Inhibition of the tissue factor pathway has been shown to attenuate the activation of coagulation and to prevent death in a gram-negative bacteremia primate model of sepsis. It has been suggested that tissue factor influences inflammatory cascades other than the coagulation system. The authors sought to determine the effects of 2 different doses of recombinant tissue factor pathway inhibitor (TFPI) on endotoxin-induced coagulant, fibrinolytic, and cytokine responses in healthy humans. Two groups, each consisting of 8 healthy men, were studied in a double-blind, randomized, placebo-controlled crossover study. Subjects were studied on 2 different occasions. They received a bolus intravenous injection of 4 ng/kg endotoxin, which was followed by a 6-hour continuous infusion of TFPI or placebo. Eight subjects received 0.05 mg/kg per hour TFPI after a bolus of 0.0125 mg/kg (low-dose group), and 8 subjects received 0.2 mg/kg per hour after a bolus of 0.05 mg/kg (high-dose group). Endotoxin injection induced the activation of coagulation, the activation and subsequent inhibition of fibrinolysis, and the release of proinflammatory and antiinflammatory cytokines. TFPI infusion induced a dose-dependent attenuation of thrombin generation, as measured by plasma F1 + 2 and thrombin–antithrombin complexes, with a complete blockade of coagulation activation after high-dose TFPI. Endotoxin-induced changes in the fibrinolytic system and cytokine levels were not altered by either low-dose or high-dose TFPI. The authors concluded that TFPI effectively and dose-dependently attenuates the endotoxin-induced coagulation activation in humans without influencing the fibrinolytic and cytokine response.
Disseminated intravascular coagulation (DIC) is a frequent complication of severe infection and, in patients with septic shock, a strong predictor of death.1 A pivotal mechanism in the pathogenesis of DIC is the activation of the (extrinsic) tissue factor/factor VIIa-dependent pathway of coagulation.2 Under physiological conditions, tissue factor (TF) cannot be detected on the luminal surface of the vascular endothelium,3 and it can be detected only in low quantities on circulating blood cells.4-6 However, during infection and after stimulation with endotoxin or tumor necrosis factor, TF can be induced rapidly on blood mononuclear cells4,7,8 and on vascular endothelium.9-11
Evidence for the role of TF/factor VIIa in activation of the coagulation system is derived from studies in primates showing that the coagulant response during bacteremia or endotoxemia could be completely blocked by monoclonal antibodies to TF12,13 or factor VIIa,14 by active site-inhibited factor VIIa,15and by infusion of the tissue factor pathway inhibitor (TFPI).16,17 Blockade of the TF-driven pathway of coagulation by TFPI16,17 or antibodies to TF13not only resulted in decreased activation of the coagulation system but also in the prevention of death. It is unlikely that inhibition of the TF pathway reduced mortality during severe bacteremia merely by preventing DIC.18 Indeed, in baboons, an alternative method of blocking the generation of thrombin by the administration of active-site blocked factor Xa did not protect against organ failure and death after Escherichia coli-induced sepsis.19 It has been suggested that TF may modulate the inflammatory response by a mechanism other than the initiation of blood coagulation.20In accordance with this hypothesis are findings that inhibiting the activity of the TF/VIIa pathway reduced the release of interleukin 6 (IL-6) and IL-8 during severe bacteremia.15 16
TFPI is a natural anticoagulant that acts by direct factor Xa inhibition and, in a factor Xa-dependent manner, by feedback inhibition of the TF/VIIa complex.21 In animal sepsis models, TFPI was able to block the coagulant response completely and to prevent death while reducing the cytokine response.16,17,22 23 Knowledge of the effect of TFPI in humans is limited. Therefore, in the current study, we used the well-characterized human model of endotoxemia to determine the effect of TFPI, given as a 6-hour infusion in 1 of 2 doses, on coagulant, fibrinolytic, and cytokine responses.
Methods
Study design
The study was performed as a randomized, double-blind, placebo-controlled crossover experiment. Written informed consent was obtained from each subject before the start of the study, and the study was approved by the institutional scientific and ethics committees. Sixteen healthy men (aged 19–29 years) volunteered to participate in the study. None had abnormalities on physical examination or routine laboratory investigation. Results of tests for hepatitis B, hepatitis C, and human immunodeficiency virus were negative. The subjects did not take any medication and did not smoke or use illicit drugs. Each was studied on two occasions 6 weeks apart. Two doses of TFPI were administered. Eight subjects were examined after the administration of endotoxin and low-dose TFPI/placebo, and 8 subjects were examined after the administration of endotoxin and high-dose TFPI/placebo. All subjects fasted overnight before endotoxin administration. At 7 am two intravenous cannulas were inserted, one for endotoxin administration and blood collection and the other for the infusion of TFPI or placebo. Endotoxin (E. colilipopolysaccharide, lot G; US Pharmacopeia, Rockville, MD) was administered at 9 am as a bolus intravenous injection at a dose of 4 ng/kg body weight. Recombinant human TFPI/SC-59 735 (Chiron, Emeryville, CA) was given immediately after the endotoxin injection as a bolus of 0.0125 mg/kg body weight. This was followed by a continuous 6-hour infusion of 0.05 mg/kg per hour (low-dose group) or as a bolus of 0.05 mg/kg body weight and then by a continuous 6-hour infusion of 0.2 mg/kg per hour (high-dose group). In the control experiments, the same solution used for diluting TFPI was given as placebo. Oral temperature, blood pressure, heart rate, and oxygen saturation were measured at half-hour intervals (Dinamap1846 SX; Critikon, Tampa, FL). Clinical symptoms such as headache, shivers, nausea, vomiting, tiredness, and malaise were recorded throughout the study periods using a graded scale (0, absent; 1, weak; 2, moderate; 3, severe).
Blood collection
Blood was obtained from an intravenous cannula at 20 minutes before endotoxin administration and at 0.5, 1, 1.5, 2, 3, 4, 5, 6, 8, 12, and 24 hours thereafter. Blood for coagulation and fibrinolysis assays was collected in siliconized Vacutainer tubes (Becton Dickinson, Plymouth, UK) containing 0.105 mol/L sodium citrate; the ratio of anticoagulant to blood was 1:9 (vol/vol). Blood for cytokine assays and leukocytes was collected in K3-EDTA–containing tubes. Leukocyte counts and differentials were assessed by a Stekker analyzer (STKS Hematology Flow Cytometer, Beckman Coulter, Buckinghamshire, UK). All blood samples, except those for the determination of leukocyte counts and differentials, were centrifuged at 3000 rpm for 15 minutes at 4°C, and plasma was stored at −20°C until assays were performed.
Assays
Plasma levels of TFPI were measured in a validated sandwich immunoassay. The assay uses a monoclonal antibody directed against the first Kunitz domain of TFPI for capture and a fluorescein-labeled polyclonal antibody to TFPI for detection. These antibodies also recognize endogenous native human TFPI. All samples were assayed in triplicate. The lower limit of quantitation was 40 ng/mL. Prothrombin time (PT) and activated partial thromboplastin time (aPTT) were measured by 1-stage clotting assays with thromboplastin PT–fibrinogen and thromboplastin APTT–SP, respectively, on an ACL 7000 analyzer (Instrumentation Laboratory, Lexington, MA). The plasma concentrations of prothrombin fragment F1 + 2 and thrombin–antithrombin complexes (TATc) were measured by enzyme-linked immunosorbent assay (ELISA; Beringwerke AG, Marburg, Germany). Tissue-type plasminogen activator (tPA) antigen and plasminogen activator inhibitor type 1 (PAI-1) antigen were assayed by ELISA (Asserachrom tPA; Diagnostica Stago, Asnieres-sur-Seine, France; and PAI-ELISA kit; Monozyme, Charlottenlund, Denmark). Plasmin-a2-antiplasmin complexes (PAPc) complexes were measured by ELISA (Enzygnost PAP micro; Behring Diagnostics GmbH, Marburg, Germany). Tumor necrosis factor (TNF), IL-6, and IL-10 were measured by ELISA according to the manufacturer's instructions (Central Laboratory of the Netherlands Red Cross Blood Transfusion Service, Amsterdam, The Netherlands). Soluble TNF receptor type 1 was measured by an enzyme-linked immunobound assay produced by Hoffmann La Roche (Basel, Switzerland) as described previously.24
Statistical analysis
Values are given as means ± SEM. Differences in results between the TFPI and control experiments were tested by repeated measurement analysis of variance. Changes in time within a group were analyzed by one-way analysis of variance. P < .05 was considered significant.
Results
TFPI plasma concentrations
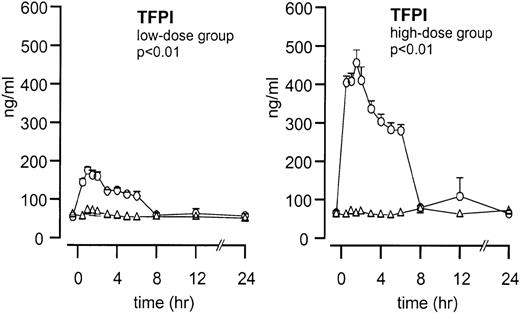
Endogenous TFPI plasma concentrations did not increase after endotoxin administration (Figure 1). After TFPI infusion, peak plasma concentrations increased from 54 ± 4 to 175 ± 8 ng/mL (P < .01; TFPI vs placebo) in the low-dose group and from 65 ± 6 to 456 ± 34 ng/ml in the high-dose group (P < .01; TFPI vs placebo).
Mean ± SEM plasma TFPI concentrations after endotoxin administration and infusion of TFPI or placebo.
Endotoxin administration was given as a bolus injection (4 ng/kg) at t = 0. Infusion of TFPI (○) and placebo (▵) started at t = 0 and was continued until t = 6 hours. P values indicate the differences in results of TFPI and placebo experiments.
Mean ± SEM plasma TFPI concentrations after endotoxin administration and infusion of TFPI or placebo.
Endotoxin administration was given as a bolus injection (4 ng/kg) at t = 0. Infusion of TFPI (○) and placebo (▵) started at t = 0 and was continued until t = 6 hours. P values indicate the differences in results of TFPI and placebo experiments.
Clinical features and hematologic responses
Injection of endotoxin induced a febrile response, peaking after 3.5 hours, together with tachycardia and transient flu-like symptoms, including headache, nausea, malaise, and chills. In addition, endotoxin administration resulted in a biphasic change in white blood cell counts, characterized by initial leukopenia followed by leukocytosis. None of these changes were influenced by TFPI (Table1 and data not shown). No adverse events attributable to TFPI infusion were observed, and no episodes of increased bleeding occurred.
Clinical features and hematologic responses
| . | Low-Dose Group . | High-Dose Group . | ||||
|---|---|---|---|---|---|---|
| TFPI . | Placebo . | P . | TFPI . | Placebo . | P . | |
| Peak temperature (°C) | 38.6 ± 0.2 | 38.8 ± 0.2 | NS | 38.3 ± 0.2 | 38.2 ± 0.2 | NS |
| Time to temperature peak (h) | 3.6 ± 0.2 | 3.6 ± 0.2 | NS | 3.9 ± 0.2 | 3.9 ± 0.4 | NS |
| Heart rate (peak, bpm) | 107 ± 3.9 | 110 ± 3.0 | NS | 102 ± 6.4 | 99 ± 3.0 | NS |
| Systolic blood pressure (nadir, mm Hg) | 103 ± 5.1 | 104 ± 2.0 | NS | 105 ± 2.7 | 106 ± 2.9 | NS |
| White blood count (×109/L) | ||||||
| Nadir | 2.5 ± 0.1 | 2.1 ± 0.2 | NS | 2.0 ± 0.2 | 1.9 ± 0.1 | NS |
| Peak | 14.1 ± 0.7 | 14.6 ± 1.4 | NS | 13.9 ± 0.8 | 13.1 ± 1.3 | NS |
| . | Low-Dose Group . | High-Dose Group . | ||||
|---|---|---|---|---|---|---|
| TFPI . | Placebo . | P . | TFPI . | Placebo . | P . | |
| Peak temperature (°C) | 38.6 ± 0.2 | 38.8 ± 0.2 | NS | 38.3 ± 0.2 | 38.2 ± 0.2 | NS |
| Time to temperature peak (h) | 3.6 ± 0.2 | 3.6 ± 0.2 | NS | 3.9 ± 0.2 | 3.9 ± 0.4 | NS |
| Heart rate (peak, bpm) | 107 ± 3.9 | 110 ± 3.0 | NS | 102 ± 6.4 | 99 ± 3.0 | NS |
| Systolic blood pressure (nadir, mm Hg) | 103 ± 5.1 | 104 ± 2.0 | NS | 105 ± 2.7 | 106 ± 2.9 | NS |
| White blood count (×109/L) | ||||||
| Nadir | 2.5 ± 0.1 | 2.1 ± 0.2 | NS | 2.0 ± 0.2 | 1.9 ± 0.1 | NS |
| Peak | 14.1 ± 0.7 | 14.6 ± 1.4 | NS | 13.9 ± 0.8 | 13.1 ± 1.3 | NS |
Activation of the coagulation system
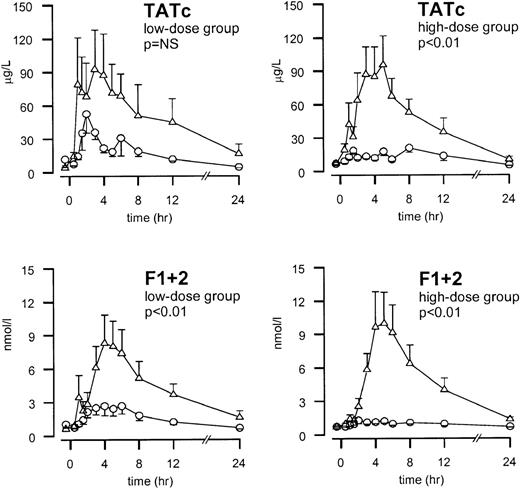
Administration of endotoxin resulted in the activation of thrombin generation, as reflected by increases in the plasma levels of the prothrombin fragment F1 + 2 and TATc (P = .001; Figure2). The endotoxin-induced increase in F1 + 2 was diminished by low-dose TFPI (peak values 2.69 ± 0.73 and 8.31 ± 2.54 nmol/L for TFPI and placebo, respectively,P < .01). It was completely abolished by high-dose TFPI (peak value 1.29 ± 0.30 and 9.95 ± 2.83 nmol/L for TFPI and placebo, respectively, P < .01). The endotoxin-induced increase in TATc was almost completely prevented by high-dose TFPI (peak values, 17.9 ± 3.9 vs 95.6 ± 30.2 μg/L;P < .01). Low-dose TFPI also decreased TATc formation, but this decrease did not reach statistical significance (peak values, 52.6 ± 17.2 and 92.6 ± 35.3 μg/L for TFPI and placebo, respectively; P = .19).
TFPI dose-dependently inhibits coagulation activation.
Mean ± SEM plasma concentrations of thrombin-antithrombin (TAT) complexes and prothrombin fragment F1 + 2 after endotoxin administration and infusion of TFPI (○) or placebo (▵). Endotoxin (4 ng/kg) was given as a bolus injection at t = 0. Infusion of TFPI started at t = 0 and was continued until t = 6 hours. Pvalues indicate the differences in results of TFPI and placebo experiments.
TFPI dose-dependently inhibits coagulation activation.
Mean ± SEM plasma concentrations of thrombin-antithrombin (TAT) complexes and prothrombin fragment F1 + 2 after endotoxin administration and infusion of TFPI (○) or placebo (▵). Endotoxin (4 ng/kg) was given as a bolus injection at t = 0. Infusion of TFPI started at t = 0 and was continued until t = 6 hours. Pvalues indicate the differences in results of TFPI and placebo experiments.
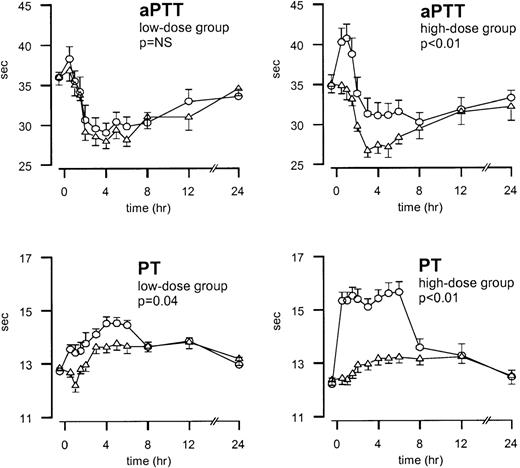
After endotoxin injection, APTT values decreased and reached a nadir after 3 hours (P < .01; Figure3). Initially, TFPI increased the aPTT values; in the high-dose experiments, it prevented the endotoxin-induced decrease in aPTT (P < .01; Figure 3). PT values slightly increased after endotoxin injection and reached the maximum value after 5 hours (P < .01; Figure 3). Treatment with TFPI resulted in the additional prolongation of PT. In the low-dose group, PT increased from 12.7 ± 0.1 to 14.5 ± 0.2 seconds during TFPI treatment versus 12.8 ± 0.1 to 13.8 ± 0.2 seconds in the placebo study period (P = .04). In the high-dose group, PT values increased from 12.2 ± 0.2 to 15.6 ± 0.4 seconds in the TFPI study period and from 12.4 ± 0.2 to 13.2 ± 0.2 seconds in the placebo study period (P < .01; Figure 3).
Mean ± SEM values of PT and aPTT after endotoxin administration and infusion of TFPI or placebo.
Endotoxin (4 ng/kg) was given as a bolus injection at t = 0. Infusion of TFPI (○) or placebo (▵) started at t = 0 and was continued until t = 6 hours. P values indicate the differences in results of TFPI and placebo experiments.
Mean ± SEM values of PT and aPTT after endotoxin administration and infusion of TFPI or placebo.
Endotoxin (4 ng/kg) was given as a bolus injection at t = 0. Infusion of TFPI (○) or placebo (▵) started at t = 0 and was continued until t = 6 hours. P values indicate the differences in results of TFPI and placebo experiments.
Activation of the fibrinolytic system
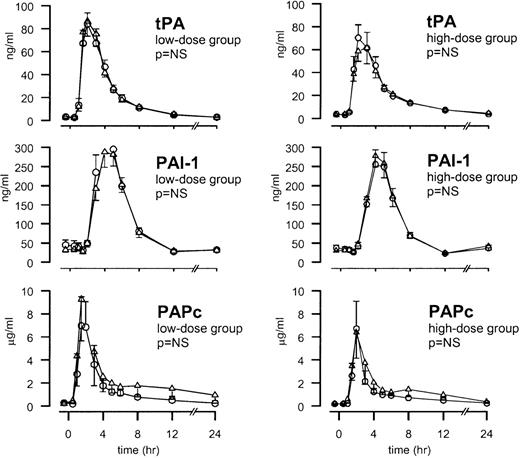
The injection of endotoxin was associated with an early release of tPA (peaking after 3 hours) followed by the appearance of PAI-1 (peaking after 4 hours) (both P < .01). Activation of the fibrinolytic system was confirmed by a transient increase in the plasma concentrations of PAPc, peaking after 2 hours (P < .01). Neither low-dose TFPI nor high-dose TFPI influenced the endotoxin-induced release of tPA, PAI-1, or PAPc (Figure4).
TFPI does not influence the fibrinolytic response.
Mean ± SEM plasma concentrations of tPA, PAI-1, and PAPc after endotoxin administration and TFPI infusion (○) or placebo (▵). Endotoxin (4 ng/kg) was given as a bolus injection at t = 0. Infusion of TFPI started at t = 0 and was continued until t = 6 hours.P values indicate the differences in results of TFPI and placebo experiments.
TFPI does not influence the fibrinolytic response.
Mean ± SEM plasma concentrations of tPA, PAI-1, and PAPc after endotoxin administration and TFPI infusion (○) or placebo (▵). Endotoxin (4 ng/kg) was given as a bolus injection at t = 0. Infusion of TFPI started at t = 0 and was continued until t = 6 hours.P values indicate the differences in results of TFPI and placebo experiments.
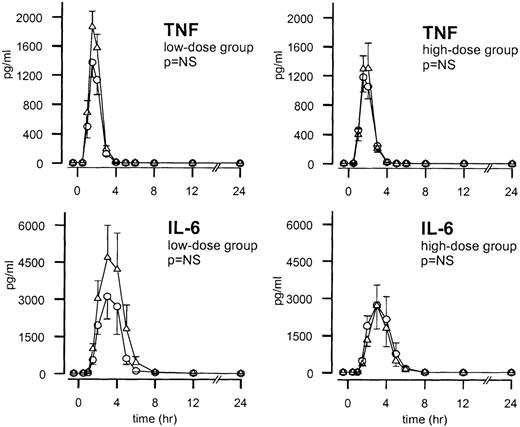
Cytokines
One hour after endotoxin administration, TNF plasma levels increased and reached peak values after 2 hours (P < .01). The TNF response on endotoxin injection was not influenced by either low- or high-dose TFPI (Figure 5). IL-6 levels increased from 90 minutes after endotoxin administration and peaked after 3 hours (P < .01). IL-6 responses after endotoxin injection were diminished after low-dose TFPI compared with after placebo, but this difference was not statistically significant. No difference was observed in IL-6 response between the high-dose TFPI group and the placebo-treated subjects (Figure 5). Endotoxin also elicited an antiinflammatory cytokine response, as reflected by transient increases in the plasma levels of the type 1 soluble TNF receptor (sTNF-R1) and IL-10. Neither of these endotoxin-induced increases was influenced by low- or high-dose TFPI (data not shown).
Mean ± SEM plasma concentrations of TNF and IL-6 after endotoxin administration and infusion of TFPI or placebo.
Endotoxin (4 ng/kg) was given as a bolus injection at t = 0. Infusion of TFPI (○) or placebo (▵) started at t = 0 and was continued until t = 6 hours. P values indicate the differences in results of TFPI and placebo experiments.
Mean ± SEM plasma concentrations of TNF and IL-6 after endotoxin administration and infusion of TFPI or placebo.
Endotoxin (4 ng/kg) was given as a bolus injection at t = 0. Infusion of TFPI (○) or placebo (▵) started at t = 0 and was continued until t = 6 hours. P values indicate the differences in results of TFPI and placebo experiments.
Discussion
Activation of the TF/VIIa pathway is considered crucial for the initiation of the coagulation system during bacteremia and endotoxemia. It has been suggested that besides its effect on coagulation, the TF/VIIa pathway can influence other inflammatory mediator systems. Therefore, we considered it of interest to determine the effect of TFPI on the coagulant, fibrinolytic, and cytokine responses during human endotoxemia. The current study is the first to show the anticoagulant effect of recombinant TFPI in humans. In the high-dose TFPI experiments, endotoxin-induced thrombin generation, as determined by increases in plasma F1 + 2 and TATc levels, was almost completely prevented; even in the low-dose TFPI studies, a reduction in thrombin production was observed. Hence, our data confirm the importance of TF in the endotoxin-induced procoagulant response in humans and further demonstrate that the effect of TFPI on thrombin generation is dose-dependent. However, TFPI was without any effect on fibrinolysis or cytokine release. The results suggest that, at least during low-grade endotoxemia, TFPI selectively attenuates coagulation activation.
The role of endogenous TFPI in sepsis and endotoxemia is not completely clear. Exposure of TF to circulating blood initiates the coagulation cascade by binding to factor VIIa, after which the TF/VIIa complex activates factor X and factor IX. Recent evidence suggests that TF may be present in an inactive, encrypted form and that the mere presence of TF on the cell surface is insufficient for initiating blood coagulation. Some additional stimulus may be required to express this latent procoagulant activity.25,26 TFPI is an approximately 43-kd, trivalent, Kunitz-type inhibitor that directly inhibits factor Xa with its second Kunitz domain. After factor Xa is bound, it rapidly inhibits the TF/VIIa complex with the first Kunitz domain.27 The third Kunitz domain has no known inhibitory role, but it may be involved in the lipoprotein binding of TFPI.28 Most of the body's TFPI is located in endothelial cells, and only 10% to 25% is found in circulating blood. Circulating TFPI is predominantly bound to lipoproteins.29 Blood platelets also carry native TFPI (approximately 10% of the plasma pool), which is released after thrombin stimulation.30 In vitro studies suggest that there may be a slight increase in TFPI produced by endothelial cells and monocytes by stimulation with endotoxin.31 Furthermore, (slightly) increased levels of TFPI have been observed in a number of illnesses, including malignancy and septicemia.32-35 In previous studies, plasma concentrations of TFPI only increased after severe injury. Thus, a sublethal dose of E. coli only induced a minimal (approximately 1.2-fold) increase in plasma TFPI concentrations, whereas infusion of an LD100 dose E. coli resulted in a 2-fold rise in plasma TFPI levels.36 In our study, endogenous TFPI levels did not increase in plasma after endotoxin administration, which could be considered a relatively mild stimulus. Together, these data suggest that the dose of endotoxin used in our human volunteer studies was insufficient to elicit an endogenous TFPI response in plasma.
In our experiments TFPI infusion was started immediately after endotoxin administration and was continued for 6 hours after a bolus injection. Two different dosages were compared. Both were chosen because of pharmacokinetic studies in healthy humans to achieve plasma TFPI levels of 86 and 346 ng/mL, respectively (investigational brochure; Searle Research & Development, Skokie, IL). In earlier studies these plasma levels were efficacious in reducing mortality in a sepsis model in rabbits.23 Low-dose TFPI infusion resulted in mean peak plasma TFPI concentrations of 175 ng/mL (3.5-fold higher than baseline) and steady state concentrations of 2.5-fold baseline. In the high-dose experiments, mean peak concentrations of 456 ng/mL (8-fold baseline) and steady state concentrations of 4.5-fold baseline were achieved. These TFPI concentrations resulted in minor prolongations of PT values of 1.8 and 3.4 seconds, respectively, that were slightly larger than the prolongation (1 second) attributable to endotoxin observed in the control experiments. Endotoxin induced a decrease in aPTT values. Previous observations and the time course of this effect suggests that the most likely explanation could be the endotoxin-induced release of von Willebrand factor (vWF) from the endothelium and the associated rise in factor VIII levels.37 38 Indeed, we could confirm a rapid release of vWF on endotoxin infusion in our study (data not shown). Interestingly, the endotoxin-induced decrease of aPTT values was attenuated by high-dose TFPI. Because vWF levels were not affected by TFPI, this probably resulted from a slight direct effect of TFPI on the aPTT.
In accordance with earlier studies,39,40 the activation of coagulation after endotoxin administration was preceded by a rapid activation and subsequent inhibition of the fibrinolytic system, as reflected by increased levels of tPA and PAPc followed by an increase in PAI-1 levels. Infusion of TFPI did not have any effect on the fibrinolytic response. These findings show that during low-grade endotoxemia in humans, the fibrinolytic response occurs independent of the generation of thrombin. Previous studies of low-grade endotoxemia in chimpanzees have revealed that blockade of endotoxin-induced coagulation activation by the administration of various anticoagulant agents, including anti-TF and anti-factor VIIa monoclonal antibodies, and the specific thrombin inhibitor hirudin, similarly did not result in the inhibition of plasmin generation.12,14,41 TNF is considered the major denominator of fibrinolysis activation in endotoxemia.42-44 Consistent with our finding that the endotoxin-induced activation of fibrinolysis in humans was not influenced by TFPI is the observation that TNF plasma concentrations were also unaffected by TFPI.
It should be noted that blocking the TF pathway in lethal E. coli sepsis in baboons not only prevented DIC, it protected against lethality.13,16,17 In addition, the administration of other physiological coagulation inhibitors has been shown to ameliorate the coagulation defect and to prevent death.45 More downstream interventions in the coagulation cascade, by the administration of active-site degraded factor Xa (DEGR-Xa), failed to influence lethality in bacteremic baboons whereas it completely inhibited the activation of coagulation.19 Because it has been suggested that the TF pathway exerts effects on other inflammatory responses besides its effects on coagulation,20 the inhibition of these effects by TFPI may have contributed to the TFPI-mediated protection against lethality. Indeed, in lethal sepsis models the inhibition of TF attenuated the IL-6 and IL-8 responses after E. coli infusion in baboons.15-17 It is uncertain how TFPI may influence cytokine production. Interestingly, clotting blood has been found to produce IL-8 but not IL-6 in vitro. The addition of endotoxin to coagulating blood resulted in a synergistic enhancement of IL-8 production that could be attenuated by the thrombin inhibitor hirudin or TFPI.46 In addition, end products of the coagulation cascade—ie, factor Xa, thrombin, and fibrin—can induce the synthesis of IL-6, IL-8, or both by various cell types in vitro.47-50Hence, the inhibition of coagulation by TFPI may reduce IL-6 and IL-8 release during sepsis. Furthermore, in vitro TFPI has been found to bind endotoxin and to block its effects on cells by interfering with its transfer to CD14.51 In the current study, we did not find any influence of TFPI on endotoxin-induced cytokine responses, as reflected by unaltered plasma concentrations of TNF, IL-6, IL-10, and sTNF-R1. IL-8 release was unchanged (data not shown). Our model differs from the lethal primate models in many important aspects. Although endotoxin infusion in healthy humans leads to the moderate activation of coagulation without organ dysfunction, lethal sepsis models induce massive thrombin generation with marked thrombus deposition at autopsy, leading to organ failure and death. The fact that the inhibition of coagulation by TFPI attenuates cytokine production in lethal sepsis models but not in the human endotoxin model could be a reflection of the amounts of thrombin formed in the different models. An alternative explanation could be that monocytes are the predominant source of cytokines during low-grade endotoxemia (which is associated with the transient release of cytokines) and that the endothelium contributes to more prolonged release, especially of IL-6 and IL-8, found in models of lethal sepsis. Indeed, endothelial cells predominantly produce IL-6 and IL-8 after stimulation.50,52 In baboons with sepsis, TFPI attenuated the IL-6 and IL-8 responses without affecting the early and transient TNF peaks.16 Hence, it can be speculated that TFPI in part attenuates the cytokine response by endothelial cells and has a much smaller effect on endotoxin-induced cytokine production by monocytes. Finally, it may be that cytokine production in the lethal sepsis model is not caused by thrombin but by ischemia and organ failure that result from occlusive thrombi in the microcirculation. If so, the effective inhibition of coagulation during sepsis may prevent the development of organ failure and thereby the secondary increase in cytokine levels.
Knowledge of the mechanisms involved in the activation of the hemostatic mechanism during severe infection has increased considerably in past years. The current study confirms in humans the pivotal role of the TF/VIIa pathway in endotoxin-induced coagulation activation and the anticoagulant potential of TFPI. Recombinant TFPI dose-dependently inhibited the activation of coagulation after endotoxin administration to healthy humans, without influencing the fibrinolytic and cytokine responses. TFPI is a selective anticoagulant drug during low-grade human endotoxemia.
Acknowledgments
The authors thank Dr Abraham van den Ende and the staff of the Hemostasis Laboratory for excellent technical assistance.
Reprints:Evert de Jonge, Department of Intensive Care, Academic Medical Center, P.O. Box 22660, 1100 DD Amsterdam, Netherlands.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal