The relative contributions of CD11a/CD18 and CD11b/CD18 to the dynamics and strength of neutrophil adhesion to intercellular adhesion molecule (ICAM)-1–transfected cells were examined over the time course of chemotactic stimulation. Suspensions of neutrophils and transfectants were sheared in a cone-plate viscometer, and formation of heterotypic aggregates was measured by 2-color flow cytometry. The 2-body collision theory was used to compute adhesion efficiency, defined as the proportion of collisions between neutrophils and target cells that resulted in capture. ICAM-1 surface density and shear rate both regulated adhesion efficiency. Target cells expressing approximately 1000 ICAM-1 sites/μm2 (Ilow) were captured with an efficiency of 0.15 at 100 s−1, which decreased to zero at 300 s−1. At 8-fold higher ICAM-1 expression (Ihigh) corresponding to levels measured on interleukin-1–stimulated endothelium, efficiency was 0.3 at 100 s−1 and remained above background to 900 s−1. Shear alone was sufficient for CD11a/CD18-mediated adhesion to ICAM-1, and stimulation with formyl-methionyl-leucyl-phenylalanine boosted capture efficiency through CD11a/CD18 by 4-fold. In comparison, CD11b/CD18 supported one third of this efficiency, but was necessary for aggregate stability over several minutes of shear and at shear stresses exceeding 5 dyne/cm2. Hydrodynamics influenced capture efficiency predominantly through the collisional contact duration, predicted to be approximately 9 milliseconds for successful capture of Ilow and 4 milliseconds for Ihigh. The implication is that an increase in ICAM-1 from resting levels to those on inflamed endothelium effectively increases the permissible shear in which capture through β2-integrins may occur. Neutrophil adhesion to ICAM-1 appears to be a cooperative and sequential process of CD11a-dependent capture followed by CD11b-mediated stabilization.
The multistep model of leukocyte emigration provides a conceptual framework describing the molecular recognition events that lead from cell capture to adhesion strengthening and transmigration. Neutrophils use selectins to tether to inflamed endothelium and subsequently arrest at specific sites in the vasculature through binding of the β2-integrins.1,2 The β2-integrin family members CD11a/CD18 (LFA-1) and CD11b/CD18 (Mac-1) are involved in firm adhesion of neutrophils to endothelial cells.3 Intravital microscopy in mice4 and rats5,6 has shown that CD11a and CD11b each contribute significantly to firm adhesion, and concurrent blocking of both β2-integrins is required for complete inhibition of human and canine neutrophil transmigration.3,7,8 ICAM-1 (CD54) is a member of the immunoglobulin superfamily that is widely expressed on vascular endothelium and certain parenchymal cells in response to activation with cytokines or endotoxin.9-11 ICAM-1 has been shown to be the primary endothelial adhesive ligand for CD11a/CD18,12 whereas CD11b/CD18 binds ICAM-1 and at least 1 other undefined ligand in supporting arrest and transmigration.13
The efficiency and location of cell recruitment are apparently regulated through the interplay of hemodynamics and the binding and mechanical properties intrinsic to adhesion receptors.2Integrin activation is required to augment cellular adhesion in numerous experimental systems.14 At shear rates characteristic of normal blood flow, selectin-mediated leukocyte rolling can be converted rapidly to shear-resistant stable adhesion through integrin binding.1,15 This pattern of adhesive interactions is observed for neutrophil emigration in response to acute inflammatory stimuli in the microcirculation16-18 and in flow chambers.19,20 Direct adhesion through β2-integrins without selectin tethering may significantly contribute to emigration under low shear conditions that predominate in microvascular beds of highly perfused organs such as liver21,22 and lung23 and more generally in the peripheral microcirculation during inflammation.24CD11b/CD18 can mediate firm attachment of neutrophils under flow conditions within seconds of chemotactic stimulation.3,25-27 CD11a/CD18 on lymphocytes can likewise effect attachment within seconds.28
Our objective in this study was to determine the hydrodynamic influences of shear rate (which determines the duration of intercellular contact) and shear stress (which translates to the compressive and tensile forces at the adhesive contact region) on adhesion mediated by CD11a/CD18 and CD11b/CD18 binding to ICAM-1 on target cells. ICAM-1 was expressed in a murine pre-B cell line29 lacking other neutrophil adhesion molecules such as selectins or their carbohydrate ligands.30 Two clones were selected that spanned an 8-fold range in ICAM-1 level, reflecting expression on resting and cytokine-stimulated endothelia. This report focuses on the sequence of molecular events that supports β2-integrin–mediated neutrophil adhesion to ICAM-1 under defined hydrodynamic shear.
We used a rotational viscometric technique to expose cell suspensions to defined shears simulating those experienced by leukocytes traversing the microcirculation. Suspensions of neutrophils and ICAM-1 target cells were exposed to a linear gradient of velocity streamlines in a cone-plate viscometer. Intercellular collisions occurred as cells traveling near the rotating cone overtook those in slower streamlines adjacent to the stationary plate. The viscometer's defined shear field allowed theoretical predictions of the frequency of cell–cell collisions, the average duration of collisions, and stresses exerted on aggregates. Stable adhesion occurred when the strength of bonds formed during collisional contact was greater than tensile forces generated as aggregates tumbled in the shear field. The numerical efficiency of this process was derived experimentally from the rate of aggregation as measured by 2-color flow cytometry.
Materials and methods
Materials
Formyl-methionyl-leucyl-phenylalanine (fMLP) and Ficoll were purchased from Sigma (St. Louis, MO); 20% formaldehyde solution was from Tousimis Research (Rockville, MD). ZK36 374, a stable prostacyclin (PGI2) derivative that inhibits platelet activation and aggregation, was a gift from Schering Company (Berlin, Germany). Fluorescein isothiocyanate (FITC)-labeled antibodies to CD45 and CD54 were from Caltag Laboratories (Burlingame, CA). Anti-CD11a monoclonal antibody (mAb) R3.1 (IgG1) and anti–ICAM-1 domain 2 mAb R6.5 (IgG2a) were generously provided by Dr Kei Kishimoto (Boehringer-Ingelheim, Ridgefield, CT). Lora Whitehouse (Repligen, Cambridge, MA) generously provided humanized anti-CD11b mAb 60.1 (denoted h60.1, IgG1). This isotype does not bind significantly to human neutrophils through FcR. Fab fragments of R6.5 and R3.1 were produced using an ImmunoPure Fab kit from Pierce (Rockford, IL). Of note, use of R6.5Fab was required to block ICAM-1 because whole IgG failed to inhibit adhesion completely. Anti-CD11c mAb Bly6 (IgG1) was from Pharmingen (San Diego, CA). Anti-CD50 mAb ICR1.1 was obtained from Dr Donald Staunton (ICOS, Bothell, WA). W6/32, an IgG2a that binds to human HLA class I, was from American Type Culture Collection (Manassas, VA). In all experiments, blocking antibodies were used at saturating concentrations: R3.1 at 10 μg/mL; h60.1, R6.5Fab, Bly6, and W6/32 at 20 μg/mL; and ICR1.1 at 30 μg/mL. The blocking ability of these mAbs at saturation concentrations has been documented previously.31 32
Cell preparation
Following the protocol approved by the Baylor Institutional Review Board, human blood was collected by venipuncture into a sterile syringe containing 10 U/mL heparin (Elkins-Sinn, Cherry Hill, NJ). Neutrophils were isolated using Mono-Poly resolving medium (ICN Biomedicals, Aurora, OH), as described previously.33 They were kept at 4°C in Ca++-free HEPES buffer (110 mmol/L NaCl, 10 mmol/L KCl, 10 mmol/L glucose, 1 mmol/L MgCl2, and 30 mmol/L HEPES, pH 7.35) containing 0.1% human serum albumin (Armour Pharmaceuticals, Kankanee, IL) and were used within 4 hours. Indicators of activation including cell shape change, L-selectin shedding, or CD11b/CD18 up-regulation were observed in less than 5% of the isolated neutrophil population after incubation of cell suspensions at 37°C for 20 minutes, indicating that they remained in a resting state. Additionally, aggregation in response to shear alone of neutrophils kept at room temperature was within 5% of that observed for those kept on ice before the experiment.
Murine pre-B lymphocytes, 300.19 cells, were stably transfected with human ICAM-1 cDNA using a modified SRa vector containing a neomycin resistance marker. Positive clones were identified by flow cytometry using R6.5 mAb. The 300.19 cells were cultured in RPMI medium (Gibco, Grand Island, NY) supplemented with 10% heat-inactivated fetal bovine serum (Hyclone, Logan, UT), 1% penicillin-streptomycin (Gibco), and 100 μmol/L 2-mercaptoethanol (Fischer, Fair Lawn, NJ). Before the experiment, the cells were pelleted and washed twice in HEPES buffer plus 0.1% human serum albumin and 1.5 mmol/L CaCl2, and were kept at room temperature for up to 4 hours.
Determination of ICAM-1 binding sites
R6.5 mAb was conjugated with FITC using the FluoReporter kit from Molecular Probes (Eugene, OR). A binding curve was generated to ascertain the concentration of R6.5-FITC sufficient for saturation of binding sites. Labeled cells were washed and analyzed on the FL1 fluorescence channel on a FACScan flow cytometer with Cell Quest analysis software (Becton Dickinson, San Jose, CA). The absolute number of cell surface binding sites was quantified using Quantum Simply Cellular microbeads (Flow Cytometry Standards, San Juan, PR), which have calibrated numbers of goat anti-mouse IgG sites, as described previously.34 ICAM-1 site densities were also confirmed using commercially available anti-CD54–FITC (Caltag, Burlingame, CA). ICAM-1 levels on 300.19 transfectants were compared with those on first-passage human umbilical vein endothelial cells (HUVEC) grown to confluence in culture dishes, as described previously.33Confluent HUVEC were brought into suspension with 0.05% trypsin and 0.02% EDTA in phosphate-buffered saline and washed twice in HEPES buffer. Unstimulated HUVEC labeled with anti-CD54–FITC expressed 2.3 × 105 sites per cell, a level comparable to the Ilow 300.19 clone. Stimulation of the HUVEC for 4 hours with interleukin-1 resulted in an increase in ICAM-1 expression to 8.5 × 105 sites per cell, a copy number approximately half that detected on the Ihigh 300.19 clone. In similar labeling experiments, expression of ICAM-2, ICAM-3, or L-selectin by 300.19 cells was not detectable (data not shown).
Cone-plate viscometry
Aggregation assays were performed at 37°C in a Haake VT550 cone-plate viscometer (Haake, Paramus, NJ), consisting of a stationary plate beneath a rotating truncated cone with an angle of 2°. This apparatus applies a uniform and linear shear field to the entire fluid sample in the gap between the cone and plate.35 Shear rate and shear stress are related through the fluid viscosity as τ = μ G, where τ is the shear stress in dyne/cm2, G is the shear rate in s−1, and μ is the viscosity in poise.36 In some experiments, buffer viscosity was increased from approximately 0.7 to 1.7 cp by the addition of 6% (wt/vol) Ficoll, a high-molecular-weight hydrophilic polymer of sucrose.
Determination of aggregation
To resolve the cellular composition of aggregates, we incubated neutrophils and 300.19 cells with spectrally distinct fluorescent labels. The 300.19 cells were stained with the vital nucleic acid dye LDS-751 (Molecular Probes) at a concentration of 0.5 μg/mL for detection in the red (FL3) channel. Neutrophils were labeled with 5 μg/mL anti-CD45–FITC for detection in the green (FL1) fluorescence channel. These labels did not affect β2-integrin expression on resting neutrophils and did not alter the increase in β2-integrin expression or adhesivity that occurred with fMLP stimulation of the cells.32,37 Neutrophils or 300.19 cells were preincubated with anti-CD11a (R3.1 Fab or whole), anti-CD11b (h60.1), anti-CD11c (Bly6), anti-CD54 (R6.5Fab), anti-CD50 (ICR1.1), or anti-HLA class 1 (W6/32) for 10 minutes at room temperature. Excess LDS-751 was removed from 300.19 cells by centrifugation. The 2 cell populations were mixed in HEPES buffer containing 0.1% human serum albumin and 1.5 mmol/L CaCl2 and were introduced into the viscometer at final concentrations of 1 × 106neutrophils/mL and 2 × 106 300.19 cells/mL. The suspension was equilibrated at 37°C for 2 minutes before shear was applied at rates from 40 s−1 to 1500 s−1. In some experiments, 1 μmol/L fMLP was added 1 second before application of shear. At prescribed time points, 40-μL samples were obtained by pipet and immediately fixed in 150 μL cold 0.5% formaldehyde in HEPES buffer. For whole-blood aggregation assays, 60 nmol/L ZK36 374 was added to the syringe with heparin before venipuncture. Neutrophils in whole blood were labeled with anti-CD45–FITC before mixing with LDS-751–labeled 300.19 cells. Whole blood/300.19 cell suspensions were diluted 1:5 with HEPES buffer before addition to the viscometer to allow adequate flow cytometric detection of binding events.38
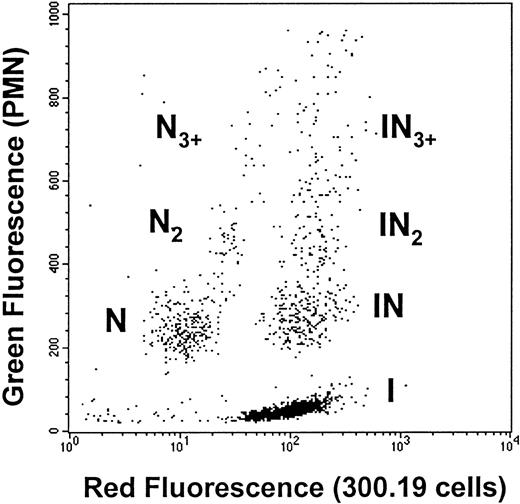
Neutrophil and 300.19 cell populations were identified by flow cytometry based on their characteristic side versus forward scatter. Quantification of heterotypic aggregation between neutrophils (N) and 300.19 cells (I) was performed by analysis of dot plots of green versus red fluorescence (Figure 1). Homotypic doublets (N2) or larger aggregates (N3+) composed solely of neutrophils, as well as heterotypic aggregates composed of 1 300.19 cell and either 1 (IN1), 2 (IN2), or 3 or more neutrophils (IN3+), were resolved. Flow cytometry and light microscopy documented that 300.19 cells did not aggregate with each other. The fraction of neutrophils in heterotypic aggregates was quantified as follows: % neutrophils in heterotypic aggregates = (IN1 + 2IN2 + 3IN3+)/(N1 + 2N2 + 3N3+ + IN1 + 2IN2 + 3IN3+). Aggregates containing more than 3 neutrophils typically represented less than 10% of the total neutrophil population and were grouped into IN3+, resulting in an estimated error of less than 3% in the preceding equation.
Flow cytometric detection of neutrophil–ICAM-1 cell aggregation.
Neutrophils (1 × 106 cells/mL) were fluorescently labeled green (CD45-FITC) and ICAM-1 300.19 cells (2 × 106 cells/mL) were labeled red (LDS-751) for 10 minutes at room temperature. The 2 cell populations were combined in the cone-plate viscometer and equilibrated to 37°C in buffer containing 1.5 mmol/L Ca++ for 2 minutes before stimulation with 1 μmol/L fMLP and initiation of fluid shear. Samples were taken at prescribed time points and immediately fixed in 0.5% cold formaldehyde. Two-color flow cytometry was used to detect distinct populations of single neutrophils (N) and 300.19 ICAM-1 cells (I); homotypic neutrophil doublets (N2) and triplets (N3); and 2-color heterotypic aggregates containing a single 300.19 cell bound to 1 (IN), 2 (IN2), or 3 or more neutrophils (IN3+). Representative dot plot depicts aggregation of neutrophils with Ihigh at 600 s−1 at 1 minute after stimulation and initiation of shear. Each dot represents a single particle event containing 1 or more cells.
Flow cytometric detection of neutrophil–ICAM-1 cell aggregation.
Neutrophils (1 × 106 cells/mL) were fluorescently labeled green (CD45-FITC) and ICAM-1 300.19 cells (2 × 106 cells/mL) were labeled red (LDS-751) for 10 minutes at room temperature. The 2 cell populations were combined in the cone-plate viscometer and equilibrated to 37°C in buffer containing 1.5 mmol/L Ca++ for 2 minutes before stimulation with 1 μmol/L fMLP and initiation of fluid shear. Samples were taken at prescribed time points and immediately fixed in 0.5% cold formaldehyde. Two-color flow cytometry was used to detect distinct populations of single neutrophils (N) and 300.19 ICAM-1 cells (I); homotypic neutrophil doublets (N2) and triplets (N3); and 2-color heterotypic aggregates containing a single 300.19 cell bound to 1 (IN), 2 (IN2), or 3 or more neutrophils (IN3+). Representative dot plot depicts aggregation of neutrophils with Ihigh at 600 s−1 at 1 minute after stimulation and initiation of shear. Each dot represents a single particle event containing 1 or more cells.
Adhesion efficiency
The probability with which colliding cells bind to each other and form stable aggregates is termed adhesion efficiency.25Mathematically, this is expressed as follows: Adhesion efficiency = (number of collisions resulting in adhesion per unit time)/(total number of collisions per unit time).
The numerator is derived from flow cytometric quantitation of the kinetics of heterotypic aggregation over the first 60 seconds after application of shear. The denominator is estimated using 2-body collision theory to predict the number of collisions per unit time, as described previously.31 34 Adhesion efficiency computed in this manner accounts for influences of the magnitude of shear rate and particle volume fraction on the frequency of collisions. As such, it is solely a function of biologic and biophysical properties of the cell, such as extent of activation, adhesion receptor affinity, receptor topography, and tensile strength of receptor-ligand bonds. For typical experiments, neutrophils (ri = 3.75 μm) were sheared with 300.19 cells (rj = 5.5 μm) at a concentration ratio (neutrophils/300.19) of approximately 0.5. Under these conditions, heterotypic collisions occur approximately 6 times more frequently than homotypic collisions.
We wish to state an erratum in a previous article.34 In previous publications using this model, the total number of collisions per unit time was calculated using a prefactor of 2/3 instead of the correct constant of 4/3 from 2-body collision theory.39 As a result, the collision frequency estimated was half the correct value, and the adhesion efficiencies were twice those actually fit to the kinetic aggregation data. This error was carried over to 2 other articles and a review published by the authors.25,31 40Therefore, a correction factor of 0.5 must be applied to the efficiencies computed in those manuscripts.
Statistics
Data are presented as mean values, and error bars indicate standard error of the mean. One-way analysis of variance was used for multiple comparisons and t tests for comparisons between 2 groups. Posttests were performed using the Newman-Keuls method.P < .05 was considered significant.
Results
Detection and kinetics of neutrophil adhesion to 300.19 cells expressing ICAM-1
Application of shear and chemotactic stimulation resulted in the formation of homotypic neutrophil aggregates containing up to 3 or more neutrophils (labeled with anti-CD45–FITC) and heterotypic aggregates consisting of a single 300.19 cell (labeled with LDS-751) bound to 1 or more neutrophils (Figure 1). The 300.19 cells were not activated after application of shear or fMLP and did not homotypically aggregate. Two 300.19 clones were selected based on their surface expression of ICAM-1. These clones were denoted Ilow and Ihigh and expressed approximately 2.2 × 105 and 1.7 × 106 ICAM-1 sites per cell, respectively, compared with HUVEC, which expressed 2.3 × 105 sites per cell before and 8.5 × 105 sites per cell after 4 hours of stimulation with interleukin-1. The nontransfected parent cell line did not express detectable surface ICAM-1.
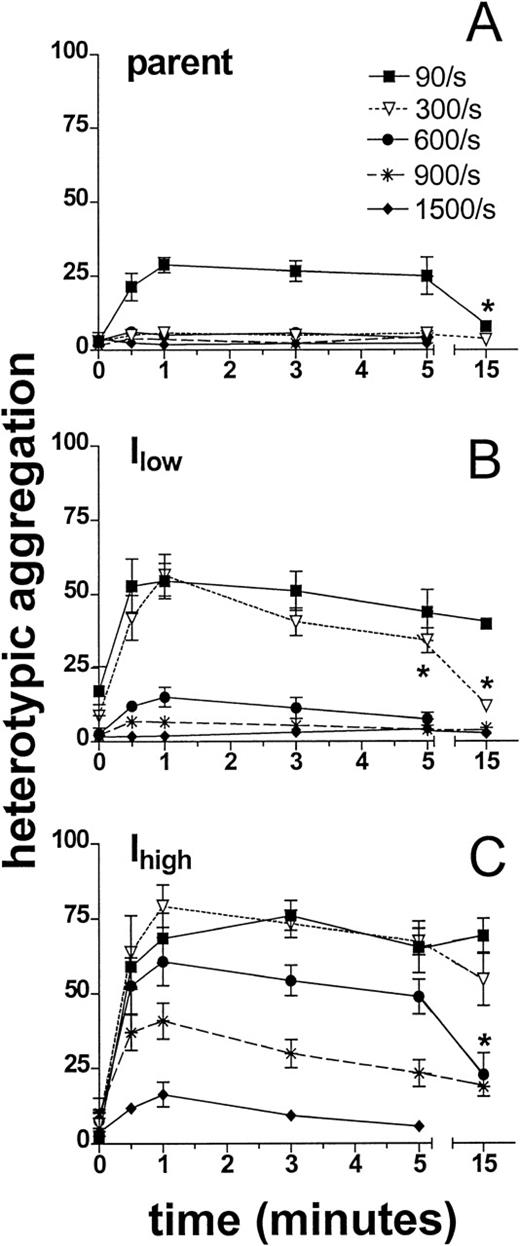
The kinetics of heterotypic aggregation were measured over a range of shear rates typical for the microcirculation (40 s−1to 1500 s−1). Neutrophil adhesion to parent cells peaked at approximately 25% singlet recruitment and was significantly above background only at the low shear rate of 90 s−1 (Figure 2A). This adhesion was inhibited by anti-CD18 but not by anti–ICAM-1 mAbs (data not shown). Adhesion to ICAM-1–transfected 300.19 cells was more rapid and extensive. For example, at 90 s−1, 50% of neutrophils adhered to Ilow and 75% to Ihighwithin 1 minute of stimulation (Figures 2B and 2C). Maximum aggregation with Ihigh was maintained at shear rates to 600 s−1 and decreased to background levels as shear was increased to 1500 s−1. Neutrophil aggregation with Ilow was maximal up to 300 s−1 and decreased to baseline at 900 s−1. Adhesion mediated by β2-integrins and ICAM-1 was more stable than neutrophil–neutrophil adhesion. Whereas breakup of homotypic aggregates began within 2 minutes and was complete by 5 minutes at shear rates above 400 s−1,25 34 neutrophil–300.19–ICAM-1 aggregates were maintained under these conditions. For example, aggregation with Ihigh was stable for 15 minutes at shear rates less than 600 s−1 (Figure 2C). At shear rates greater than 600 s−1, the peak extent of heterotypic aggregation was diminished.
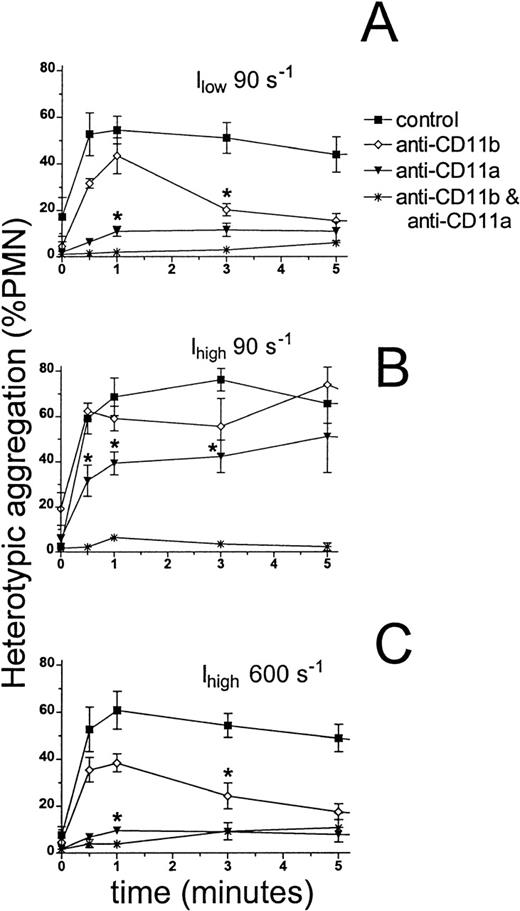
The kinetics of heterotypic aggregation following shear and fMLP stimulation.
Neutrophils (1 × 106 cells/mL) and 300.19 cells (2 × 106 cells/mL) were labeled, stimulated, and sheared as described in Figure 1. Aggregation was monitored as described in “Materials and Methods.” Heterotypic aggregation is presented as the percentage of total neutrophils bound to a 300.19 cell. Aggregation kinetics over a range of shear rates as denoted are plotted for the parent 300.19 cell line (A) and for transfected clones expressing ICAM-1 at (B) low levels (Ilow) and (C) 8-fold higher levels (Ihigh). *P < .05 compared with peak aggregation at the same shear rate. Data are presented as mean ± SEM from 3 to 7 separate experiments.
The kinetics of heterotypic aggregation following shear and fMLP stimulation.
Neutrophils (1 × 106 cells/mL) and 300.19 cells (2 × 106 cells/mL) were labeled, stimulated, and sheared as described in Figure 1. Aggregation was monitored as described in “Materials and Methods.” Heterotypic aggregation is presented as the percentage of total neutrophils bound to a 300.19 cell. Aggregation kinetics over a range of shear rates as denoted are plotted for the parent 300.19 cell line (A) and for transfected clones expressing ICAM-1 at (B) low levels (Ilow) and (C) 8-fold higher levels (Ihigh). *P < .05 compared with peak aggregation at the same shear rate. Data are presented as mean ± SEM from 3 to 7 separate experiments.
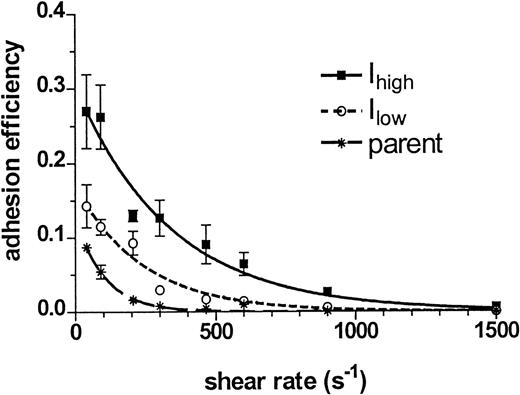
The efficiency of neutrophil capture of ICAM-1 cells provides a measure of the intrinsic properties of the cells that determine its adhesivity by accounting for physical parameters that regulate collision frequency, such as shear rate, aggregate size, and concentrations of neutrophils and 300.19 cells. Figure 3shows the dependence of adhesion efficiency on shear rate and ICAM-1 expression. Peak efficiency occurred at the lowest shear rate and diminished with increased shear. For example, stable adhesion between neutrophils and Ihigh at 40 s−1 occurred in 27 of 100 collisions, versus 15 of 100 for Ilow. These efficiencies were significantly higher than the 9 in 100 collisions between neutrophils and the parent cells that resulted in capture. Comparison of exponential decay functions used to fit efficiency versus shear rate indicated that neutrophils captured Ihigh twice as effectively as Ilow. Furthermore, the efficiency of adhesion to Ihigh was significantly above baseline up to 900 s−1, as compared with 300 s−1for Ilow. The efficiency of adhesion to non–ICAM-1 ligands expressed on parent 300.19 cells approached zero at 200 s−1. Adhesion efficiency decreased directly with increased shear rate over a log range in ICAM-1 from approximately 2 × 105 to 2 × 106 receptors per cell. Together these data indicate that the efficiency and extent of 300.19 cell recruitment into aggregates were dependent upon ICAM-1 expression and the magnitude of applied shear rate.
Adhesion efficiency as a function of shear rate and ICAM-1 expression level.
The kinetics of heterotypic aggregation over the first 60 seconds of fMLP stimulation and initiation of shear were fit with a mathematical model at each shear rate, as described in “Materials and Methods.” Figure shows the efficiency of neutrophil capture of the parent 300.19 cell line and of transfected clones expressing low (Ilow) and high (Ihigh) levels of ICAM-1. The experimental data were fit as smooth curves with a first-order exponential decay function. Data are presented as mean ± SEM from 3 to 7 separate experiments.
Adhesion efficiency as a function of shear rate and ICAM-1 expression level.
The kinetics of heterotypic aggregation over the first 60 seconds of fMLP stimulation and initiation of shear were fit with a mathematical model at each shear rate, as described in “Materials and Methods.” Figure shows the efficiency of neutrophil capture of the parent 300.19 cell line and of transfected clones expressing low (Ilow) and high (Ihigh) levels of ICAM-1. The experimental data were fit as smooth curves with a first-order exponential decay function. Data are presented as mean ± SEM from 3 to 7 separate experiments.
Shear-induced adhesion of unstimulated neutrophils to ICAM-1
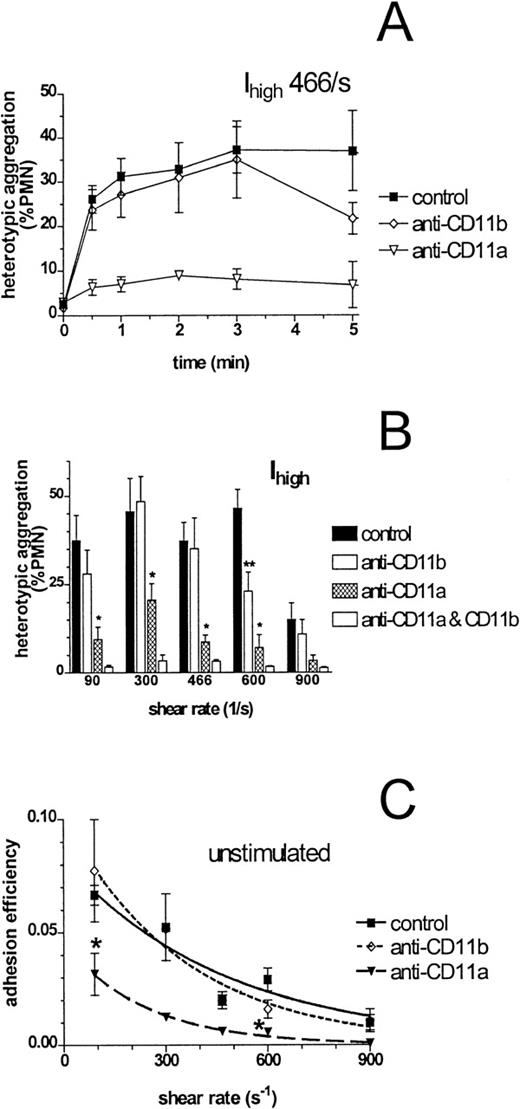
Unstimulated neutrophils have been shown to adhere to purified ICAM-1 in a parallel plate flow chamber.3 In the absence of chemotactic stimulation, application of shear resulted in significant formation of heterotypic aggregates that plateaued within 1 minute and remained stable to 5 minutes (Figure4A). Most aggregates comprised a single neutrophil bound to a single 300.19 cell, as compared with the multicellular aggregates formed after fMLP stimulation (Figure 1). Subsequent activation by fMLP addition at 2 or 3 minutes after initiation of shear boosted aggregation to the same peak level (approximately 75%) observed for concurrent application of shear and fMLP. Preincubation of neutrophils with anti-CD11a significantly inhibited shear-induced adhesion at all shear rates tested (Figure 4B). Significant inhibition of unstimulated adhesion with anti-CD11b was detected only at 600 s−1. However, CD11b/CD18 did contribute to the stability of adhesion because disaggregation occurred by 5 minutes with anti-CD11b (Figure 4A). Further, complete inhibition of aggregation at each shear rate required concomitant blocking of CD11a and CD11b (Figure 4B).
Shear-induced adhesion of unstimulated neutrophils to ICAM-1 transfectants.
Neutrophils (1 × 106 cells/mL) were preincubated for 10 minutes with saturating concentrations of monoclonal antibodies to CD11a or CD11b. They were then mixed with Ihigh(2 × 106 cells/mL), added to the cone-plate viscometer, and allowed to equilibrate for 2 minutes before initiation of fluid shear. Samples were taken at prescribed time points, fixed, and detected by flow cytometry as described in “Materials and Methods.” (A) Kinetics of aggregation are plotted for Ihigh at a shear rate of 466 s−1. (B) Peak extent of shear-induced aggregation occurred within 1 minute of initiation of shear and is plotted for Ihigh over a range of shear rates and antibody blocking conditions. *P < .01 compared with control at the same shear rate; **P < .05 compared with control and with concurrent block of CD11a and CD11b at the same shear rate. (C) Data from Figure 4B were modeled to calculate adhesion efficiency over the first 60 seconds after initiation of shear, as described in “Materials and Methods.” Smooth lines represent curves fit to experimental data with a first-order exponential decay function. *P < .05 compared with control at the same shear rate. Data are presented as mean ± SEM from 3 to 6 separate experiments.
Shear-induced adhesion of unstimulated neutrophils to ICAM-1 transfectants.
Neutrophils (1 × 106 cells/mL) were preincubated for 10 minutes with saturating concentrations of monoclonal antibodies to CD11a or CD11b. They were then mixed with Ihigh(2 × 106 cells/mL), added to the cone-plate viscometer, and allowed to equilibrate for 2 minutes before initiation of fluid shear. Samples were taken at prescribed time points, fixed, and detected by flow cytometry as described in “Materials and Methods.” (A) Kinetics of aggregation are plotted for Ihigh at a shear rate of 466 s−1. (B) Peak extent of shear-induced aggregation occurred within 1 minute of initiation of shear and is plotted for Ihigh over a range of shear rates and antibody blocking conditions. *P < .01 compared with control at the same shear rate; **P < .05 compared with control and with concurrent block of CD11a and CD11b at the same shear rate. (C) Data from Figure 4B were modeled to calculate adhesion efficiency over the first 60 seconds after initiation of shear, as described in “Materials and Methods.” Smooth lines represent curves fit to experimental data with a first-order exponential decay function. *P < .05 compared with control at the same shear rate. Data are presented as mean ± SEM from 3 to 6 separate experiments.
Modeling of adhesion efficiency of unstimulated neutrophils to 300.19 cells revealed that 7 of 100 collisions at the lowest shear rate resulted in capture of Ihigh (Figure 4C), a significantly greater level than that observed for adhesion to parent cells (< 2%). As indicated earlier, shear-induced capture of ICAM-1–expressing cells was attributed entirely to CD11a/CD18 because no significant difference from control efficiency was detected upon blocking of CD11b.
To determine whether constitutive adhesion attributed to CD11a/CD18 was due to activation of neutrophils during isolation, we assessed heterotypic aggregation in whole blood. Venous blood was drawn into a syringe containing heparin and the prostaglandin analogue ZK36 374, which inhibits activation and aggregation of platelets.38Whole blood was diluted 1:5 in HEPES buffer, mixed with Ihigh cells (2 × 106/mL), and sheared in a manner identical to that in the isolated cell suspension experiments. Kinetics of shear-induced heterotypic aggregation were comparable to those of isolated neutrophils, with a peak heterotypic aggregation of 50% ± 6% at 600 s−1 (n = 3). Anti-CD11a blocked this aggregation and anti-CD11b had no effect. We also examined the possibility of activation through release of chemotactic stimuli by 300.19 cells. Pretreatment of neutrophils with pertussis toxin to block Giα-mediated signal transduction did not inhibit shear-induced adhesion to Ihigh, but did block the boost in avidity after fMLP stimulation (data not shown). Together these data indicate that neutrophils can constitutively bind ICAM-1 through CD11a/CD18 without chemotactic activation.
Relative contributions of CD11a/CD18 and CD11b/CD18 in neutrophil adhesion to ICAM-1 with stimulation
β2-Integrins were required for neutrophil adhesion to Ilow and Ihigh cells over the time course of fMLP stimulation (Figure 5). For adhesion to Ilow at a shear rate of 90 s−1, blocking of CD11b did not significantly alter the rate of aggregation, and CD11a/CD18 alone supported adhesion up to the peak level observed for untreated control at 1 minute (Figure 5A). However, after maximum aggregation at 1 minute, disaggregation proceeded more rapidly in the presence of anti-CD11b as compared with untreated control. In comparison, CD11b/CD18-dependent adhesion (in the presence of anti-CD11a) proceeded more slowly and reached a level only approximately 20% of control. These aggregates remained stable over 5 minutes (Figure 5A).
Contributions of CD11a and CD11b to neutrophil–ICAM-1 adhesion over the time course of fMLP stimulation.
Neutrophils (1 × 106 cells/mL) were preincubated with saturating concentrations of anti-CD11a, anti-CD11b, or both concurrently. They were then mixed with Ihigh(2 × 106 cells/mL), added to the cone-plate viscometer, and allowed to equilibrate for 2 minutes before stimulation with 1 μmol/L fMLP and initiation of fluid shear. Samples were taken at prescribed time points, fixed, and detected by flow cytometry as described in “Materials and Methods.” The contribution of each β2-integrin subunit to capture of 300.19–ICAM-1 was assessed under the following conditions: (A) Ilow at 90 s−1, (B) Ihigh at 90 s−1, and (C) Ihigh at 600 s−1. *P < .05 compared with control at the same time point. Data are presented as mean ± SEM from 3 to 6 separate experiments.
Contributions of CD11a and CD11b to neutrophil–ICAM-1 adhesion over the time course of fMLP stimulation.
Neutrophils (1 × 106 cells/mL) were preincubated with saturating concentrations of anti-CD11a, anti-CD11b, or both concurrently. They were then mixed with Ihigh(2 × 106 cells/mL), added to the cone-plate viscometer, and allowed to equilibrate for 2 minutes before stimulation with 1 μmol/L fMLP and initiation of fluid shear. Samples were taken at prescribed time points, fixed, and detected by flow cytometry as described in “Materials and Methods.” The contribution of each β2-integrin subunit to capture of 300.19–ICAM-1 was assessed under the following conditions: (A) Ilow at 90 s−1, (B) Ihigh at 90 s−1, and (C) Ihigh at 600 s−1. *P < .05 compared with control at the same time point. Data are presented as mean ± SEM from 3 to 6 separate experiments.
Adhesion to Ihigh at 90 s−1 was also dependent on β2-integrins and, as indicated earlier, blocking of CD11b did not significantly alter the initial boost in aggregate formation (Figure 5B). Anti-CD11a effectively slowed the rate of aggregation as demonstrated for Ilow. However, the increased availability of ICAM-1 on Ihigh markedly increased the extent of CD11b/CD18-dependent capture. In contrast to Ilow, aggregation with Ihigh was sustained for more than 5 minutes, demonstrating persistence of adhesion through either CD11a/CD18– or CD11b/CD18–ICAM-1 bonds at this relatively low shear rate.
An increase in shear rate up to 600 s−1 resulted in a pattern of adhesion to Ihigh resembling that to Ilow at 90 s−1 (Figure 5C). A significant contribution to the initial boost in aggregation was again evident only for CD11a/CD18-dependent adhesion. Aggregation was reversible in the presence of anti-CD11b, as observed for the low shear and low ICAM-1 condition. Together these data suggest that CD11a/CD18 alone is sufficient for the initial boost in neutrophil adhesion at low and high ICAM-1 surface density over a wide range of shear rates. The contribution of CD11b/CD18 was most apparent in maintaining the stability of formed aggregates after initial tethering through CD11a/CD18. A distinct contribution from CD11b/CD18 in capture of ICAM-1 cells was detected only at high ICAM-1 surface density or at low shear rates corresponding to relatively long intercellular contact durations.
Specific molecular interactions support neutrophil adhesion to ICAM-1
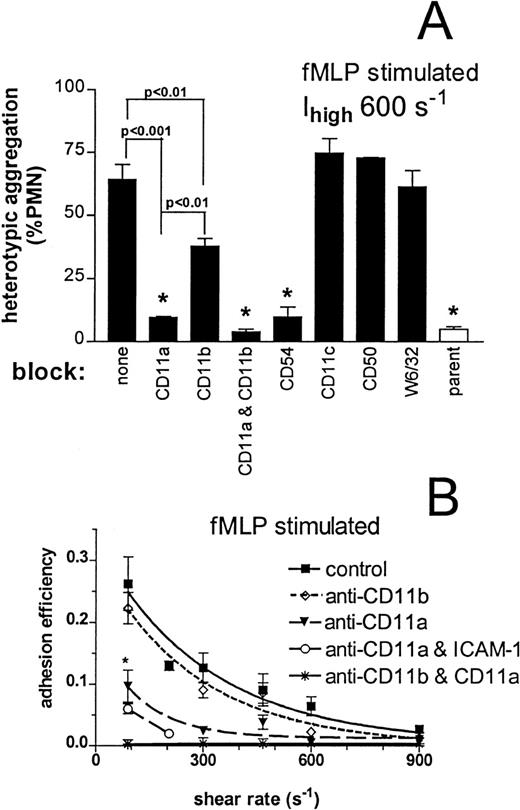
We next determined which neutrophil adhesion molecules bound ICAM-1 expressed on 300.19 cells. The peak extent of aggregation after fMLP stimulation at an optimal shear rate of 600 s−1 is plotted in Figure 6A for samples pretreated with a panel of antibodies. Under these conditions, approximately 65% of the neutrophil population formed aggregates with Ihigh, as compared with 7% for adhesion to parent cells. Addition of anti–ICAM-1decreased aggregation to background, as did simultaneously blocking CD11a and CD11b; these results indicate that adhesion through non–ICAM-1 ligands was minimal. Addition of anti-CD11a alone also decreased heterotypic aggregation to background. In contrast, blocking CD11b decreased aggregation by a lesser but significant amount (approximately 40%). In control experiments, we blocked CD11c, a third β2-integrin subunit that supports leukocyte adhesion to stimulated endothelium,32 ICAM-3 (CD50), which serves as a major ligand for LFA-1 in homotypic neutrophil aggregation,40 and the neutrophil HLA class 1 receptor. None of these molecules contributed to heterotypic aggregation. Under these conditions, CD11a/CD18 is necessary and sufficient for collisional adhesion to ICAM-1; however, both CD11a/CD18 and CD11b/CD18 are required for optimal adhesion.
Receptors that support neutrophil adhesion to ICAM-1.
Neutrophils (1 × 106 cells/mL) were preincubated with saturation concentrations of blocking antibodies, mixed with either Ihigh or parent 300.19 cells (2 × 106 cells/mL), stimulated with 1 μmol/L fMLP, and sheared in the cone-plate viscometer. (A) Peak heterotypic aggregation at 1 minute after stimulation with fMLP and initiation of shear at 600 s−1 is compared for saturating concentrations of blocking antibodies as denoted. The open bar depicts binding to the nontransfected parent 300.19 cell. *P > .05 compared with other bars with the same symbol. (B) Adhesion efficiency for aggregation mediated through CD11a/CD18 and CD11b/CD18. The kinetics of heterotypic aggregation over the first 60 seconds after stimulation with fMLP and initiation of shear were fit with a mathematical model at each shear rate. Smooth lines represent curves fit to experimental data with a first-order exponential decay function. *P < .05 compared with control at the same shear rate. Data are presented as mean ± SEM from 3 to 6 separate experiments.
Receptors that support neutrophil adhesion to ICAM-1.
Neutrophils (1 × 106 cells/mL) were preincubated with saturation concentrations of blocking antibodies, mixed with either Ihigh or parent 300.19 cells (2 × 106 cells/mL), stimulated with 1 μmol/L fMLP, and sheared in the cone-plate viscometer. (A) Peak heterotypic aggregation at 1 minute after stimulation with fMLP and initiation of shear at 600 s−1 is compared for saturating concentrations of blocking antibodies as denoted. The open bar depicts binding to the nontransfected parent 300.19 cell. *P > .05 compared with other bars with the same symbol. (B) Adhesion efficiency for aggregation mediated through CD11a/CD18 and CD11b/CD18. The kinetics of heterotypic aggregation over the first 60 seconds after stimulation with fMLP and initiation of shear were fit with a mathematical model at each shear rate. Smooth lines represent curves fit to experimental data with a first-order exponential decay function. *P < .05 compared with control at the same shear rate. Data are presented as mean ± SEM from 3 to 6 separate experiments.
Activation boosted the efficiency of neutrophil adhesion to 300.19 ICAM-1 cells approximately 4-fold above unstimulated cell suspensions (Figures 4 and 6B). As observed for unstimulated neutrophils, adhesion efficiency over the first minute of stimulation was supported almost entirely by CD11a/CD18. Only at the lowest shear did CD11b/CD18 significantly bind 300.19-Ihigh cells, at approximately one third the efficiency of CD11a/CD18. Concurrent blocking of CD11a and ICAM-1 indicated that adhesion was largely attributable to binding through CD11b/CD18–ICAM-1.
Effects of shear stress on neutrophil adhesion to ICAM-1
The efficiency of neutrophil adhesion to ICAM-1 300.19 cells was found to decrease with increasing shear rate (Figures 4 and 6B). According to 2-body collision theory, as shear rate is increased, cells in suspension collide more frequently and with shorter encounter duration, which could limit adhesion by constraining the number of integrin–ICAM-1 bonds formed. Alternatively, the predominant effect of increased shear rate may be the accompanying increase in fluid drag and translation of tensile force to the intercellular contact region. This may independently limit capture efficiency by quickly rupturing the bonds initially formed during transient contact. To examine the effects of an increase in shear stress, we raised the buffer viscosity by adding Ficoll. The increase in viscosity from 0.7 to 1.7 cp is expected to result in approximately a 2.5-fold increase in shear stress at a given shear rate.
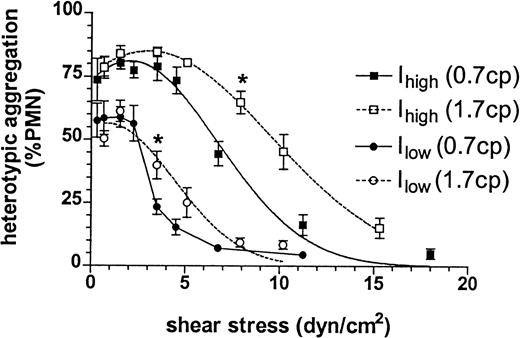
Plots of peak heterotypic aggregation versus shear stress assumed similar patterns in low- and high-viscosity buffers. Aggregation remained constant to a threshold level of shear stress of approximately 5 dyne/cm2 for Ihigh and approximately 2.5 dyne/cm2 for Ilow (Figure7). The predominant influence of stress on adhesion was revealed above these respective thresholds, where adhesion to each cell line steadily decreased as stress was increased. The influence of shear rate was revealed by the significantly increased aggregation of suspensions in the high-viscosity as compared with the low-viscosity buffer, as the latter was sheared approximately 2.5 times faster at a given shear stress.
The effect of shear stress on neutrophil adhesion to ICAM-1.
Heterotypic aggregation of neutrophils (1 × 106cells/mL) and 300.19 cells (2 × 106 cells/mL) was measured as described previously in normal HEPES buffer of viscosity 0.7 cp (filled symbols) or in buffer augmented with 6% Ficoll to increase viscosity to 1.7 cp (open symbols). Peak heterotypic aggregation at 1 minute after stimulation with 1 μmol/L fMLP and initiation of shear is plotted versus shear stress for Ilow(circles) and Ihigh (squares). *P < .05 compared with maximum value on the same curve. Data are presented as mean ± SEM from 3 to 6 separate experiments.
The effect of shear stress on neutrophil adhesion to ICAM-1.
Heterotypic aggregation of neutrophils (1 × 106cells/mL) and 300.19 cells (2 × 106 cells/mL) was measured as described previously in normal HEPES buffer of viscosity 0.7 cp (filled symbols) or in buffer augmented with 6% Ficoll to increase viscosity to 1.7 cp (open symbols). Peak heterotypic aggregation at 1 minute after stimulation with 1 μmol/L fMLP and initiation of shear is plotted versus shear stress for Ilow(circles) and Ihigh (squares). *P < .05 compared with maximum value on the same curve. Data are presented as mean ± SEM from 3 to 6 separate experiments.
Strength of adhesion through CD11a/CD18– and CD11b/CD18–ICAM-1 bonds
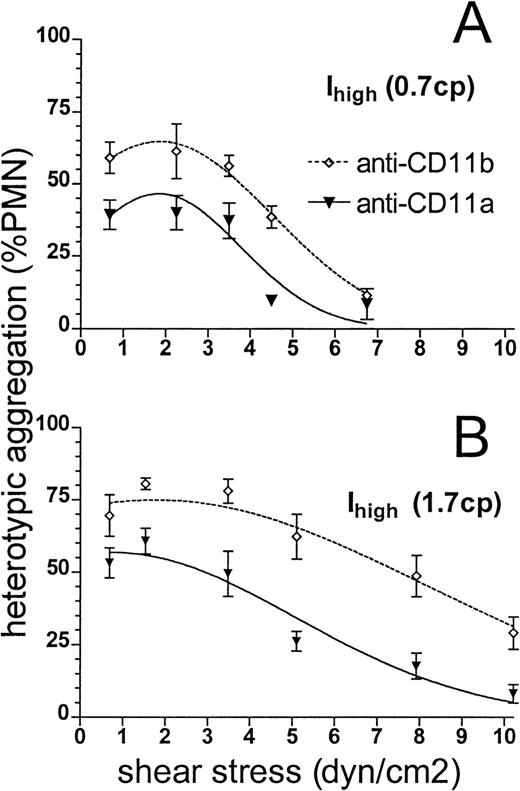
The effects of increased stress on aggregates formed through CD11a/CD18 or CD11b/CD18 were examined by blocking each with antibody. Plots of peak aggregation versus shear stress indicated that either CD11a/CD18 or CD11b/CD18 can support adhesion at stresses less than 4 dyne/cm2 (Figure 8A). At stresses above this threshold, adhesion in low-viscosity buffer through either subunit alone decreased at similar rates, reaching background by 7 dyne/cm2. In high-viscosity buffer, adhesion through CD11a/CD18 was potentiated and supported 3-fold more aggregation than CD11b/CD18 at stresses above 4 dyne/cm2. Analysis of adhesion efficiency indicated that CD11a/CD18-mediated capture of ICAM-1 cells was actually more efficient in high-viscosity as compared with low-viscosity buffer (0.37 versus 0.22 at 90 s−1). Together the data suggest that shear stress can limit β2-integrin–ICAM-1 bond formation and that CD11a/CD18 can support adhesion at 3-fold higher stress than CD11b/CD18.
Strength of adhesion through CD11a/CD18 and CD11b/CD18.
Neutrophils (1 × 106 cells/mL) were preincubated with anti-CD11a (▾) or anti-CD11b (⋄) for 10 minutes at saturating concentrations and then mixed with 300.19 Ihigh cells (2 × 106 cells/mL). Suspensions were stimulated with 1 μmol/L fMLP and sheared in the cone-plate viscometer over a range of shears. Peak extent of heterotypic aggregation in the presence of anti-CD11b or anti-CD11a was measured and plotted at each shear stress. Aggregation is compared for experiments performed in (A) normal HEPES buffer of viscosity 0.7 cp or (B) buffer of viscosity 1.7 cp augmented with 6% Ficoll. Data are presented as mean ± SEM from 3 to 6 separate experiments.
Strength of adhesion through CD11a/CD18 and CD11b/CD18.
Neutrophils (1 × 106 cells/mL) were preincubated with anti-CD11a (▾) or anti-CD11b (⋄) for 10 minutes at saturating concentrations and then mixed with 300.19 Ihigh cells (2 × 106 cells/mL). Suspensions were stimulated with 1 μmol/L fMLP and sheared in the cone-plate viscometer over a range of shears. Peak extent of heterotypic aggregation in the presence of anti-CD11b or anti-CD11a was measured and plotted at each shear stress. Aggregation is compared for experiments performed in (A) normal HEPES buffer of viscosity 0.7 cp or (B) buffer of viscosity 1.7 cp augmented with 6% Ficoll. Data are presented as mean ± SEM from 3 to 6 separate experiments.
Discussion
The major findings of this study are as follows: (1) Neutrophils constitutively adhered to ICAM-1 cells through CD11a/CD18 in isolated suspensions and in whole blood; (2) CD11a/CD18 accounted for most of the capture efficiency under shear conditions, whereas CD11b/CD18 supported stable adhesion over minutes of chemotactic stimulation; and (3) chemotactic stimulation increased the efficiency of CD11a/CD18-dependent adhesion by 4-fold. This efficiency was optimal at the lowest applied shear and decreased as shear was increased. Thus, we provide evidence that cooperation between CD11a/CD18 and CD11b/CD18 is based at least in part on CD11a/CD18 functioning as a tether to sustain contact duration sufficient for CD11b/CD18 binding.
Efficiency of β2-integrin–dependent neutrophil adhesion
Previous studies using rotational viscometry have found that the efficiency of homotypic neutrophil adhesion was 0.1 at 100 s−1 and increased to a peak level of 0.4 between 400 s−1 and 800 s−1.34 When L-selectin was blocked with antibody, adhesion was solely dependent on CD11a/CD18 and CD11b/CD18 and the efficiency was again 0.1 at 100 s−1, but in this case it dropped steadily to 0 by 400 s−1.40 In the present study, efficiency of neutrophil adhesion to 300.19-Ilow was also approximately 0.1 at 100 s−1 and dropped to 0 by 400 s−1. Efficiency of capture of 300.19-Ihigh at 100 s−1 was increased to approximately 0.3 and remained above 0 at 900 s−1. These data demonstrate that increases in ICAM-1 site density increase neutrophil adhesion efficiency in the absence of selectin tethering.
Chemotactic stimulation boosts the efficiency of ICAM-1 capture predominantly through CD11a/CD18
Neutrophils exhibited a capacity to adhere to 300.19–ICAM-1 cells in the presence of shear and absence of chemotactic stimulation. Both isolated neutrophils and those in whole blood constitutively bound 300.19–ICAM-1 cells over a range of shear. The binding of CD11a/CD18 alone was sufficient for neutrophil capture, as addition of anti-CD11b produced minimal inhibition at most shears. Chemotactic stimulation increased the efficiency of adhesion almost 4-fold from the unstimulated case. This boost was attributed to CD11a/CD18 because anti-CD11b again had little effect. However, total inhibition of adhesion required blocking of both β2-integrins. A contribution of CD11b/CD18 to aggregate stability was apparent within several minutes of shear in the presence and absence of fMLP stimulation. This pattern of β2-integrin–mediated adhesion is different from that reported in a previous study that described adhesion to a murine melanoma cell line expressing ICAM-1 (E3–ICAM-1).31 37 Neutrophil adhesion to the E3–ICAM-1 cells was supported equally by CD11a/CD18 binding of ICAM-1 and CD11b/CD18 binding of undefined ligands. In contrast, adhesion to 300.19–ICAM-1 was almost entirely dependent on expression of ICAM-1, with less than a 15% contribution of CD11b/CD18 binding to non–ICAM-1 ligands.
Chemotactic stimulation increases the avidity of CD11a/CD18 to bind ICAM-1
Recently published data have shown that chemotactic stimulation can induce activation of CD11a/CD18 on lymphocytes over a time scale of seconds, resulting in a transition from rolling to firm arrest through binding to ICAM-1.28 The current study provides the first evidence of a rapid increase in the avidity of CD11a/CD18 on neutrophils after chemotactic stimulation. The precise mechanisms underlying this boost in avidity remain unknown but appear to involve a rapid increase in the binding of CD11a/CD18 to ICAM-1 at the site of collisional contact rather than diffusion of constitutive or newly activated receptor. This hypothesis is based on the fact that CD11a expression, which is comparable to CD11b on resting neutrophils, remains unchanged after chemotactic stimulation. Moreover, the predicted minimum contact duration at which adhesion to 300.19-Ihigh cells was detected is on the order of 4 milliseconds at 600 s−1 (e.g., tcontact≈ 2.6/shear rate).34,41 This interval is insufficient for significant numbers of CD11a/CD18 sites to diffuse into the region of cell–cell contact.42 43
Sequential recognition events characterize the kinetics and stability of adhesion of β2-integrins to ICAM-1
Shear rate and ICAM-1 availability were the predominant parameters regulating the formation and stability of heterotypic aggregates. At low shear rate and high ICAM-1 expression, either CD11a/CD18 or CD11b/CD18 alone supported adhesion to 300.19 cells. However, differences in function became apparent when these parameters were varied to limit receptor-ligand availability and collisional contact duration. CD11a/CD18 exhibited greater avidity in both stimulated and unstimulated states and was sufficient for optimal capture of ICAM-1 at high and low expression levels over a range of shears. Adhesion in the presence of CD11a/CD18 alone, however, was not stable over time in shear. Conversely, CD11b/CD18 was less than half as efficient as CD11a/CD18 in capture but supported stable adhesion for up to 15 minutes of shear. When CD11b was blocked and shear was greater than 300 s−1, adhesion was reversible within 2 minutes (Figure5). These data suggest that within minutes of fMLP stimulation, CD11a/CD18 undergoes a reversal in its ability to both form and maintain bonds with ICAM-1 and that CD11b/CD18 stabilizes adhesion over time. The mechanism underlying the cooperation between CD11a and CD11b in mediating optimum adhesion to ICAM-1 remains unknown. One possibility is that initial high-avidity binding of CD11a/CD18 facilitates subsequent engagement of lower-avidity CD11b/CD18 sites by prolonging contact duration and increasing membrane contact area. Alternatively, CD11a/CD18 binding may initiate intracellular signaling, leading to a shear-dependent increase in CD11b/CD18 avidity. It has been reported that CD11a/CD18 directly signals the release of reactive oxygen species in adherent neutrophils.44
Shear stress and shear rate differentially influence adhesion through CD11a/CD18 and CD11b/CD18
A distinct threshold in stress was evident for each 300.19–ICAM-1 clone (Figure 7). Maximal adhesion to Ihigh was maintained to 5 dyne/cm2, twice the level for Ilow. Drag force exerted on an aggregate suspended in a newtonian fluid is predicted to increase linearly with an increase in shear rate or viscosity.36 At stresses above the respective threshold values, peak aggregation decreased in a linear manner (Figure 7). In contrast, the nonlinear relation between shear rate and collisional contact duration correlated with an exponential decline in adhesion efficiency as shear rate was increased (Figure 3).34 41 The dominant influence of contact duration on adhesion is illustrated in the peak aggregation versus shear stress plots of Figure 8. At a given stress, cells in high-viscosity buffer were sheared at a rate approximately 2.5 times slower than in low-viscosity buffer. The resulting prolonged collisional contact duration in high-viscosity buffer doubled the extent of aggregation. CD11a/CD18 supported aggregation at stresses greater than 10 dyne/cm2, whereas adhesion through CD11b/CD18 fell to background above 5 dyne/cm2. At comparable bond density, the greater strength of adhesion through CD11a/CD18 may be attributed to greater tensile strength of CD11a/CD18–ICAM-1 bonds than of CD11b/CD18–ICAM-1 bonds. Alternatively, CD11a/CD18 may bind more rapidly and form more individual bonds during collision. The latter hypothesis appears more likely given the capacity of CD11a/CD18 to support adhesion at higher shear rates and low ICAM-1 surface density.
On the basis of the kinematics of 2 spherical particles in a linear shear field,41 we estimated the minimum contact duration necessary for neutrophil capture of 300.19 cells to be approximately 13 milliseconds for Ilow and less than 4 milliseconds for Ihigh. These predictions do not account for cell deformation, which could prolong collision duration. The implication for neutrophil emigration is that an increase in ICAM-1 expression from levels at rest to those in inflamed endothelium could effectively raise the permissible shear rate (i.e., decrease the minimum encounter duration) at which β2-integrin–mediated capture is likely to occur. Kinematic analysis of a spherical cell colliding with a planar substrate predicts that the contact duration in the absence of subsequent adhesion is approximately 0.1/shear rate.45 This is 25 times shorter than for 2 cells colliding in suspension. Flow chamber studies of activated neutrophils arresting on purified ICAM-1 have indicated that adhesion occurred at shear rates less than 28 s−1.46 This corresponds to a minimum contact duration of approximately 4 milliseconds, a value in close agreement with the current estimate for CD11a/CD18-supported capture of 300.19-Ihigh cells in suspension.
In this study, we demonstrate that CD11a/CD18 on neutrophils is constitutively active and increases its avidity for ICAM-1 within seconds of chemotactic stimulation. Optimum adhesion was dependent on chemotactic activation of CD11a/CD18 and CD11b/CD18, which bind sequentially to support neutrophil capture and stable adhesion. Efficiency of capture was influenced separately by ICAM-1 density and fluid shear rate and stress. The implication is that during ischemic or inflammatory events in the microcirculation, the efficiency of neutrophil capture and arrest may be regulated by hydrodynamic and molecular parameters.
Acknowledgments
We would like to thank Dr Robert Rothlein and Dr Kei Kishimoto of Boehringer-Ingelheim Pharmaceuticals for generously supplying the antibody reagents used.
Supported by National Institutes of Health Grant Nos. AI31652, AI19031, HL42550, HL18672, and NS23326; and grant no. C-938 from the Robert A. Welch Foundation. S.I.S. and G.S.K. are Established Investigators of the American Heart Association. S.I.S. is a fellow of the Whitaker Biomedical Foundation. This material is based upon work supported under a National Science Foundation Graduate Fellowship to E.R.H.
Reprints:Scott I. Simon, University of California at Davis, School of Engineering, 1 Shields Avenue, Davis, CA 95616-5294; e-mail:sisimon@ucdavis.edu.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal