The platelet glycoprotein (GP) Ib–IX-V complex mediates adhesion to von Willebrand factor (vWf) in (patho)physiologic thrombus formation. The vWf-binding site on GP Ib–IX-V is within the N-terminal 282 residues of GP Ib, which consist of an N-terminal flanking sequence (His-1–Ile-35), 7 leucine-rich repeats (Leu-36–Ala-200), a C-terminal flank (Phe-201–Gly-268), and a sulfated tyrosine sequence (Asp-269–Glu-282). We have used mammalian cell expression of canine–human chimeras of GP Ib, corresponding to precise structural boundaries, to demonstrate the first specific requirement for individual leucine-rich repeats for binding of vWf either induced by a modulator, ristocetin, or under hydrodynamic flow. Implicit in this approach was that the GP Ib chimeras retained a functional conformation, a supposition confirmed by analyzing restoration of function to reversed human–canine chimeras and demonstrating that all chimeras bound vWf activated by botrocetin, a modulator that is indiscriminate between species. Leucine-rich repeats 2, 3, and 4 of GP Ib were identified as being critical for vWf adhesion to GP Ib–IX-V.
Thrombosis is an acute form of cardiovascular disease wherein sudden aggregation of blood-borne platelets occludes the arterial blood supply, leading to tissue infarction. Platelet aggregation is also critical in normal hemostasis, which is triggered by exposure of platelets to the subendothelial matrix after vessel-wall injury. Thrombus formation at high shear stress in hemostasis and thrombosis is initiated when the platelet membrane adhesive receptor, the glycoprotein (GP) Ib–IX-V complex, binds to its adhesive ligand, von Willebrand factor (vWf), in the subendothelium or plasma.1-5 This interaction allows the initial tethering and rolling of platelets before their firm adhesion and activation.6-8 In thrombosis, the interaction of GP Ib–IX-V with vWf is induced when platelets and plasma vWf are exposed to pathologic shear stress in arteries occluded by atherosclerotic plaque, or when circulating platelets are exposed to subendothelial vWf after atherosclerotic plaque rupture. Binding of vWf to GP Ib–IX-V transduces signals across the plasma membrane that activate the platelets, coinciding with secretion of agonists such as ADP, elevation of cytosolic Ca++, and Ca++-dependent activation of the integrin αIIbβ3 (GP IIb-IIIa), which mediates platelet aggregation through adhesion to vWf or fibrinogen.3,5,8 The GP Ib–IX-V complex consists of 2 GP Ib-IX units (GP Ibα disulfide-linked to GP Ibβ and noncovalently associated with GP IX) and GP V; the 4 polypeptides GP Ibα, GP Ibβ, GP IX, and GP V are present in a 2:2:2:1 stoichiometry.1vWf binds to the extracellular N-terminal domain of GP Ibα (His-1–Glu-282), which contains 7 tandem leucine-rich repeats, their conserved N- and C-terminal disulfide-looped flanking sequences, and an anionic sequence, Asp-269–Glu-282, containing sulfated tyrosines at Tyr-276, Tyr-278, and Tyr-279.9-12
Leucine-rich repeats have been found in more than 50 mammalian, invertebrate, bacterial, or yeast proteins.1 Crystal structures have been determined for the leucine-rich repeat protein ribonuclease inhibitor and its ligand ribonuclease A,13,14and for the leucine-rich spliceosomal U2B′′-U2A′ proteins.15 In ribonuclease inhibitor, each repeat contains a β-strand-loop-α helix structure; the 15 tandem repeats together form a horseshoe shape with the helical regions of each repeat forming the outer convex surface and the β strands lining the central ligand-binding pocket. The leucine-rich repeats in GP Ibα have also been implicated in regulating vWf binding to platelet GP Ib–IX-V. For instance, single amino acid substitutions at either Leu-57/Phe16 within the first leucine-rich repeat or Ala-156/Val17 within the sixth repeat are associated with a form of the congenital bleeding disorder Bernard-Soulier syndrome,18 where GP Ibα is expressed in a dysfunctional form that does not bind vWf. Whether these mutations disrupt adhesive sites or conformation of the domain, however, is unknown. In contrast, point mutations within the C-terminal flank domain, Gly-233/Val or Met-239/Val, manifest as a gain-of-function phenotype,19suggesting that this domain also regulates vWf binding. The aim of the present study was to identify discrete adhesive sites within the vWf-binding domain of GP Ib–IX-V that constitute potential antithrombotic targets.
Although there is compelling evidence that vWf binds to at least 2 sites within the first 282 residues of GP Ibα, 1 site involving the sulfated tyrosine(s) and an additional site within His-1–Leu-275 involving the leucine-rich repeats and/or flanking sequences,9-12,20 delineation of adhesive sites on GP Ibα for vWf is limited by lack of defined epitopes for functional antibodies and the potential of either natural or synthetic mutations of GP Ibα to disrupt receptor conformation. As an alternative approach to defining binding sites within GP Ibα for both vWf and anti–GP Ibα antibodies, we used Chinese hamster ovary (CHO) cells to express canine–human chimeras of GP Ibα corresponding to precise boundaries between structural regions. Murine monoclonal antibodies against the N-terminal domain of human GP Ibα are highly species specific and do not bind canine GP Ibα; canine and human amino acid sequences are only 65.2% identical in the region His-1–Glu-282.21,22 We also took advantage of the fact that the vWf activator, ristocetin, induces binding of human vWf only to human platelets, but not to canine platelets.23 In contrast, the snake venom modulator, botrocetin, does not discriminate between the species and induces the binding of human vWf to human and canine platelets. Botrocetin-dependent vWf binding to all the chimeras provided evidence that the GP Ibα chimeras retained a functional conformation. These combined studies allowed detailed mapping of binding sites for anti–GP Ibα monoclonal antibodies and vWf and identification of specific leucine-rich repeats that are critical for vWf binding to the GP Ib–IX-V complex induced by ristocetin or under hydrodynamic flow.
Materials and methods
Cell lines, antibodies, and reagents
Na125I was purchased from Amersham (Castle Hill, Australia). Ristocetin was purchased from Boehringer-Mannheim (Mannheim, Germany). Oligonucleotides (20-25 base pairs) based on canine or human cDNA sequences21,22 were obtained from Beckman (Melbourne, Australia). Human factor VIII concentrate was a gift from the Commonwealth Serum Laboratories (Melbourne, Australia). vWf was purified from human factor VIII concentrate and radioiodinated where appropriate using the chloramine T method, as described previously.24-26 The cobra venom metalloproteinase, mocarhagin, was purified from Naja mocambique mocambique venom (Sigma, St Louis, IL) as described previously.12 CHO cells stably transfected with GP Ibβ and GP IX (CHO βIX cells) were prepared as reported.9,10,27 Murine monoclonal antibodies directed against GP Ibα were purified as described in detail elsewhere.25 HPL7 was purchased from Sapphire (Alexandria, Australia), AN51 from Dako (Carpinteria, CA), Hip1 from Pharmingen (San Diego, CA), and SZ2 from Immunotech (Westbrook, ME). 6D1 was generously provided by Dr B. Coller (New York, NY), LJIb10 by Dr Z. Ruggeri (San Diego, CA), and C-34 from Dr J. Miller (Syracuse, NY). MB45 was obtained from the Vth International Workshop on Leukocyte Typing. The following antibodies are all directed against the N-terminal, vWf-binding domain of GP Ibα and have been characterized elsewhere: AK2, SZ2, and C-3412,25,28; LJIb1029-31; AP125,32; 6D133; Hip126; TM6034; and VM16d.12,35 WM23 is directed against an epitope within the extracellular macroglycopeptide region of GP Ibα.25
Botrocetin was purified by dissolving 5 g lyophylized Bothrops jararaca venom (Sigma) in 30 mL 0.01 mol/L Tris, 0.15 mol/L sodium chloride, pH 7.4 (TS buffer), and fractionating by 0% to 60% ammonium sulfate at 22°C. The precipitate was resuspended in 100 mL TS buffer, dialyzed against the same buffer, and loaded at 30 mL/h onto a 2.5 × 40-cm column of DEAE-Sephacel (Pharmacia, Uppsala, Sweden). Protein was eluted with a 400-mL, linear 0.15- to 1.0-mol/L sodium chloride gradient in 0.01 mol/L Tris, pH 7.4. Peak fractions containing botrocetin were pooled, made 1.2 mol/L in ammonium sulfate, and loaded at 30 mL/h onto a 1.5 × 20-cm column of phenyl-Sepharose (Pharmacia). Protein was eluted with a 400-mL, linear 1.2- to 0-mol/L ammonium sulfate gradient in 0.01 mol/L Tris, pH 7.4. Peak fractions were pooled, dialyzed against 5 mmol/L sodium phosphate, pH 6.8, loaded onto a 1.5 × 20-cm column of hydroxyapatite (BioRad, Richmond, CA) in the same buffer, and eluted with a 200-mL, linear 5- to 200-mmol/L sodium phosphate gradient, pH 6.8. Botrocetin was dialyzed into TS buffer and concentrated using an Amicon ultrafiltration device (Danvers, MA) fitted with a YM10 membrane. This form of 2-chain botrocetin differs from that previously purified in our laboratory26 based on its activity in platelet-rich plasma (half-maximal aggregation at approximately 1 μg/mL) and is comparable to that reported by Fujimura et al.36
Platelet aggregation
Platelet aggregation of citrated platelet-rich plasma stirred at 900 rpm was performed in a whole blood Lumiaggregometer (Chronolog, Havertown, PA) at 37°C, as described previously.12,26 37 Aggregation was initiated by the addition of ristocetin (1.5 mg/mL final concentration) or botrocetin (2.5 μg/mL final concentration).
Binding of 125I-labeled vWf to washed platelets
Washed human and canine platelets were prepared by published methods.12,37 The binding of 125I-vWf to platelets in the presence of 1 mg/mL ristocetin or 2 μg/mL botrocetin was measured as described previously.12,26 37 Washed platelets (5 × 108/mL) in TS buffer containing 0.1% (w/v) bovine serum albumin (BSA) were equilibrated with 1 μg/mL125I-labeled vWf in the presence of ristocetin (1.5 mg/mL final concentration) or botrocetin (2.5 μg/mL final concentration) in a final volume of 100 μL. After 30 minutes at 22°C, the samples were centrifuged at 8750 × g for 2 minutes, and radioactivity associated with the pellet was counted in a γ-counter after aspiration of the supernatant. In some assays, platelets were preincubated with mocarhagin at 10 μg/mL for 30 minutes at 22°C, washed once in TS buffer, and resuspended at the original concentration before measuring ristocetin- and botrocetin-dependent vWf binding. The effect of monoclonal antibodies on vWf binding was determined by preincubating platelets with antibodies at a final concentration of 10 μg/mL for 5 minutes before the addition of 125I-vWf and ristocetin or botrocetin.
Preparation of expression vectors for canine–human chimeras of GP Ib
Canine–human chimeras containing successively more N-terminal canine sequence at domain junctions were generated using cDNA of canine22 and human21 GP Ibα. The expression vector consisting of the full-length GP Ibα cDNA inserted at anEcoR1 site of the plasmid pDX has been described.9,10,27 38 The strategy for generating chimeric constructs involved polymerase chain reaction amplification of the appropriate GP Ibα constructs using pfu DNA polymerase to avoid 3′ T extension on the amplification products (0.3-1.1 kb for canine and 1.7-0.9 kb for human fragments), blunt-end ligation of the canine and human DNA at domain junctions, and subcloning back into the human GP Ibα expression plasmid usingXhoI and XmaI restriction sites. The XhoI site in pDX is 0.25 kb upstream of the GP Ibα coding region, and theXmaI site is 1.8 kb into the GP Ibα coding region (2.42 kb total). Where necessary, blunt-end splice sites containing 1- to 3-bp deletions (presumably due to ragged 5′ ends of amplification primers) were corrected by site-directed mutagenesis (Transformer system; Clontech, Palo Alto, CA). Each construct was verified by sequencing.
Expression of canine–human chimeras in CHO βIX cells
Canine–human GP Ibα expression vectors (1-3 μg) were stably transfected into CHO βIX cells using Lipofectamine (Gibco BRL, Gaithersburg, MD) by established methods.9,10,27 CHO cells contain no endogenous GP Ibα, GP Ibβ, GP IX, or GP V, but cotransfection with GP Ibβ and GP IX facilitates stable surface expression of GP Ibα; GP V is not necessary for functional GP Ibα expression.27,38 Cells expressing surface GP Ibα were selected using the anti–GP Ibα monoclonal antibody WM23 conjugated to magnetic beads (Dynal, Oslo, Norway), according to the manufacturer's instructions. WM23 has an epitope downstream of Glu-28225 that is present in all of the chimeras.
Binding of vWf and monoclonal antibodies to CHO cells
CHO βIX cells lacking GP Ibα or CHO βIX cells cotransfected with wild-type human GP Ibα or canine–human chimeras of GP Ibα were assessed for the ability to bind human vWf and monoclonal antibodies against human GP Ibα, using previously described methods.9,10,27,38 Monoclonal antibody binding to transfected CHO cells was analyzed using fluorescein isothiocyanate (FITC)-labeled secondary antibody and flow cytometry. Cells were harvested by 10-minute treatment of cultures with EDTA (0.53 mmol/L), washed by centrifugation (10 minutes at 700 × g), and resuspended in 0.01 mol/L sodium phosphate, 0.15 mol/L sodium chloride, pH 7.4, containing 0.1% (w/v) BSA (PBS-BSA buffer). Cells were then incubated with monoclonal antibodies (5 μg/mL) for 40 minutes at 22°C, washed twice, incubated in the presence of FITC-labeled rabbit anti-mouse antibody for a further 40 minutes, washed, and resuspended in PBS-BSA buffer. The geometric mean fluorescence of each sample was analyzed using a FACscan flow cytometer (Becton Dickinson, San Jose, CA), fitted with an argon-ion laser that stimulates at 488 nm and records fluorescence above 530 nm, and Cellquest software (Becton Dickinson). For measuring vWf binding, CHO βIX cells or CHO βIX cells expressing wild-type GP Ibα or chimeras were first suspended in PBS-BSA buffer to a final concentration of 105/mL. The cells were then incubated with purified 125I-labeled human vWf (1 μg/mL final concentration) and either ristocetin (1 mg/mL final concentration) or botrocetin (2.5 μg/mL final concentration). After 30 minutes at 22°C, the cells were centrifuged through 20% (w/v) sucrose in PBS-BSA buffer (4 minutes at 8750g), and label associated with the pellet was counted in a γ-counter.27Nonspecific binding was measured in the absence of ristocetin or botrocetin in a parallel assay.
Flow-dependent adhesion of CHO cells to vWf
Cells in the flow chamber were visualized by phase contrast video microscopy and analyzed by digital image processing, as described previously.39 Cells in PBS (5 × 106/mL) were passed over a glass slide coated with vWf (50 μg/mL) at a shear stress of 10 dynes/cm2. The velocity of rolling cells was calculated by overlapping sequential images snapped at 30 frames/s and determining the distance the cells rolled during the snaps. Mean velocities were determined from a minimum of 100 cells per experiment. The number of rolling cells was calculated from a single field of view, counting each cell that rolled on the vWf surface during a 4-minute flow period. Three to 5 experimental runs were performed using different populations of cells.
Results
Ristocetin- and botrocetin-dependent vWf binding to canine and human platelets
The use of canine–human chimeras of the N-terminal domain of GP Ibα (His-1–Glu-282) is based on previous studies demonstrating that although botrocetin induced vWf binding to both canine and human platelets, ristocetin induced vWf binding only to human platelets.23,40 We confirmed this specifically for our preparation of botrocetin because canine platelet-rich plasma aggregated in the presence of 2.5 μg/mL botrocetin but not with 1.5 mg/mL ristocetin (data not shown). In addition, washed canine platelets supported the binding of purified 125I-labeled human vWf in the presence of 2.5 μg/mL botrocetin but not with 1 mg/mL ristocetin, whereas washed human platelets supported binding in the presence of either modulator (Figure 1A). The specificity of vWf binding to platelet GP Ibα was confirmed because botrocetin-dependent vWf binding to canine platelets and human platelets was abolished by pretreating the platelets with the cobra venom metalloproteinase mocarhagin (Figure 1B), previously shown to cleave human GP Ibα between Glu-282 and Asp-283 and to abrogate vWf binding.12
Species-specific binding of vWf to human or canine platelets.
(A) Specific binding of 125I-labeled vWf (1 μg/mL) to washed human or canine platelets (5 × 107) in the presence of either ristocetin (1 mg/mL final concentration) or botrocetin (2.5 μg/mL final concentration) for 30 minutes at 22°C. Nonspecific binding was determined in the absence of modulator in a parallel assay. Data are the means of triplicate determinations (± SEM) and are representative of 3 separate experiments with different populations of cells. (B) Botrocetin-dependent binding of 125I-labeled vWf (1 μg/mL) to washed human or canine platelets (5 × 108/mL). Platelets were pretreated with TS buffer only or with 10 μg/mL mocarhagin (MOC) for 30 minutes at 22°C, washed once in TS buffer, and resuspended to the original concentration.
Species-specific binding of vWf to human or canine platelets.
(A) Specific binding of 125I-labeled vWf (1 μg/mL) to washed human or canine platelets (5 × 107) in the presence of either ristocetin (1 mg/mL final concentration) or botrocetin (2.5 μg/mL final concentration) for 30 minutes at 22°C. Nonspecific binding was determined in the absence of modulator in a parallel assay. Data are the means of triplicate determinations (± SEM) and are representative of 3 separate experiments with different populations of cells. (B) Botrocetin-dependent binding of 125I-labeled vWf (1 μg/mL) to washed human or canine platelets (5 × 108/mL). Platelets were pretreated with TS buffer only or with 10 μg/mL mocarhagin (MOC) for 30 minutes at 22°C, washed once in TS buffer, and resuspended to the original concentration.
Binding of monoclonal antibodies to GP Ib chimeras on CHO cells
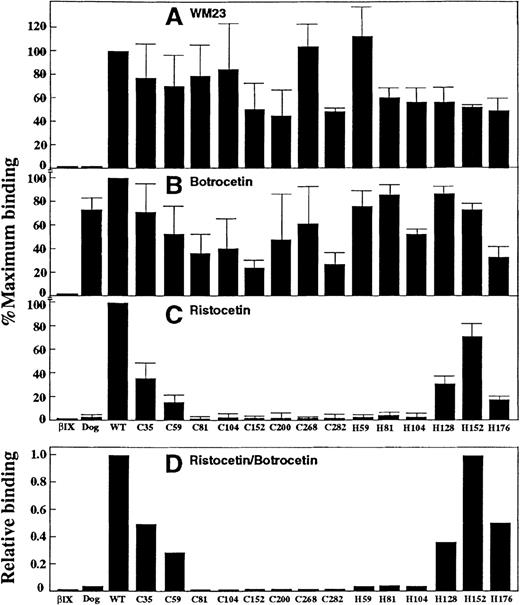
Expression vectors for canine–human chimeras of GP Ibα were prepared from human and canine cDNA using a blunt-end ligation method to facilitate replacement of structural domains at precise boundaries (Figure 2A). In the first set of chimeras, the human sequence was incrementally replaced with canine sequence from the N-terminus (Figure 2B). All of the constructs were correctly made and transfected into CHO βIX cells except for those corresponding to the boundaries between leucine-rich repeats 4/5 (C128) and 6/7 (C176), which could not be made for technical reasons. Analysis of the expressed chimeras for vWf binding (discussed later) pointed to a role for the leucine-rich repeats. Consequently, a second series of chimeras was prepared using C282 as template, with incremental replacement of canine with human sequence at leucine-rich repeat boundaries, again from the N-terminus (Figure 2C). Epitopes for a panel of 12 anti–GP Ibα monoclonal antibodies were evaluated by flow cytometry of wild-type GP Ibα– or chimeric GP Ibα–expressing CHO βIX cells (CHO βIX cells are stably transfected with GP Ibβ and GP IX to facilitate GP Ibα expression). For each population of cells, binding to the test antibody was compared with binding of WM23, a monoclonal antibody whose epitope is downstream of Glu-28212 25 and therefore present on all of the chimeras (Figures 2B and 2C). Representative histograms for WM23, AP1, and AK2 binding to the series of canine–human chimeras are shown in Figure3A. Table 1summarizes antibody binding to all of the chimeras tested. WM23 bound to cells expressing wild-type human GP Ibα and all the chimeras, but not to CHO βIX cells, which lack GP Ibα. Although we cannot entirely exclude the possibility that monoclonal antibody epitopes were lost because of conformational effects when human sequence was replaced by canine sequence, we addressed this issue experimentally by measuring not only loss of binding as the human sequence was replaced by canine sequence, but also regain of binding when the canine sequence was restored to human (Table 1). For all of the monoclonal antibodies tested, epitopes were regained either at the same domain boundary from which they were initially lost or at adjacent downstream domains. These results argue against loss of epitopes due to gross conformational disruption at cross-species boundaries. As the simplest interpretation of the antibody-binding data, binding domains for each antibody (Figure3B) were assigned from the first domain at which replacement of human with canine sequence abolished binding, to the last domain where replacement of canine by human sequence recovered binding. For example, AK2 bound to C35 but not to C59, C81, C104, C152, C200, or C268; and bound to H59, H81, H104, H152, and H176 (Table 1). The AK2 epitope was thus localized to the first leucine-rich repeat (residues 36-59). It is important to note the possibility, however, that when a series of human residues is replaced by the canine counterpart, diminished antibody binding may not necessarily mean that the binding site is contained within these residues because cross-species chimeras may affect the formation or stabilization of a particular epitope.
Canine–human chimeras of GP Ib.
(A) Protocol for generating canine–human GP Ibα chimeras from wild-type canine GP Ibα cDNA (solid box) and human GP Ibα cDNA (open box) by a blunt-end ligation method (see “Materials and Methods” for details). (B) Canine–human chimeras of the GP Ibα N-terminal 282 residues. Residue numbers at domain boundaries correspond to the human GP Ibα sequence.21 Chimeras corresponding to boundaries between leucine-rich repeats 4/5 (C128) and 6/7 (C176) were not included in the study. (C) Human–canine chimeras of GP Ibα corresponding to boundaries between leucine-rich repeats.
Canine–human chimeras of GP Ib.
(A) Protocol for generating canine–human GP Ibα chimeras from wild-type canine GP Ibα cDNA (solid box) and human GP Ibα cDNA (open box) by a blunt-end ligation method (see “Materials and Methods” for details). (B) Canine–human chimeras of the GP Ibα N-terminal 282 residues. Residue numbers at domain boundaries correspond to the human GP Ibα sequence.21 Chimeras corresponding to boundaries between leucine-rich repeats 4/5 (C128) and 6/7 (C176) were not included in the study. (C) Human–canine chimeras of GP Ibα corresponding to boundaries between leucine-rich repeats.
Binding of anti–GP Ib monoclonal antibodies to CHO cells.
(A) Representative sets of flow cytometry data using an FITC-labeled secondary antibody measuring the binding of anti–GP Ibα monoclonal antibodies WM23, AK2, and AP1 to CHO βIX cells or CHO βIX cells expressing wild-type GP Ibα or canine–human GP Ibα chimeras. (B) Epitopes of anti–GP Ibα monoclonal antibodies based on the flow cytometry data represented in panel A. (C) The effect of antibodies (10 μg/mL final concentration) on specific binding of125I-labeled vWf (1 μg/mL final concentration) to washed human platelets (5 × 107/mL final concentration) in the presence of 1 mg/mL ristocetin [R] or 2.5 μg/mL botrocetin [B]: AN51, 6D1, AK2, HPL7, AP1, TM60, MB45, LJIB10 (this study); C-34, VM16d, SZ2,12 Hip126 (this study). Inhibition of ristocetin-dependent vWf binding was tested by platelet aggregation in citrated platelet-rich plasma.
Binding of anti–GP Ib monoclonal antibodies to CHO cells.
(A) Representative sets of flow cytometry data using an FITC-labeled secondary antibody measuring the binding of anti–GP Ibα monoclonal antibodies WM23, AK2, and AP1 to CHO βIX cells or CHO βIX cells expressing wild-type GP Ibα or canine–human GP Ibα chimeras. (B) Epitopes of anti–GP Ibα monoclonal antibodies based on the flow cytometry data represented in panel A. (C) The effect of antibodies (10 μg/mL final concentration) on specific binding of125I-labeled vWf (1 μg/mL final concentration) to washed human platelets (5 × 107/mL final concentration) in the presence of 1 mg/mL ristocetin [R] or 2.5 μg/mL botrocetin [B]: AN51, 6D1, AK2, HPL7, AP1, TM60, MB45, LJIB10 (this study); C-34, VM16d, SZ2,12 Hip126 (this study). Inhibition of ristocetin-dependent vWf binding was tested by platelet aggregation in citrated platelet-rich plasma.
Binding of anti-GP Ib monoclonal antibodies to GP Ib chimeras
| . | AN51 . | C-34 . | MB45 . | AK2 . | HPL7 . | Hip1 . | 6D1 . | AP1 . | VM16d . | TM60 . | SZ2 . | LJIb10 . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C35 | − | − | − | + | + | + | + | + | + | + | + | + |
| C59 | − | − | − | − | − | + | + | + | + | + | + | + |
| C81 | − | − | − | − | − | − | + | + | + | + | + | + |
| C104 | − | − | − | − | − | − | + | + | + | + | + | + |
| C152 | − | − | − | − | − | − | − | + | + | + | + | + |
| C200 | − | − | − | − | − | − | − | + | + | + | + | + |
| C268 | − | − | − | − | − | − | − | − | − | − | + | + |
| C282 | − | − | − | − | − | − | − | − | − | − | − | − |
| H59 | + | − | + | + | − | − | − | − | − | − | − | − |
| H81 | + | + | + | + | − | + | − | − | − | − | − | − |
| H104 | + | + | + | + | + | + | − | − | − | − | − | − |
| H128 | + | + | + | + | + | + | + | − | − | − | − | − |
| H152 | + | + | + | + | + | + | + | − | − | − | − | − |
| H176 | + | + | + | + | + | + | + | − | − | − | − | − |
| . | AN51 . | C-34 . | MB45 . | AK2 . | HPL7 . | Hip1 . | 6D1 . | AP1 . | VM16d . | TM60 . | SZ2 . | LJIb10 . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C35 | − | − | − | + | + | + | + | + | + | + | + | + |
| C59 | − | − | − | − | − | + | + | + | + | + | + | + |
| C81 | − | − | − | − | − | − | + | + | + | + | + | + |
| C104 | − | − | − | − | − | − | + | + | + | + | + | + |
| C152 | − | − | − | − | − | − | − | + | + | + | + | + |
| C200 | − | − | − | − | − | − | − | + | + | + | + | + |
| C268 | − | − | − | − | − | − | − | − | − | − | + | + |
| C282 | − | − | − | − | − | − | − | − | − | − | − | − |
| H59 | + | − | + | + | − | − | − | − | − | − | − | − |
| H81 | + | + | + | + | − | + | − | − | − | − | − | − |
| H104 | + | + | + | + | + | + | − | − | − | − | − | − |
| H128 | + | + | + | + | + | + | + | − | − | − | − | − |
| H152 | + | + | + | + | + | + | + | − | − | − | − | − |
| H176 | + | + | + | + | + | + | + | − | − | − | − | − |
Binding of vWf to canine–human chimeras of GP Ib on CHO cells
Because ristocetin and botrocetin differentially modulate vWf binding to human and canine GP Ib–IX-V on platelets, we tested CHO βIX cells expressing canine–human chimeras of GP Ibα to determine whether they retained the capacity for ristocetin-dependent vWf binding. Like canine platelets23 (and in this study), CHO βIX cells expressing wild-type canine GP Ibα supported binding of human vWf only in the presence of botrocetin, but not ristocetin (Figure 4). The levels of botrocetin-dependent vWf binding to different chimeras correlated with the relative binding of WM23 to the same cells, at least within a factor of 2 (Figure 4B cf. Figure 4A), suggesting that differences in botrocetin-dependent binding to different cells reflected differences in surface expression of GP Ibα. Therefore, although these results do not discount the possibility that at least part of the variation in botrocetin-dependent vWf binding may be due to conformational differences between chimeras, the correlation between levels of binding of vWf in the presence of botrocetin and WM23 binding suggest that all of the chimeras were expressed in a functional form without gross conformational disruption occurring.
Binding of the anti–GP Ib monoclonal antibody WM23 and vWf to GP Ib chimeras.
(A) Relative binding of WM23 to CHO βIX cells (5 × 106/mL) or CHO βIX cells expressing wild-type canine GP Ibα, human GP Ibα, or canine–human chimeras of GP Ibα, as indicated by mean fluorescence intensity in flow cytometric analysis (see “Materials and Methods”). Data were normalized to CHO βIX cells expressing wild-type human GP Ibα. (B) Specific binding of 125I-labeled vWf (1 μg/mL) to the cells described in the legend to panel A in the presence of botrocetin (2.5 μg/mL final concentration) for 30 minutes at 22°C. Nonspecific binding was determined in the absence of modulator. (C) Binding of vWf to the cells described in the legend to panel A, but in the presence of ristocetin (1 mg/mL final concentration) instead of botrocetin. (D) Relative vWf binding in the presence of ristocetin (C) compared with botrocetin (B). Data in A-C are the means of triplicate determinations (± SEM) and are representative of 3 separate experiments with different populations of cells.
Binding of the anti–GP Ib monoclonal antibody WM23 and vWf to GP Ib chimeras.
(A) Relative binding of WM23 to CHO βIX cells (5 × 106/mL) or CHO βIX cells expressing wild-type canine GP Ibα, human GP Ibα, or canine–human chimeras of GP Ibα, as indicated by mean fluorescence intensity in flow cytometric analysis (see “Materials and Methods”). Data were normalized to CHO βIX cells expressing wild-type human GP Ibα. (B) Specific binding of 125I-labeled vWf (1 μg/mL) to the cells described in the legend to panel A in the presence of botrocetin (2.5 μg/mL final concentration) for 30 minutes at 22°C. Nonspecific binding was determined in the absence of modulator. (C) Binding of vWf to the cells described in the legend to panel A, but in the presence of ristocetin (1 mg/mL final concentration) instead of botrocetin. (D) Relative vWf binding in the presence of ristocetin (C) compared with botrocetin (B). Data in A-C are the means of triplicate determinations (± SEM) and are representative of 3 separate experiments with different populations of cells.
Compared with cells expressing wild-type human GP Ibα, ristocetin-dependent binding of 125I-labeled vWf was diminished in cells expressing the C35 and C59 chimeras and was not detectable in cells expressing C81, C104, C128, C152, C200, C268, or C282 (Figure 4C). Figure 4D shows ristocetin-dependent vWf binding relative to that in the presence of botrocetin. The results suggested that (1) elements within the N-terminal flanking sequence and the adjacent leucine-rich repeat were important for ristocetin-dependent vWf recognition because their replacement by canine sequence resulted in the progressive loss of vWf binding, and (2) downstream domains were essential for vWf binding because replacement of sequences beyond the first leucine-rich repeat completely abolished ristocetin-dependent vWf binding. To analyze the specific contributions of other domains, we prepared a second series of chimeras with progressive substitution of canine by human sequence in C282 corresponding to boundaries between leucine-rich repeats (Figure 2C). These chimeras all bound 125I-labeled vWf in the presence of botrocetin (Figure 4B), but ristocetin-dependent vWf binding was rescued only when the sequence was made human to the end of the fourth repeat (Figure4C). These data imply that leucine-rich repeats 2, 3, and 4 were critical for the adhesive function of GP Ibα because only chimeras containing all 3 of these human domains (C35, C59, H128, H152, and H176) showed measurable vWf binding in the presence of ristocetin. As for binding of antibodies to the chimeras, it should be noted that when a series of human residues is replaced by the canine counterpart, diminished vWf binding to the chimeras may reflect an effect on the formation or stabilization of the binding site. Ristocetin binds human vWf within proline-rich anionic sequences flanking the vWf A1 domain.37,41 42 However, the mechanism by which ristocetin induces vWf binding to GP Ibα, including the requirement, if any, for binding receptor, is unknown. We therefore examined vWf binding to the canine–human GP Ibα chimeras in the absence of either ristocetin or botrocetin by evaluating the adhesion of chimera-expressing cells to vWf under hydrodynamic shear stress.
Adhesion of CHO cells to vWf under flow
Under hydrodynamic shear stress, heterologous cells expressing wild-type GP Ibα adhere to immobilized vWf in the absence of modulators.39 43 Cells roll on the surface, or display saltatory translocation, depending on the applied shear stress and the avidity of the GP Ibα–vWf interaction. Saltatory translocation occurs when cells jump along the direction of flow, where there is an increased off-rate/on-rate for adhesion compared with rolling cells. At 10 dynes/cm2, conditions in which human GP Ibα–expressing cells exhibit rolling on immobilized vWf, CHO βIX cells expressing wild-type canine GP Ibα failed to roll (Figure5; Table 2). We therefore tested whether CHO βIX cells expressing canine–human GP Ibα chimeras interacted with vWf under the same conditions. Only C35 and C59 chimeras, containing canine instead of human sequence to the end of the N-terminal flank or first leucine-rich repeat, respectively, rolled on vWf, albeit at higher velocity than cells expressing wild-type GP Ibα (Figure 5; Table 2). C200 showed saltatory translocation on vWf, indicating a weaker interaction with ligand (Figure 5). Replacing canine sequence with human to the end of the first, second, or third leucine-rich repeat (H59, H81, or H104) failed to restore vWf binding under flow, but chimeras H128, H152, and H176 displayed saltatory motion, with H152 cells showing distinct rolling (Table 2).
Binding of chimera-expressing CHO cells to vWf under flow.
Representative results for binding of CHO βIX cells and CHO βIX cells expressing wild-type GP Ibα (αβIX) or the canine–human chimeras C35, C200, and C282. Upper panels show the cells in PBS buffer (5 × 106/mL) in the flow chamber before the application of flow. Lower panels show overlayed sequential images snapped at 30 frames/s during a 4-minute period of perfusion in the direction of the arrow at 10 dynes/cm2 over a glass surface coated with 50 μg/mL vWf. Results are representative of 3 to 5 experimental runs performed using different populations of cells. Arrowheads show points of adhesion for translocating cells expressing chimera C200.
Binding of chimera-expressing CHO cells to vWf under flow.
Representative results for binding of CHO βIX cells and CHO βIX cells expressing wild-type GP Ibα (αβIX) or the canine–human chimeras C35, C200, and C282. Upper panels show the cells in PBS buffer (5 × 106/mL) in the flow chamber before the application of flow. Lower panels show overlayed sequential images snapped at 30 frames/s during a 4-minute period of perfusion in the direction of the arrow at 10 dynes/cm2 over a glass surface coated with 50 μg/mL vWf. Results are representative of 3 to 5 experimental runs performed using different populations of cells. Arrowheads show points of adhesion for translocating cells expressing chimera C200.
Adhesion of GP Ib chimera-expressing CHO cells to vWf under flow
| . | Cell Rolling* . | Rolling Velocity (μm/s)† . | Saltatory Translocation* . |
|---|---|---|---|
| Canine | − | − | − |
| βIX | − | − | − |
| αβIX | +++ | 42 ± 1 | + |
| C35 | ++ | 105 ± 4 | ++ |
| C59 | ++ | 110 ± 6 | ++ |
| C81 | − | − | − |
| C104 | − | − | − |
| C152 | − | − | − |
| C200 | − | − | +++ |
| C268 | − | − | − |
| C282 | − | − | − |
| H59 | − | − | − |
| H81 | − | − | − |
| H104 | − | − | − |
| H128 | − | − | +++ |
| H152 | − | 126 ± 7 | +++ |
| H176 | − | − | + |
| . | Cell Rolling* . | Rolling Velocity (μm/s)† . | Saltatory Translocation* . |
|---|---|---|---|
| Canine | − | − | − |
| βIX | − | − | − |
| αβIX | +++ | 42 ± 1 | + |
| C35 | ++ | 105 ± 4 | ++ |
| C59 | ++ | 110 ± 6 | ++ |
| C81 | − | − | − |
| C104 | − | − | − |
| C152 | − | − | − |
| C200 | − | − | +++ |
| C268 | − | − | − |
| C282 | − | − | − |
| H59 | − | − | − |
| H81 | − | − | − |
| H104 | − | − | − |
| H128 | − | − | +++ |
| H152 | − | 126 ± 7 | +++ |
| H176 | − | − | + |
Grades are defined as follows: +, 1% to 10% of cells; ++, 11% to 79% of cells; +++, >80% of cells.
Mean ± SEM.
Discussion
In the normal physiologic process of hemostasis in response to vascular injury and in the pathologic process of thrombosis in stenosed or sclerotic arteries, platelet adhesion and aggregation are initiated by a specific adhesion receptor, the platelet membrane GP Ib–IX-V complex. Under conditions of high shear stress, GP Ib–IX-V binds to the multimeric adhesive glycoprotein vWf in the subendothelial matrix or plasma, triggering platelet activation and precipitating aggregation mediated by the αIIbβ3 integrin GP IIb-IIIa.1-5 The vWf-binding domain within the N-terminal 282 amino acids of GP Ibα is composed of 10 distinct structural regions: an N-terminal flanking sequence (His-1–Ile-35), 7 tandem leucine-rich repeats (Leu-36–Ala-200), a C-terminal flank (Phe-201–Gly-268), and an anionic sequence (Asp-269–Glu-282) containing sulfated tyrosines at Tyr-276, Tyr-278, and Tyr-279. The conformational dependence of ligand recognition by GP Ibα, combined with a lack of structural information on the GP Ibα ligand-binding domain, has meant that the mechanism(s) by which the ligand or receptor (or both) becomes adhesive in a physiologic or pathologic context has remained obscure. Structural information on members of the leucine-rich protein family is currently limited to 2 examples, ribonuclease inhibitor13,14 and the RNA-binding spliceosomal protein U2B′′-U2A′,15 neither of which contains the conserved disulfide-looped N- and C-terminal flanking sequences found in GP Ibα. A comparison of primary sequences for the N-terminal domains of human and canine GP Ibα (His-1–Glu-282) shows an overall identity of 65.2%.21 22 Of the 85 nonidentical residues, only 14 are conservative substitutions. For the structural domains of His-1–Glu-282, the 7 leucine-rich repeats combined have 66.1% identity, with the identity within individual repeats ranging from 58.3% to 70.8%. The N-terminal and C-terminal flanking sequences are 68.6% and 63.2% identical, respectively. Within the anionic domain (Asp-269–Glu-282), 8 of 14 residues (57.1%) are identical. In the present study, we investigated the potential functional roles of individual structural regions within the N-terminal 282 amino acid residues of GP Ibα.
A striking feature of the GP Ibα–vWf interaction is the subtle cross-species differences in vWf binding to platelet GP Ibα. In vitro, human vWf is activated to bind human receptor in the absence of shear by ristocetin or botrocetin. In contrast, only botrocetin, but not ristocetin, induces human vWf binding to the canine receptor. Botrocetin binds to distinct sequences within the vWf A1 domain that also binds GP Ibα; the vWf-botrocetin complex then forms a ternary complex with receptor.26,40,44 Ristocetin binds proline-rich sequences flanking the A1 domain in human vWf.37,41 42 We have exploited the functional differences between human and canine GP Ibα to define adhesive sites on GP Ibα that interact with vWf by expressing on the surface of CHO cells canine–human chimeras of GP Ibα with the interspecies junctions corresponding to precise structural domains. This approach has allowed detailed assessment of binding sites for vWf and anti–GP Ibα monoclonal antibodies. Two lines of evidence demonstrated that the GP Ibα chimeras were stably expressed on the surface of CHO βIX cells. First, we showed that all of the chimera-expressing cell lines bound the anti–GP Ibα monoclonal antibody, WM23, with an epitope downstream of Glu-282. Second, all of the chimeras were expressed in a functional form because they supported binding of vWf in the presence of botrocetin, which does not discriminate between human and canine GP Ibα. Although the possibility of conformational differences between chimeras cannot be ruled out, botrocetin-dependent vWf binding to different populations of cells generally reflected the binding of WM23 (within a factor of 2). vWF bound to all the chimeras in the presence of botrocetin, consistent with a lack of substantial conformational effects on replacing human with canine sequence. In this regard, molecular modeling of the 7 leucine-rich repeats of canine and human GP Ibα, based on the structure of the U2B′′-U2A′ protein leucine-rich repeats, suggested that core residues conferring structural conformity within the leucine-rich domains of canine and human sequence were highly conserved, whereas only surface residues differed markedly between species (Whisstock et al, manuscript in preparation).
The chimera-expressing cells were used to map 12 anti–GP Ibα monoclonal antibodies. Six of the antibodies (AN51, MB45, AK2, Hip1, HPL7, and C-34) recognized 1 or more domains within the first 104 residues of GP Ibα containing the N-terminal flank and the first 3 leucine-rich repeats; 6D1 recognized the fourth repeat (Leu-105–Glu-128). None of the antibodies mapped into repeats 5 to 7. Three antibodies recognized the C-terminal flank sequence Phe-201–Gly-268 (AP1, VM16d, and TM60), and 2 antibodies mapped into the anionic sulfated tyrosine region (SZ2 and LJIb10). These results are consistent with earlier findings that AK2 and VM16d immunoprecipitated the His-1–Leu-275 GP Ibα fragment,12that SZ212 and LJIb1031 recognized the anionic-sulfated tyrosine sequence, and that TM60 recognized Asp-249–Asp-274.45 Our ability to map antibodies to defined regions within the N-terminal 282 residues of GP Ibα demonstrates the utility of the chimera-expressing cells for analysis of murine anti–GP Ibα antibodies. It is interesting that there was no direct correlation between the domains recognized by the antibodies and their functional effects on ristocetin- and botrocetin-dependent vWf binding to GP Ibα. In particular, epitopes for antibodies that strongly inhibited (> 90%) both ristocetin- and botrocetin-dependent vWf binding (Figure 3C) were widely distributed: AK2 recognized the first leucine-rich repeat, Hip1 the second repeat, 6D1 the fourth repeat, and AP1 mapped to the C-terminal flank sequence (Figure 3B). Other antibodies that inhibited binding induced by both modulators by more than 50%—C-34 and TM60—overlapped binding sites for AK2 and AP1, respectively. Monoclonal antibodies VM16d and SZ2, which preferentially inhibited botrocetin-dependent vWf binding compared with ristocetin-dependent binding (Figure 3C),12mapped to the C-terminal flank (VM16d) or the sulfated tyrosine sequence (SZ2). In contrast, antibodies reported to inhibit the interaction of α-thrombin with platelet GP Ibα, including TM60,34 VM16d,35 SZ2,12 and LJIb10,31 all mapped to the C-terminal flank (TM60, VM16d) or the sulfated tyrosine sequence (SZ2, LJIb10). This is consistent with previous evidence that α-thrombin recognizes the sulfated tyrosine sequence.4,11 12
vWf binding to the GP Ibα chimeras was investigated in 2 ways. First, ristocetin-dependent binding of vWf relative to binding in the presence of botrocetin was measured under static conditions. These assays showed that ristocetin-dependent binding was progressively lost through replacement of the N-terminal flank and the adjacent leucine-rich repeat, and there was no detectable binding to subsequent chimeras. Binding was not rescued until the canine sequence was made human to the end of the fourth repeat, suggesting that repeats 2 to 4 were critical for binding (residues 60-128). Studies with synthetic peptides based on GP Ibα have identified sequences Thr-81–Leu-95, Leu-136–Leu-150, Asp-235–Lys-262, Ser-251–Tyr-279, and Gly-271–Glu-285 as potentially mediating ristocetin-dependent vWf binding to platelets.30,45 These sequences are all within or C-terminal to repeats 2 to 4 and may partly define vWf binding sequences; however, interpretation of their inhibitory effect is complicated because the peptides are at least an order of magnitude less inhibitory than larger proteolytic fragments of native GP Ibα. It is interesting that in the absence of modulators, interaction of the chimera-expressing cells with immobilized vWf under flow closely correlated with ristocetin-dependent vWf binding. Like cells expressing the wild-type human receptor, chimeras containing canine sequence to the end of the N-terminal flank or first leucine-rich repeat rolled on vWf, but with significantly higher mean velocities, indicating a diminished binding avidity (increased off-rate/on-rate). Again similar to ristocetin-dependent vWf binding, interaction with vWf under flow was not recovered until human sequence was reinstated to the end of the fourth repeat. In this case, there was saltatory translocation rather than rolling. When human sequence was returned to the end of the fifth repeat (H152), rolling was also observed. Subsequent loss of rolling in the receptor containing even more human sequence, to the end of the sixth repeat, paralleled the diminished ristocetin-dependent binding to this chimera under static conditions (Figure 4C; cf. Table 2). The chimera containing canine sequence to the end of the seventh repeat (C200), unlike the C152 or C268 chimeras, also showed saltatory adhesion to vWf. These results suggest the contribution of interdomain interactions to optimal ligand recognition and would be consistent with a model in which multiple elements within the N-terminal flank, the first 4 leucine-rich repeats, the C-terminal flank, and the sulfated tyrosine sequence together confer optimal vWf binding to GP Ibα. We found that shear-dependent binding of GP Ibα to vWf under flow more closely resembled ristocetin- than botrocetin-dependent vWf binding. The ability of the anti–GP Ibα antibody AK2 to inhibit both ristocetin-induced binding of vWf to GP Ibα and adhesion to vWf under flow12,43 and the lack of inhibition in both systems by SZ212 39 are consistent with this supposition.
In conclusion, we have investigated the functional role of specific structural domains within the platelet membrane vWf receptor, the GP Ib–IX-V complex, by analyzing a series of canine–human chimeras of GP Ibα that contain the vWf-binding site. These chimeras involved sequential interchange of human and canine sequence at precise boundaries between structural regions. Cell lines expressing GP Ibα chimeras were used to map binding sites for functional anti–GP Ibα monoclonal antibodies and for vWf either induced by ristocetin or under flow. Analysis of antibody and vWf binding to canine–human GP Ibα chimeras indicated the following: (1) The N-terminal flank and first leucine-rich repeat are important for vWf binding because their replacement by canine sequence showed a progressive loss of function; (2) leucine-rich repeats 2 to 4 are critical for function because only chimeras lacking these human domains showed no detectable vWf binding; (3) consistent with the vWf binding data, the majority of inhibitory anti–GP Ibα monoclonal antibodies mapped into the N-terminal flank and 4 adjacent leucine-rich repeats (AN51, AK2, Hip1, C-34, 6D1); and (4) the C-terminal flank apparently regulates vWf binding because 3 inhibitory antibodies (AP1, VM16d, and TM60) mapped into that region. These combined studies provide the basis for understanding the molecular mechanism of GP Ib–IX-V–dependent thrombus formation.
Acknowledgments
We thank Ms Carmen Llerena and Ms Andrea Aprico for outstanding technical assistance.
Supported by the National Heart Foundation of Australia, the National Institutes of Health, the American Heart Association, U.S. Public Health Service Grant No. HL 56027 (D.K.), and the National Health and Medical Research Council of Australia Grant No. 997144 (J.C.W.).
Present address of Dermot Kenny: Department of Clinical Pharmacology, Royal College of Surgeons in Ireland, 123 St Stephen's Green, Dublin 2, Ireland.
Y.S. and G.M.R. are co-first authors.
Reprints:Robert K. Andrews, Baker Medical Research Institute, P.O. Box 6492, St Kilda Road Central, Melbourne, Australia, 8008; e-mail: rkandrews@hotmail.com.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.



![Fig. 3. Binding of anti–GP Ib monoclonal antibodies to CHO cells. / (A) Representative sets of flow cytometry data using an FITC-labeled secondary antibody measuring the binding of anti–GP Ibα monoclonal antibodies WM23, AK2, and AP1 to CHO βIX cells or CHO βIX cells expressing wild-type GP Ibα or canine–human GP Ibα chimeras. (B) Epitopes of anti–GP Ibα monoclonal antibodies based on the flow cytometry data represented in panel A. (C) The effect of antibodies (10 μg/mL final concentration) on specific binding of125I-labeled vWf (1 μg/mL final concentration) to washed human platelets (5 × 107/mL final concentration) in the presence of 1 mg/mL ristocetin [R] or 2.5 μg/mL botrocetin [B]: AN51, 6D1, AK2, HPL7, AP1, TM60, MB45, LJIB10 (this study); C-34, VM16d, SZ2,12 Hip126 (this study). Inhibition of ristocetin-dependent vWf binding was tested by platelet aggregation in citrated platelet-rich plasma.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/95/3/10.1182_blood.v95.3.903.003k37_903_910/6/m_bloo00337003x.jpeg?Expires=1765913509&Signature=XIkEzqDT-mahS2hhPplqfeJYX6j7mMGn2uMyVy-sycydMww33Zg5n8NLCK28Y8yEEbD0bG48ZZzg9YiaQyPQJTrXGTLdAbdqI~AHsoCXW~LE6bv1mBdQ5TRun0fNHCImRwQaTY4-oEJaFugtwMRQbGkP4k75jau4Jb7Wa3irhbtfxcNAR7c6piFM-9AL9nh2C5ObHYfDgyuei8sSiaGQYhcQZoLV7f1z8XGiA-kL1~H4b8hCTauqGIQHSq6KnncBNEvMgXVVjk~vV4xGAQIFeSG~NtiwAylsA0LedEZfAXbmEjaW~fJftIfxLC8UUiH6ql37LUtSfAlSSI1wsRyanA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal