Phosphatidylinositide 3-kinase (PI3K) is a key enzyme implicated in intracellular signaling of diverse cellular responses including receptor-mediated responses and neutrophil activation. Several PI3K subunits have been cloned and shown to be localized to plasma membrane receptors, the cytosol, or intracellular vesicles or caveolae. We report the localization of PI3K to a distinct intracellular site, cytoplasmic lipid bodies, in leukocytes. In U937 monocyte cells, PI3K p85 regulatory and p110β catalytic subunits were localized to lipid bodies by immunocytochemistry and/or immunoblotting and enzyme assays of subcellular fractions. In RAW murine macrophages, p55, p85, and p85β PI3K subunits were present at isolated lipid bodies. PI3K p85 was also shown to colocalize and, by co-immunoprecipitation, to be physically associated with phosphorylated Lyn kinase in lipid bodies induced to form in human polymorphonuclear leukocytes. These findings, therefore, indicate a novel site for PI3K compartmentalization and suggest that PI3K-mediated signaling is active within cytoplasmic lipid bodies in leukocytes.
Lipid bodies are distinct lipid-rich cytoplasmic inclusions that may be present in many cell types;1,2 in particular, leukocytes are engaged in inflammatory, atherosclerotic, and neoplastic processes.1,3-5 Lipid bodies are intracellular depots of esterified arachidonate1,4,5 and also are discrete sites for localization of eicosanoid-forming enzymes including cyclooxygenase,6-8 5-lipoxygenase, and leukotriene C4 synthase.8 The formation of lipid bodies can be rapidly induced in leukocytes by signaling pathways activated by platelet activating factor (PAF) orcis-unsaturated fatty acids.4,8-10 The quantitative induction of new lipid body formation in intact and anucleate leukocytes correlates with the priming of these cells for increased generation of eicosanoid mediators.8-10Conversely, inhibition of lipid body formation correlates with suppression of the capacity for enhanced eicosanoid formation.8-10 Thus, lipid bodies may have roles in the formation of eicosanoid mediators by leukocytes. Moreover, the finding that cytosolic phospholipase A2(cPLA2) and microtubule-associated proteins (MAP) kinases (also known as extracellular signal-regulated kinases [ERKs])11 are present at lipid bodies suggests that regulatory signal transduction responses occur at lipid body domains.
Phosphatidylinositide 3-kinase (PI3K) is a key lipophilic enzyme implicated in intracellular lipid signaling of diverse cellular responses, including receptor-mediated mitogenesis,12neutrophil activation,13-17 cell migration,18glucose transport,19 vesicular sorting,20,21membrane ruffling,22 and cytoskeleton reorganization.23 A number of PI3K subunits have been purified and cloned in the last few years. Active PI3K is a heterodimeric enzyme consisting of a 110-kd (p110) catalytic subunit and an 85-kd (p85) regulatory subunit.24 PI3K phosphorylates phosphatidylinositol (PI), PI 4-phosphate (PI(4)P), or PI4,5-bisphosphate (PI(4,5)P2) on the D3 position of the inositol ring to produce PI(3)P, PI(3,4)P2, or PI(3,4,5)P3, respectively.25,26 The PI3K products, PtdIns(3,4)P2 and PtdIns(3,4,5)P3, have been shown to activate several isoforms of calcium-insensitive protein kinase C27,28 and serine-threonine kinaseAkt (also referred to as PKBα or Racα).29
There are 2 isoforms of the PI3K p85 subunit, p85α and p85β.30 The p85 subunit is tyrosine phosphorylation–dependent and is composed of a Bcr homology domain, a Src homology 3 (SH3) domain, 2 proline-rich regions, and 2 SH2 domains.31,32 The catalytic p110 subunit has 3 isozymes, p110α, p110β, and p110γ. P110α and p110β are p85-dependent. The interaction of p85 with p110 is required for the enzymatic activity of p110α and p110β.33,34 It is possible that the role of p85 is to target p110 to the membrane, where its lipid substrates reside.23 However, p110γ is a p85-independent but G-protein-activated isozyme.35 Several 55-kd alternative splicing products of the p85α gene have also been identified.36 37
PI3K has been shown to be a cytosolic enzyme in resting cells24,38,39 and to localize to low-density intracellular membrane vesicles in adipocytes,38 clathrin-coated vesicles in 3T3-L1 cells,23 and caveolae in fibroblasts40 and endothelial cells.41 Although PI3K signaling has been extensively studied in leukocytes and other myeloid-derived cells,17 42-45 the intracellular localization of the lipid kinase in these cells is unclear. Thus, we investigated the subcellular distribution of PI3K in human monocytic U937 cells, murine macrophage RAW 264.7 cells, and PAF- and arachidonate-primed human polymorphonuclear (PMN) leukocytes. Studies using immunocytochemistry and subcellular fractionation demonstrated that PI3K localizes in part to cytoplasmic lipid bodies in these cells. In addition, PI3K p85 was also shown to colocalize with phosphorylated Lyn kinase in lipid bodies of stimulated human PMN leukocytes. These findings suggest that PI3K transduces cellular responses within lipid body domains in leukocytes.
Materials and methods
We obtained the following as noted (brand names given in parentheses): Monoclonal antibodies (mAbs) specific for PI3K p85, MAP kinases (pan-ERKs), caveolin, annexin VI, phosphotyrosine (PY-20) (Transduction Laboratory, Lexington, KY); polyclonal antibodies (pAbs) specific for PI3K p85α, p85β, p110β, ERK3, Lyn, and 14-3-3β and protein A and protein G agarose beads (Santa Cruz Biotech, Santa Cruz, CA); antimitochondria p60 mAb (Calbiochem, San Diego, CA); antiphosphotyrosine4G10 (Upstate Biotechnology, Lake Placid, NY); mouse nonimmune immunoglobulin G (IgG) isotype controls (Organon Teknika, Durham, NC); biotinylated secondary antibodies and a glucose oxidase avidin-biotinylated enzyme complex kit (Vectastain ABC kit; Vector Laboratories, Burlingame, CA); fluorescent fatty acid 1-pyrenedodecanoic acid (Molecular Probes, Eugene, OR); horseradish peroxidase–conjugated (HRP-conjugated) secondary antibodies and recombinant human granulocyte-macrophage colony-stimulating factor (GM-CSF) (Biosource, Camarillo, CA); protein assay microbicinchoninic acid (BCA) kit and detection solution (Supersignal ECL; Pierce, Rockford, IL); PI (Avanti Polar Lipid, Alabaster, AL); PI(4)P, PI(4,5)P2, phosphatidyl-L-serine, and arachidonic acid (Sigma, St Louis, MO); [γ-32P] adenosine 5′-triphosphate (ATP; 37 × 1010 Bq/mmol [10 Ci/mmol]) (Du-Pont NEN, Boston, MA); and [14C]-arachidonic acid (2035 M Bq/mmol [55 mCi/mmol]) (American Radiolabeled Chemicals, St Louis, MO).
Culture of U937, RAW cells, and endothelial cells
Additional materials used were the human monocytic leukemia U937 cell line, mouse macrophage cell line RAW 264.7, and a spontaneously transformed human umbilical vein endothelial cell line (ECV 304) (American Type Culture Collection, Rockville, MD) and tissue culture media (Life Technologies, Gaithersburg, MD) supplemented with 10% fetal calf serum (FCS), 10 mmol/L L-glutamine, 50 units/mL penicillin, and 50 μg/mL streptomycin. The U937 and RAW cells were grown in RPMI 1640 medium, endothelial cells in M199 medium; the cells were grown at 37°C in a humidified 5% carbon dioxide incubator. Cells were grown to a density of approximately 1 × 106/mL (U937) or to subconfluence (RAW cells and endothelial cells) and serum-starved overnight in medium with 0.5% FCS before being used for immunostaining or isolation of lipid bodies. Lysates of cells, including 3T3-L1–derived adipocytes (courtesy of Dr Jeffrey Flier), rat mast cell line RBL-2H3 cells (American Type Culture Collection), and human epidermoid carcinoma cell line A431 cells (Transduction Laboratories), were used as controls for immunoblotting studies.
Purification of human PMN leukocytes
PMN leukocytes were purified as previously described.2In brief, using acidified citrate as an anticoagulant, fresh blood was obtained by venipuncture from volunteer donors. After the addition of 6% dextran 70 (McGaw, Irvine, CA), erythrocytes were allowed to sediment for 1 hour at room temperature. The leukocyte-rich supernatant was overlaid onto an equal volume of gradient (Ficoll-Paque; Pharmacia, Piscataway, NJ) and centrifuged at 400g for 20 minutes. PMN leukocytes were recovered from the pellet and washed in Ca++/Mg++–free Hank's balanced salt solution (HBSS). Residual erythrocytes were lysed with hypotonic saline. The PMN leukocytes usually contained approximately 90% neutrophils and 5%-10% eosinophils with very few monocytes and lymphocytes.
Lipid body staining and immunofluorescent/ immunocytochemical microscopy
Lipid bodies in U937 cells were stained with oil red O2,46 or labeled with fluorescent fatty acid 1-pyrenedodecanoic acid (10 μmol/L) for 2 hours at 37°C.11 Dual immunofluorescent/immunocytochemical staining was completed as described.8 11 In brief, pyrenedodecanate-labeled cells were cytospun and fixed in 3% paraformaldehyde in phosphate-buffered saline solution at room temperature for 10 minutes. Fixed cells were permeabilized with 0.05% saponin in HBSS, and nonspecific reactive sites were blocked with 10% normal goat serum for 1 hour. After washing, cells were incubated for 1 hour at room temperature with mouse anti-PI3K p85 mAb (0.5 μg/mL) or nonimmune mouse IgG control as primary antibodies and with biotin-conjugated goat antimouse (1/100 dilution) as secondary antibodies. Immunoreactive PI3K was identified with the glucose oxidase kit (Vectastain ABC kit, Vector Laboratories) following the manufacturer's instruction. Cytoplasmic lipid bodies were visualized under excitation at 340 nm, whereas PI3K immunostainings were examined under light microscopy. The cells were photographed using either × 100 or × 63 objectives.
Isolation of lipid bodies by subcellular fractionation
Lipid bodies were isolated essentially as previously described.4,11 In brief, U937, RAW cells, or endothelial cells were washed twice with Ca++/Mg++–free HBSS and resuspended in 3 mL of disruption buffer25: 25 mmol/L Tris-HCl (tris[hydroxymethyl]aminomethane–hydrogen chloride); 100 mmol/L potassium chloride; 1 mmol/L EDTA (ethylenediaminetetraacetic acid); and 5 mmol/L EGTA (ethyleneglycotetraacetic acid), pH 7.4, supplemented with 10 μg/mL leupeptin, 0.7 μg/mL pepstatin A, and 0.1 mmol/L phenylmethylsulfonyl fluoride. Cells were disrupted by nitrogen cavitation at 800 psi for 10 minutes at 4°C. The cavitate was collected dropwise and mixed with an equal volume of disruption buffer containing 1.08 mol/L sucrose. After centrifugation at 1500g for 10 minutes to pellet nuclei, the supernatant was transferred to a 12-mL ultracentrifugation tube and overlaid sequentially with 2.0 mL each of 0.27 mol/L sucrose buffer, 0.135 mol/L sucrose buffer, and Top solution (25 mmol/L Tris-HCl, 1 mmol/L EDTA, and 1 mmol/L EGTA, pH 7.4). Following centrifugation at 150 500g for 60 minutes, 8 fractions of 1.5 mL were collected from top to bottom: the buoyant lipid bodies (Nos. 1 and 2), the mid-zone (Nos. 3 and 4) between lipid bodies and cytosol, and the cytosol (Nos. 5-8). The microsomal pellet (No. 9) and nuclei (No. 10) were washed and resuspended in 1.5 mL Top solution by sonication. The protein content in each fraction was measured by micro BCA assay using bovine serum albumin as a standard. The activities of lactate dehydrogenase (LDH)47 and arylsulfatase C48 were measured as cytosolic and microsomal markers, respectively. Lipid bodies were detected microscopically by Nile red fluorescent staining.2
Lipid bodies were also isolated from PAF-primed or arachidonate-primed PMN leukocytes (3-5 × 106/mL). The cells were pretreated with 0.5 μmol/L PAF for 1 hour or 20 μmol/L arachidonic acid for 30 minutes to induce lipid body formation;10 in some experiments, as noted, the cells were then stimulated with GM-CSF (60 ng/mL) before subcellular fractionation as described above.
[14C]-Arachidonic acid labeling of subcellular fractions
Cells were incubated with [14C]-arachidonic acid (0.074 M Bq/108 cells [2 μCi/108 cells]) for 24 hours in RPMI 1640 supplemented with 0.5% FCS. Cells were washed twice in HBSS before subcellular fractionation as described above. After ultracentrifugation, aliquots of each fraction were counted for radioactivity to determine lipid labeling in subcellular compartments.
Immunoblot and immunoprecipitation
Proteins from cellular fractions were concentrated by precipitation with 10% TCA overnight at 4°C. The precipitates were washed twice with ice- cold acetone. Protein concentrations were normalized in each fraction after micro BCA assay. Samples (20 μg protein each) were prepared in Laemmli sample buffer (125 mmol/L Tris, pH 6.8; 20% glycerol; 4% SDS; and 2% 2-ME plus bromphenol blue) in denaturing conditions, and proteins were separated by electrophoresis in 10% SDS-PAGE gels. After transfer onto nitrocellulose membranes, nonspecific binding sites were blocked with 5% nonfat milk (Bio-Rad, Hercules, CA) in Tris-buffered saline-Tween (TBST; 50 mmol/L Tris-HCl, 150 mmol/L sodium chloride (NaCl), and 0.1% Tween-20, pH 7.4). Membranes were probed with primary antibodies of interest and HRP-conjugated secondary antibodies in TBST with 3% milk. Detection of antigen-antibody complexes was performed by chemiluminescence (Supersignal ECL, Pierce). When the same membrane was sequentially probed with different antibodies, the blot was stripped in stripping buffer (62.5 mmol/L Tris-HCl, pH 6.8; 2% SDS; 100 mmol/L 2-ME) for 10 minutes at 70°C.
For immunoprecipitation, 1 mL of lipid body fractions from arachidonate-stimulated PMN leukocytes were sequentially immunoprecipitated with 2 rounds of nonimmune rabbit serum followed by 5 μg of anti-Lyn rabbit pAb. Antigen-antibody complexes were immunoprecipitated as described.13 The anti-Lyn immunoprecipitates were resolved by SDS-PAGE, transferred to membranes, and immunoblotted with mAb specific for p85 PI3K.
PI3K activity assay
U937 cells or PAF primed-PMN leukocytes (10 × 106/mL) were incubated with GM-CSF (60 ng/mL) for 5 minutes at 37°C. Cells were spun down and fractionated as described above in disruption buffer supplemented with protease inhibitors and phosphatase inhibitors (1 mmol/L Na3VO4 and 50 mmol/L sodium fluorine). Subcellular fractions were assayed for PI3K activity as described.49 In brief, a mixture of PI and phosphatidylserine was dispersed in kinase buffer (20 mmol/L Tris-HCl, pH 7.5; 100 mmol/L NaCl; and 0.5 mmol/L EGTA) at a concentration of 1 mg/mL by sonication. The kinase substrate solution (50 μL) was added to 0.5 mL aliquots of each subcellular fraction. The reaction was initiated by the addition of 10 μL of kinase buffer containing 0.074 M Bq (2 μCi) of [γ-32P]ATP as well as 100 μmol/L ATP and 20 mmol/L magnesium chloride (MgCl2) at final concentrations. After incubation for 10 minutes at room temperature, reactions were terminated by adding 3 mL of chloroform/methanol.12 Lipids were extracted as described,13 and reaction products were separated on potassium oxalate–coated TLC plates in 1 part propanol to 2 parts N acetic acid (65:35 [vol/vol]) and visualized and quantitated (Instant Imager; Packard, Meriden, CT). The PI3K PI(3)P product was identified by comparison with nonlabeled standards.
Results
Immunocytochemical localization of PI3K to cytoplasmic lipid bodies
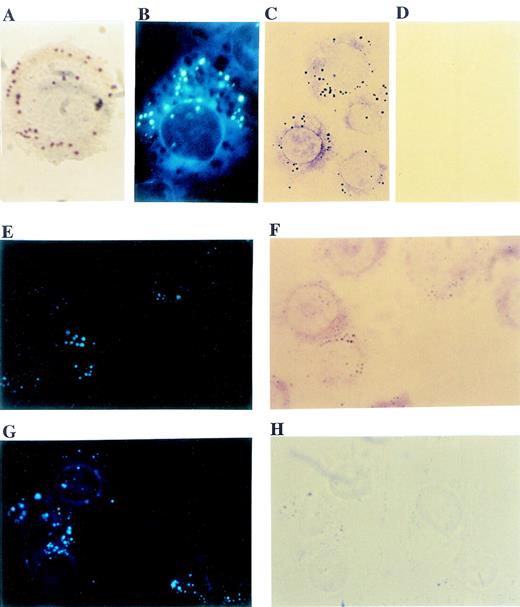
Like activated leukocytes and various neoplastic cells,1,2 5 human monocytic U937 cells contained numerous cytoplasmic lipid bodies that were easily identifiable either with oil red O staining (Figure 1A) or with fluorescent fatty acid labeling using 1–pyrenedodecanoic acid (Figure1B). To evaluate the intracellular localization of PI3K in U937 cells, immunocytochemistry with anti-p85 subunit mAb was used. Distinct anti-p85 immunostaining of punctate structures within the cytoplasm (Figure 1C) were similar in size and numbers to cytoplasmic lipid bodies (Figures 1A and B). In control cells stained with nonimmune mouse IgG, there was no immunocytochemical staining. (Figure 1D). To confirm that the punctate cytoplasmic structures were lipid bodies, the same cells were labeled with both pyrenedodecanoic acid (Figures 1E and G) and p85 mAb (Figure 1F) or nonimmune mouse IgG (Figure 1H). The punctate immunocytochemical staining for PI3K (Figure 1E) perfectly matched the fluorescent-labeled lipid bodies (Figure 1F). In contrast, although there were punctate fluorescent fatty acid–labeled lipid bodies in control cells (Figure 1G), no immunostaining was seen with nonimmune mouse IgG (Figure 1H). These findings indicate that PI3K localizes at cytoplasmic lipid bodies of U937 cells. It should be noted that in addition to the punctate lipid body stainings, PI3K also displayed diffuse cytoplasmic distribution (Figures 1C and F).
Immunocytochemical localization of PI3K to cytoplasmic lipid bodies in U937 cells.
Cytoplasmic lipid bodies in U937 cells were stained with oil red O (A) or labeled with the fluorescent fatty acid, 1-pyrenedodecanoic acid (B). PI3K localization within cells was detected immunocytochemically with an mAb specific for the p85 isoforms of PI3K and avidin:biotinylated enzyme complex glucose oxidase immunocytochemistry, which yields colored reaction product at sites of p85 PI3K localization, including distinct punctate intracellular structures (C). In contrast, comparable immunocytochemistry with a control nonimmune mouse IgG yielded no staining (D). For dual labeling, U937 cells were labeled by incorporation of fluorescent fatty acid, 1-pyrenedodecanoic acid (E, G), and by immunocytochemistry with p85 mAb (F) or nonimmune mouse IgG (H). The punctate immunolocalization PI3K in (E) matched perfectly with fluorescent fatty acid–labeled lipid bodies (F). In contrast, although there were punctate fluorescent lipid bodies in control cells (G), no immunostaining was seen with nonimmune mouse IgG (H). It should be noted that fluorescent lipid body labelings in cells stained with PI3K (E) were weaker than in controls (G). This was likely due to quenching of fluorescence by the glucose oxidase product formed in the immunostaining (F) and the fact that larger lipid bodies in (H) were visualized as refractile, darker structures that lacked any specific glucose oxidase immunostaining. Objective magnification ×100 for (A) and (B) and × 63 for (C)-(H).
Immunocytochemical localization of PI3K to cytoplasmic lipid bodies in U937 cells.
Cytoplasmic lipid bodies in U937 cells were stained with oil red O (A) or labeled with the fluorescent fatty acid, 1-pyrenedodecanoic acid (B). PI3K localization within cells was detected immunocytochemically with an mAb specific for the p85 isoforms of PI3K and avidin:biotinylated enzyme complex glucose oxidase immunocytochemistry, which yields colored reaction product at sites of p85 PI3K localization, including distinct punctate intracellular structures (C). In contrast, comparable immunocytochemistry with a control nonimmune mouse IgG yielded no staining (D). For dual labeling, U937 cells were labeled by incorporation of fluorescent fatty acid, 1-pyrenedodecanoic acid (E, G), and by immunocytochemistry with p85 mAb (F) or nonimmune mouse IgG (H). The punctate immunolocalization PI3K in (E) matched perfectly with fluorescent fatty acid–labeled lipid bodies (F). In contrast, although there were punctate fluorescent lipid bodies in control cells (G), no immunostaining was seen with nonimmune mouse IgG (H). It should be noted that fluorescent lipid body labelings in cells stained with PI3K (E) were weaker than in controls (G). This was likely due to quenching of fluorescence by the glucose oxidase product formed in the immunostaining (F) and the fact that larger lipid bodies in (H) were visualized as refractile, darker structures that lacked any specific glucose oxidase immunostaining. Objective magnification ×100 for (A) and (B) and × 63 for (C)-(H).
Subcellular localization of PI3K proteins and enyzme activities
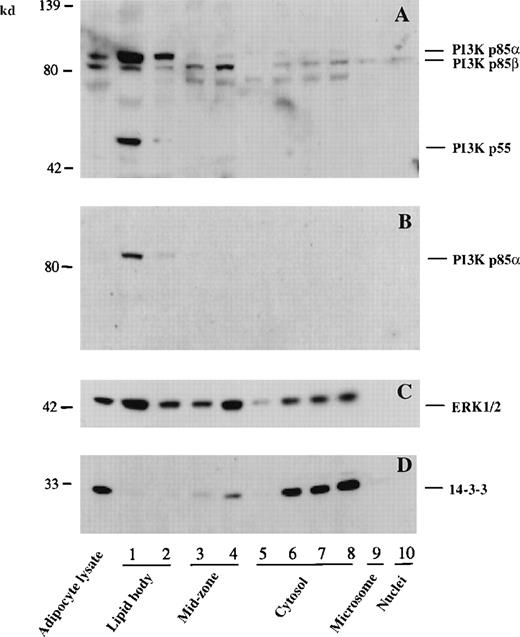
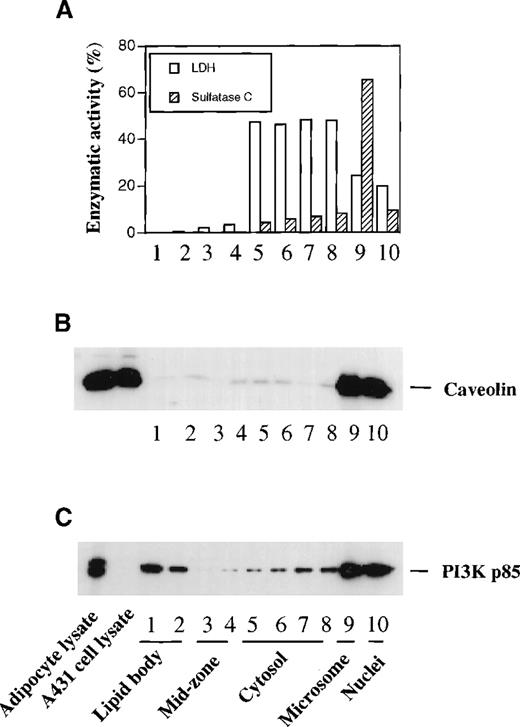
To confirm the immunocytochemical localization of PI3K to lipid bodies in U937 cells, cells were subjected to subcellular fractionation using nitrogen cavitation disruption and sucrose gradient centrifugation specifically designed to isolate buoyant lipid bodies.4,11 The separations of subcellular fractions were indicated by distributions of various markers (Figure2). Microsomal sulfatase C was enriched in fraction No. 9, and cytosolic fractions Nos. 5-8 contained LDH and most cell protein (Figure 2A). Bouyant lipid bodies, identified by staining with the lipophilic fluorescent stain, Nile red, were largely present in the uppermost (Nos. 1 and 2) fractions, as previously characterized.4,11 These fractions were enriched with [14C]-AA–labeled lipids (Figure 2B). Fraction Nos. 3 and 4, the mid-zone fractions, contained fewer numbers of lipid bodies, as assessed by Nile red staining, as well as low-density endosomal vesicles, as evidenced by localization of annexin VI. Annexin VI was absent from the uppermost lipid body fractions but present in mid-zone fractions (Figure 2C), as previously reported.11
Subcellular localization of PI3K to cytoplasmic lipid bodies of U937 cells.
Lipid body and other cellular fractions were isolated from U937 cells as described in “Materials and Methods.” Lipid body fractions were identified microscopically by their content of Nile red staining lipid bodies. Fractions were assayed for LDH and sulfatase C activities as cytosolic and microsomal markers, respectively (A). (B) Proteins in cellular fractions were quantified by micro BCA assay. For lipid labeling of lipid bodies, cells were preincubated with [14C]-AA before subcellular fractionation, and results represent the total [14C]-labeled lipid present in each fraction. Data are representative of 3 independent experiments. (C) Western blotting of specific proteins present in subcellular fractions. Proteins (20 μg) concentrated from each subcellular fraction by TCA precipitation were electrophoresed on a 10% SDS-PAGE gel and immunoblotted with anti-PI3K p85 mAb, anti-PI3K p110β pAb, anti-annexin VI mAb, anti-MAP kinase ERK3 pAb, and an anti-MAP kinase anti-pan ERK mAb. (D) PI3K specific activities in freshly isolated subcellular fractions from GM-CSF stimulated U937 cells. PI3K activity with PI as substrate was measured by the formation of PI3P as described in “Materials and Methods.”
Subcellular localization of PI3K to cytoplasmic lipid bodies of U937 cells.
Lipid body and other cellular fractions were isolated from U937 cells as described in “Materials and Methods.” Lipid body fractions were identified microscopically by their content of Nile red staining lipid bodies. Fractions were assayed for LDH and sulfatase C activities as cytosolic and microsomal markers, respectively (A). (B) Proteins in cellular fractions were quantified by micro BCA assay. For lipid labeling of lipid bodies, cells were preincubated with [14C]-AA before subcellular fractionation, and results represent the total [14C]-labeled lipid present in each fraction. Data are representative of 3 independent experiments. (C) Western blotting of specific proteins present in subcellular fractions. Proteins (20 μg) concentrated from each subcellular fraction by TCA precipitation were electrophoresed on a 10% SDS-PAGE gel and immunoblotted with anti-PI3K p85 mAb, anti-PI3K p110β pAb, anti-annexin VI mAb, anti-MAP kinase ERK3 pAb, and an anti-MAP kinase anti-pan ERK mAb. (D) PI3K specific activities in freshly isolated subcellular fractions from GM-CSF stimulated U937 cells. PI3K activity with PI as substrate was measured by the formation of PI3P as described in “Materials and Methods.”
When these subcellular fractions were subjected to Western blotting with antibodies specific for PI3K subunits, the p85 subunit of PI3K was detected in the cytosolic and mid-zone fractions and was especially enriched in lipid body fractions (Figure 2C). This finding is fully in accord with the immunocytochemical localization of p85 PI3K, especially to punctate lipid bodies as well as other cytoplasmic locales (Figure 1). In addition, the catalytic p110b subunit of PI3K was highly enriched in the lipid body fractions and also present in mid-zone and cytosolic fractions (Figure 2C). Of note, lipid body fractions were essentially free of endosomal marker annexin VI. As we reported previously,11 MAP kinase ERK3 was predominantly localized to the nuclear fraction, whereas ERK1/2 was present in multiple subcellular compartments including lipid bodies, the mid-zone, and the cytosol (Figure 2C). Thus, in U937 cells, lipid bodies were prominent sites of localization of both p85 and p110b subunits of PI3K.
Next we examined PI3K activity in subcellular fractions isolated from GM-CSF–stimulated U937 cells. GM-CSF is known to activate PI3K in U937 cells.42 Coincident with the distribution of immunoactive PI3K in subcellular fractions, high specific PI3K activity was present in the lipid body fractions (Figure 2D). Therefore, enzymatically active PI3K was localized to lipid bodies. In addition, mid-zone fractions exhibited high PI3K-specific activity. This may reflect the localization of low-density endosomal vesicles (noted by the marker annexin VI) in the mid-zone fractions (Figure 2C). Lipid body fractions contained only p85α subunits of PI3K, whereas the mid-zone fractions also contained p85β of PI3K (Figure 2C as well as Figure3). Therefore, it is possible that the p85β subunit of PI3K in the mid-zone may contribute to the significant acitivity in this fraction. This finding seems to be consistent with the report on the localization of PI3K in low-density intracellular membranes in adipocytes.38
Colocalization of PI3K p55, p85, and p85β to lipid bodies of murine RAW cells.
Lipid body and other subcellular fractions were isolated from RAW cells as described in “Materials and Methods.” A positive control adipocyte lysate (100 μg) and proteins (20 μg) from each subcellular fraction of RAW cells were electrophoresed and immunoblotted with anti-PI3K p85 mAb (A), a PI3K p85α specific pAb (B), an anti-MAP kinase anti-pan ERK mAb (C), and an anti-protein 14-3-3β pAb (D).
Colocalization of PI3K p55, p85, and p85β to lipid bodies of murine RAW cells.
Lipid body and other subcellular fractions were isolated from RAW cells as described in “Materials and Methods.” A positive control adipocyte lysate (100 μg) and proteins (20 μg) from each subcellular fraction of RAW cells were electrophoresed and immunoblotted with anti-PI3K p85 mAb (A), a PI3K p85α specific pAb (B), an anti-MAP kinase anti-pan ERK mAb (C), and an anti-protein 14-3-3β pAb (D).
Colocalization of PI3K p85, p85β, and p55 to lipid bodies
To examine whether PI3K also localizes to intracellular lipid-body domains of other myeloid-derived cells, the subcellular distribution of PI3K in a murine macrophage cell line was investigated (Figure 3). In these experiments, electrophoresis was continued for 1 hour after the dye exited the gels to better separate PI3K p85α and p85β isoforms. Under these conditions, the anti-PI3K p85 mAb recognized 3 distinct bands in lipid-body fractions of RAW cells. Two of the bands were approximately 85 kd, and the third band was 55 kd (Figure 3A). The upper 85-kd band was identified as PI3K p85α by immunoblot, with a specific antibody against this isoform of the p85 subunit (Figure 3B). The lower 85-kd band was therefore the p85β isoform, whereas the 55-kd band may be the recently identified 55-kd regulatory subunit of PI3K.36 37 Of note, all 3 regulatory PI3K subunits, p85α, p85βb, and p55, were richly compartmentalized to lipid bodies of RAW cells. As in U937 cells, MAP kinase ERK1/2 was localized to the cytosol as well as lipid bodies (Figure 3C). As a control, protein 14-3-3β was found principally in the cytosol (Figure 3D).
Association of PI3K with Lyn at lipid bodies of human leukocytes
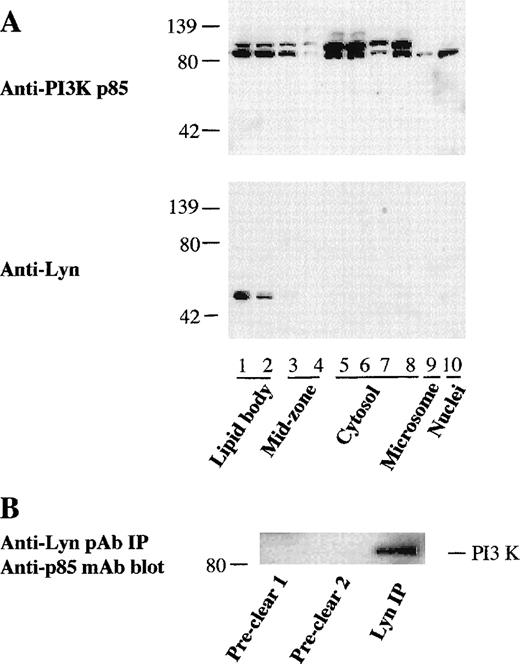
Since the findings presented above demonstrated a distinct association of PI3K with cytoplasmic lipid bodies of myeloid-derived cells, we evaluated whether this was also true with human leukocytes. In resting human PMN leukocytes, there are only a few lipid bodies,2,4 and accordingly we cannot recover enough lipid bodies from normal PMN leukocytes for study (data not shown). However, specific stimuli, including PAF and arachidonic acid, can induce increased lipid body formation in leukocytes.8,9 10Therefore, to enable recovery of enough lipid bodies for our subcellular localization study, PMN leukocytes were pretreated with PAF for 1 hour or arachidonate for 30 minutes to induce lipid body formation before subcellular fractionation. As shown in Figure4A, significant amounts of PI3K p85 α and β were found in the isolated lipid bodies of PAF-primed PMN leukocytes. Strikingly, src-type phosphotyrosine kinase Lyn was also highly concentrated in PMN leukocyte lipid body fractions (Figure 4A). Lyn localization to PMN leukocyte lipid bodies was comparable by Western blotting of subcellular fractions whether or not PAF-primed PMN leukocytes were treated with GM-CSF (not shown). To examine whether PI3K was physically associated with Lyn at lipid bodies in activated PMN leukocytes, Lyn kinase in lipid body fractions isolated from arachidonate-stimulated PMN leukocytes was immunoprecipitated. The immunoprecipitates were subjected to Western blotting for the detection of p85 PI3K. As shown in Figure 4B, immunoprecipitates with anti-Lyn pAbs coprecipitated from the lipid body fraction anti-p85 mAb-detectable PI3K. Conversely, anti-p85 mAb coprecipitated immunodetectable Lyn from lipid body fractions (not shown). These results suggest the physical association of Lyn with PI3K in lipid bodies of activated PMN leukocytes.
Association of PI3K with Lyn kinase in lipid bodies of PMN leukocytes.
(A) Localization of PI3K and Lyn kinase in lipid bodies of PAF-primed PMN leukocytes. PMN leukocytes were preincubated with PAF (0.5 μmol/L) for 1 hour at 37°C to induce lipid body formation before subcellular fractionation. Proteins were then concentrated from each subcellular fractions by TCA precipitation, and 20 μg from each fraction were electrophoresed and immunoblotted with an anti-PI3K p85 mAb and anti-Lyn kinase pAb. (B) Physical association of Lyn kinase with PI3K in lipid bodies of activated PMN leukocytes. PMN leukocytes were pretreated with arachidonic acid (20 μmol/L) for 30 minutes before subcellular fractionation. Lyn kinase in the lipid body fraction (1.0 mL) was sequentially immunoprecipitated with nonimmune control rabbit antibody twice (preclear 1 and 2), followed by immunoprecipitation with 5 μg of anti-Lyn kinase rabbit pAb. The precipitates were electrophoresed and immunoblotted with anti-PI3K p85 mAb and developed with HRP-conjugated goat antimouse antibody.
Association of PI3K with Lyn kinase in lipid bodies of PMN leukocytes.
(A) Localization of PI3K and Lyn kinase in lipid bodies of PAF-primed PMN leukocytes. PMN leukocytes were preincubated with PAF (0.5 μmol/L) for 1 hour at 37°C to induce lipid body formation before subcellular fractionation. Proteins were then concentrated from each subcellular fractions by TCA precipitation, and 20 μg from each fraction were electrophoresed and immunoblotted with an anti-PI3K p85 mAb and anti-Lyn kinase pAb. (B) Physical association of Lyn kinase with PI3K in lipid bodies of activated PMN leukocytes. PMN leukocytes were pretreated with arachidonic acid (20 μmol/L) for 30 minutes before subcellular fractionation. Lyn kinase in the lipid body fraction (1.0 mL) was sequentially immunoprecipitated with nonimmune control rabbit antibody twice (preclear 1 and 2), followed by immunoprecipitation with 5 μg of anti-Lyn kinase rabbit pAb. The precipitates were electrophoresed and immunoblotted with anti-PI3K p85 mAb and developed with HRP-conjugated goat antimouse antibody.
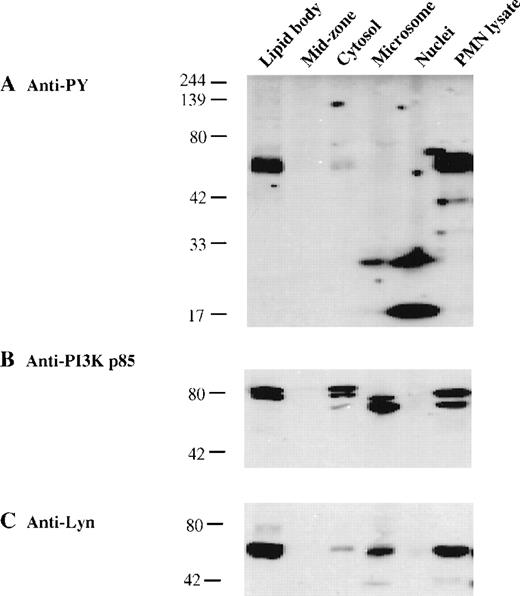
To evaluate whether lipid body–associated kinases were phosphorylated, proteins concentrated from subcellular fractions and cell lysate concentrated from GM-CSF–stimulated PMN leukocytes were resolved by SDS-PAGE; transferred to membranes; and immunoblotted with antibodies specific for phosphotyrosine, PI3K p85, and Lyn. Immunoblots with anti-phosphotyrosine antibody detected very strong signals at approximately 55 kd only in the lipid body fraction and whole cell lysate (Figure 5A), which perfectly matched the bands identified by specific Lyn pAbs (Figure 5C). In addition, antiphosphotyrosine antibody immunoprecipitates contained Lyn detectable on Western blotting (not shown). In contrast, the Lyn present in microsomes and cytosol was not predominantly tyrosine-phosphorylated. Other low molecular weight phosphotyrosine proteins were concentrated in the nuclei and microsomes (Figure 5A). In line with prior reports on the activation of Lyn and the physical association of phosphorylated Lyn with PI3K in GM-CSF–stimulated human PMN42 and activated mononuclear leukocytes,43,44,50 51 our findings suggest that phosphorylated Lyn (Figure 5A and C) colocalizes with PI3K (Figure 5B) in the lipid bodies of activated PMN leukocytes.
Compartmentalization of phosphorylated Lyn kinase with PI3K in lipid bodies of human PMN leukocytes.
PAF-pretreated PMN leukocytes were stimulated with GM-CSF (60 ng/mL) for 10 minutes before subcellular fractionation. Proteins from the lipid body and other subcellular fractions (20 μg each) and a PMN leukocyte cell lysate (200 μg) were electrophoresed and transferred to membranes for immunoblotting. (A) Phosphotyrosine proteins were detected with antiphosphotyrosine mAb 4G10. PI3K p85 (B) was detected with anti-PI3K p85 mAb, and Lyn kinase (C) was detected with an anti-Lyn kinase pAb.
Compartmentalization of phosphorylated Lyn kinase with PI3K in lipid bodies of human PMN leukocytes.
PAF-pretreated PMN leukocytes were stimulated with GM-CSF (60 ng/mL) for 10 minutes before subcellular fractionation. Proteins from the lipid body and other subcellular fractions (20 μg each) and a PMN leukocyte cell lysate (200 μg) were electrophoresed and transferred to membranes for immunoblotting. (A) Phosphotyrosine proteins were detected with antiphosphotyrosine mAb 4G10. PI3K p85 (B) was detected with anti-PI3K p85 mAb, and Lyn kinase (C) was detected with an anti-Lyn kinase pAb.
Association of PI3K with lipid bodies in endothelial cells
It has been shown that PI3K is enriched in caveolae of fibroblasts40 and endothelial cells.41 In accord with reports by others,52 we did not find immunoreactive caveolin in human leukemic cell lines and PMN leukocytes (data not shown). Like myeloid-derived cells, endothelial cells also contain numerous cytoplasmic lipid bodies that can be easily identified by oil red O or osmium staining (data not shown). To ascertain that PI3K-bearing lipid bodies were distinct from the lipid-rich caveolae-like structures, we evaluated cells in which caveolae are identifiable by their content of immunodetectable caveolin. We examined the subcellular localization of caveolin in human umbilical vein endothelial cells using our lipid body isolation scheme. As shown in Figure 6A, the buoyant lipid body fractions were essentially free of the cytosolic marker LDH and the microsomal enzyme arylsulfatase C. Proteins concentrated from these subcellular fractions were immunoblotted for caveolin and PI3K. Caveolin was highly concentrated in the microsomal and nuclear fractions of endothelial cells under our subcellular fractionation conditions (Figure 6B). Whereas caveolin was hardly detectable in lipid bodies (Figure 6B), significant amounts of PI3K p85 were present in the highly buoyant lipid body fractions (Figure 6C), indicating that lipid bodies are different from caveolae, and PI3K association with lipid bodies was not due to caveolar contamination (Figure 6A and B). Of note, a pool of PI3K was also found to be highly concentrated in the caveolin-rich microsomal and nuclear fractions of endothelial cells. In summary, we demonstrate that PI3K is highly associated with cytoplasmic lipid bodies in PMN leukocytes and other myeloid-derived cells and also in endothelial cells, in which lipid bodies can be distinguished from the lipid-rich domains of caveolae.
Association of PI3K with lipid bodies independent of caveolae in endothelial cells.
Endothelial cells were subjected to subcellular fractionation as described in “Materials and Methods.” Lipid body fractions were identified microscopically by their content of Nile red staining lipid bodies. Fractions were assayed for LDH and sulfatase C activities as cytosolic and microsomal markers, respectively (A). Equal amounts of protein (20 μg) concentrated from each subcellular fraction and from control cell lysates were electrophoresed and immunoblotted with anti-caveolin mAb (B) or anti-PI3K p85 mAb (C). Similar results were obtained from 3 independent experiments.
Association of PI3K with lipid bodies independent of caveolae in endothelial cells.
Endothelial cells were subjected to subcellular fractionation as described in “Materials and Methods.” Lipid body fractions were identified microscopically by their content of Nile red staining lipid bodies. Fractions were assayed for LDH and sulfatase C activities as cytosolic and microsomal markers, respectively (A). Equal amounts of protein (20 μg) concentrated from each subcellular fraction and from control cell lysates were electrophoresed and immunoblotted with anti-caveolin mAb (B) or anti-PI3K p85 mAb (C). Similar results were obtained from 3 independent experiments.
Discussion
PI3K is involved in a number of cellular responses and acts by generating specific lipid products that act in signal transduction pathways.53,54 For some of these PI3K-mediated cellular responses, stimulatory agonist molecules bind to the exterior of cells and activate PI3K at cellular membranes. Correspondingly, PI3K can be found to associate with specific plasma membrane receptors55 and to participate in their receptor-mediated signal transduction. PI3K can be found in the cytosol24,38and also localizes to low-density intracellular membrane vesicles23,39 and caveolae40 41 in adipocytes, fibroblasts, and endothelial cells. In each of these sites, PI3K is implicated in generating important signal-transducing phosphoinositide messengers.
We have been interested in the regulated biochemical events that occur at cytoplasmic lipid bodies in varied cells including leukocytes. In the present study, we have used methods of immunocytochemistry and subcellular fractionation that specifically preserve and recover lipid bodies, respectively, to evaluate the localization of PI3K at sites, including lipid bodies, in myeloid-derived cells. In the U937 monocyte cell line, immunocytochemistry localized PI3K p85 to punctate lipid bodies as well as other intracytoplasmic locations (Figure 1). Confirmation of this localization was obtained by subcellular fractionation, which demonstrated that both the p85α and p85β regulatory isoforms of PI3K and the p110β catalytic subunit of PI3K were localized to buoyant lipid bodies (Figure 2). The catalytically active PI3K enzyme was demonstrable in isolated bouyant lipid body fractions of U937 cells (Figure 2). In the RAW murine macrophage cell line, each of the 3 regulatory PI3K subunits, p55, p85α, and p85β, were localized to bouyant lipid bodies on subcellular fractionation (Figure 3). In PAF- and arachidonate-primed human PMN leukocytes, the PI3K p85α and p85β isoforms were recovered with lipid bodies (Figure 4). Thus, PI3K localization to lipid bodies was present in several types of leukocytes. It should be noted that although normal PMN leukocytes contain only a few lipid bodies, inflammatory stimuli, including PAF and cis-unsaturated fatty acids (eg, arachidonic acid), may induce rapid formation of lipid bodies in neutrophils and eosinophils.8,9 10
PI3K also colocalized with the phosphorylated, src-like protein-tyrosine kinase, Lyn, at lipid bodies of stimulated-PMN leukocytes. Subcellular fractionation of PMN leukocytes, induced to form lipid bodies by PAF- or arachidonate-stimulation, demonstrated that Lyn kinase protein was detectable by Western blotting in isolated lipid body fractions (Figure 4A). As demonstrated by coimmunoprecipitation of p85 PI3K with Lyn kinase pAbs, Lyn kinase was physically associated with PI3K in lipid body fractions of stimulated PMN leukocytes (Figure 4B). Lyn kinase present in PMN leukocyte lipid body fractions was tyrosine-phosphorylated (Figure 5B). The findings of phosphorylated Lyn kinase association with PI3K are in accord with prior reports. GM-CSF has been shown to induce both increased Lyn phosphorylation and the association of PI3K with phosphorylated Lyn in human PMN leukocytes.42 Likewise, in B lymphocytes following antigen-receptor50 or PAF43stimulation, in monocytes following lipopolysaccharide stimulation,44 and in Daudi cells following CD40 cross-linking,51 tyrosine-phosphorylated Lyn kinase has been associated with PI3K. Activation of PI3K can be dependent on its association with activated tyrosine-phosphorylated Lyn since inhibition of Lyn activation with the tyrosine kinase inhibitor, herbimycin, abrogates activation of PI3K.44 Therefore, our demonstration of the association of PI3K with phosphorylated Lyn at lipid bodies of stimulated PMN leukocytes is fully compatible with the possibility that PI3K-mediated signaling may be active within cytoplasmic lipid bodies of activated leukocytes.
PI3K has been shown previously to colocalize with caveolin at caveolae in endothelial cells41 and fibroblasts.40 In addition, in human umbilical vein endothelial cells, PI3K p85 has also now been localized to buoyant lipid bodies, independent of caveolin (Figure 6). Thus, PI3K localization to lipid bodies in these cells was not attributable to caveolar contributions. Thus, cytoplasmic lipid bodies represent an additional subcellular compartment in which PI3K may mediate intracellular signaling.
The functional roles of lipid bodies within leukocytes remain incompletely understood. Lipid bodies may be induced to form rapidly within several minutes by intracellular signaling processes that are activatable by PAF and cis-unsaturated fatty acids and are dependent on new protein synthesis.4,8-10 Lipid bodies, moreover, appear to have roles in the formation of arachidonate-derived eicosanoids.8-10 Lipid bodies are sites at which key eicosanoid-forming enzymes (cyclooxygenase, 5- and 15-lipoxygenase, and leukotriene C4 synthase) are localized.6-8 The arachidonate-releasing enzyme cPLA2 and its activating MAP kinases also localizes to lipid bodies.11 It is likely that regulated signal transduction responses occur within lipid body domains. In this context, our findings that PI3K, including its regulatory and catalytic subunits, localize to lipid bodies of myeloid-derived cells would support a role for PI3K in generating phosphoinositide signaling molecules within lipid bodies. Moreover, the localization of PI3K and its physical association with phosphorylated Lyn kinase in human PMN leukocyte lipid bodies further support the functioning of PI3K in lipid bodies. These findings indicate that PI3K may participate in signal transduction responses within cytoplasmic lipid bodies in leukocytes and in myeloid-derived and other cells.
Acknowledgments
We thank Dr Jeffrey Flier for providing adipocytes; Drs Anne Nicholson-Weller, Lewis C. Cantley, Lucia E. Rameh, and Patricia T. Bozza for helpful discussions; and Jennifer P. Gray for excellent technical assistance with immunocytochemistry.
Supported by grants AI22571 and AI20241 from the National Institutes of Health, Bethesda, MD (P.W.).
Reprints:Peter F. Weller, Beth Israel Deaconess Medical Center, DA-617, 330 Brookline Ave, Boston, MA 02215; e-mail:pweller@caregroup.harvard.edu.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

![Fig. 2. Subcellular localization of PI3K to cytoplasmic lipid bodies of U937 cells. / Lipid body and other cellular fractions were isolated from U937 cells as described in “Materials and Methods.” Lipid body fractions were identified microscopically by their content of Nile red staining lipid bodies. Fractions were assayed for LDH and sulfatase C activities as cytosolic and microsomal markers, respectively (A). (B) Proteins in cellular fractions were quantified by micro BCA assay. For lipid labeling of lipid bodies, cells were preincubated with [14C]-AA before subcellular fractionation, and results represent the total [14C]-labeled lipid present in each fraction. Data are representative of 3 independent experiments. (C) Western blotting of specific proteins present in subcellular fractions. Proteins (20 μg) concentrated from each subcellular fraction by TCA precipitation were electrophoresed on a 10% SDS-PAGE gel and immunoblotted with anti-PI3K p85 mAb, anti-PI3K p110β pAb, anti-annexin VI mAb, anti-MAP kinase ERK3 pAb, and an anti-MAP kinase anti-pan ERK mAb. (D) PI3K specific activities in freshly isolated subcellular fractions from GM-CSF stimulated U937 cells. PI3K activity with PI as substrate was measured by the formation of PI3P as described in “Materials and Methods.”](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/95/3/10.1182_blood.v95.3.1078.003k16_1078_1085/6/m_bloo00316002w.jpeg?Expires=1767709825&Signature=mOCg~koAruq1PFMQCMd1U6t6r9fBTIvDZfTH4X5D66ADrbVzEWhex154nx1d2DOE5-kn-ovmS3CyY--jN8cngCpiJ-twFm5iv~SuC38XBpfKn-jGbQ3DfOyvcd-ro3eKyjUBPyEFifOYOMwjBS9bs5Raekqyc2FQepmdaOZCGO3HdeR~r7KlcZa0pmKFDLUHR164i4w4tR7qYitl1Hv-CQbsWIdFgsXKcBsSmGZoE~rqljlWp3sd8CZ97SsCIvgJY~IVfmnj~i5RBxUGYM44SoJKvxY7fsM5I3BvLg75CRlEOfSW7wEmPk5jiSwsyPB6KFIsM68CIZaOJ9DO9O~GfA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal