine-conjugated antibodies against rabbit IgG. The green color, in all the pictures shown, represents the expression of rab11, as distinguished by the staining with mouse antibodies against rab11, followed by fluorescein isothiocipnate (FITC)-conjugated antibodies against mouse IgG. The yellow color indicates the colocalization of CXCR2 with rab 11 expression. (A) The expression of CXCR2 and rab11 prior to exposure to GCP-2. (B, C, D) The localization of CXCR2 and rab11 expression following the exposure of the cells to GCP-2 for 1, 5, and 60 minutes, respectively. (E) The expression of CXCR2 and rab11 after induction of internalization and recovery at 37°C for 1.5 hours in the absence of cycloheximide. (F) The same as in (E), in the presence of cycloheximide. Reference bars in the lower right corners of selected sections represent 10 μm. A representative experiment of 2 to 4 performed is shown.
Localization of CXCR2 expression and of the expression of rab11+ endosomes by confocal analysis.
CXCR2-expressing HEK 293 cells were incubated with or without 1000 ng/mL GCP-2, at 37°C for 1, 5, and 60 minutes. The GCP-2–exposed cells were subdivided into 2 groups. One group of cells was washed and stained immediately after exposure to GCP-2 with anti-CXCR2 and anti-rab11–specific antibodies, as described in “Materials and methods.” The cells of the other group were washed and allowed to recover at 37°C for 90 minutes, in the presence or absence of 10 μg/mL cycloheximide. The cells were washed and stained with anti-CXCR2 and anti-rab11–specific antibodies, and subjected to confocal analysis as described in “Materials and methods.” In all the pictures shown, the red color represents the expression of CXCR2, as distinguished by the staining with rabbit antibodies against CXCR2, followed by rhodamine-conjugated antibodies against rabbit IgG. The green color, in all the pictures shown, represents the expression of rab11, as distinguished by the staining with mouse antibodies against rab11, followed by fluorescein isothiocipnate (FITC)-conjugated antibodies against mouse IgG. The yellow color indicates the colocalization of CXCR2 with rab 11 expression. (A) The expression of CXCR2 and rab11 prior to exposure to GCP-2. (B, C, D) The localization of CXCR2 and rab11 expression following the exposure of the cells to GCP-2 for 1, 5, and 60 minutes, respectively. (E) The expression of CXCR2 and rab11 after induction of internalization and recovery at 37°C for 1.5 hours in the absence of cycloheximide. (F) The same as in (E), in the presence of cycloheximide. Reference bars in the lower right corners of selected sections represent 10 μm. A representative experiment of 2 to 4 performed is shown.
A functional hierarchy exists between IL-8, GCP-2, and NAP-2, as manifested by their abilities to induce receptor internalization
Previous studies on the interactions of IL-8 and NAP-2 with CXCR2 have indicated that the 2 chemokines have similar affinities to CXCR2 and that IL-8 very potently displaced NAP-2, as well as binding to this receptor. However, despite their similarly high affinities for CXCR2, NAP-2 could not effectively block IL-8 binding to this receptor.22,23 The different displacement abilities of IL-8 and NAP-2 were shown to be functionally relevant, as indicated by the ability of IL-8 to functionally dominate the ability of NAP-2 to induce CXCR2 internalization.35 However, the entire functional hierarchical relationships between the 3 closely related ELR+-CXC chemokines, IL-8, GCP-2, and NAP-2, was not yet established.
To further characterize the functional relationships between IL-8, GCP-2, and NAP-2, we have extended our study to determine the hierarchy between the 3 chemokines with regard to their ability to induce receptor internalization. To this end, 2 approaches were taken: (1) Based on the ability of IL-8 to induce higher CXCR1 internalization than GCP-2 (Figure 1A and Feniger-Barish et al35), the ability of IL-8 to functionally compete with or displace GCP-2 was determined using CXCR1-expressing cells exposed to GCP-2. (2) Based on the ability of GCP-2 to induce higher CXCR2 internalization as compared to NAP-2 (Figure 1B and Feniger-Barish et al35), the potential of GCP-2 to functionally compete with or displace NAP-2 was determined by its ability to induce CXCR2 internalization in CXCR2-expressing cells that were exposed to NAP-2.
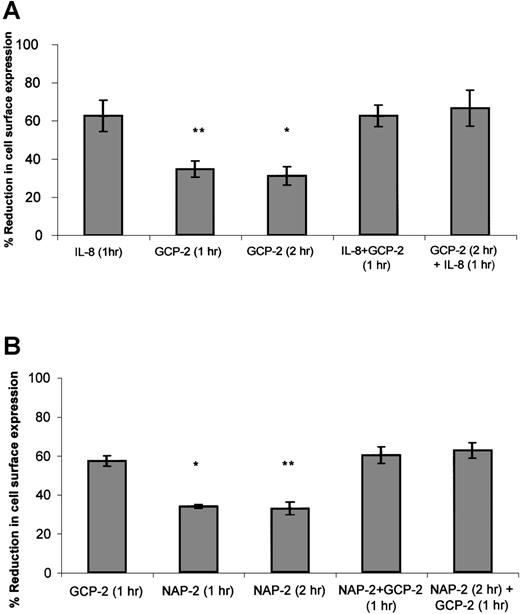
As suggested in approach no. 1 above, to determine the functional hierarchy between IL-8 and GCP-2, CXCR1-expressing cells were exposed to 2 different procedures. In the first, the ability of IL-8 to compete with GCP-2 was assayed by the exposure of the cells to concomitant stimulation with both IL-8 and GCP-2. In the second, the ability of IL-8 to displace GCP-2 was evaluated by exposing the cells to GCP-2, followed by their treatment with IL-8. As shown in Figure4A, exposure of the cells to IL-8 in the presence, or following the administration of GCP-2, resulted in the induction of potent internalization of CXCR1 that was significantly higher than GCP-2–induced internalization (P ≤ .001), and similar to internalization levels induced by IL-8 alone, suggesting the functional dominance of IL-8 over GCP-2.
The functional hierarchy between IL-8, GCP-2, and NAP-2, as manifested by their abilities to induce CXCR1 or CXCR2 internalization.
(A) IL-8 functionally competes for and displaces GCP-2–induced internalization of CXCR1 expression. CXCR1-transfected HEK 293 cells were incubated with IL-8 (1000 ng/mL) or GCP-2 (3000 ng/mL) for the indicated time at 37°C. GCP-2 + IL-8 (1 hour) indicates a concomitant exposure to both chemokines for 1 hour. GCP-2 (2 hours) + IL-8 (1 hour) indicates an exposure to GCP-2 for 2 hours, to which IL-8 was added during the second hour of incubation with GCP-2. The cells were washed and stained with anti-CXCR1–specific antibodies, and subjected to fluorescence-activated cell sorting (FACS) analysis as described in “Materials and methods.” Each value represents the mean ± SD of 3 independent experiments. *P = .001 for GCP-2 exposure for 2 hours vs the GCP-2 (2 hours) + IL-8 (1 hour) treatment. **P < .001 for GCP-2 exposure for 1 hour vs the GCP-2 + IL-8 (1 hour) treatment. (B) GCP-2 functionally competes for and displaces NAP-2–induced downmodulation of CXCR2 expression. CXCR2-transfected HEK 293 cells were incubated with GCP-2 (1000 ng/mL) or NAP-2 (2000 ng/mL) for the indicated time at 37°C. NAP-2 + GCP-2 (1 hour) indicates a concomitant exposure to both chemokines for 1 hour. NAP-2 (2 hours) + GCP-2 (1 hour) indicates an exposure to NAP-2 for 2 hours, to which GCP-2 was added during the second hour of incubation with NAP-2. The cells were washed and stained with anti-CXCR2–specific antibodies, and subjected to FACS analysis as described in “Materials and methods.” Each value represents the mean ± SD of 3 independent experiments. * P = .01 for GCP-2 exposure for 1 hour vs the NAP-2 + GCP-2 (1 hour) treatment. **P = .006 for NAP-2 exposure for 2 hours vs the NAP-2 (2 hours) + GCP-2 (1 hour) treatment.
The functional hierarchy between IL-8, GCP-2, and NAP-2, as manifested by their abilities to induce CXCR1 or CXCR2 internalization.
(A) IL-8 functionally competes for and displaces GCP-2–induced internalization of CXCR1 expression. CXCR1-transfected HEK 293 cells were incubated with IL-8 (1000 ng/mL) or GCP-2 (3000 ng/mL) for the indicated time at 37°C. GCP-2 + IL-8 (1 hour) indicates a concomitant exposure to both chemokines for 1 hour. GCP-2 (2 hours) + IL-8 (1 hour) indicates an exposure to GCP-2 for 2 hours, to which IL-8 was added during the second hour of incubation with GCP-2. The cells were washed and stained with anti-CXCR1–specific antibodies, and subjected to fluorescence-activated cell sorting (FACS) analysis as described in “Materials and methods.” Each value represents the mean ± SD of 3 independent experiments. *P = .001 for GCP-2 exposure for 2 hours vs the GCP-2 (2 hours) + IL-8 (1 hour) treatment. **P < .001 for GCP-2 exposure for 1 hour vs the GCP-2 + IL-8 (1 hour) treatment. (B) GCP-2 functionally competes for and displaces NAP-2–induced downmodulation of CXCR2 expression. CXCR2-transfected HEK 293 cells were incubated with GCP-2 (1000 ng/mL) or NAP-2 (2000 ng/mL) for the indicated time at 37°C. NAP-2 + GCP-2 (1 hour) indicates a concomitant exposure to both chemokines for 1 hour. NAP-2 (2 hours) + GCP-2 (1 hour) indicates an exposure to NAP-2 for 2 hours, to which GCP-2 was added during the second hour of incubation with NAP-2. The cells were washed and stained with anti-CXCR2–specific antibodies, and subjected to FACS analysis as described in “Materials and methods.” Each value represents the mean ± SD of 3 independent experiments. * P = .01 for GCP-2 exposure for 1 hour vs the NAP-2 + GCP-2 (1 hour) treatment. **P = .006 for NAP-2 exposure for 2 hours vs the NAP-2 (2 hours) + GCP-2 (1 hour) treatment.
When similar experiments were performed on CXCR2-expressing cells (Figure 4B), determining the ability of GCP-2 to compete with and displace NAP-2 (as suggested in approach no. 2 above), it was observed that exposure of the cells to GCP-2, in the presence or following the administration of NAP-2, resulted in the ability of GCP-2 to induce the same levels of internalization that were observed without the prior exposure to NAP-2, and induce levels significantly higher than those induced by NAP-2 alone (P = .01 and P = .006 depending on the assay conditions; see the legend to Figure 4B). These results demonstrate that GCP-2 is functionally dominant over NAP-2. In addition, although both GCP-2 and IL-8 induced high levels of CXCR2 internalization, the somewhat higher levels of CXCR2 internalization induced by IL-8 as compared to GCP-2 allowed us to analyze the hierarchy between the 2 chemokines, indicating that IL-8 is functionally dominant over GCP-2 in the context of CXCR2 (data not shown). Therefore, our findings suggest a functional hierarchy, according to which IL-8 > GCP-2 > NAP-2 in their ability to induce receptor internalization.
The role of G protein coupling and of signaling events in GCP-2–induced internalization of CXCR2
Analysis of the dose response curve of GCP-2–induced internalization of CXCR2 (Figure 2) indicated that potent internalization of CXCR2 was induced by high (>1000 ng/mL) and partially desensitizing concentrations of the chemokine (38.2 ± 7.2% of chemotactic desensitization was induced by such concentrations of GCP-2; data not shown and Wolf et al26). On the other hand, significantly lower levels of internalization were induced by activating doses of GCP-2 (ie, 100 ng/mL). Activation was determined by chemotaxis assays (Table 1, Wuyts et al,25 and Wolf et al26) (Figure 2). This observation is in agreement with other studies,40-43suggesting the significant interdependence between GPCR desensitization and internalization. These findings raise the possibility that GCP-2–induced G protein coupling to the receptors, and/or the resulting intracellular signaling events, should be partially turned off to allow for potent and maximal internalization processes to take place.
Inhibition of GCP-2–induced migration of CXCR2-expressing cells by pertussis toxin
| . | −PTx . | +PTx . |
|---|---|---|
| Control | 1.33 ± 0.23 | 0.47 ± 0.23 |
| GCP-2 100 ng/mL | 142 ± 20.4 | 1.2 ± 0.72 |
| . | −PTx . | +PTx . |
|---|---|---|
| Control | 1.33 ± 0.23 | 0.47 ± 0.23 |
| GCP-2 100 ng/mL | 142 ± 20.4 | 1.2 ± 0.72 |
CXCR2-transfected HEK 293 cells were incubated with 100 ng/ml Pertussis toxin for 2 hours, washed and subjected to chemotaxis assays in response to 100 ng/ml GCP-2, as described in “Materials and methods.” The mean ± SD of a representative experiment of 3 performed is shown. P < .001 for migration after the treatment, vs without treatment, by pertussis toxin.
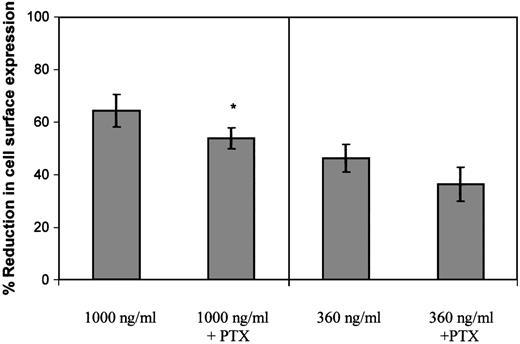
To determine the role of G protein coupling in the regulation of GCP-2–induced internalization of CXCR2, CXCR2-expressing cells were exposed to PTx. PTx was shown to efficiently modify members of the Gαi subclass of G proteins, which were shown to be key mediators of IL-8–, as well as of GCP-2–induced migration of neutrophils.24,44 45 First, the ability of the toxin to uncouple the binding of Gαi to CXCR2 was determined, as manifested by its ability to inhibit GCP-2–induced migration of CXCR2-expressing cells. Different concentrations of PTx, in the range of 100 to 1000 ng/mL, were assayed for their inhibitory potential, and all proved to abolish GCP-2–induced chemotactic responses. As shown in Table 1, 100 ng/mL PTx completely inhibited GCP-2–induced migration of CXCR2-expressing cells (P < .001), indicating that in our system this concentration of PTx was a potent inhibitor of GCP-2–induced coupling of Gαi to CXCR2.
Following determination of the ability of PTx to inhibit Gαi coupling, the effect of 100 ng/mL PTx on GCP-2–induced CXCR2 internalization was evaluated. When CXCR2-expressing cells were exposed to 360 ng/mL GCP-2, a concentration that potently induced G protein coupling and effectively activated cellular migration (similar to that induced by 100 ng/mL; data not shown and Wolf et al26), PTx did not significantly affect the level of CXCR2 internalization (P > .05; Figure5). However, a minimal, but statistically significant inhibitory effect of the toxin was observed on CXCR2 internalization, when the cells were exposed to 1000 ng/ml GCP-2 (Figure 5; reduction of 16.3%; P < .002), a concentration that partly desensitized migration and induced optimal internalization of the receptor. Therefore, our data suggest that the actual event of Gαi protein coupling has only a minor role, if at all, in the regulation of GCP-2–induced internalization of CXCR2.
The role of G protein coupling in the regulation of GCP-2–induced CXCR2 internalization.
CXCR2-expressing HEK 293 cells were incubated with 360 ng/mL or 1000 ng/mL GCP-2 for 2 hours at 37°C. Prior to (for 2 hours) and during the exposure to GCP-2, the cells were treated with 100 ng/mL pertussis toxin (PTx) at 37°C. The cells were washed and stained with anti-CXCR2–specific antibodies, and subjected to fluorescence activated cell sorting (FACS) analysis as described in “Materials and methods.” Each value represents the mean ± SD of over 4 independent experiments. * P < .002 for internalization induced by 1000 ng/mL GCP-2 in the absence of, vs in the presence of, pertussis toxin (PTx).
The role of G protein coupling in the regulation of GCP-2–induced CXCR2 internalization.
CXCR2-expressing HEK 293 cells were incubated with 360 ng/mL or 1000 ng/mL GCP-2 for 2 hours at 37°C. Prior to (for 2 hours) and during the exposure to GCP-2, the cells were treated with 100 ng/mL pertussis toxin (PTx) at 37°C. The cells were washed and stained with anti-CXCR2–specific antibodies, and subjected to fluorescence activated cell sorting (FACS) analysis as described in “Materials and methods.” Each value represents the mean ± SD of over 4 independent experiments. * P < .002 for internalization induced by 1000 ng/mL GCP-2 in the absence of, vs in the presence of, pertussis toxin (PTx).
PTx, as a potent inhibitor of Gαi coupling to CXCR2, blocks the initiation of Gαi-dependent signaling events in response to GCP-2 stimulation. The fact that the toxin had only a minimal effect on GCP-2–induced internalization of CXCR2 does not exclude the possibility that signaling events, once initiated, regulate the internalization process. To examine this possibility, the effect of wortmannin on GCP-2–induced internalization of CXCR2 was evaluated. Wortmannin is a potent inhibitor of phosphatidylinositol (PI) 3 kinases and PI4 kinases, major mediators of IL-8–induced signaling.46 47 First, the ability of wortmannin to inhibit signals that are required for GCP-2–induced migration was determined. The results shown in Figure 6A indicate that wortmannin induced a dose-dependent and significant reduction in GCP-2–induced migratory responses of CXCR2-expressing cells (reduction of 53 ± 17.4% in migration by 1000 nmol/L wortmannin. This value is the mean ± SD of 4 independent experiments;P < .01). Although potent and significant, the inhibitory effect of wortmannin on GCP-2–induced migration of CXCR2-expressing cells was partial, as expected from the possible participation of other signaling events, except for PI3 and PI4 kinases, in this process.
The role of signaling events in the regulation of GCP-2–induced CXCR2 internalization.
(A) Inhibition of GCP-2–induced chemotaxis of CXCR2-expressing HEK 293 cells by wortmannin. The cells were treated with 10, 100, or 1000 nmol/L wortmannin for 1 hour at 37°C, and washed and subjected to a chemotaxis assay in response to 100 ng/mL GCP-2, as described in “Materials and methods.” A representative experiment of 4 performed is shown. *P < .05, **P < .01 for the response after the treatment, vs without treatment, by wortmannin. (B) Enhancement of GCP-2–induced internalization of CXCR2-transfected HEK 293 cells by wortmannin. CXCR2-transfected cells were incubated with 360 ng/mL GCP-2 for 2 hours at 37°C. Prior to (for 1 hour) and during the exposure to GCP-2, the cells were treated with 10, 100, or 1000 nmol/L wortmannin, washed, stained with anti-CXCR2-specific antibodies, and subjected to fluorescence-activated cell sorting (FACS) analysis, as described in “Materials and methods.” Each value represents the mean ± SD of 4 to 6 independent experiments. ***P < .001 for the response in the presence, vs the response in the absence of wortmannin.
The role of signaling events in the regulation of GCP-2–induced CXCR2 internalization.
(A) Inhibition of GCP-2–induced chemotaxis of CXCR2-expressing HEK 293 cells by wortmannin. The cells were treated with 10, 100, or 1000 nmol/L wortmannin for 1 hour at 37°C, and washed and subjected to a chemotaxis assay in response to 100 ng/mL GCP-2, as described in “Materials and methods.” A representative experiment of 4 performed is shown. *P < .05, **P < .01 for the response after the treatment, vs without treatment, by wortmannin. (B) Enhancement of GCP-2–induced internalization of CXCR2-transfected HEK 293 cells by wortmannin. CXCR2-transfected cells were incubated with 360 ng/mL GCP-2 for 2 hours at 37°C. Prior to (for 1 hour) and during the exposure to GCP-2, the cells were treated with 10, 100, or 1000 nmol/L wortmannin, washed, stained with anti-CXCR2-specific antibodies, and subjected to fluorescence-activated cell sorting (FACS) analysis, as described in “Materials and methods.” Each value represents the mean ± SD of 4 to 6 independent experiments. ***P < .001 for the response in the presence, vs the response in the absence of wortmannin.
Similar doses of wortmannin were analyzed for the regulation of GCP-2-induced internalization of CXCR2. Internalization was induced by concentrations of GCP-2 that provoked potent activation of migration (360 ng/mL), as well as by doses that partially desensitized GCP-2–induced chemotactic responses (1000 ng/mL). As shown in Figure6B, when internalization was induced by 360 ng/mL GCP-2, a notable increase, of 49 ± 13%, in receptor internalization was induced by 1000 nmol/L wortmannin (This value is the mean ± SD of 4 independent experiments; P < .001). The dose response of wortmannin-mediated inhibition of chemotaxis was in full agreement with that of wortmannin-induced enhancement of CXCR2 internalization. The potent, but partial, increase in CXCR2 internalization that was induced following the treatment with wortmannin may be the result of the only partial inhibition of GCP-2–induced migratory responses that was induced by this compound (Figure 6A). Similar stimulating effects of wortmannin on CXCR2 internalization were observed when receptor downmodulation was induced by 1000 ng/mL GCP-2 (data not shown). These results suggest that PI3/PI4 kinase-mediated signaling events, once initiated following receptor activation, are effective regulators of the internalization process.
Determination of mechanisms regulating CXCR2 re-expression on the cell surface
Studies of neutrophils, as well as our previous findings on CXCR1- and CXCR2-expressing HEK 293 cells, have shown that CXCR1 and CXCR2 are recycled back to the plasma membrane on free ligand (IL-8) removal and recovery of the cells at 37°C.35 37 To determine the mechanisms involved in such a process, we first analyzed by FACS the kinetics of re-expression of CXCR2 on GCP-2 removal, and recovery of the cells at 37°C. These experiments were performed in the presence or in the absence of cycloheximide, to determine whether receptor recycling or receptor de novo synthesis accounted for receptor re-expression on the plasma membrane. Receptor expression levels following ligand removal were compared to the levels of their expression in control cells in which GCP-2–induced CXCR2 internalization was not elicited.
As shown in Figure 7, following the removal of GCP-2 and recovery of the cells at 37°C, CXCR2 was re-expressed on the cell membrane. On incubation at 37°C for 90 minutes, CXCR2 expression was restored to 81.9 ± 5.3% of the control level in cells that had no exposure to GCP-2. Recovery of receptor expression to 92.1 ± 5.1% of control cells was observed following 4 hours of incubation. Treatment with cycloheximide did not affect the recovery of CXCR2 at 90 minutes (re-expression of 74.1 ± 5.2%;P = .14 when recovery in the absence of cycloheximide was compared to recovery in the presence of the compound), but significantly reduced the level of receptor re-expression when incubations were extended to 4 hours (re-expression of 62.7 ± 12.3%, P = .03). (All the above values are the mean ± SD of 3 independent experiments.)
Re-expression of CXCR2 on the cell membrane.
CXCR2-expressing HEK 293 cells were either not incubated with GCP-2 (control cells) or incubated with 1000 ng/mL GCP-2 at 37°C for 1 hour. The GCP-2–exposed cells were subdivided into 2 groups. One group of cells was washed and stained immediately after exposure to GCP-2 with anti-CXCR2–specific antibodies, as described in “Materials and methods.” The cells of the other group were washed and allowed to recover at 37°C for various intervals, in the presence or absence of 10 μg/mL cycloheximide. The cells were washed and stained with anti-CXCR2-specific antibodies, and subjected to fluorescence-activated cell sorting (FACS) analysis as described in “Materials and methods.” No recovery indicates cells undergoing internalization and no recovery at 37°C; 90 minutes and 4 hours indicate the time of recovery at 37°C. Counts indicates relative cell number; and baseline, cells stained with cell sorter buffer instead of antibodies to CXCR2. A representative experiment of 3 performed is shown.
Re-expression of CXCR2 on the cell membrane.
CXCR2-expressing HEK 293 cells were either not incubated with GCP-2 (control cells) or incubated with 1000 ng/mL GCP-2 at 37°C for 1 hour. The GCP-2–exposed cells were subdivided into 2 groups. One group of cells was washed and stained immediately after exposure to GCP-2 with anti-CXCR2–specific antibodies, as described in “Materials and methods.” The cells of the other group were washed and allowed to recover at 37°C for various intervals, in the presence or absence of 10 μg/mL cycloheximide. The cells were washed and stained with anti-CXCR2-specific antibodies, and subjected to fluorescence-activated cell sorting (FACS) analysis as described in “Materials and methods.” No recovery indicates cells undergoing internalization and no recovery at 37°C; 90 minutes and 4 hours indicate the time of recovery at 37°C. Counts indicates relative cell number; and baseline, cells stained with cell sorter buffer instead of antibodies to CXCR2. A representative experiment of 3 performed is shown.
These results indicate that the membrane expression of CXCR2 is quickly recovered following GCP-2-induced internalization, and that cycloheximide affected the recovery of CXCR2 only at later time points subsequent to the internalization process. Therefore, “short-term” recovery of CXCR2 expression was mainly the result of receptor recycling.
To further investigate the regulation of receptor re-expression on the cell surface following GCP-2-induced internalization of CXCR2, we determined the endocytic machinery involved in the recycling of these receptors to the plasma membrane. The intracellular localization of CXCR2 was determined by confocal analysis in relation to the distribution of recycling endosomes, identified by antibodies directed against rab11 (a protein that regulates receptor trafficking through recycling endosomes and serves as a marker of early/recycling endosomes48-52). The localization of CXCR2 and of rab11+ endosomes was determined prior to and after the induction of receptor internalization, and subsequently after removal of the ligand and recovery of CXCR2-expressing cells at 37°C for 90 minutes (the time point at which receptor recycling accounted for receptor re-expression on the cell surface).
As shown in Figures 3A1 and 3A2, prior to GCP-2–induced CXCR2 internalization, CXCR2 was localized primarily on the cell membrane and showed notable colocalization with the expression of rab11+ endosomes. Internalization of CXCR2 was induced by the exposure of CXCR2-expressing cells to 1000 ng/mL GCP-2 for different time points, 1, 5, 15, and 60 minutes. At 1 minute following induction of internalization, the majority of CXCR2 and rab11 staining was still localized on the cell membrane (Figures 3B1 and 3B2). However, starting at 5 minutes after induction of CXCR2 internalization, the receptor could be distinguished in the cytoplasmic regions of the cell (Figure 3C1). The number of cells in which intracellular localization of the receptor could be observed was increased as the exposure time to GCP-2 was elongated. Analysis of CXCR2 internalization at the 5-, 15-, and 60-minute time points indicated that the internalization of the CXCR2 and its cytoplasmic localization were accompanied by the cytoplasmic expression and colocalization of rab11+ endosomes (Figures3C1-2, 3D1-3; the data for 15 minutes are not shown).
Following removal of the ligand, and recovery of the cells at 37°C for 90 minutes, the expression of CXCR2 on the plasma membrane was restored (Figure 3E1). However, at this stage, the distribution of rab11+ endosomes was primarily cytoplasmic and only in part membranous (Figure 3E2). The expression of CXCR2 on the cell membrane and the dissociation of its localization from that of rab11 were distinguished not only in the absence, but also in the presence of cycloheximide (Figure 3F). To conclude, the colocalization of CXCR2 with rab11+ endosomes prior to and after the induction of internalization by GCP-2 suggests that rab11+ endosomes are involved in CXCR2 intracellular trafficking.
Discussion
To gain insight into the coordinated activity of ELR+-CXC chemokines, and to determine the fine tuning of their ability to interact with their receptors, our study focused on the regulation of cell surface expression of CXCR1 and CXCR2 by GCP-2. Due to possible intracellular interactions between CXCR1 and CXCR2 following their interaction with ELR+-CXC chemokines, we utilized a well-characterized model system in which each of the receptors was solely expressed. This system was shown to highly resemble neutrophils, in terms of ligand binding affinity, activation, homologous desensitization, and internalization of CXCR1 and CXCR2.23,30,35 39
Our study provides evidence for the following novel findings:
(1) Although GCP-2 is considered as an effective ligand of both CXCR1 and CXCR2, the interaction of this chemokine with the 2 receptors is different. This is manifested by the low ability of GCP-2 to induce CXCR1 internalization, whereas a highly potent CXCR2 internalization is induced by this chemokine. These results are supported by previous observations, demonstrating that GCP-2 is a less potent activator of CXCR1 than of CXCR2.24-26 In contrast to GCP-2, IL-8 is a potent inducer of activation, as well as of internalization, of both CXCR1 and CXCR2.22,23,35,53 54 The divergent abilities of IL-8 and GCP-2 to induce chemotactic signals and receptor internalization may represent the existence of fine control mechanisms that regulate the migration of neutrophils to inflammatory sites.
(2) A distinct functional hierarchy exists between GCP-2 and 2 closely related ELR+-CXC chemokines, IL-8 and NAP-2, in their abilities to interact with CXCR1 and CXCR2, as manifested by their relative abilities to induce receptor internalization. Our previous results35 and the present study (Figure 4B) indicated that in the context of CXCR2, IL-8 and GCP-2 competed with and displaced NAP-2, suggesting that both IL-8 and GCP-2 are functionally dominant over NAP-2. Our results also indicated that IL-8 dominated the GCP-2–induced internalization of CXCR2. In addition, when the regulation of CXCR1 was studied, IL-8 proved to govern GCP-2–induced responses (Figure 4A). On the whole, a hierarchy between these chemokines emerges, and its dominance order is IL-8 > GCP-2 > NAP-2. Therefore, our results suggest that the interrelationships between these chemokines are highly regulated, and that on the concomitant exposure of neutrophils to these 3 chemokines, the potency of the response will be dictated primarily by IL-8. However, if conditions at a certain inflammatory site do not allow for IL-8 expression, GCP-2 will be the dominant chemokine, resulting in a potent interaction with IL-8 receptors, primarily with CXCR2 and evoking a sufficiently effective acute inflammatory response. Although dominated by the other 2 ELR+-CXC chemokines, NAP-2 may have a pathophysiological role, being a cleavage product of Connective Tissue Activating Peptide III that reaches high serum concentrations during injury.18
(3) Our findings are the first to suggest the contribution of signaling events to the regulation of CXCR2 internalization. The regulation of CXCR2 internalization by G protein coupling was shown to be clearly dissociated from the downstream signaling events that follow receptor activation. Whereas the actual event of Gαicoupling to CXCR2 did not have a major role in the regulation of its internalization, the subsequent signaling cascade that was induced by chemokine-receptor interactions had a noticeable contribution to the regulation of CXCR2 internalization. The ability of wortmannin to promote CXCR2 internalization was correlated with its ability to inhibit signaling events that give rise to chemotactic responses. These results suggest that once induced, potent activation of certain signaling components results in a partial decrement of internalization processes. Such a mechanism may explain our findings, as well as observations made by other researchers, demonstrating the tightly regulated inter-relationships between the processes of internalization and homologous desensitization of GPCR.40-43
Our observations on the enhancing effect of wortmannin on GCP-2-induced CXCR2 internalization is supported by similar findings on the internalization-stimulating activities of this compound with regard to receptors that are not G protein–coupled.55,56 The optimal internalization-promoting ability of wortmannin was induced by 1000 nmol/L of the compound. Such high concentrations of wortmannin are considered to preferentially inhibit PI4 kinases.55,57,58 In that respect, it is interesting to note that Ins-1,4,5-P3, and primarily PI(4,5)P2, have been implicated to be most potent inhibitors of the organization of AP-2 adaptin complexes.59 AP-2 adaptins are major participants in the internalization of receptors via clathrin-coated pits.60,61 Indeed, IL-8–induced internalization of CXCR2 was previously shown to be mediated by clathrin-coated structures.62 Therefore, our results suggest that the potent activation of CXCR2 by GCP-2 results in activation of PI3 kinases and PI4 kinases, whose products partially inhibit the self-association activity of AP-2, and therefore do not allow for maximal levels of clathrin-coated pits-mediated internalization of CXCR2 to occur. However, on exposure to desensitizing concentrations of GCP-2, the activation of these kinases is partially turned off, and the ability of their products to inhibit AP-2 organization is reduced, giving rise to maximal and potent internalization of CXCR2.
(4) This study is the first to suggest evidence for the nature of the endocytic machinery involved in the recycling of the chemokine receptor CXCR2. A significant membranous colocalization of CXCR2 and rab11 was identified before induction of internalization, followed by complete cytoplasmic colocalization of the 2 components after elicitation of receptor internalization. Since the majority of internalized CXCR2 is targeted for re-expression on the plasma membrane, our results suggest that rab11+ endosomes participate in the first stages of receptor trafficking to the cell surface. In agreement with this idea, rab11 was partially co-localized with the membranous CXCR2 expression following receptor recycling back to the plasma membrane. Therefore, the potential involvement of rab11+ endosomes in the recycling process of CXCR2 was demonstrated. However, the partial dissociation between rab11 expression and the localization of CXCR2 following re-expression of the receptor on the cell membrane suggests that additional endosomes, not expressing the rab11+phenotype, may also mediate the final stages of CXCR2 delivery to the cell surface.
Acknowledgments
The authors greatly appreciate the assistance of Dr Leonide Mittelman in the performance of confocal analysis.
Reprints:Adit Ben-Baruch, Department of Cell Research and Immunology, The George S. Wise Faculty of Life Sciences, Tel Aviv University, Tel Aviv 69978, Israel; e-mail: aabb@post.tau.ac.il.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal