Previous experiments suggest that actin disassembly, perhaps at a specific site, is required for platelet secretion. Platelet stimulation by phorbol 12-myristate 13-acetate (PMA) induced pleckstrin phosphorylation, platelet aggregation, and secretion. Inhibition of protein kinase C (PKC) is accompanied by inhibition of pleckstrin phosphorylation and serotonin secretion. Here, we demonstrate the presence of myristoylated alanine-rich C kinase substrate (MARCKS), another PKC substrate, in platelets and its phosphorylation during PMA stimulation. MARCKS is known to bind actin and to cross-link actin filaments; the latter is inhibited by PKC-induced MARCKS phosphorylation. MARCKS phosphorylation and serotonin release from permeabilized platelets have the same time course and were blocked by a peptide (MPSD) with the amino acid sequence corresponding to the phosphorylation site domain of MARCKS. Pleckstrin and myosin light chain phosphorylation was not modified. A peptide (Ala-MPSD) in which the four serine residues of MPSD were substituted by alanines was ineffective. These results provide the first evidence that MARCKS may play a role in platelet secretion. Moreover, pleckstrin phosphorylation has a different time course than that of MARCKS or serotonin release and was not modified when MARCKS phosphorylation and serotonin release were inhibited, suggesting that pleckstrin is either not directly involved in secretion or that it might only be involved upstream in the cascade of events leading to exocytosis.
In response to vessel injury or exposure to different substances, platelets undergo activation that consists of shape change, formation of pseudopodia, aggregation, and secretion.1-4These changes are also accompanied by the rearrangement of cytoskeleton components,4-6 together with the translocation of several proteins from cytosol to cytoskeleton.7-9 Actin polymerization-depolymerization cycles take place in different areas (ie, pseudopodia and central contractile gel) during platelet activation.4 Previous work has shown the presence of gelsolin10,11 and scinderin12 in platelets, 2 Ca++-dependent F-actin severing proteins that control actin network dynamics. It has been suggested that, during platelet aggregation, actin polymerizes and the content of the secretory granules is released to the cell exterior.1,4Opposite to this view are the results of experiments from our laboratory showing that recombinant scinderin potentiates Ca++-induced release of serotonin from permeabilized platelets.13 This work suggests that, similar to what is observed in other secretory systems (ie, chromaffin cell),14,15 actin disassembly is required for platelet secretion.13
Stimulation of protein kinase C (PKC) by phorbol 12-myristate 13-acetate (PMA) induces platelet aggregation and secretion.16-20 This is accompanied by phosphorylation of pleckstrin, a major substrate of PKC.16-20 It has also been postulated that pleckstrin is involved in platelet secretion, because inhibition of PKC decreases pleckstrin phosphorylation, and this is accompanied by inhibition of secretion.19,20 Thrombin stimulation of platelets produces PKC activation and phosphorylation of pleckstrin.21,22 Therefore, the effects of thrombin on platelets seem to be mediated, at least in part, through the activation of PKC.21-23 Activation of PKC in other secretory systems, such as the chromaffin cell system,15,24 potentiates secretion, and this effect of PKC activation is due to disassembly or disruption of cortical actin networks.15 This allows a large number of secretory vesicles to move to release sites on the plasma membrane.15 In view of this and of our previous work on the effects of recombinant scinderin on platelets,13 we have hypothesized that PKC activation in platelets produces actin filament disassembly enhancing the secretory response. In addition to pleckstrin, myristoylated alanine-rich C kinase substrate (MARCKS) is a PKC substrate that has the properties of binding actin and crosslinking actin filaments.25 26 Phosphorylation of MARCKS by PKC inhibits its ability to crosslink actin filaments.25Therefore, it becomes of interest to know whether MARCKS phosphorylation participates in the PKC effects on platelet secretion.
In this report, we describe the presence of MARCKS in platelets and its phosphorylation in response to PMA stimulation. We present evidence that a peptide (MPSD) with the amino acid sequence corresponding to the phosphorylation site domain of MARCKS blocks both MARCKS phosphorylation and serotonin release from permeabilized platelets in response to PMA stimulation. The MPSD peptide was specific for MARCKS and, in permeabilized platelets, pleckstrin and myosin light chain phosphorylation induced by PMA stimulation was unchanged. Therefore, the results described here provide the first evidence for a role of MARCKS in platelet secretion.
Materials and methods
Materials
Peptides that correspond to the phosphorylation site domain of MARCKS (MPSD, KKKKKRFSFKKSFKLSGFSFKKNKK) and to the same domain but with the serine residues replaced by alanine residues (Ala-MPSD, KKKKKRFAFKKAFKLAGFAFKKNKK) were custom-made by Research Genetics (Huntsville, AL). PMA, digitonin, and adenosine 5[prime]-triphosphate (ATP; disodium salt) were obtained from Sigma (Oakville, ON, Canada). PMA was prepared as a stock solution in DMSO and stored at −20°C. [3H]serotonin (5-HT) was purchased from DuPont (Boston, MA). All chemical and solvents were of analytical grade. Antibodies were obtained from the following sources: mouse monoclonal immunoglobulin (Ig)G raised against the C-terminal domain of human MARCKS (Upstate, Lake Placid, NY); goat polyclonal raised against a peptide mapping at the C-terminus of human MARCKS (Santa Cruz Biotechnology, Santa Cruz, CA); rabbit polyclonal IgG raised against a synthetic peptide corresponding to amino acids 641-673 of C-terminus of PKC (recognizes all PKC isoforms) (Upstate); mouse monoclonal raised against the N-terminus of human pleckstrin (Transduction Laboratories, Lexington, KY); horseradish peroxidase (HRP)-conjugated goat antimouse IgG (Bio-Rad, Mississauga, ON, Canada); HRP-conjugated affinity purified F(ab′)2 fragment rabbit antigoat F(ab′)2 fragment specific, HRP-conjugated goat antirabbit IgG, HRP-conjugated F(ab′)2 fragment donkey antimouse IgG, CY3-conjugated affinity purified F(ab′)2 fragment donkey antimouse IgG (all from Jackson Immuno Research Laboratories Inc, West Grove, PA); and mouse monoclonal IgG raised against human CD41a (PharMingen, Mississauga, ON, Canada).
Source of platelets
Platelet-rich plasma was obtained from the blood bank of Ottawa Red Cross and centrifuged at 200g for 15 minutes to eliminate red blood cells. The supernatant thus obtained was centrifuged at 800g for 15 minutes to obtain a platelet sediment.
Platelet permeabilization and labeling of serotonin stores
The platelet pellet was resuspended in Ca++-free Locke's solution (NaCl, 154 mM; KCl, 2.6 mM; K2HPO4, 2.14 mM; KH2PO4, 0.85 mM; MgCl2, 1.2 mM; glucose, 10 mM; and EGTA, 2.0 mM; pH 7.2). After a wash with Locke's solution, the platelet concentration was adjusted to 7.5 × 108/mL. Platelets were then incubated at 37°C for 90 minutes with 0.6 nmol [3H]5-HT/mL (specific activity = 0.94TBq/mmol; DuPont, Boston, MA).17 After incubation, the [3H]5-HT–labeled platelets were washed by incubation with 6 changes of 1 mL Ca++-free Locke's solution for 60 minutes before the experiments were commenced. [3H]5-HT–labeled platelets were permeabilized by treatment during 5 minutes with 15 μM of digitonin in K+-glutamate buffer (MgCl2, 12.5 mM; K+-glutamate, 160 mM; EGTA, 2.5 mM; EDTA, 2.5 mM; ATP, 5 mM; HEPES, 20 mM; pH 7.4).18 After permeabilization, platelets were centrifuged at 900g for 2 minutes (4°C) and then resuspended in K+-glutamate buffer. Ca++ concentrations required to give appropriate pCa (−log [Ca2+]) values were calculated as previously described.13 27 The K+-glutamate buffer used in the experiments has a pCa value of less than 9. The degree of permeabilization was determined using rhodamine-phalloidin (a probe for filamentous actin). Intact and permeabilized platelets were centrifuged onto polylysine-coated glass slides using a bench-top cytospin centrifuge (Cytofuge 2, Stat Spin Inc, Norwood, MA). Platelets were then fixed in 3.7% formaldehyde for 20 minutes and stained with rhodamine-phalloidin (1:200 dilution; Molecular Probes, Eugene, OR) for 15 minutes at room temperature, washed 3 times with phosphate-buffered saline (PBS; NaCl 130, mM; Na-phosphate, 100 mM; pH 7.2), and mounted in 50% glycerol/PBS. Platelet preparations were examined using incident fluorescent light, pictures were taken, and images were processed as described under “Fluorescence Microscopy.” The percentage of rhodamine-phalloidin–positive cells (permeablized platelets) was then determined from the prints.
Serotonin release studies
These studies were performed as described previously.13Briefly, samples (100 μL) containing 7.5 × 107permeabilized platelets in K+-glutamate buffer were stimulated with 100 nM of PMA for 45 seconds in the absence or presence of 10 μM of either MPSD or alanine-substituted MARCKS phosphorylation site domain peptide (Ala-MPSD). Release experiments were terminated by the addition of an equal volume of 6% glutaraldehyde in 0.1 mol/L phosphate buffer (pH 7.4). Preparations were centrifuged at 900g for 2 minutes. Sediments were extracted with 200 μL of 10% trichloroacetic acid (TCA), and radioactivity in supernatants and TCA extracts was measured in a liquid spectrometer (Beckman Instruments, Fullerton, CA). Total [3H]5-HT platelet content was determined by adding the radioactivity present in the incubation medium to the TCA extract. [3H]5-HT output was expressed as a percentage of total content after subtraction of values for spontaneous release. A minimum of 8 samples per condition were measured, and mean ± SEM was plotted.
[32P]Pi labeling of platelets
Platelets (7.5 × 108/mL) suspended in Ca++-free Locke's solution were centrifuged at 800g for 2 minutes. The platelet sediment was resuspended in a phosphate-free solution (buffer P)28 of the following composition: NaCl, 145 mM; KCl, 5 mM; MgSO4, 1 mM; glucose, 10 mM; HEPES, 25 mM; EGTA, 0.5 mM; pH 7.3, to give a platelet concentration of 5 × 108/mL. Platelets were incubated for 60 minutes in buffer P containing 5.5 GBg carrier-free [32P]Pi/mL (Amersham, Oakville, ON, Canada). Platelets were then sedimented by centrifugation at 800g for 2 minutes and washed twice with the same buffer.
Protein phosphorylation studies
[32P]Pi-labeled platelets were permeabilized with digitonin as indicated above. During the 5-minute permeabilization period, aliquots of platelets were incubated alone or in the presence of 10 μM of either MPSD or Ala-MPSD. PMA was present in the permeabilization media during the last 3 minutes. When total proteins (heat-stable and heat-sensitive) were studied, incubation was terminated by addition of an equal volume of twice-concentrated Laemmli's loading buffer (Tris-HCl, 125 mM; glycerol, 20%; SDS, 4%; 2β mercaptoethanol, 10%; bromophenol blue, 0.05%; pH 6.8) followed by incubation at 95°C for 7 minutes. When heat-stable proteins were studied, incubation was stopped by the addition of twice-concentrated RIPA buffer (NaCl, 140 mM; KCl, 2.6 mM; Ha2PO4, 10 mM; KH2PO4, 1.8 mM; NP-40, 1%; sodium deoxycholate, 0.5%) containing 1 μg of aprotinin/mL, 1 μg leupeptin/mL, 1 mM PMSF, 1 mM NaVO4, 1 mM NaF, and 50 mM benzamidine followed by boiling for 10 minutes. Boiled platelet extracts were then centrifuged at 16 000g for 2 minutes. Supernatants thus obtained were mixed with equal volumes of twice-concentrated Laemmli's loading buffer. The preparations were then heated to 95°C for 7 minutes.
Electrophoresis and immunoblotting
All protein samples were analyzed by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Monodimensional SDS-PAGE was performed according to Doucet and Trifaró.29 For Western blotting, proteins were electrotransferred to nitrocellulose membranes (pore size: 0.45 μm, Bio-Rad), and immunoblotting was performed with antibodies raised against different antigens (MARCKS, PKC, and pleckstrin). This was followed by incubation with the corresponding HRP-conjugated secondary antibodies (goat antimouse IgG, goat antirabbit IgG, and rabbit antigoat IgG).
Autoradiography and densitometric analysis
Coomassie brilliant blue stained gels or nitrocellulose membranes were exposed to Hyperfilm ECL (Amersham). The intensity of the autoradiograph bands was analyzed using Scion Image Beta 2 software (Scion Corp, Frederick, MD). The areas under the peaks were integrated using the same program, and results were expressed in arbitrary units.
Fluorescence microscopy
Platelets were centrifuged onto polylysine-coated glass slides using a bench-top cytospin centrifuge (Cytofuge 2). Platelets were immediately fixed in 3.7% formaldehyde in PBS for 20 minutes. Preparations were washed several times with PBS, permeabilized with 1% Triton X-100 for 3 minutes, washed again with PBS, and incubated with 1% bovine serum albumin and 1% donkey pre-immune serum in PBS for 1 hour at room temperature to block nonspecific binding sites. Platelets were then washed with PBS and incubated with either nonspecific mouse IgG (control, 1:250 dilution), human MARCKS mouse monoclonal antibody (1:250 dilution), or human CD41a mouse monoclonal antibody (1:200 dilution) for 1 hour at room temperature. All preparations were then washed 3 times with PBS and incubated for 1 hour with (secondary antibody) affinity-purified CY3-conjugated donkey Fab2 fragment raised against mouse IgG (1:200 dilution). Preparations were then washed with PBS and mounted in Slowfade buffer containing 50% glycerol (Molecular Probes). Preparations were examined using incident fluorescent light under a Zeiss Axoplan microscope equipped with an HBO 50 mercury lamp and an oil immersion objective (100×; 1.3 aperture). Pictures were taken with a Sony digital camera, and the images were saved using a Northern Eclipse software (Empix, Mississauga, ON, Canada). Images were then digitally imported into Adobe Photoshop software for further analysis. Images were printed on Epson quality paper using an Epson Stylus Photo EX color printer (Epson America Inc, Torrance, CA).
Results
Characterization of the permeabilized platelet preparation
Platelets were permeabilized with 15 μM of digitonin as indicated above and incubated for different periods of time in the presence or absence of 100 nM of PMA. Intact platelets were also incubated under similar conditions. The time course of the spontaneous serotonin release was quite similar for intact and permeabilized preparations, with the exception of the 15-minute incubation period, where the spontaneous release was higher for permeabilized platelets (Figure1A). Time courses of serotonin release for permeabilized and intact platelets stimulated by PMA for different periods of time were also quite similar. Here again, release from permeabilized platelets after 15-minute incubation with PMA was higher than for intact platelets (Figure 1A). Platelets were also permeabilized by different periods of time, and they were stimulated for 45 seconds with 100 nM of PMA. Under these conditions, all secretory responses to PMA were similar (Figure 1B).
Effect of permeabilization on [3H]5-HT output from platelets.
(A) Time course of [3H]5-HT output from intact and permeabilized cells in the presence or absence of PMA. [3H]5-HT–labeled platelets were used and were permeabilized for 4 different periods of time with 15 μM of digitonin in K+-glutamate buffer. When indicated, PMA (100 nM) was present during the entire incubation period. After incubation, platelets were recovered by centrifugation, and the content of [3H]5-HT was measured in the medium and in the platelets. [3H]5-HT outputs were expressed as a percentage of total content. Values represent the mean ± SEM of at least 8 different preparations. (B) Effect of permeabilization is shown for different periods of time on the responses to a short period of stimulation with PMA. [3H]5-HT–labeled platelets were permeabilized as indicated in A, and they were stimulated for 45 seconds with 100 nM of PMA. Platelets were recovered by stimulation, and [3H]5-HT output was measured. Bars represent the mean ± SEM of 4 different preparations.
Effect of permeabilization on [3H]5-HT output from platelets.
(A) Time course of [3H]5-HT output from intact and permeabilized cells in the presence or absence of PMA. [3H]5-HT–labeled platelets were used and were permeabilized for 4 different periods of time with 15 μM of digitonin in K+-glutamate buffer. When indicated, PMA (100 nM) was present during the entire incubation period. After incubation, platelets were recovered by centrifugation, and the content of [3H]5-HT was measured in the medium and in the platelets. [3H]5-HT outputs were expressed as a percentage of total content. Values represent the mean ± SEM of at least 8 different preparations. (B) Effect of permeabilization is shown for different periods of time on the responses to a short period of stimulation with PMA. [3H]5-HT–labeled platelets were permeabilized as indicated in A, and they were stimulated for 45 seconds with 100 nM of PMA. Platelets were recovered by stimulation, and [3H]5-HT output was measured. Bars represent the mean ± SEM of 4 different preparations.
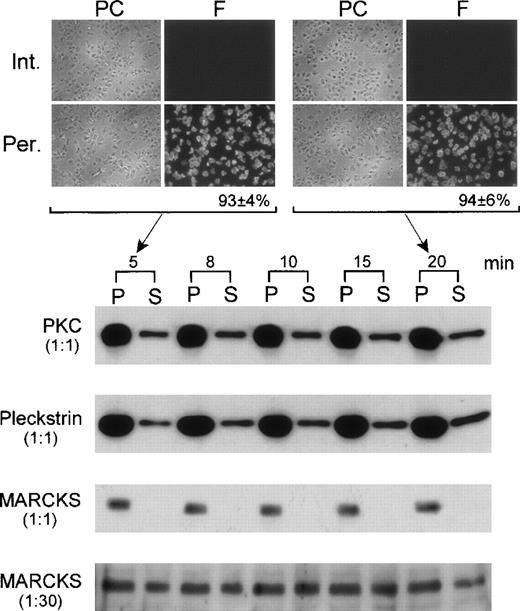
The effect of permeabilization on the leakage of proteins was also investigated. Permeabilized platelets were centrifuged after different periods of incubation. Platelets were then resuspended in volumes equal to that of the incubation media (supernatants). Equal aliquots of platelets, suspensions, and supernatants were taken and subjected to SDS-PAGE, followed by immunoblotting using antibodies against PKC, pleckstrin, and MARCKS (see below for characterization of MARCKS in platelets). PKC and pleckstrin were both detected in the medium under these conditions (Figure 2). However, MARCKS was not detected in the medium except when this was concentrated 30 times (Figure 2). The content of PKC in the medium after 5 minutes of treatment with digitonin was 24% of the total content and 25% after 20 minutes. Similarly, the amount of pleckstrin in the medium was 19% and 28% after 5- and 20-minute treatment with digitonin, respectively. Therefore, most of the protein leakage occurred during the first 5 minutes of permeabilization. In the case of MARCKS, the leakage into the medium corresponded to 2% and 4% of the total content after 5 and 20 minutes of permeabilization, respectively. Furthermore, the degree of digitonin permeabilization was monitored during the experiments using rhodamine-phalloidin (a probe for filamentous actin) as indicated in “Materials and Methods.” After 5 and 20 minutes of digitonin treatment, the percentages of permeabilized platelets were 93 ± 4% (n = 3) and 94 ± 6% (n = 3), respectively (Figure 2). This rules out the possibility that platelets were resealed during the 20-minute incubation period.
Leakage of proteins from digitonin-permeabilized platelets.
Platelets were incubated with 15 μM of digitonin in K+-glutamate buffer for different periods of times. Platelets were then recovered by centrifugation, and pellets (P) were resuspended in K+-glutamate buffer to the same volumes of the corresponding supernatants (S; incubation media). Equal aliquots (1:1) of P and S were run on SDS-PAGE, followed by immunoblotting with antibodies against PKC, pleckstrin, and MARCKS. Under these conditions, MARCKS was not detected in the medium (S). Therefore, S was concentrated 30 times; aliquots were run again and tested for the presence of MARCKS (1:30). In this case, a strong band was observed, and this is shown at the bottom of the figure. The degree of platelet permeabilization in this experiment was determined by incubating fixed platelets for 15 minutes with rhodamine-phalloidin. Results obtained for intact (Int.) and permeabilized (Per.) platelets after 5 and 20 minutes of digitonin treatment are shown at the top of the figure. Phase contrast (PC) and fluoresence (F) fields of the preparations are shown at left and right, respectively.
Leakage of proteins from digitonin-permeabilized platelets.
Platelets were incubated with 15 μM of digitonin in K+-glutamate buffer for different periods of times. Platelets were then recovered by centrifugation, and pellets (P) were resuspended in K+-glutamate buffer to the same volumes of the corresponding supernatants (S; incubation media). Equal aliquots (1:1) of P and S were run on SDS-PAGE, followed by immunoblotting with antibodies against PKC, pleckstrin, and MARCKS. Under these conditions, MARCKS was not detected in the medium (S). Therefore, S was concentrated 30 times; aliquots were run again and tested for the presence of MARCKS (1:30). In this case, a strong band was observed, and this is shown at the bottom of the figure. The degree of platelet permeabilization in this experiment was determined by incubating fixed platelets for 15 minutes with rhodamine-phalloidin. Results obtained for intact (Int.) and permeabilized (Per.) platelets after 5 and 20 minutes of digitonin treatment are shown at the top of the figure. Phase contrast (PC) and fluoresence (F) fields of the preparations are shown at left and right, respectively.
Identification of MARCKS in platelets and its phosphorylation during PKC activation
Platelets were sedimented on glass slides using a cytospin centrifuge as described in “Materials and Methods,” and they were immunostained using MARCKS and CD41a (fibrinogen receptor) antibodies. Platelets reacted with both antibodies and showed a strong fluorescence, indicating the presence of both antigens (Figure3). Permeabilized platelets, previously labeled with [32P]Pi, were also incubated for 3 minutes in the absence or presence of 100 nM of PMA. Heat-resistant proteins prepared as indicated in “Materials and Methods” were separated by SDS-PAGE. Proteins were then electrotransferred to nitrocellulose membranes, and this was followed by autoradiography and immunoblotting with MARCKS monoclonal antibodies. PMA treatment increased the phosphorylation of several heat-stable proteins (Figure4). Two of these bands were identified as MARCKS by the antibody. These bands had the same electrophoretic mobility of protein bands present in neuroblastoma and Meg 01 cell lines, which cross-react with MARCKS antibody (data not shown). There was an increase in the phosphorylation of these 2 protein bands upon stimulation of PKC by PMA, especially in the band with lowest electrophoretic mobility (Figure 4). This protein band was easily detected by the antibody in the immunoblots (Figure 4). Densitometric scanning of the immunoblots also indicated that the concentration of MARCKS in platelets was 68 ± 3% (n = 4) of that in Meg 01 cells, a cell line derived from a megakaryocyte leukemia.
Immunostaining of platelets with MARCKS and CD41a antibodies.
Intact platelets were fixed, permeabilized, and immunostained with either human MARCKS mouse monoclonal antibody (C and D) or human CD41a (fibrinogen receptor) mouse monoclonal antibody (E and F). The second antibody used in both cases was affinity-purifed Cy3-conjugated donkey Fab2 fragment raised against mouse IgG (dilution 1:200). Panels A and B show control platelets incubated only with the second antibody.
Immunostaining of platelets with MARCKS and CD41a antibodies.
Intact platelets were fixed, permeabilized, and immunostained with either human MARCKS mouse monoclonal antibody (C and D) or human CD41a (fibrinogen receptor) mouse monoclonal antibody (E and F). The second antibody used in both cases was affinity-purifed Cy3-conjugated donkey Fab2 fragment raised against mouse IgG (dilution 1:200). Panels A and B show control platelets incubated only with the second antibody.
Detection of MARCKS in platelet extracts and its phosphorylation during PKC activation.
Platelets labeled with [32P]Pi were permeabilized with 15 μM of digitonin in K+-glutamate buffer for 5 minutes. During the last 3 minutes of permeabilization, 100 nM of PMA in 0.05% DMSO (final concentration) or 0.05% DMSO (control) were present in the incubation medium. Reactions were stopped with RIPA buffer containing protease and phosphatase inhibitors. SDS-PAGE was performed on boiled extracts of these preparations to separate heat-stable proteins. Proteins were electrotransferred to nitrocellulose membranes, and autoradiography was first performed. This was followed by Western blotting with a mouse monoclonal antibody (4 μg/mL) raised against the C-terminal domain of human MARCKS. Two bands of 83 and 85 kd were detected by the antibody, which corresponded to 2 phosphorylated protein bands found in the autoradiography. Although PMA increased the phosphorylation of both MARCKS bands, the upper band was heavily phosphorylated, increasing the amount of slow-moving MARCKS (85 kd), as indicated by a much heavier immunoreactivity of this band in the PMA-treated preparations.
Detection of MARCKS in platelet extracts and its phosphorylation during PKC activation.
Platelets labeled with [32P]Pi were permeabilized with 15 μM of digitonin in K+-glutamate buffer for 5 minutes. During the last 3 minutes of permeabilization, 100 nM of PMA in 0.05% DMSO (final concentration) or 0.05% DMSO (control) were present in the incubation medium. Reactions were stopped with RIPA buffer containing protease and phosphatase inhibitors. SDS-PAGE was performed on boiled extracts of these preparations to separate heat-stable proteins. Proteins were electrotransferred to nitrocellulose membranes, and autoradiography was first performed. This was followed by Western blotting with a mouse monoclonal antibody (4 μg/mL) raised against the C-terminal domain of human MARCKS. Two bands of 83 and 85 kd were detected by the antibody, which corresponded to 2 phosphorylated protein bands found in the autoradiography. Although PMA increased the phosphorylation of both MARCKS bands, the upper band was heavily phosphorylated, increasing the amount of slow-moving MARCKS (85 kd), as indicated by a much heavier immunoreactivity of this band in the PMA-treated preparations.
MARCKS phosphorylation site domain (MPSD) peptide blocks serotonin release induced by PKC activation
Because MARCKS is a major substrate of PKC, experiments were performed to test the possibility of the involvement of MARCKS in the secretory response induced by PKC activation. The approach followed was the use of peptide MPSD of 25 amino acids with a sequence corresponding to the domain containing the phosphorylation site and also the calmodulin (CaM)- and actin-binding sites (Figure5A).30 In addition, a similar 25–amino acid peptide, in which the serine residues were substituted by alanine (Ala-MPSD), was also tested (Figure 5A). Permeabilized platelets were always stimulated for 45 seconds. During this short period, not only a good secretory response to stimulation was obtained,13 but this is also the time that, as shown in other well-studied secretory systems,15 is required for secretion from the release-ready vesicle pool.15 PMA (100 nM), as expected, induced a significant secretory response from permeabilized platelets (Figure 5B). This increase in serotonin output was blocked in the presence of 10 μM of MPSD. On the other hand, when Ala-MPSD was present at the same concentration, PMA-induced serotonin release was not affected (Figure 5B). The inhibitory effect of MPSD on PMA-induced serotonin release was also concentration dependent (Figure6). Moreover, it is known that MPSD binds to CaM and phosphatidylinositol 4,5-bisphosphate (PIP2), and that its phosphorylation decreases the binding to these molecules.31 32 To rule out the possibility that the inhibitory effects of MPSD observed were due to either displacement or inhibition of CaM or PIP2 effects, experiments were performed with these molecules. The presence in incubation medium of 10 μM of either CaM or PIP2 neither modified serotonin spontaneous release nor affected nor reversed the inhibition by MPSD of PMA-induced serotonin release (Figure 7). In another set of experiments, concentrations of PIP2 up to 100 μM failed to modify the inhibitory effect of MPSD- on PMA-induced secretion (data not shown).
Effects of peptides MPSD and Ala-MPSD on PKC-induced [3H]5-HT release from permeabilized platelets.
[3H]5-HT–labeled platelets were permeabilized for 5 minutes with 15 μM of digitonin in K+-glutamate buffer. Permeabilization was done in the absence or presence of 10 μM of either peptide MPSD or peptide Ala-MPSD. MPSD has a 25–amino acid sequence corresponding to the phosphorylation site domain of MARCKS (A). This is the site for the binding of F-actin and calmodulin (A). In Ala-MPSD, the 4 serine residues of MPSD have been substituted by alanines (A). When indicated, PMA (100 nM) was present in the permeabilization medium during the last 3 minutes. Platelets were recovered by centrifugation and resuspended in the same medium for 45 seconds. At the end of this stimulation period, [3H]5-HT content was measured in the medium and in platelets. [3H]5-HT outputs were expressed as a percentage of total content after subtraction of spontaneous release (B). Each bar represents the mean ± SEM of results obtained from 3 different experiments (bottom). A minimum of 24 samples per condition were measured.
Effects of peptides MPSD and Ala-MPSD on PKC-induced [3H]5-HT release from permeabilized platelets.
[3H]5-HT–labeled platelets were permeabilized for 5 minutes with 15 μM of digitonin in K+-glutamate buffer. Permeabilization was done in the absence or presence of 10 μM of either peptide MPSD or peptide Ala-MPSD. MPSD has a 25–amino acid sequence corresponding to the phosphorylation site domain of MARCKS (A). This is the site for the binding of F-actin and calmodulin (A). In Ala-MPSD, the 4 serine residues of MPSD have been substituted by alanines (A). When indicated, PMA (100 nM) was present in the permeabilization medium during the last 3 minutes. Platelets were recovered by centrifugation and resuspended in the same medium for 45 seconds. At the end of this stimulation period, [3H]5-HT content was measured in the medium and in platelets. [3H]5-HT outputs were expressed as a percentage of total content after subtraction of spontaneous release (B). Each bar represents the mean ± SEM of results obtained from 3 different experiments (bottom). A minimum of 24 samples per condition were measured.
Concentration-dependent inhibition by MPSD of [3H]5-HT output in response to PKC activation.
Platelets were labeled with [3H]5-HT, permeabilized with digitonin, and incubated with 100 nM of PMA in the absence or presence of either MPSD or Ala-MPSD, as indicated in the legend to Figure 5. Inhibition of PMA-induced [3H]5-HT output by different concentrations of MPSD was expressed as a percentage of the [3H]5-HT output in the presence of PMA. Ala-MPSD at the higher concentration tested (10 μM) did not modify the secretory response induced by PMA. Values represent mean ± SEM of 8 different platelet preparations.
Concentration-dependent inhibition by MPSD of [3H]5-HT output in response to PKC activation.
Platelets were labeled with [3H]5-HT, permeabilized with digitonin, and incubated with 100 nM of PMA in the absence or presence of either MPSD or Ala-MPSD, as indicated in the legend to Figure 5. Inhibition of PMA-induced [3H]5-HT output by different concentrations of MPSD was expressed as a percentage of the [3H]5-HT output in the presence of PMA. Ala-MPSD at the higher concentration tested (10 μM) did not modify the secretory response induced by PMA. Values represent mean ± SEM of 8 different platelet preparations.
Effect of calmodulin and PIP2 on the inhibition by MPSD of [3H]5-HT output in response to PKC activation.
Platelets were labeled with [3H]5-HT, permeabilized with digitonin, and incubated with 100 nM of PMA in the absence or presence of 10 μM of MPSD. When indicated, 10 μM of either calmodulin or PIP2 were present in the incubation medium. [3H]5-HT outputs were expressed as a percentage of total content. Values represent mean ± SEM of at least 8 different platelet preparations.
Effect of calmodulin and PIP2 on the inhibition by MPSD of [3H]5-HT output in response to PKC activation.
Platelets were labeled with [3H]5-HT, permeabilized with digitonin, and incubated with 100 nM of PMA in the absence or presence of 10 μM of MPSD. When indicated, 10 μM of either calmodulin or PIP2 were present in the incubation medium. [3H]5-HT outputs were expressed as a percentage of total content. Values represent mean ± SEM of at least 8 different platelet preparations.
The fact that MPSD blocked PKC-induced release of serotonin suggests the involvement of MARCKS in the release reaction. This, together with the lack of effect of Ala-MPSD, also suggests that 1 or more of the serine residues present in MPSD are necessary for the inhibitory effect of the peptide. Because these serine residues are the phosphorylation sites of MARCKS as a result of PKC activation, the effects of MPSD and Ala-MPSD on platelet protein phosphorylation were investigated next.
Effects of MPSD and Ala-MPSD on protein phosphorylation induced by PKC activation
Platelets previously labeled with [32P]Pi were incubated with 100 nM of PMA in the absence or presence of either MPSD or Ala-MPSD. This was followed by separation of heat-stable proteins by SDS-PAGE. The autoradiographies of the gels and their corresponding scannings are depicted in Figure 8. Activation of PKC by PMA increased the phosphorylation of both MARCKS and myosin light chain (MLC) (Figure 8A and B). Pleckstrin, a major PKC substrate, is not heat-stable (see below). In the presence of 10 μM of MPSD, there was a significant inhibition of PKC-induced phosphorylation of MARCKS (Figure 8A). However, and as proof that PKC activity was intact under these conditions, there was no inhibition of PMA-induced phosphorylation of MLC (Figure 8A). Moreover, the phosphorylation of 4 other unidentified heat-stable proteins (p25, p31, p50, and p66) increased upon PMA stimulation. Here again, there was no inhibition of this effect by MPSD (Figure 4A). Phosphorylations of p25, p31, p50, and p66 induced by PMA stimulation in the presence of MPSD were 102% ± 7%, 104% ± 4%, 99% ± 8% and 95% ± 7%, respectively, of phosphorylations induced by PMA in the absence of MARCKS inhibitor peptide. Further proof of PKC activation in the presence of MPSD was the fact that this peptide was phosphorylated (Figure 8A). A much smaller level of phosphorylation of MPSD was observed in the absence of PMA (Figure 8A). Furthermore, when 10 μM of Ala-MPSD was present in the medium, there was no inhibition of PKC-induced MARCKS phosphorylation (Figure 8B). The increase in MLC phosphorylation was not affected and, as expected, Ala-MPSD was not phosphorylated (Figure 8B). Because pleckstrin, a major PKC substrate, has been implicated in serotonin release, it was important to determine the level of pleckstrin phosphorylation under conditions in which PMA-induced serotonin release was blocked (ie, presence of MPSD). Therefore, proteins present in unheated platelet extracts were separated by SDS-PAGE, and the autoradiography and scanning of one such gel are shown in Figure 9A. The increase in the phosphorylation of pleckstrin observed in the presence of 100 nM of PMA was not modified with the presence of either MPSD or Ala-MPSD at concentrations at which MPSD inhibits MARCKS phosphorylation and serotonin release (Figure 9A). Here, again, the phosphorylation of MLC was not affected by the peptides, and phosphorylation of MPSD was observed when PMA was present (Figure 9A). Figure 9B shows mean ± SEM of phosphorylation of heat-stable and heat-sensitive proteins of 4 different experiments.
Effects of MPSD and Ala-MPSD on the phosphorylation of MARCKS induced by PKC activation.
Platelets were labeled with [32P]Pi, permeabilized with digitonin, incubated with 100 nM PMA in the absence or presence of 10 μM of either MPSD or Ala-MPSD, and subsequently stimulated as described in the legend to Figure 5. At the end of the stimulation periods, heat-stable platelet extracts were prepared, and their proteins were separated by SDS-PAGE as indicated in “Materials and methods.” Proteins were then electrotransferred to nitrocellulose membranes; these were exposed to hyperfilm, and the autoradiographies thus obtained were scanned as indicated in “Materials and methods.” Panel A shows the autoradiography of an experiment carried out to test the effect of MPSD on protein phosphorylation, and panel B shows a similar experiment performed with Ala-MPSD. Double arrows in A and B show the position of MARCKS, and single arrowheads indicate the position of myosin light chain (MLC). The open triangles in A indicate the position of proteins p25, p31, p50, and p66. The asterisk in A indicates the position of peptide MPSD, which was less phosphorylated in the absence (basal PKC activity) than in the presence of PMA (basal + stimulated PKC activity). Note the absence of a phosphorylated band when the experiment was performed with Ala-MPSD (B). On the right side in both panels, scannings of the autoradiographies are shown. The numbers beside the MARCKS and MLC peaks are arbitrary units obtained from computer integration of peak areas. Similar results were obtained in 3 other experiments (see Figure9B).
Effects of MPSD and Ala-MPSD on the phosphorylation of MARCKS induced by PKC activation.
Platelets were labeled with [32P]Pi, permeabilized with digitonin, incubated with 100 nM PMA in the absence or presence of 10 μM of either MPSD or Ala-MPSD, and subsequently stimulated as described in the legend to Figure 5. At the end of the stimulation periods, heat-stable platelet extracts were prepared, and their proteins were separated by SDS-PAGE as indicated in “Materials and methods.” Proteins were then electrotransferred to nitrocellulose membranes; these were exposed to hyperfilm, and the autoradiographies thus obtained were scanned as indicated in “Materials and methods.” Panel A shows the autoradiography of an experiment carried out to test the effect of MPSD on protein phosphorylation, and panel B shows a similar experiment performed with Ala-MPSD. Double arrows in A and B show the position of MARCKS, and single arrowheads indicate the position of myosin light chain (MLC). The open triangles in A indicate the position of proteins p25, p31, p50, and p66. The asterisk in A indicates the position of peptide MPSD, which was less phosphorylated in the absence (basal PKC activity) than in the presence of PMA (basal + stimulated PKC activity). Note the absence of a phosphorylated band when the experiment was performed with Ala-MPSD (B). On the right side in both panels, scannings of the autoradiographies are shown. The numbers beside the MARCKS and MLC peaks are arbitrary units obtained from computer integration of peak areas. Similar results were obtained in 3 other experiments (see Figure9B).
Effects of MPSD and Ala-MPSD on the phosphorylation of pleckstrin (p47) and myosin light chain (p20) induced by PKC activation.
(A) An experiment similar to that described in the legend to Figure 8was performed on [32P]PI–labeled platelets except that, here, total platelet extracts (heat-stable plus heat-sensitive proteins) were prepared. SDS-PAGE, electrotransfers, autoradiography, scanning, and integration of peak areas (arbitrary units) were performed as indicated in the legend to Figure 8 and in “Materials and methods.” The arrow and the arrowhead indicate the position of pleckstrin and myosin light chain (MLC), respectively. The asterisk shows the position of phosphorylated MPSD, which in this gel system migrated with another unknown phosphoprotein present in all lines. Pleckstrin and MLC phosphorylations were not modified by either MPSD or Ala-MPSD. (B) Cumulative data on protein phosphorylation obtained from experiments carried out on different platelet preparations are shown. Experiments were performed as described above in A for pleckstrin and MLC and as indicated in legend to Figure 8 for MARCKS. Bars represent mean ± SEM of [32P]Pi incorporation, expressed as a percentage of control (absence of PMA), obtained from 3 to 4 different experiments for each condition tested.
Effects of MPSD and Ala-MPSD on the phosphorylation of pleckstrin (p47) and myosin light chain (p20) induced by PKC activation.
(A) An experiment similar to that described in the legend to Figure 8was performed on [32P]PI–labeled platelets except that, here, total platelet extracts (heat-stable plus heat-sensitive proteins) were prepared. SDS-PAGE, electrotransfers, autoradiography, scanning, and integration of peak areas (arbitrary units) were performed as indicated in the legend to Figure 8 and in “Materials and methods.” The arrow and the arrowhead indicate the position of pleckstrin and myosin light chain (MLC), respectively. The asterisk shows the position of phosphorylated MPSD, which in this gel system migrated with another unknown phosphoprotein present in all lines. Pleckstrin and MLC phosphorylations were not modified by either MPSD or Ala-MPSD. (B) Cumulative data on protein phosphorylation obtained from experiments carried out on different platelet preparations are shown. Experiments were performed as described above in A for pleckstrin and MLC and as indicated in legend to Figure 8 for MARCKS. Bars represent mean ± SEM of [32P]Pi incorporation, expressed as a percentage of control (absence of PMA), obtained from 3 to 4 different experiments for each condition tested.
Similar PMA concentration-dependency for MARCKS phosphorylation and serotonin release
Serotonin output, pleckstrin, and MARCKS phosphorylation were measured in platelets stimulated with increasing concentrations (1-500 nM) of PMA. Figure 10A shows one such phosphorylation experiment. Arrows indicate the first protein band for each type, showing a significant increase in phosphorylation. Figure10B shows cumulative data from 6 experiments. MARCKS phosphorylation and serotonin release curves were almost identical (EC50[median effective concentration] of 85- and 80-nM PMA, respectively), whereas the pleckstrin phosphorylation curve (EC50of 45-nM PMA), although of similar shape to the other 2, was shifted to the left. In other words, it was necessary to reach a concentration of PMA of 100 nM to observe significant and parallel increases in MARCKS phosphorylation and serotonin release, whereas concentrations of PMA of 50 nM or less significantly increased pleckstrin phosphorylation (Figure 10B).
PMA–concentration-dependent responses.
Platelets were labeled with either [32P]Pi or [3H]5-HT, permeabilized with digitonin, and stimulated with increasing concentrations (1-500 nM) of PMA. [3H]5-HT release and protein phosphorylation were measured. (A) Autoradiography of SDS-PAGE gels of heat-stable platelet extracts (MARCKS) and whole platelet extracts (pleckstrin) is shown. The concentrations of PMA used are indicated at the bottom of the figure. Arrowheads indicate the smallest concentrations of PMA producing a significant increase in the phosphorylation of each protein. (B) PMA–concentration-dependent curves for [3H]5-HT release and pleckstrin and MARCKS phosphorylation are shown. Values represent the mean ± SEM of 6 different platelet preparations.
PMA–concentration-dependent responses.
Platelets were labeled with either [32P]Pi or [3H]5-HT, permeabilized with digitonin, and stimulated with increasing concentrations (1-500 nM) of PMA. [3H]5-HT release and protein phosphorylation were measured. (A) Autoradiography of SDS-PAGE gels of heat-stable platelet extracts (MARCKS) and whole platelet extracts (pleckstrin) is shown. The concentrations of PMA used are indicated at the bottom of the figure. Arrowheads indicate the smallest concentrations of PMA producing a significant increase in the phosphorylation of each protein. (B) PMA–concentration-dependent curves for [3H]5-HT release and pleckstrin and MARCKS phosphorylation are shown. Values represent the mean ± SEM of 6 different platelet preparations.
Discussion
The present experiments provide the first demonstration of the presence of MARCKS (a major PKC substrate) in platelets and the first demonstration that stimulation of platelets by PMA increases MARCKS phosphorylation. We have made use of digitonin-permeabilized platelets13 to test the involvement of MARCKS in platelet secretion. A complete description and characterization of the permeabilized preparation is reported here. The results show that the degree of permeabilization obtained with digitonin is high; the leakage of proteins into the medium was low, between 2% and 28%; and a good secretory response was observed up to 15 minutes following permeabilization. As in previous experiments on platelet secretion,13 45-second stimulation was used in most experiments with this preparation, a period showing a significant rate and the highest rate of secretion. It has been shown in the well-characterized chromaffin cell system that secretion during the first 45 to 60 seconds of stimulation corresponds to release from the so-called “release-ready vesicle pool.”14,15 The secretory behavior of permeabilized platelets, especially in the presence of recombinant scinderin,13 suggests that platelets also have a pool of release-ready vesicles that may be involved in the initial phase of fast release. In permeabilized platelets, 100 nM of PMA induces phosphorylation of pleckstrin and MARCKS, responses that are accompanied by an increase in serotonin release. However, PMA at concentrations between 1 to 50 nM induces only the phosphorylation of pleckstrin, suggesting some differences in the phosphorylation pathways for the 2 proteins. Previous work with other secretory tissues has demonstrated that the release of hormones or neurotransmitters is accompanied by the increase in the phosphorylation of several proteins, including MARCKS.33-38 However, although these experiments show some degree of correlation between an increase in MARCKS phosphorylation and hormone or neurotransmitter release, they did not provide a cause-effect relationship between the 2 events. In the present experiments, we have provided a direct relationship between MARCKS phosphorylation and serotonin release in response to PKC activation using the peptide MPSD (with sequence corresponding to the phosphorylation sites of MARCKS). Preincubation of permeabilized platelets with MPSD blocks both MARCKS phosphorylation and serotonin release, whereas control peptide Ala-MPSD (a peptide in which the serine residues were substituted by alanine residues) was without effect. MPSD not only inhibited these 2 PKC-dependent responses, but it was also phosphorylated in the process, suggesting that PKC activity remained intact when the peptide was present in the medium. The observations that the increase in pleckstrin phosphorylation (also a major substrate of PKC) was not affected in the presence of MPSD also indicated that PKC was active. Additional proof of the selectivity of MPSD inhibition is that other unidentified heat-stable proteins (p25, p31, p50, and p66) were also phosphorylated in response to PMA, but these phosphorylations were not modified by MPSD. Increased phosphorylation of pleckstrin and MLC in the face of MPSD-induced inhibition of MARCKS phosphorylation and serotonin release suggests that phosphorylation of these proteins is unrelated to the transduction pathway in which MARCKS is involved or that their involvement in serotonin release is upstream of MARCKS in the cascade of events leading to exocytosis. In this case, the possibility should be considered that a threshold concentration of phosphorylated pleckstrin is required for this protein to be involved in secretion and that this threshold is reached only at 100 nM of PMA. One possibility is that MLC phosphorylation is involved in platelet-shape change and/or aggregation39,40—steps that cannot be studied separately from secretion in intact platelets. Similarly, pleckstrin phosphorylation could play a role in platelet-shape change and aggregation. Furthermore, there is some dissociation between the PMA concentration-dependent curves of the 2 protein phosphorylations, with the phosphorylation curve for MARCKS being most identical to the serotonin release curve. The suggestion that pleckstrin is involved in secretion in response to PKC activation comes from a large number of publications, all showing increases or decreases in both pleckstrin phosphorylation and platelet release reaction during stimulation or inhibition of PKC activity.16-29 23 However, in these publications, cause-effect has not been demonstrated; nor has the agonist-concentration dependence for MARCKS phosphorylation and serotonin release between these 2 PKC responses been provided. An alternative explanation for the different phosphorylation patterns observed between pleckstrin and MARCKS might be that different PKC isozymes are involved in their phosphorylations. If this were the case, the same PKC isozyme should be involved in both the phosphorylation of MARCKS and the release of serotonin because the 2 processes have a similar EC50.
The possibility that the inhibitory effect of MPSD on secretion was due to displacement or inhibition of CaM and PIP2effects31,32 was tested in experiments performed in the presence of these molecules. CaM and PIP2, in concentrations of up to 10 and 100 μM, respectively, were ineffective in blocking the inhibitory effect of MPSD. PIP2 at the same concentrations used here was shown to be a powerful inhibitor of the potentiation of Ca++-induced serotonin release by recombinant scinderin.13 However, the present experiments do not rule out the possibility that endogenous PIP2, present in high concentrations at specific membrane sites, may regulate exocytosis.
In view of the present results, a question that immediately comes to mind is how MARCKS is involved in platelet secretion. Experiments with recombinant scinderin in digitonin-permeabilized platelets suggest that F-actin disassembly, perhaps at a specific site, is important for platelet secretion.13 We demonstrated that recombinant scinderin (a Ca++-dependent F-actin severing protein) potentiated Ca++-induced release, an effect blocked by peptides with sequences corresponding to either of the 2 actin-binding sites of scinderin,13,41 suggesting the requirement of F-actin disassembly in the release process. Cortical actin disassembly required for hormone and neurotransmitter release has been demonstrated in other secretory tissues, such as chromaffin cells and laptotropes,14,15,42 and cytochalasin E treatment decreases actin polymerization and inhibits platelet aggregation without affecting granular secretion.43 Work on chromaffin cells has also demonstrated cortical F-actin disassembly and/or filament rearrangement during PKC activation by phorbol esters.15 In these studies, an increase in the initial rate of noradrenaline release was observed and demonstrated to be due to an increase in the number of secretory vesicles at release sites.15 Additional work from our laboratory on digitonin-permeabilized chromaffin cells has demonstrated that the MPSD peptide used in the present studies also blocked cortical F-actin disassembly and/or filament rearrangement in response to activation of PKC by PMA.44 All of these observations seem to suggest that filament disassembly or rearrangement might be involved in the secretory response to PMA. MARCKS is an actin filament binding protein that cross-links actin filaments in some cell types.45 Phosphorylation of MARCKS by PKC decreases its affinity for F-actin, and phospho-MARCKS cannot cross-link actin filaments,25 a property reserve to dephospho-MARCKS.25 Therefore, it is possible that PMA activation of platelet PKC increases the phosphorylation of MARCKS, decreases actin filament cross-linking, and decreases the local density of F-actin networks. This would facilitate, as in other secretory systems,15,44 the movement of secretory vesicles to release sites increasing serotonin release. It has been further suggested that the Ca++-induced– and PMA-evoked platelet release reactions are distinct mechanisms.20 Data from our laboratory13,46 suggest that, as in chromaffin cell secretion,14 15 Ca++-induced platelet secretion involves scinderin activation followed by actin disassembly. Secretion dependent on PKC activation has now been shown to involve MARCKS phosphorylation, which would rearrange F-actin networks by decreasing actin crosslinking. These mechanisms are not mutually exclusive and, in response to different secretagogues (which in some cases produce both PKC stimulation and Ca++ entry or release from intracellular stores) both mechanisms could operate. The presence of parallel or sequential mechanisms involved in platelet release allows different possibilities to modulate platelet secretion.
Acknowledgment
We are grateful to S. J. Dunn for typing the manuscript and to the Ottawa Red Cross for providing platelet-rich plasma.
Support by a grant from the Ontario Heart and Stroke Foundation.
Reprints:José-Marı́a Trifaró, Secretory Process Research Program, Department of Cellular and Molecular Medicine, Faculty of Medicine, University of Ottawa, Ottawa, Ontario, Canada K1H 8M5.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

![Fig. 1. Effect of permeabilization on [3H]5-HT output from platelets. / (A) Time course of [3H]5-HT output from intact and permeabilized cells in the presence or absence of PMA. [3H]5-HT–labeled platelets were used and were permeabilized for 4 different periods of time with 15 μM of digitonin in K+-glutamate buffer. When indicated, PMA (100 nM) was present during the entire incubation period. After incubation, platelets were recovered by centrifugation, and the content of [3H]5-HT was measured in the medium and in the platelets. [3H]5-HT outputs were expressed as a percentage of total content. Values represent the mean ± SEM of at least 8 different preparations. (B) Effect of permeabilization is shown for different periods of time on the responses to a short period of stimulation with PMA. [3H]5-HT–labeled platelets were permeabilized as indicated in A, and they were stimulated for 45 seconds with 100 nM of PMA. Platelets were recovered by stimulation, and [3H]5-HT output was measured. Bars represent the mean ± SEM of 4 different preparations.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/95/3/10.1182_blood.v95.3.894.003k15_894_902/6/m_bloo00315001x.jpeg?Expires=1765896670&Signature=ibKg9yHTI5pPlAaHE13imMzzPsGlIkQDtBvWGYb2YSSe9WmBnOTxnsbin-q4yHy9iLCV65D0a7~Ogln5bGzA4gE3dHBc25HpD6UjgZWyt~zwcRizG~H0Q4tlOpKV2IXyyf2XAPryODgtfmoEtETsIWoZbaCc9~E6q6OQqa8zJJW4dKpMQiQB2hNiGD~VPnBiu40Xv9j-cskLM5DBw1UQiPxrWkXNmq-RS~HTH42PsJpVdt11E08R6ovvpzjwqWGhZzXOENecm9xxcY6dBUL3es0j-Vg2usu95kPeLC-0JXNnYYWbN0QqVRQg6m5pF9f6fiKVY9d07DzYukP9bymenw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Fig. 4. Detection of MARCKS in platelet extracts and its phosphorylation during PKC activation. / Platelets labeled with [32P]Pi were permeabilized with 15 μM of digitonin in K+-glutamate buffer for 5 minutes. During the last 3 minutes of permeabilization, 100 nM of PMA in 0.05% DMSO (final concentration) or 0.05% DMSO (control) were present in the incubation medium. Reactions were stopped with RIPA buffer containing protease and phosphatase inhibitors. SDS-PAGE was performed on boiled extracts of these preparations to separate heat-stable proteins. Proteins were electrotransferred to nitrocellulose membranes, and autoradiography was first performed. This was followed by Western blotting with a mouse monoclonal antibody (4 μg/mL) raised against the C-terminal domain of human MARCKS. Two bands of 83 and 85 kd were detected by the antibody, which corresponded to 2 phosphorylated protein bands found in the autoradiography. Although PMA increased the phosphorylation of both MARCKS bands, the upper band was heavily phosphorylated, increasing the amount of slow-moving MARCKS (85 kd), as indicated by a much heavier immunoreactivity of this band in the PMA-treated preparations.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/95/3/10.1182_blood.v95.3.894.003k15_894_902/6/m_bloo00315004w.jpeg?Expires=1765896670&Signature=DaVfJToErbAUNY7Oupr3gIjyZuoI3MMohesvUa6kGsN~stIpCZqADUcETFJLNYFcMe49SNOZ00aruJYg3PpHjUQqOIry6NehdmgKTgI6Y0HKoEBS9QD-2AUdglNpV4DbtJYukeLX9CwVJuby1Vt2ovyuNFYOfjtoL1RdaZT3mdjYwVdFaaCR0pR6esHPmnAwNU~wOz9r7JS4CJ0mqpzukZXi-LnQVyOXxgYu5iJE8oYkZYuPdZCNiWVGw67kLBieYxmNrg8HtKqRkG7U4s5laDVxphuJvKZeJ4NSTIX8js~f9jzW9otcxy7X7PmK7WYbV0vDAK0VEqECs~ZVL6dceg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 5. Effects of peptides MPSD and Ala-MPSD on PKC-induced [3H]5-HT release from permeabilized platelets. / [3H]5-HT–labeled platelets were permeabilized for 5 minutes with 15 μM of digitonin in K+-glutamate buffer. Permeabilization was done in the absence or presence of 10 μM of either peptide MPSD or peptide Ala-MPSD. MPSD has a 25–amino acid sequence corresponding to the phosphorylation site domain of MARCKS (A). This is the site for the binding of F-actin and calmodulin (A). In Ala-MPSD, the 4 serine residues of MPSD have been substituted by alanines (A). When indicated, PMA (100 nM) was present in the permeabilization medium during the last 3 minutes. Platelets were recovered by centrifugation and resuspended in the same medium for 45 seconds. At the end of this stimulation period, [3H]5-HT content was measured in the medium and in platelets. [3H]5-HT outputs were expressed as a percentage of total content after subtraction of spontaneous release (B). Each bar represents the mean ± SEM of results obtained from 3 different experiments (bottom). A minimum of 24 samples per condition were measured.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/95/3/10.1182_blood.v95.3.894.003k15_894_902/6/m_bloo00315005x.jpeg?Expires=1765896670&Signature=HTjHMf2YFd-kUZNXLwGsds8OXmUftRjXteWxPWqPtR52uSZtv6nbtC8Pa1PlKYv7bsmFI~oFPmDL3NAK~DQYOtswa5n5DyHynhgD~o7fevtrH8DWxDYRGvoSl4k4apMIwasXaiyoxwExZ1PX5z6V-KThEXMI2cMCq7YQ0BSIFyl5Oo84WXbuJMK05rV~gFdOXoAJBLLhS3awO1xNSFKqh7wmTtQ0BEW6sk4qgGV8wg-G88~5nb8Rl-A3p61TxAyOxUojOF2e7Q97srI5Ad4ZZBZMLkVpTeP2-ROdKonQnje3WG8X1Yb-Cfy1kouViYrwHo9jsAypjZjQvc-ZxNBdjQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 6. Concentration-dependent inhibition by MPSD of [3H]5-HT output in response to PKC activation. / Platelets were labeled with [3H]5-HT, permeabilized with digitonin, and incubated with 100 nM of PMA in the absence or presence of either MPSD or Ala-MPSD, as indicated in the legend to Figure 5. Inhibition of PMA-induced [3H]5-HT output by different concentrations of MPSD was expressed as a percentage of the [3H]5-HT output in the presence of PMA. Ala-MPSD at the higher concentration tested (10 μM) did not modify the secretory response induced by PMA. Values represent mean ± SEM of 8 different platelet preparations.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/95/3/10.1182_blood.v95.3.894.003k15_894_902/6/m_bloo00315006x.jpeg?Expires=1765896670&Signature=LHZggvokKkfBvQblHyiO5vEZITaPA--Ywx7HNeMxExNgBzDZPdIccwuskpiz~P74pQQVF6GL8j3Jl-RGvL5klhkOibwDvv9-ym184O5~ya29toRYI~7PoP~R19bHSoCmuaRJllRMsb3n~GGHj4dNkEqWDixmVEmJtMUVXOUlf5epv4uL5RsXg1GEBooVvdMUpB-UHTyEtMRkNOccr8Fd5MO5fVZv7Ysl1Xkw8zb0XjoPECVJRZGAazBIlLY3XQZ4UVPEzLQsxKvr4gBFy9pvg~VL45a54n8crw-fFe5JWrkR207SCovWGxSbzmIzlMT26WEc5lBMVWTYbDXXHD1zsQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 7. Effect of calmodulin and PIP2 on the inhibition by MPSD of [3H]5-HT output in response to PKC activation. / Platelets were labeled with [3H]5-HT, permeabilized with digitonin, and incubated with 100 nM of PMA in the absence or presence of 10 μM of MPSD. When indicated, 10 μM of either calmodulin or PIP2 were present in the incubation medium. [3H]5-HT outputs were expressed as a percentage of total content. Values represent mean ± SEM of at least 8 different platelet preparations.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/95/3/10.1182_blood.v95.3.894.003k15_894_902/6/m_bloo00315007x.jpeg?Expires=1765896670&Signature=TF14F10wRxYDKQW9K9zPGF7gpJ0HaIA~lNSpmk~s97ztNAZ3e2OhqbXcl0-y0okx5RTaff4cfI3S6VTxoITLRDZP75dFLENXzo8lvF5QukxARb5ae5gce8U5wbQHJLHbbMKuU8c3aVhu~HCfUXftqARjcl9TlvG45nllr64HyKDwCs1yOPM2c0KQEMCkYyISRU2o-S1k~y~LHe1LJ6G3UlaIhu3mtD4LLvPAEhh1-Jc1aLCkPOezHGHKJ7jg8scCk2cWtHMjXQcW77u4jtoaegi17TDPMSh6LLEiGw4iOYwfe5u0LMwzISq~yfnd8Kgf6iOAXyu7N1WkE0IhOjcYnA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 8. Effects of MPSD and Ala-MPSD on the phosphorylation of MARCKS induced by PKC activation. / Platelets were labeled with [32P]Pi, permeabilized with digitonin, incubated with 100 nM PMA in the absence or presence of 10 μM of either MPSD or Ala-MPSD, and subsequently stimulated as described in the legend to Figure 5. At the end of the stimulation periods, heat-stable platelet extracts were prepared, and their proteins were separated by SDS-PAGE as indicated in “Materials and methods.” Proteins were then electrotransferred to nitrocellulose membranes; these were exposed to hyperfilm, and the autoradiographies thus obtained were scanned as indicated in “Materials and methods.” Panel A shows the autoradiography of an experiment carried out to test the effect of MPSD on protein phosphorylation, and panel B shows a similar experiment performed with Ala-MPSD. Double arrows in A and B show the position of MARCKS, and single arrowheads indicate the position of myosin light chain (MLC). The open triangles in A indicate the position of proteins p25, p31, p50, and p66. The asterisk in A indicates the position of peptide MPSD, which was less phosphorylated in the absence (basal PKC activity) than in the presence of PMA (basal + stimulated PKC activity). Note the absence of a phosphorylated band when the experiment was performed with Ala-MPSD (B). On the right side in both panels, scannings of the autoradiographies are shown. The numbers beside the MARCKS and MLC peaks are arbitrary units obtained from computer integration of peak areas. Similar results were obtained in 3 other experiments (see Figure9B).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/95/3/10.1182_blood.v95.3.894.003k15_894_902/6/m_bloo00315008w.jpeg?Expires=1765896670&Signature=zgcR6BT4pIEv09Fgt94R0AhA139btxFPa3EKFVbQ0lzeMMspBdSCd8H4uT06A~7fgN~6UVp8xwR69o8qbQnmA8RDudqGNxkAFZ8XMv3TtP9gU9tRzBIY5zYTx0PDdNtMmKT7VWseHidbMfgkePLfrxyfEv82RsS13J5LDWu7EbrkpzVQh0TN-ZUlqwsicH8094~So8HzD9EIFOH-wdRg5HZhmsXli8P-FiCdpmS9YdS-KKPGfLNWexQfvkBini0GburB75Cd9aIGP2LkSNU~sYgXhNRqvXyacVwgzycdUOq7WPlqgSKSyzUzh14l1ZDn48UxV0Qk2hDFB-5rn6lVMA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 9. Effects of MPSD and Ala-MPSD on the phosphorylation of pleckstrin (p47) and myosin light chain (p20) induced by PKC activation. / (A) An experiment similar to that described in the legend to Figure 8was performed on [32P]PI–labeled platelets except that, here, total platelet extracts (heat-stable plus heat-sensitive proteins) were prepared. SDS-PAGE, electrotransfers, autoradiography, scanning, and integration of peak areas (arbitrary units) were performed as indicated in the legend to Figure 8 and in “Materials and methods.” The arrow and the arrowhead indicate the position of pleckstrin and myosin light chain (MLC), respectively. The asterisk shows the position of phosphorylated MPSD, which in this gel system migrated with another unknown phosphoprotein present in all lines. Pleckstrin and MLC phosphorylations were not modified by either MPSD or Ala-MPSD. (B) Cumulative data on protein phosphorylation obtained from experiments carried out on different platelet preparations are shown. Experiments were performed as described above in A for pleckstrin and MLC and as indicated in legend to Figure 8 for MARCKS. Bars represent mean ± SEM of [32P]Pi incorporation, expressed as a percentage of control (absence of PMA), obtained from 3 to 4 different experiments for each condition tested.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/95/3/10.1182_blood.v95.3.894.003k15_894_902/6/m_bloo00315009w.jpeg?Expires=1765896670&Signature=QUBYPLP3yfOiOfoQa5iIW-Lqs7ACoIS446odpxTpH~-pTiNODCuHwLAyhdVv2gg8j5VaSJjgrEGpMjxZefVK1G3xm6d9KY3P2~~eXf7RZTTv0kMFlyEwK5y5gJztlqEyUCj0ryOQCY8z~hBOks9NalaraDfsFFTZqRqWifWkfmM9dqH17P3z3YzAfC7KRs9ZvH72Bh4SW4uYgZ~YU9uFuwuelEMckq4mXsMPANBDkoemaYFzvNUPIyt8gSR3qxtxBrjunQYxy3fkTxr~dN5fIU8HJKJesxp9y0T0pRUkxdW3mh~ZsgQcvdzxpi94mEKppkD7~SFuazxSWCGwsQ1cIw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 10. PMA–concentration-dependent responses. / Platelets were labeled with either [32P]Pi or [3H]5-HT, permeabilized with digitonin, and stimulated with increasing concentrations (1-500 nM) of PMA. [3H]5-HT release and protein phosphorylation were measured. (A) Autoradiography of SDS-PAGE gels of heat-stable platelet extracts (MARCKS) and whole platelet extracts (pleckstrin) is shown. The concentrations of PMA used are indicated at the bottom of the figure. Arrowheads indicate the smallest concentrations of PMA producing a significant increase in the phosphorylation of each protein. (B) PMA–concentration-dependent curves for [3H]5-HT release and pleckstrin and MARCKS phosphorylation are shown. Values represent the mean ± SEM of 6 different platelet preparations.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/95/3/10.1182_blood.v95.3.894.003k15_894_902/6/m_bloo00315010w.jpeg?Expires=1765896670&Signature=i5U7aNx~AJSmgoxzRdgRo7MxJb~clWvx4qw~2YJXVG0Gbn77BieANgHBSNaplgXWlbLzZ~VgSuw7kH-Zx790OJfosTOD4RStmhhyQDot2HhBjYMNWK9ArM1~0Cc629Y6P~1hmLkkbfxUYIKlaRZCnkwnuOAhritLvXCZZE~OQaMJFghuJ5Vus6~zfRoyVbmanhxa84nRuFLdp5Ru7ZyA4EDXWtbNgjXZ~-RNEwv0WD3--J6M4JD4KjEKQP5hSTdh4AkkMAbvgYRuyptoRe-ElgOVKJB2-E3MOQMAj2gxJwT2UQqv9AqNX55fdJKRYPl2pUG8T58eb7V6QD6q9MprVQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal