Polytrauma (PT) leads to systemic activation of polymorphonuclear neutrophils (PMNs). Organ damage commonly found in these patients is ascribed to respiratory bursts of activated PMNs. With the use of sodium dodecyl sulfate–polyacrylamide gel electrophoresis, PMN extracts from PT patients were found to contain a clear protein band not seen in control PMNs from healthy volunteers. This band was identified by amino acid sequencing and Western blotting as pyruvate kinase (PK). Enzymatic assays revealed a 600-fold increase in PK activity in PMNs of PT patients, with the highest levels occurring between the fifth and seventh posttraumatic day. In lymphocytes, no such increase was detectable. As PK is a major regulatory enzyme in glycolysis, glucose-dependent lactate production in PMNs from PT patients was assayed. These cells showed a higher glycolytic lactate production than controls. It was additionally demonstrated that acute activation of respiratory burst activity depends mainly on breakdown of glucose to lactate via the pentose-phosphate pathway and glycolysis. In PMNs from PT patients, this glucose-dependent respiratory burst activity was more than twofold higher than in controls. The increase in expression and activity of PK in PMNs from PT patients may contribute to the high glucose-dependent respiratory burst activity seen in these cells.
Severe polytrauma (PT) remains a major cause of morbidity and mortality despite modern techniques of resuscitation and intensive care and an ever-increasing number of powerful and effective antibiotics.1 In PT patients, a local inflammatory process spills over and causes an exaggerated systemic response, with inflammatory damage to otherwise healthy cells and organs distant to the primary site of injury. Severe traumatic injury leads to the systemic activation of various humoral systems and cellular systems2; it also induces metabolic changes in protein catabolism3 and reduces the endogenous storage of glutamine,4 an important immunonutrient.5
Polymorphonuclear neutrophils (PMNs) play an important role in this inflammatory process. Mediators released by activated PMNs, such as oxygen radicals, proteinases, and phospholipid-derived products, cause organ damage that often results in multiple failure of vital organs.6 Additionally, these mediators affect the hydration state of cells, leading to abnormalities in protein catabolism in these patients.7 The first step in organ damage, injury to vascular endothelial cells, is mediated by the toxic oxygen metabolites of activated PMNs.8
When exposed to appropriate stimuli, PMNs change their pattern of oxygen metabolism, increasing their oxygen uptake sharply while releasing large amounts of superoxide anion into the environment. The key reaction in this respiratory burst is the 1-electron reduction of oxygen to superoxide anion using the reduced form of nicotinamide adenine dinucleotide phosphate (NADPH), catalyzed by the membrane-bound NADPH oxidase.9 NADP+ is then reconverted to NADPH by the pentose phosphate pathway (PPP) by breakdown of glucose. Thus, respiratory burst activity of PMNs depends on glucose metabolism. In the present study, it is described, for the first time: an increase in expression of pyruvate kinase (PK) in PMNs of PT patients, compared with healthy volunteers. PK catalyzes a major regulatory step in the glycolytic pathway, the conversion of phosphoenolpyruvate (PEP) to pyruvate. PMNs of PT patients show an enhanced breakdown of PEP to pyruvate, which correlates with increased glycolytic lactate production in these cells. It is suggested that the increased expression of PK in PMNs of PT patients results in a higher PPP rate for higher NADPH production and is therefore a requirement for the increased respiratory burst activity found in these cells.
Materials and methods
If not otherwise indicated, all chemicals used were obtained from Sigma (St. Louis, MO).
We studied 16 patients with PT (11 male, 5 female), with a mean age of 37.7 years (range, 21-73). The trauma involved the head in 12 patients, the thorax in 9 patients, the spine in 3 patients, the abdomen in 4 patients, the pelvis in 7 patients, and the extremities in 13 patients. All patients had trauma in at least 2 of the above-mentioned areas. The disease was graded according to the Injury Severity Score.10 Patients had an average Injury Severity Score of 44 (range, 25-58). All patients needed mechanical ventilation and showed the signs of a trauma-induced systemic inflammatory response syndrome upon arrival in the intensive care unit. Throughout the study, blood loss frequently had to be substituted with erythrocyte transfusions, a mean 19.4 red packed cell units (range, 0-101). At the time of investigation, no patient was classified as septic according to standard clinical and laboratory parameters. Blood cultures were negative in all patients. The control group comprised 22 healthy volunteers (10 male, 12 female), aged 31 ± 8 years. The protocol for obtaining human blood was approved by the Ethics Committee of the University of Vienna.
Leukocytes of 14 mL anticoagulated (ethylenediaminetetraacetic acid [EDTA]–treated) fresh venous blood were purified, as described,11 within 20 minutes after blood samples were collected. Briefly, PMNs were separated from mononuclear cells by centrifugation of blood cells loaded on Ficoll-Paque (Pharmacia, Uppsala, Sweden). PMNs were collected from the pellet of the Ficoll gradient, washed in 0.9% NaCl, and separated from the erythrocytes by dextran sedimentation. Remaining red blood cells were removed by hypotonic lysis. PMN recovery was greater than 92%, and red blood cell contamination was less than 3%. To isolate lymphocytes, cells were collected from the interface region of the Ficoll gradient, washed twice in phosphate buffered saline (PBS) (10 mmol/L Na2HPO4/NaH2PO4, pH = 7.4; 135 mmol/L NaCl; 2.7 mmol/L KCl; 5 mmol/L EDTA; 0.5% bovine serum albumin), and resuspended in PBS containing magnetically labeled anti-CD14 antibodies. Monocytes were separated from lymphocytes by magnetic cell sorting (MACS, Miltenyi Biotec, Bergisch Gladbach, Germany) according to the manufacturer's instructions. The purity of the lymphocyte fraction was usually 90%, as determined by flow cytometry. The remaining 10% were mostly thrombocytes and monocytes.
Cells were lysed by hypotonic treatment. The lysis buffer consisted of 10 mmol/L Tris-HCl, pH = 7.8; 1 mmol/L EDTA; 10 mmol/L KCl; 0.3% Triton X-100; and 1 mmol/L phenylmethylsulfonyl fluoride (PMSF). After homogenization, the cell lysate was collected and clarified from the nuclei by centrifugation at 800g for 3 minutes. The cell lysate was centrifuged a second time (17 000g for 3 minutes), and the supernatant was stored in aliquots at −70°C. The protein content was quantified as described by Bradford.12
Sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) was performed according to Laemmli.13 Briefly, 10 μg of cell lysate was loaded per lane on a 10% SDS–polyacrylamide gel and run at 160 V for 50 minutes. Protein bands were visualized by Coomassie staining. Two-dimensional PAGE was performed as described by O'Farrell.14 Protein spots were visualized by silver staining according to Merril.15
For Western blot analysis, proteins from a 10 μg cell lysate sample were separated by SDS-PAGE and transferred to a nitrocellulose membrane by electroblotting. PK expression was revealed with goat antibodies against rabbit PK-M (Rockland, Gilbertsville, PA), which shows strong cross-reactivity with human PK-M. Bound antibodies were detected with a peroxidase-conjugated anti-goat immunoglobulin (Ig) G antibody (Sigma, St. Louis, MO) in the presence of Supersignal substrate (Pierce, Rockford, IL).
Proteins were separated by SDS-PAGE and blotted onto a polyvinylidene difluoride protein sequencing membrane (PVDF) (Immobilon-P, Millipore, Bedford, MA) with the use of a BioRad electroblotting unit. Protein bands were visualized by Coomassie staining, and the band of interest was excised. The piece of membrane was destained in 50% methanol, blocked for 30 minutes at 37°C with 0.5% polyvinylpyrrolidone–40 in 0.1 mol/L acetic acid, and then extensively washed with water. The membrane was incubated overnight with 1μg trypsin (sequencing grade, Boehringer-Mannheim, Mannheim, Germany) in 200 μL 100 mmol/L Tris/Cl buffer, pH = 8.0, 10% acetonitrile. The membrane was stripped twice with 200 μL 50% acetonitrile. The supernatants were pooled, acidified with 6 μL 1 mol/L HCl and subjected to reversed phase high performance liquid chromatography (Nucleosil 300, 5 μm, C18, 4 × 250 mm). The peptides were eluted at 1 mL/min with the use of a 0 to 50% acetonitrile gradient in 0.1% trifluoroacetic acid (1% per minute), and 0.5 mL fractions were collected. The absorbance was measured at 214 nm. Sequence analysis of peak fractions was carried out by automated sequencing (model 477A sequenator, Applied Biosystems, Foster City, CA).
PK activity was assayed by the widely used standard method described by Fujii and Miwa.16 Briefly, cell lysate was diluted in tri-potassium-magnesium-EDTA (TKME)-buffer (100 mmol/L Tris-Cl, pH = 8.0; 100 mmol/L KCl; 10 mmol/L MgCl2; 0.5 mmol/L EDTA) containing 0.2 mmol/L nicotinamide adenine dinucleotide (reduced form) (NADH), 1.5 mmol/L adenosine diphosphate, 5 mmol/L PEP, and 60 milliunits(mU)/mL lactate dehydrogenase (LDH) and incubated at 37°C for up to 60 minutes. In this assay, the conversion of PEP to pyruvate (catalyzed by PK) is combined with the reduction of pyruvate to lactate (catalyzed by LDH). The latter reaction is accompanied by an oxidation of NADH. LDH activity added to the assay mixture was in excess and does not interfere with the results of this combined assay. The conversion of 1 μmol PEP to pyruvate per minute, as determined by the decrease in absorbance at 340 nm due to oxidation of NADH, was defined as 1 unit. Triton X-100 contained in the cell lysate reduces the sensitivity of the PK assay but also leads to higher reproducibility.17
To determine lactate production, we incubated cells at 37°C for up to 4 hours and measured the lactate content of the medium photometrically by the lactate oxidase-peroxidase assay (Sigma Diagnostics, St. Louis, MO). To exclude any negative effects of the long incubation time without any metabolic substrate on the viability of PMNs, we analyzed cell viability and function before and after incubation. After 4 hours of incubation without substrate, more than 90% of cells were still viable. To determine cell function, we stimulated cells with phorbol myristate acetate (PMA) and measured the superoxide anion production as described below. Neither PMNs from PT patients nor those from healthy volunteers showed a significant reduction in superoxide anion formation by the 4-hour incubation.
Superoxide anion production by isolated PMNs was determined by measuring the superoxide dismutase (SOD)–inhibitable reduction of ferricytochrome c to the ferrous form, as described previously.18 Briefly, 106 cells were incubated in 1 mL PBS (138 mmol/L NaCl, 2.7 mmol/L KCl, 0.6 mmol/L CaCl2, 1.0 mmol/L MgCl2, 10 mmol/L NaH2PO4/Na2HPO4, pH = 7.4) containing 40 μmol/L cytochrome c with or without 20 μg/mL SOD. Samples were equilibrated for 5 minutes at 37°C, and the reaction was initiated by adding 100 ng/mL PMA. Absorbance was measured at 550 nm every 2 minutes for up to 1 hour. After this incubation, still more than 91% of cells were viable as assayed by trypan blue exclusion. The reference sample containing SOD allowed estimation of the superoxide specific cytochrome c reduction. The absorbance determined in the reference was subtracted from that in the sample; the resulting value was the rate of SOD-inhibitable cytochrome c reduction. The maximum rate was calculated from the maximum slope (usually between the sixth and the 25th minute after addition of PMA) by the use of the specific millimolar extinction coefficient of reduced cytochrome c of 21.1 [(mmol/L−1·cm−1)].
Results
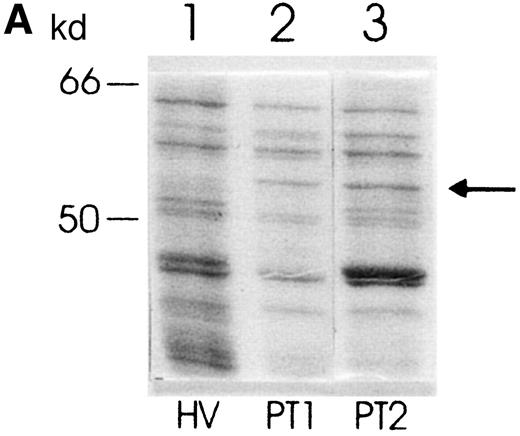
Peripheral blood PMNs of patients with polytrauma (PT-PMNs) express a set of proteins different from that of PMNs of healthy volunteers (HV-PMNs). When these proteins are separated by molecular weight by means of SDS-PAGE, a sharp protein band that does not appear in HV-PMN samples is found in PT-PMNs (Figure1a). This additional band migrates at a molecular weight of about 56 to 58 kd. It showed rather strong staining in every PT-PMN extract analyzed (n = 10). However, in most of the analyzed HV-PMN extracts, no band was seen at the corresponding position. The PT-PMN–specific band was seen in all samples taken from patients within 48 hours after trauma. Between the fifth and the seventh day after trauma, the band became even stronger in 4 out of 10 patients (data not shown).
Protein expression in PMNs from PT patients and healthy volunteers.
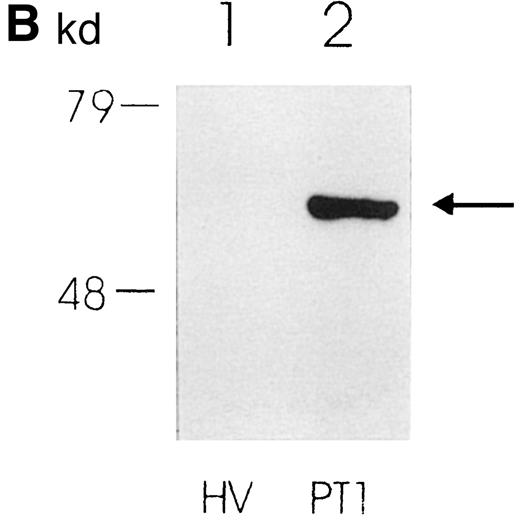
(A) Coomassie blue–stained SDS-polyacrylamide gel showing protein pattern of lysates (10 μg per lane) from PMNs from a healthy volunteer (lane 1) and 2 PT patients (lanes 2 and 3). The arrow indicates the additional band in lanes 2 and 3. (B) Western blot showing expression of PK in PMNs from a healthy volunteer (lane 1) and a PT patient (lane 2).
Protein expression in PMNs from PT patients and healthy volunteers.
(A) Coomassie blue–stained SDS-polyacrylamide gel showing protein pattern of lysates (10 μg per lane) from PMNs from a healthy volunteer (lane 1) and 2 PT patients (lanes 2 and 3). The arrow indicates the additional band in lanes 2 and 3. (B) Western blot showing expression of PK in PMNs from a healthy volunteer (lane 1) and a PT patient (lane 2).
To investigate whether this protein band, which was found only in PT-PMN extracts, corresponded to a single protein or consisted of different proteins, 2-dimensional gel electrophoresis was performed. A PT-PMN extract was separated by SDS-PAGE, the PT-PMN–specific band was excised, and the protein was eluted from the gel. After a 2-dimensional electrophoretical separation of the eluate (first dimension, isoelectric focusing; second dimension, SDS-PAGE), only 1 spot could be seen in the silver stained gel, which demonstrated that the PT-PMN–specific band consisted of a single protein. This spot corresponded to a protein with a molecular weight between 56 and 58 kd, with a pI between 7.4 and 7.6 (data not shown).
In order to identify this protein, we analyzed the amino acid sequence of 2 trypsinized fragments as described in the “Materials and methods” section. Both sequences were identical to the glycolytic enzyme PK-M (PK; EC 2.7.1.40; SWISSPROT Acc. No. P14 618): the sequence of trypsin-fragment 1 (A-P-I-I-A-V-T-R) corresponded to the PK sequence at position 446-454, and the sequence of trypsin-fragment 2 (G-P-E-I-R-T-G-L-I-K-G-S) corresponded to the PK sequence at position 114-126. The theoretical molecular weight of PK (57 746 d) and the calculated pI (7.57) corresponds to the values obtained in the 2-dimensional PAGE (MW = 56-58 kd; pI = 7.4-7.6).
For further confirmation of these data, we analyzed PK expression by Western blotting using a specific antibody. In Figure 1B, a much stronger staining is clearly observed in the PT-PMN lane than in the HV-PMN lane. In sum, these results strikingly illustrate that PK expression is much greater in PT-PMNs than in HV-PMNs.
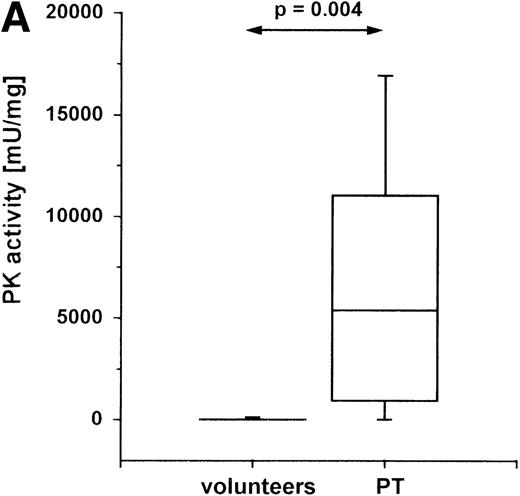
PK catalyzes the essentially irreversible transfer of the phosphate group from PEP to ADP, yielding adenosine triphosphate (ATP) and pyruvate. To investigate whether the high expression of PK in PT-PMNs causes an increased breakdown of PEP to pyruvate, we assayed the enzyme activity in these cells. Peripheral blood from PT patients and from healthy volunteers was collected, and PK activity in the lysate of isolated PMNs was measured. In PT-PMNs (Figure2A), the PK activity was much higher than in HV-PMNs. The PK activities found in HV-PMNs were in the same range as described previously.19
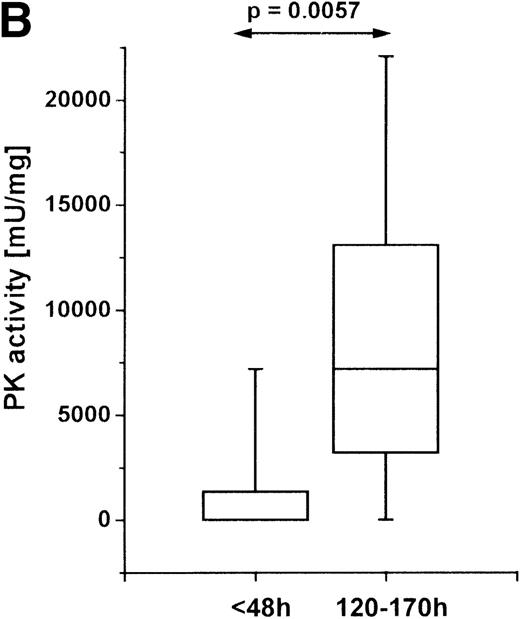
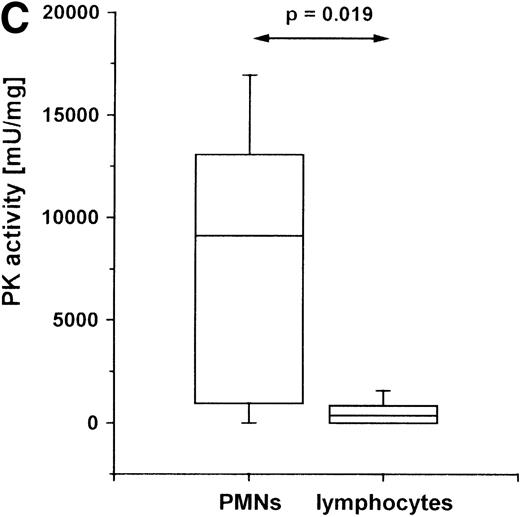
PK activity in PMNs of PT patients.
Cells were isolated from venous blood, and PK activity was measured in cell lysates. PK activity is expressed as milliunits (mU) per mg protein. (A) PK activity in PMNs of healthy volunteers (n = 19) and PT patients (n = 9) between the second and fifth posttraumatic day. (B) PK activity in PMNs at different times after trauma: within the first 48 hours (n = 5) after trauma, and between 120 and 170 hours after trauma (n = 9). (C) PK activity in PMNs and lymphocytes of PT patients. PMNs and lymphocytes were isolated simultaneously from venous blood of PT patients (n = 6) between the second and fifth posttraumatic day. The data are shown in a box-chart plot, with markings that indicate the 0, 25th 50th, 75th, and 100th percentiles.
PK activity in PMNs of PT patients.
Cells were isolated from venous blood, and PK activity was measured in cell lysates. PK activity is expressed as milliunits (mU) per mg protein. (A) PK activity in PMNs of healthy volunteers (n = 19) and PT patients (n = 9) between the second and fifth posttraumatic day. (B) PK activity in PMNs at different times after trauma: within the first 48 hours (n = 5) after trauma, and between 120 and 170 hours after trauma (n = 9). (C) PK activity in PMNs and lymphocytes of PT patients. PMNs and lymphocytes were isolated simultaneously from venous blood of PT patients (n = 6) between the second and fifth posttraumatic day. The data are shown in a box-chart plot, with markings that indicate the 0, 25th 50th, 75th, and 100th percentiles.
PT triggers a complex cascade of posttraumatic events that leads to a multifocal pathophysiologic process. The sequence of events following trauma has been divided into an acute early phase of traumatic shock and a later phase of systemic inflammation.2 To determine whether the increase in PK activity is an early or a late event in the pathogenesis of polytrauma, we measured the enzyme activity in PMNs within the first 2 posttraumatic days and between the fifth and the seventh day after trauma (Figure 2B). In the early stage after injury, the PK activity was higher in some patients than in healthy controls, but when we considered the whole group, we found no significant elevation. In the later phase, however, a strong and highly significant increase in PK activity was detected. In 4 patients, we investigated PK activity in PMNs at both times and found a similar increase between the early and the late phases. Since later posttrauma periods were not investigated, it is not clear when the values return to normal.
To investigate whether the increase of PK in PMN is a cell type–specific event or a general phenomenon of leukocytes, we measured the enzyme activity in extracts of PMNs and lymphocytes within the same PT patients. Unfortunately, the low number of monocytes isolated from the 14 mL blood available from each patient do not allow PK activity assays. In lymphocytes, a lower PK activity was observed than in PMNs (Figure 2C). In comparison with healthy volunteers, the PK activity in lymphocytes of PT patients is not significantly elevated (data not shown).
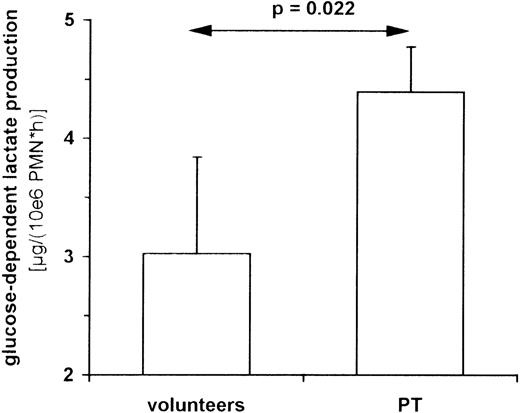
The catalysis of PEP to pyruvate by PK is 1 of the 2 major regulatory steps in glycolysis. The other locus of control is at the level of phosphofructokinase. In PMNs, PK has been described to correlate closely to the lactate production and is assumed to be rate-limiting for glycolysis.20 To delineate the consequences of an increased PK activity on the metabolism of PMNs, we measured glycolysis rate. PMNs have been described as converting glucose almost completely to lactate21 (Figure 3). Therefore, we analyzed lactate production of PT-PMNs between the second and fifth posttraumatic day and lactate production of HV-PMNs. Cells were incubated in PBS in the presence or absence of glucose, and lactate production was measured for up to 4 hours. PT-PMNs showed a glucose-dependent lactate production rate that was higher than that of HV-PMNs (Figure 4). This indicates that the higher expression of PK in PT-PMNs is associated with an elevated glycolysis rate. An increase of lactate production by about 50% appears to be modest in comparison with the 600-fold increase in PK activity. However, the assay systems used to measure both parameters are completely different and do not allow direct stoichiometric comparisons (PK activity was measured in vitro in cell lysates, whereas the lactate production was analyzed in vivo in intact cells).
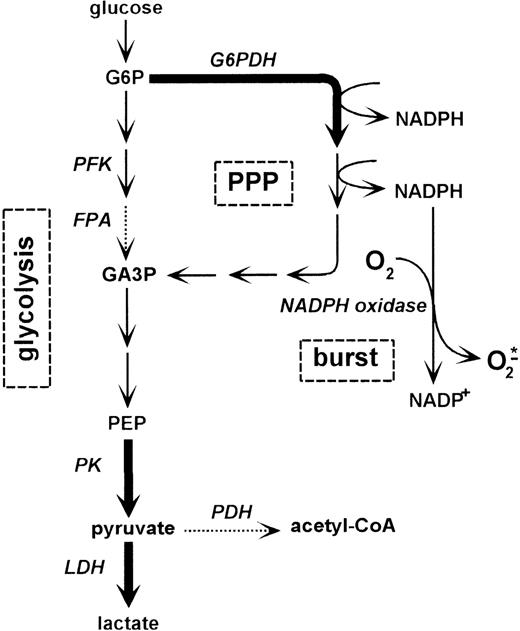
Schematic representation of the relationship between glycolysis, pentose phosphate pathway, and formation of oxygen radicals by NADPH oxidase.
The form of arrows indicates the specific enzyme activities in PMNs as determined previously by Fauth et al17: thick arrows correspond to an activity of 400 to 2000 mU/mg; dashed arrows correspond to an activity lower than 100 mU/mg. Abbreviations: PPP, pentose phosphate pathway; G6P, glucose 6-phosphate; G6PDH, glucose 6-phosphate dehydrogenase; PFK, phosphofructokinase; FPA, fructose-1,6-bis-phosphate aldolase; GA3P, glyceraldehyde 3-phosphate; PEP, phosphoenolpyruvate; PK, pyruvate kinase; LDH, lactate dehydrogenase; PDH, pyruvate dehydrogenase.
Schematic representation of the relationship between glycolysis, pentose phosphate pathway, and formation of oxygen radicals by NADPH oxidase.
The form of arrows indicates the specific enzyme activities in PMNs as determined previously by Fauth et al17: thick arrows correspond to an activity of 400 to 2000 mU/mg; dashed arrows correspond to an activity lower than 100 mU/mg. Abbreviations: PPP, pentose phosphate pathway; G6P, glucose 6-phosphate; G6PDH, glucose 6-phosphate dehydrogenase; PFK, phosphofructokinase; FPA, fructose-1,6-bis-phosphate aldolase; GA3P, glyceraldehyde 3-phosphate; PEP, phosphoenolpyruvate; PK, pyruvate kinase; LDH, lactate dehydrogenase; PDH, pyruvate dehydrogenase.
Lactate production of PMNs from PT patients and healthy volunteers.
PMNs were isolated from venous blood of healthy volunteers (n = 5) and PT patients (n = 5) between the second and fifth posttraumatic day. Cells were cultured in PBS with and without 11 mmol/L glucose. Lactate in the medium was measured every hour for up to 4 hours. Glucose-dependent lactate production is determined by the difference between lactate production in the presence and the absence of glucose. Data shown represent the median ± standard deviation.
Lactate production of PMNs from PT patients and healthy volunteers.
PMNs were isolated from venous blood of healthy volunteers (n = 5) and PT patients (n = 5) between the second and fifth posttraumatic day. Cells were cultured in PBS with and without 11 mmol/L glucose. Lactate in the medium was measured every hour for up to 4 hours. Glucose-dependent lactate production is determined by the difference between lactate production in the presence and the absence of glucose. Data shown represent the median ± standard deviation.
PK catalyzes the final step in glycolysis (Figure 3). Glucose breakdown via phosphofructokinase, as well as by the PPP, results in the conversion of PEP to pyruvate via PK. In PK-deficient red blood cells, the PPP was shown to be inhibited.22 The PPP provides cells with NADPH, which serves as the substrate of respiratory burst activity in PMNs. To investigate whether glucose breakdown via the PPP or glycolysis directly affects superoxide anion production in PMNs, we examined respiratory burst activity rates in the presence and absence of glucose. HV-PMNs were isolated and treated with PMA in a medium consisting of PBS with and without glucose. Superoxide anion production was determined as SOD-inhibitable cytochrome c reduction. Superoxide anion production was observed to be higher in the presence than in the absence of glucose (Figure 5). In addition, PMNs in the glucose-containing medium exhibited a lactate production rate of 4.13 ± 0.82 μg/(106PMN·hour), whereas in the absence of glucose, less lactate was produced (0.76 ± 0.67 μg/(106PMN·hour), P = .0001, n = 5). These data clearly show that inclusion of glucose in the activation medium further increases respiratory burst activity and that this increase is associated with a higher level of glucose breakdown to lactate.
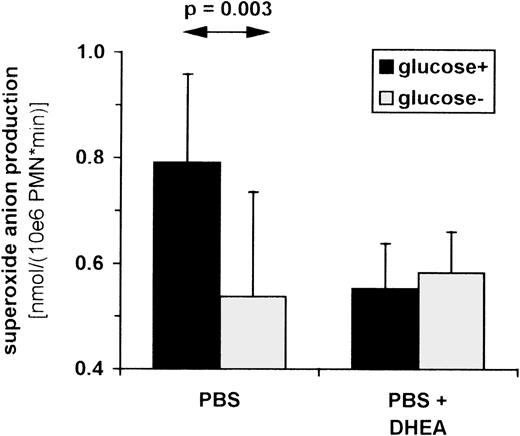
Influence of glucose breakdown on superoxide anion production in PMNs.
Superoxide anion production was determined as SOD-inhibitable cytochrome c reduction. PMNs were isolated from venous blood of healthy volunteers (n = 7) incubated in PBS with or without 11 mmol/L glucose (left 2 bars) and 500 μmol/L DHEA (right 2 bars). Cells were stimulated with 100 ng/mL PMA in the presence and absence of SOD. Cytochrome c reduction was measured photometrically (550 nm) every 2 minutes for up to 1 hour, and maximum slope was used to calculate superoxide anion production per minute. Data shown represent the median ± standard deviation.
Influence of glucose breakdown on superoxide anion production in PMNs.
Superoxide anion production was determined as SOD-inhibitable cytochrome c reduction. PMNs were isolated from venous blood of healthy volunteers (n = 7) incubated in PBS with or without 11 mmol/L glucose (left 2 bars) and 500 μmol/L DHEA (right 2 bars). Cells were stimulated with 100 ng/mL PMA in the presence and absence of SOD. Cytochrome c reduction was measured photometrically (550 nm) every 2 minutes for up to 1 hour, and maximum slope was used to calculate superoxide anion production per minute. Data shown represent the median ± standard deviation.
To determine whether the glucose-mediated increase in superoxide anion production depends on the breakdown by means of PPP, we examined the effect of the specific inhibitor of glucose 6-phosphate dehydrogenase: dehydroepiandrosterone (DHEA). As is well established, glucose 6-phosphate dehydrogenase is the rate-limiting step in the PPP (Figure3). The addition of DHEA to PMNs incubated in PBS with glucose reduced superoxide anion production to levels seen without glucose (Figure 5). In PMNs incubated in PBS without glucose, however, DHEA had no effect on superoxide anion production. Thus, the glucose-dependent increase in respiratory burst activity can be inhibited by blocking the PPP. Blockage of the PPP is likely to result in a decreased level of available NADPH. These data show that respiratory burst activity of PMNs depends, at least in part, on the rate of glucose breakdown via the PPP/glycolysis pathway where PK plays a regulatory role.
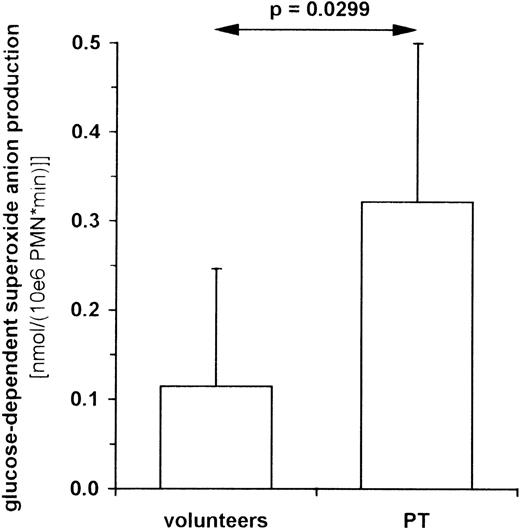
To investigate whether the increase of PK activity and glycolytic lactate formation found in PT-PMNs is accompanied by higher glucose-dependent respiratory burst activity, we examined superoxide anion formation in the presence and absence of glucose. Blood samples were taken from patients 48 to 120 hours after trauma and from healthy volunteers. PMNs were isolated and treated with PMA in a medium consisting of PBS with and without glucose. Superoxide anion production was determined as SOD-inhibitable cytochrome c reduction. In the absence of glucose, both groups showed similar respiratory burst rates (PT-PMNs = 0.495 ± 0.190 nmol/(106PMN·minute), n = 7; HV-PMNs = 0.549 ± 0.217 nmol/(106 PMN·minute), n = 9). The glucose-dependent superoxide anion production, however, was more than twofold higher in PT-PMNs than in HV-PMNs (Figure 6).
Glucose-dependent respiratory burst activity in PMNs from PT patients and healthy volunteers.
Superoxide anion production was determined as SOD-inhibitable cytochrome c reduction. PMNs were isolated from venous blood of healthy volunteers (n = 7) and polytrauma patients between the second and the fifth posttraumatic day (n = 6) and incubated in PBS with or without 11 mmol/L glucose. Cells were stimulated with 100 ng/mL PMA in the presence and absence of SOD. Cytochrome c reduction was measured photometrically (550 nm) every 2 minutes for up to 1 hour, and maximum slope was used to calculate superoxide anion production per minute. The superoxide anion production in the presence of glucose minus that in its absence was defined as glucose dependent. Data shown represent the median ± standard deviation.
Glucose-dependent respiratory burst activity in PMNs from PT patients and healthy volunteers.
Superoxide anion production was determined as SOD-inhibitable cytochrome c reduction. PMNs were isolated from venous blood of healthy volunteers (n = 7) and polytrauma patients between the second and the fifth posttraumatic day (n = 6) and incubated in PBS with or without 11 mmol/L glucose. Cells were stimulated with 100 ng/mL PMA in the presence and absence of SOD. Cytochrome c reduction was measured photometrically (550 nm) every 2 minutes for up to 1 hour, and maximum slope was used to calculate superoxide anion production per minute. The superoxide anion production in the presence of glucose minus that in its absence was defined as glucose dependent. Data shown represent the median ± standard deviation.
Discussion
This study demonstrates that after severe trauma, a dramatic increase in expression of PK is seen to occur in PMNs. PK, a key enzyme in the glycolytic pathway, is found as at least 3 tissue-specific isozymes in mammals.23 By amino acid sequencing, it was shown that the form expressed in PMNs of PT patients is the isozyme PK-M. This is in accordance with data from Tsutsumi et al, which showed that in leukocyte preparations from healthy volunteers the isozyme PK-M is predominantly expressed.24
PK-M has been further found to exist as 2 different isotypes: M1 and M2, produced by alternative splicing of the same primary transcript from the M gene.25 The amino acid sequences of these isotypes are almost identical and differ by only 45 residues coded by exons specific for the isotype. Sequencing data, however, did not enable us to definitively distinguish whether the PK found in PMNs was of the M1 or the M2 type. However, since PK-M1 is expressed predominantly in skeletal muscle, heart, and brain and the M2 isotype is widely distributed in such adult tissues as leukocytes (as well as in the fetus and in undifferentiated or proliferating tissues26 27), the PK-isotype found in PMNs is most likely the M2 type.
PK-M2 was shown to be identical with the thyroid hormone binding protein p58.28 Binding of 3.3′,5-triiodo-L-thyronine (T3) and its analogues results in the inhibition of the activity of PK-M2, preventing its conversion to an active tetrameric form.29 The increased PK activity in PMNs of PT patients, however, is associated with increased amounts of PK protein. Thus, the up-regulation of PK activity after trauma is probably due to protein expression rather than to increased tertramerization. Therefore, a major involvement of T3 can be excluded.
The activity as well as the messenger RNA level of M2-type PK has been reported to increase in rat thymocytes after induction of proliferation by concanavalin A and interleukin 2.30 In contrast to thymocytes, mature PMNs have lost proliferative ability. Normally, only mature PMNs circulate in the blood, but in times of stress, progressively earlier neutrophilic forms can be seen in blood smears.31 This suggests that the increase in PK activity of PT-PMNs, as reported here, might be due to an increased pool of immature precursor cells. However, these PT-PMNs display an increased glucose-dependent respiratory burst activity. Thus, since respiratory burst is a late manifestation of PMN maturation,31 an increased pool of PMN precursor cells in these preparations is unlikely. The increase of PK expression found in the PMNs of PT patients therefore seems not to be related to a maturation effect.
An increase in PK activity in PMNs was also found in patients with psoriasis vulgaris and psoriasis arthropathica.32 The activity of the enzyme was significantly increased during the active stage of the disease and returned to normal during remission. In PT patients, the PK activity was also shown to change during the course of illness. PMNs of PT patients display an increase in PK activity within the first 48 hours after trauma when compared with HV-PMNs. During the course of illness, PK activity (Figure 2B) as well as PK expression increases further. Even during remission, such changes are seen to persist: a lower, but still elevated, PK activity level is detectable. The reason for this increased PK expression remains unclear. Trauma induces a complex cascade of pathophysiological events. Immediately after trauma, various humoral and cellular systems become activated. Platelets and PMNs are the cells chiefly affected. An increased number of PMNs can be found in the liver, kidney, heart, spleen, and brain.6 Mediators released by activated PMNs cause a systemic inflammatory response syndrome. This commonly occurs within the first 2 days after trauma.2 The results presented here show that at this stage PK activity in PMNs is already elevated.
Within the first week after trauma, the exaggerated systemic response leads to a severe impairment of organ function.2 This accounts for the typical host response, which causes a further massive activation of the monocyte-macrophage axis, endothelial cells, and PMNs. Between the fifth and the seventh posttraumatic day, PK activity in PMNs is even higher than in the first 2 days after trauma. In lymphocytes, in contrast, no increase in PK activity is ever detected. These results indicate that PK activity is up-regulated in peripheral blood PMNs and seems to be related to the activation state of these cells during pathogenesis.
The circulating PMN pool reflects the balance between synthesis on the one hand and margination and breakdown on the other. Biologically activated PMNs marginate and leave the circulation. Therefore, increased PK expression in PMNs may not be a direct result of increased stimulation of these cells, but rather a response of the storage compartment in the bone marrow to the pathological stimuli. The PMNs produced after severe trauma may differ from those produced under normal conditions. Additional corroboration for such a notion comes from the observation that abnormalities are found in the functional characteristics of circulating PMNs after serious injury. PMNs' adherence increases proportionally in severely traumatized patients.33 In addition, PMN chemotaxis is impaired following major traumatic injury.34
It may be hypothesized that the increase in PK activity in PMNs after trauma is closely related to the glucose metabolism of these cells. Very little information is available on the glucose metabolism of PMNs. In whole leukocyte preparations, which are 60% to 70% PMNs, only a slight conversion of glucose to CO2 (5%) is described.21 Correspondingly, Ahmed and coworkers found that only 2% of consumed glucose is converted to CO2 in dimethyl sulfoxide–differentiated neutrophilic-like HL60 cells.35 Of the glucose consumed, 82% was converted to lactate via glycolysis. The major rate-limiting step of glycolysis is the conversion of PEP to pyruvate catalyzed by PK.20 The increased PK activity in PT-PMNs coincides with increased glucose-specific lactate production, indicating that these cells exhibit a higher glycolysis rate than HV-PMNs. Glycolysis supplies cells not only with ATP, but also with intermediates and cofactors. In the course of glucose breakdown, glucose 6-phosphate can be metabolized to glyceraldehyde-3-phosphate either via phosphofructokinase and fructose-1,6-bis-phosphate aldolase or via the PPP (Figure 3). Glyceraldehyde-3-phosphate is then metabolized to pyruvate via PK. PMNs have been reported to show a low fructose-1, 6-bis-phosphate aldolase activity, whereas the activity of glucose 6-phosphate dehydrogenase in these cells is about 6 times higher.17 Since glucose 6-phosphate dehydrogenase is the rate-limiting step in the PPP,21 PMNs seem to metabolize glucose preferentially via the PPP and PK to lactate. The PPP provides cells with NADPH, which is the substrate of respiratory burst activity in PMNs. It was demonstrated in this study that the glucose-dependent respiratory burst can be almost completely suppressed by inhibiting glucose 6-phosphate dehydrogenase. This indicates that in PMNs a direct connection exists between the glucose breakdown via the pentose-phosphate-PK pathway and respiratory burst activity. Additional corroboration for this comes from Tan et al,36 who have recently reported that PMA-stimulated superoxide anion production of PMNs was seen to increase with the concentration of exogenous glucose and to plateau at 5 to 10 mmol/L glucose. In addition, PMN activation involved increased glucose transport and intrinsic activation of glucose transporter molecules. The superoxide anion production rate of PMNs observed by Tan and coworkers, however, was remarkably higher than in our experiments. This could be due to the different PMN isolation procedures used. Tan et al isolated PMNs by density gradient centrifugation as opposed to hypotonic lysis, which was used in the present study. They observed a fivefold decrease in superoxide anion production after hypertonic lysis, which correlates with the level of superoxide anion production measured in the present study.
An increase in PK activity would permit higher glucose consumption, which in turn would provide an additional substrate for respiratory burst activity. Indeed, in PT-PMNs, elevated PK activity correlates with a twofold increase of glucose-dependent superoxide anion production after stimulation with PMA. To our knowledge, there are no publications selectively describing the glucose-dependent respiratory burst activity in PMNs from PT patients. However, several studies were performed analyzing the overall superoxide anion production in the presence of glucose, which measures the sum of glucose-dependent and glucose-independent superoxide anion production. All groups found an activation of PMNs and an increased superoxide anion production after severe injury. However, their results differ with respect to the time window when activation occurs. Botha and coworkers showed that PMNs from patients with severe torso trauma are primed and activated in the first 24 hours postinjury, but become unresponsive to PMA activation after 48 hours.37 In contrast, other groups found an elevated superoxide anion production in trauma patients up to 72 hours after injury.38,39 Similar results were published recently by Ogura and coworkers.40 They found an enhanced response to fMLP from days 2 to 21 after injury. In the present study, the elevated glucose-dependent superoxide anion production was measured between the second and the fifth posttraumatic day, which is in accordance with the latter publications.
Our data demonstrate that after trauma, an increase in expression and activity of the glycolytic enzyme PK is seen to occur in PMNs. This elevation is associated with an increased glucose-dependent superoxide anion production. Since PK is the rate-limiting step in glycolysis in PMNs, it might be hypothesized that the increased activity of this enzyme in PT patients contributes to the higher glucose-dependent respiratory burst activity of these cells.
Acknowledgments
We are grateful to Susanne Oehler for helpful discussions and proofreading the manuscript. We also thank Joachim Seipelt for providing the PK-specific antibodies.
Reprints:Rudolf Oehler, Surgical Research Laboratories, Allgemeines Krankenhaus 8G9.05, Waehringer-Guertel 18-20, A-1090 Vienna, Austria (EU); e-mail: rudolf.oehler@akh-wien.ac.at.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal