Neutrophils express 2 low-affinity FcγR, FcγRIIIB (CD16B), and FcγRIIA (CD32A). CD16B is a glycosyl-phosphatidyl inositol-anchored molecule, whereas CD32A is a polypeptide-anchored molecule. These 2 receptors also differ in their signaling. The biological significance of coexpression of 2 FcγRs with distinct membrane anchors and signaling capacities is not clearly understood. Using neutrophils from a CD16B-deficient donor and normal neutrophils treated with anti-CD16 monoclonal antibodies, the authors demonstrated that affinity modulation of CD32A is one of the mechanisms by which neutrophils regulate their FcγR-dependent functions. Neutrophils isolated from a CD16B− donor rosetted poorly with sheep erythrocytes opsonized with rabbit IgG (EA) (12% ± 2% versus 80% ± 6% for control) and were unable to mediate immunophagocytosis. However, activation of CD16B−neutrophils with fMLP, a bacterial chemotactic peptide, increased the CD32A-dependent EA rosetting to 58%. The CD32A-dependent rosetting of fMLP-activated normal neutrophils also increased nearly 5-fold, but there was no increase in CD32A expression. The CD32A-dependent immune complex (IC) binding was also increased in activated neutrophils. This affinity regulation was not observed with CD32A expressed on Chinese hamster ovary cells. These results suggest that in resting neutrophils CD32A is in a low-affinity state and that these cells primarily engage CD16B for IC binding. However, once the neutrophils are activated, the CD32A is converted to a high-affinity state that leads to CD32A-dependent ligand binding and signaling. These results suggest that neutrophils adopt a novel strategy to engage the 2 different FcγR selectively during physiologic and pathologic conditions to carry out their functions efficiently.
The peripheral blood phagocytes, neutrophils, and monocytes recognize foreign organisms and eliminate them from the body by antibody-dependent cellular cytotoxicity and phagocytosis. FcγR plays a major role in mediating antibody-dependent cellular cytotoxicity and immunophagocytosis and in clearing immune complexes (IC) from the circulation.1-6 The occupancy of FcγR by IC induces secretion of various inflammatory mediators and lymphokines. Therefore, knowledge of the structure and function of Fcγ receptors is fundamental for understanding inflammatory processes and defense by the immune system.
The structure and binding properties of 3 types of FcγR have been described.1-5 FcγRI (CD64) is a high-affinity receptor of 70 kd expressed on monocytes, tissue macrophages, and activated neutrophils. FcγRII (CD32), a low-affinity receptor for monomeric IgG, is a 40-kd protein expressed on monocytes, macrophages, neutrophils, B cells, platelets, epithelial cells, and endothelial cells. The FcγRIII (CD16), which is also a low-affinity receptor for monomeric IgG, is a glycoprotein of 50 to 70 kd expressed on neutrophils, natural killer cells, eosinophils, and macrophages. The CD16 expressed on natural killer cells and macrophages (CD16A) has a classical polypeptide membrane anchor, whereas the neutrophil CD16 (CD16B) is glycosyl-phosphatidyl inositol (GPI)-anchored.7-11
The 2 low-affinity Fc receptors for IgG, CD32A and CD16B, bind ligands with overlapping specificity.2-5 The density of CD16B expressed on neutrophils is 4 to 5 times higher than that of CD32A (135 000 versus 31 000 molecules/cell).7 The 2 low-affinity FcγRs expressed on neutrophils differ in their signaling capacities. Cross-linking of neutrophil CD16B induces Ca++mobilization, chicken E cytotoxicity, and degranulation, but it is unable to signal for respiratory burst, tumor cell cytotoxicity, and phagocytosis.9,12-18 However, under similar conditions, CD32A is capable of signaling for all these Fcγ-dependent functions in neutrophils.15,19 These studies show that the polypeptide-anchored form of CD32A is a potent trigger molecule compared with GPI-anchored CD16B.15 It is intriguing to note that neutrophils express CD16B at 4 to 5 times higher density with overlapping ligand specificity, yet CD16B is not as potent as CD32A in triggering functions. It would be interesting to know why neutrophils express 2 Fcγ receptors differing in their membrane anchor and signaling capacity. We have identified a blood donor with no CD16B expression but with normal CD32A expression. Using neutrophils from this donor and normal neutrophils blocked with anti-CD16B monoclonal antibody (mAb), we demonstrated that the ligand-binding function of CD32A was modulated from a low-affinity state in resting neutrophils to a high-affinity state in activated neutrophils. Our findings suggest that such an affinity regulation could play an important role in the preferential use of 2 FcγRs by neutrophils to carry out its functions under physiologic and pathologic conditions.
Materials and methods
Reagents
Human IgG subtypes, rabbit anti-DNP IgG, and crystalline bovine serum albumin were purchased from Sigma Chemical Company (St. Louis, MO). Human transferrin and rabbit antihuman transferrin IgG were purchased from Boehringer Mannheim (Indianapolis, IN). Sheep erythrocytes (SRBC) were from Colorado Serum Company (Denver, CO). Na125I was from Amersham (Arlington Heights, IL), and Iodogen was from Pierce (Rockford, IL). IgG-free fetal bovine serum (FBS) and other tissue culture media were purchased from GIBCO BRL (Grand Island, NY).
Cell lines and antibodies
Anti-CD16 (3G8 and CLBFcgran-1) mAbs were described previously.7 Monoclonal antibodies specific for NA1 (CLBFcgran 11) and NA2 (GRM1) allotypes of CD16B were kindly provided by Drs T. Huizinga (Amsterdam, Netherlands) and F. Jarred (Virgen de las Nieves AVD, Grenada, Spain), respectively. Fluorescein isothiocyanate (FITC)-conjugated-F(ab')2 sheep antimouse IgG was purchased from Tago Immunochemicals (Burlingame, CA). Mouse anti-DAF (IA10) and anti-CD59 (10G10) were gifts of Drs E. M. Medof (Case Western Reserve University, Cleveland, OH) and W. F. Rosse (Duke University, Durham, NC), respectively. Mouse hybridoma cell lines secreting antibody against CD32 (IV.3), CD64,32.2 CD11a (TS1/22), CD11b (LM2/1), CD18 (TS1/18), LFA3 (TS2/9), CR1 (mAb 543), and HLA 1 (W6/32) were obtained from American Type Culture Collection (Rockville, MD). Mouse antihuman CD11c (SHCL3) was a gift from Dr C. Parkos (Emory University, Atlanta, GA). CHOK1 cell transfectant expressing CD32A was established by cotransfecting CD32A cDNA20 in a pCDM8 vector (kindly provided by Dr Brian Seed, Massachusetts General Hospital, Boston, MA) with a plasmid containing hygromycin selection marker pSVhygro. Transfectants expressing high levels of CD32A were selected by panning and immunofluorescent flow cytometry sorting. Chinese hamster ovary (CHO) cell transfectants were maintained in 200 μg/mL hygromycin B.
Isolation and flow cytometric analysis of neutrophils
Neutrophils and mononuclear cells were isolated from human peripheral blood using the dextran sedimentation method as described previously.9 Neutrophils were prepared at room temperature and used as quickly as possible. All the washing media and centrifuges were also kept at room temperature to avoid temperature fluctuations during neutrophil preparation.
For flow cytometry analysis, cells (5 × 105) were incubated with the mouse mAbs or control mIgG1 (X63), followed by staining with FITC-conjugated-F(ab')2 sheep antimouse IgG. The samples were analyzed in FACScan flow cytometer (Becton Dickinson, San Jose, CA).
Rosetting of human IgG-conjugated erythrocytes to neutrophils
Human IgG subtypes and antibodies were coupled to SRBC by the chromium chloride method.21 Neutrophils (5 × 105/well) in Hanks' balanced salt solution/1% IgG-free FBS were incubated with human IgG-coupled SRBC (2 × 107/well) for 4 hours at 4°C in the presence and absence of Fab fragments of anti-CD16 mAb, CLBFcgran-1, or anti-CD32A mAb, IV.3. At least 200 cells were examined under light microscopy for rosetting. Neutrophils with a minimum of 5 SRBC attached were scored as rosettes. Rosetting assays were also carried out using SRBC coated with TNP-rabbit anti-DNP IgG. Rosetting was also conducted in the presence of mAbs against CD11a, CD11b, CD11c, and CD18 to determine whether integrins contributed to the rosette formation of neutrophils with rabbit anti-DNP IgG-opsonized SRBC. Aggregate RBCs, occasionally seen during the preparation of IgG-coated SRBC, were removed immediately before rosetting by being passed through a nylon mesh.
Polymerase chain reaction amplification of CD16 gene-specific sequences
CD16 gene-specific sequences8 were analyzed using genomic DNA isolated from peripheral blood mononuclear cells.22 The region of exon 5 (corresponding to 611-825 bp of cDNA) with gene-specific restriction endonuclease sites (Dra I and Taq I) was amplified from genomic DNA by polymerase chain reaction (PCR) as described.23 An aliquot of PCR-amplified product was subjected to Taq I or Dra I restriction enzyme digest, and the digested products were analyzed on 2% NuSieve agarose gels.
Phagocytosis assay
Phagocytosis of rabbit anti-DNP IgG-opsonized SRBC (EA) was determined as described.24 Briefly, neutrophils (1 × 106) in 100 μL RPMI/2% IgG-free FBS/10 mmol/L HEPES, pH 7.3, were incubated on ice with 8 × 107 of EA in 50 μL of the same buffer for 30 minutes in duplicate tubes in the presence or absence of Fab fragments of anti-CD16 and anti-CD32 mAbs. One set of tubes was transferred to 37°C and incubated for another 30 minutes. Surface-bound and unbound EA were removed by hypotonic lysis in H2O for 20 seconds. The cells were washed in cold phosphate-buffered saline and lysed in 200 μL of 10 mmol/L phosphate buffer, pH 6.5, containing 0.1% sodium dodecyl sulphate and 0.1% Triton X-100. Pseudoperoxidase activity of hemoglobin from the ingested E was assayed as described,24 using a 20 μL aliquot of the cell lysate, o-tolidine (50 μg/mL) in 50 mmol/L acetate buffer, pH 5.5, and 0.12% H2O2. The color developed was read at 405 nm in a Bio-Rad enzyme-linked immunosorbent assay plate reader. Neutrophils incubated with unopsonized TNP-E were used as controls.
Immune complex binding assay
Human transferrin was iodinated with Na125I using Iodogen,25 and a soluble immune complex was prepared as described.26 Briefly, 125I-transferrin (50 μg/mL) was mixed with rabbit antihuman transferrin IgG (50 μg/mL) for 4 hours at 4°C. The complex was centrifuged at 15 000 rpm for 30 minutes at 4°C, and the supernatant was used for an125I-IC binding assay. No visible pellet was seen after centrifugation of the IC. Formyl-methionyl-leucyl-phenylalanine (fMLP)-activated and control neutrophils (5 × 105/well) were preincubated with Fab fragment of indicated mAbs (50 μL of 100 μg/mL) for 30 minutes at 4°C.125I-IC was then added, and incubation continued for 45 minutes at 4°C. After the cells were washed with cold Hanks' balanced salt solution/1% fetal bovine serum, the 125I-IC bound to cells was counted in a gamma counter. The IC bound to neutrophils in binding buffer was taken as 100%. The IC binding in the presence of a 50-fold excess of Fab fragments of anti-CD16 mAb (CLBFcgran-1) and anti-CD32 mAb (IV.3) was taken as nonspecific binding.
The molecular size of IC was determined by size exclusion chromatography using Sephacryl S300-HR (Pharmacia, Piscataway, NJ).125I-IC (100 μg/mL) was passed through Sephacryl S300-HR, protein peaks were identified by monitoring absorbance at 280 nm, and the radioactivity in each fraction was counted in a gamma counter. The protein peak corresponding to 125I-IC was identified by binding of purified CD16 to 125I-IC. Calculations using the specific activities of radiolabeled transferrin and the molecular size of the IC showed that IC was made up of 3 transferrin and 2 IgG molecules.
Results
Characterization of CD16B deficiency in blood donor neutrophils
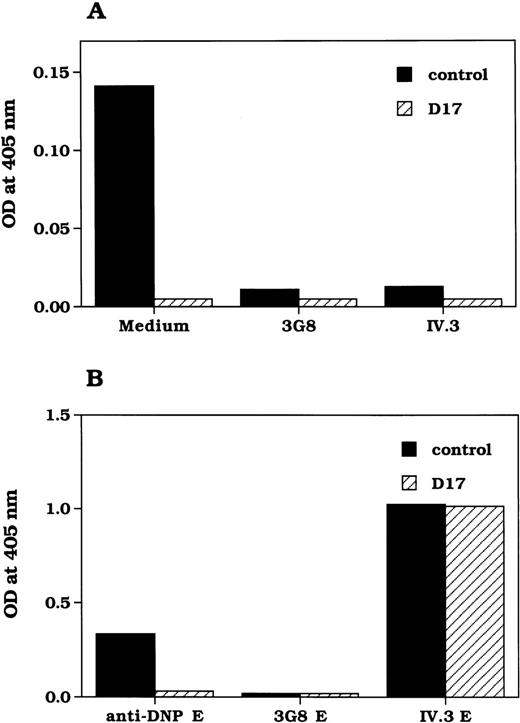
The expression of CD16B and CD32A on neutrophils from donor 17 were analyzed by flow cytometry. As shown in Figure1, a CD16-specific mAb, CLBFcgran-1 did not bind to neutrophils from a blood donor (donor 17, or D17). In addition, anti-CD16 mAbs 3G8, CLBFcgran-11, and GRM1 also did not bind to neutrophils from the same donor (data not shown). However, the expression of CD32A on neutrophils was similar to that observed in neutrophils from the control donor (Figure 1). CD16B is expressed as a GPI-anchored form on neutrophils. Because the cell surface expression of GPI-anchored proteins such as CD16B, CD55, and CD59 is defective in patients with paroxysmal nocturnal hemoglobinuria27 because of a defect in the biosynthesis of the GPI-anchor, the expression of other GPI-anchored proteins was analyzed. The expression of CD55 and CD59 in neutrophils from D17 were normal (data not shown), indicating that the deficient CD16B expression in D17 was not caused by paroxysmal nocturnal hemoglobinuria. Neither control nor D17 neutrophils expressed CD64. The expression of other cell surface proteins, such as LFA-3, CD11a, CD11b, CD11c, CR1, and HLA class I, was not altered in neutrophils from D17 (data not shown).
Immunofluorescent flow cytometry analysis of neutrophils from donor 17 and control.
Cells were stained with indicated mAbs followed by FITC-conjugated-F(ab')2 sheep antimouse IgG. CLBFcgran-1 is an antiCD16 mAb; IV.3 is an anti-CD32A mAb. X63 is a nonbinding mouse myeloma IgG.
Immunofluorescent flow cytometry analysis of neutrophils from donor 17 and control.
Cells were stained with indicated mAbs followed by FITC-conjugated-F(ab')2 sheep antimouse IgG. CLBFcgran-1 is an antiCD16 mAb; IV.3 is an anti-CD32A mAb. X63 is a nonbinding mouse myeloma IgG.
The expression of CD16A in the LGL fraction of the mononuclear cell fraction from D17 was analyzed by flow cytometry, and the expression of CD16A in LGL was normal (data not shown), as reported in persons deficient in CD16B.28 CD16A and CD16B are products of 2 different highly homologous genes,8 and the failure in expression of CD16B in neutrophils from D17 might have been caused by a defect in the gene or in the transcription of the gene specific for CD16B. Because similar deficiencies in CD16B expression resulting from a CD16B gene defect have been reported in a patient with systemic lupus erythematosus23 and in a healthy mother,29 we performed a similar PCR amplification of CD16 genes23 using genomic DNA from D17 and a control donor. A 215-bp product was obtained by PCR amplification of DNA from D17 and the control (Figure2B, lanes 1 and 4). The restriction sites for Dra I and Taq I were present only in the CD16A gene6; therefore, CD16A or CD16B gene-specific fragments can be identified by differential susceptibility to these restriction endonucleases (Figure2A). All the 215-bp PCR product obtained from D17 was completely susceptible to Taq I and Dra I restriction endonuclease digestion, whereas only part of the 215-bp PCR product from the control was susceptible (Figure 2B). These results indicated that the CD16A gene was normal and that the lack of the expression of CD16B in neutrophils resulted from partial or complete deletion of the CD16B gene.
Analysis of CD16A and CD16B genes expressed in donor 17 by PCR.
(A) Restriction map of CD16B (neutrophils) and CD16A (natural killer [NK] cells). Gene-specific restriction endonuclease sites Dra I (D) and Taq I (T) are indicated in a box. Also shown are the positions of the primers used for PCR amplification of the segment of the exon 5 (corresponding to 611-825 bp of cDNA). (thick bars) Coding regions of cDNA. (thin bars) Noncoding regions of cDNA. (B) Restriction endonuclease analysis of PCR products recovered from donor 17 and control. The exon 5 segment was amplified with primers 1 (forward, gtttggcagtgtcaa) and 2 (reverse, gctcttattactcccatggga) using genomic DNA from control (left panel) and D17 (right panel). The PCR product was treated without (lanes 1 and 3) or with Dra I (lanes 2 and 4) or with Taq I (lanes 3 and 6), and then it was analyzed by 2% NuSieve agarose gel.
Analysis of CD16A and CD16B genes expressed in donor 17 by PCR.
(A) Restriction map of CD16B (neutrophils) and CD16A (natural killer [NK] cells). Gene-specific restriction endonuclease sites Dra I (D) and Taq I (T) are indicated in a box. Also shown are the positions of the primers used for PCR amplification of the segment of the exon 5 (corresponding to 611-825 bp of cDNA). (thick bars) Coding regions of cDNA. (thin bars) Noncoding regions of cDNA. (B) Restriction endonuclease analysis of PCR products recovered from donor 17 and control. The exon 5 segment was amplified with primers 1 (forward, gtttggcagtgtcaa) and 2 (reverse, gctcttattactcccatggga) using genomic DNA from control (left panel) and D17 (right panel). The PCR product was treated without (lanes 1 and 3) or with Dra I (lanes 2 and 4) or with Taq I (lanes 3 and 6), and then it was analyzed by 2% NuSieve agarose gel.
Deficient IgG binding by D17 neutrophils
The functional status of FcγR on neutrophils from D17 was analyzed for its ability to bind to human IgG subtypes. The binding of human IgG subtypes to neutrophils was analyzed using the IgG subtypes coupled to SRBC (E-IgG), and subsequent rosette formation was quantified. Neutrophils from a control donor showed approximately 60%, 43%, and 55% rosette formation with E-IgG1, E-IgG2, and E-IgG3, respectively (Figure 3). No E-IgG rosetting to normal neutrophils was observed in the presence of an anti-CD16 mAb, CLBFcgran1, whereas in the presence of an anti-CD32 mAb, IV.3, 44%, 20%, and 43% rosetting was observed with E-IgG1, E-IgG2 and E-IgG3, respectively. In contrast, the binding of E-IgG subtypes to the neutrophils from D17 was reduced significantly (Figure 3); only 9%, 5%, and 8.5% rosetting with E-IgG1, E-IgG2, and E-IgG3, respectively. The low-level rosetting of E-IgG subtypes to D17 neutrophils was mediated through CD32A, as evident from the complete inhibition of binding by IV.3 (Figure 3). E-IgG4 did not bind to neutrophils from either the control or D17 (Figure 3). We also analyzed the binding of the rabbit IgG by determining the rosette formation of neutrophils with rabbit anti-DNP IgG-opsonized, TNP-conjugated SRBC (EA). The rosette formation of D17 neutrophils with EA was only approximately 12% ± 2% (mean ± SD from 4 different blood donations) compared with 79% ± 6% rosette formation with the control neutrophils.
Rosetting of human IgG-coupled SRBC with neutrophils from donor 17.
Neutrophils from control (closed bar) or donor 17 (hatched bar) were incubated with human IgG subtype-coupled SRBC in the absence or presence of 5 μg/mL Fab fragments of anti-CD16 mAb, CLBFcgran-1, or anti CD32A mAb, IV.3. The rosetting assay was performed as described in “Materials and Methods.” Cells were incubated with IgG-coupled SRBC at 4°C, and the rosettes formed were counted by light microscopy. Experiments were performed in duplicate.
Rosetting of human IgG-coupled SRBC with neutrophils from donor 17.
Neutrophils from control (closed bar) or donor 17 (hatched bar) were incubated with human IgG subtype-coupled SRBC in the absence or presence of 5 μg/mL Fab fragments of anti-CD16 mAb, CLBFcgran-1, or anti CD32A mAb, IV.3. The rosetting assay was performed as described in “Materials and Methods.” Cells were incubated with IgG-coupled SRBC at 4°C, and the rosettes formed were counted by light microscopy. Experiments were performed in duplicate.
Neutrophils from donor 17 do not phagocytose rabbit IgG opsonized SRBC
The consequence of the CD16B deficiency on the capacity of D17 neutrophils to phagocytose EA was analyzed. Neutrophils from the control were able to phagocytose the EA efficiently, but neutrophils from D17 were not phagocytic (Figure 4A), despite the normal expression of CD32A. This was not surprising considering that D17 neutrophils bound poorly to EA. To confirm that the defective phagocytosis was caused by lack of binding and to determine whether CD32A retained its signaling capacity to mediate phagocytosis in the absence of CD16B, Fab fragments of anti-CD32A mAb were used as a ligand to coat SRBC (E-IV.3). When IV.3 was used as the ligand, neutrophils from D17 and the control showed approximately 80% rosette formation. The level of phagocytosis of the E-IV.3 by neutrophils from D17 was similar to the level seen in control neutrophils (Figure 4B), and it was much higher than that of IgG-coated E. This suggested that CD32A is capable of phagocytic signaling when stable binding is achieved by the use of high-affinity ligands such as mAb. These results indicated that in the absence of CD16B expression, D17 neutrophils cannot mediate phagocytosis of EA because of inefficient ligand binding by CD32A. These results also showed that the signal produced by CD16B was not required for phagocytosis. Under similar conditions, Fab fragments of anti-CD16-coated E (E-3G8) were not phagocytosed, though they bound normal neutrophils as efficiently as IV.3-coated E, which is in agreement with the observations by others.30 31
Phagocytosis of rabbit IgG-opsonized SRBC and FcγR-specific, mAb-coated SRBC by neutrophils from donor 17.
Neutrophils isolated from control (closed bar) or D17 (hatched bar) were analyzed for the phagocytosis of EA (A) or Fab fragments of indicated mAb-coated SRBC (B) as described in “Materials and Methods.” The pseudoperoxidase activity of the hemoglobin from the ingested E was assayed using o-tolidine, and the color formed was measured at 405 nm in an enzyme-linked immunosorbent assay reader.
Phagocytosis of rabbit IgG-opsonized SRBC and FcγR-specific, mAb-coated SRBC by neutrophils from donor 17.
Neutrophils isolated from control (closed bar) or D17 (hatched bar) were analyzed for the phagocytosis of EA (A) or Fab fragments of indicated mAb-coated SRBC (B) as described in “Materials and Methods.” The pseudoperoxidase activity of the hemoglobin from the ingested E was assayed using o-tolidine, and the color formed was measured at 405 nm in an enzyme-linked immunosorbent assay reader.
fMLP treatment up-regulates CD32A ligand binding function on D17 neutrophils
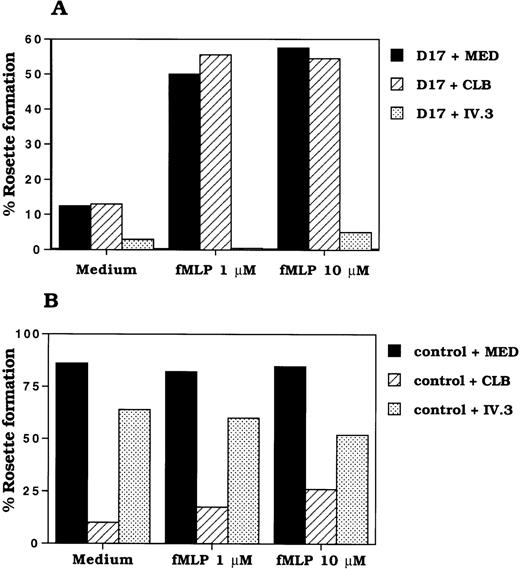
The affinity of receptors such as integrins is known to be modulated by the activation state of the cell.32 33 Therefore, we have tested whether the activation of D17 neutrophils increases the ligand-binding function of CD32A. IgG binding was determined by rosetting of EA with neutrophils. The rosette formation of EA with neutrophils from D17 was only 12% compared with 86% seen in the control (Figure 5A). However, the rosetting of EA with D17 neutrophils was increased from 12% to 58% after fMLP treatment (Figure 5A). The binding of EA to D17 neutrophils was completely inhibited by anti-CD32 mAb (IV.3), whereas anti-CD16 mAbs did not have any effect on the EA rosetting. Furthermore, fMLP treatment did not affect the level of CD32A expression in D17 neutrophils. In fact, it reduced the level of expression of CD32A by 35% (data not shown). Moreover, CD64 was not expressed in resting and fMLP-activated D17 or control neutrophils (data not shown). These results suggested that the activation of neutrophils converted the low-affinity form of CD32A to a high-affinity form.
Effect of fMLP treatment of neutrophils on rosette formation with EA.
Neutrophils (1 × 107/mL) from control and donor 17 were incubated with RPMI 1640 medium without serum in the absence or presence of fMLP (1 μmol/L, 10 μmol/L) for 30 minutes at 37°C. After washing the cells in RPMI 1640/2% IgG free fetal bovine serum, the rosette formation of neutrophils from donor 17 (A) and control (B) with E was performed as described in “Materials and Methods.” Experiments were performed in duplicate.
Effect of fMLP treatment of neutrophils on rosette formation with EA.
Neutrophils (1 × 107/mL) from control and donor 17 were incubated with RPMI 1640 medium without serum in the absence or presence of fMLP (1 μmol/L, 10 μmol/L) for 30 minutes at 37°C. After washing the cells in RPMI 1640/2% IgG free fetal bovine serum, the rosette formation of neutrophils from donor 17 (A) and control (B) with E was performed as described in “Materials and Methods.” Experiments were performed in duplicate.
Up-regulation of CD32A-dependent EA rosetting in normal neutrophils by fMLP treatment
Next we determined whether CD32A expressed on normal neutrophils is in a low-affinity state and can be modulated by cell activation. The CD32A-dependent ligand binding was determined by treating normal neutrophils with Fab fragments of anti-CD16 mAb, CLBFcgran1, followed by rosetting with EA. As shown in Table 1, normal neutrophils rosetted 70% to 80% with EA, whereas under the same conditions 5% to 20% of the anti-CD16 mAb-treated neutrophils formed rosettes. Activation of neutrophils with fMLP increased CD32A-dependent rosetting to 25% to 60%, suggesting that ligand-binding capacity of CD32A is modulated in normal neutrophils. This rosette formation was completely blocked when both anti-CD16 and anti-CD32 mAbs were added together during rosetting. Addition of mAbs against α- (CD11a, CD11b, and CD11c) and β-chains (CD18) of integrins did not influence the rosette formation (data not shown), suggesting that integrins are not involved in the rosette formation between EA- and fMLP-activated neutrophils. This finding is in agreement with the reports from Brown et al34 that FcγR-mediated EA rosette formation of neutrophils was not inhibited by anti-CD11b/CD18 mAb. Moreover, EA rosette formation was normal in neutrophils from patients with leukocyte adhesion deficiency patients who lack integrin expression.34 These results are in contrast to those of Kusunoki et al,35 who demonstrated that CD11b integrin is involved in neutrophil adhesion. It is possible that CD11b integrin may influence the downstream signaling events of CD32A by associating with FcγR, as has been shown for CD16B. However, we did not see any effect of integrin mAbs on EA rosetting. Rosette formation was not caused by CD64 because there was no expression of CD64 in unactivated and activated neutrophils. In some donors, even the incubation of neutrophils at 37°C up-regulated CD32A-dependent EA rosetting. The increase in CD32A-dependent rosetting in fMLP-treated normal neutrophils was not caused by the increased surface expression of CD32A because the FACS analysis showed a decrease in the expression (data not shown). In control and D17 neutrophils, fMLP treatment resulted in a nearly 35% to 40% decrease in CD32A expression and a 3-fold increase in the level CD11b expression. fMLP treatment also reduced the level of expression of CD16B by 80% in normal neutrophils. The modulation of level of expression of these receptors by fMLP was consistent with the previously reported observations.36 37These results showed that the activation-induced modulation of CD32A ligand binding was not unique to the CD32A expressed on CD16B negative neutrophils.
Effect of fMLP treatment on the EA rosetting by neutrophils from normal donors
| Treatment . | Medium . | +anti-CD16 mAb (CLBFcgran-1) . | ||||
|---|---|---|---|---|---|---|
| 4°C . | 37°C . | fMLP . | 4°C . | 37°C . | fMLP . | |
| Donor HE | 70 | 76 | 75 | 7 | 33 | 45 |
| Donor AN | 74 | 66 | 71 | 3 | 27 | 44 |
| Donor MA | 72 | 77 | 69 | 23 | 31 | 63 |
| Donor PA | 72 | 71 | 65 | 10 | 26 | 47 |
| Donor DI | 78 | 75 | 67 | 2 | 6 | 22 |
| Treatment . | Medium . | +anti-CD16 mAb (CLBFcgran-1) . | ||||
|---|---|---|---|---|---|---|
| 4°C . | 37°C . | fMLP . | 4°C . | 37°C . | fMLP . | |
| Donor HE | 70 | 76 | 75 | 7 | 33 | 45 |
| Donor AN | 74 | 66 | 71 | 3 | 27 | 44 |
| Donor MA | 72 | 77 | 69 | 23 | 31 | 63 |
| Donor PA | 72 | 71 | 65 | 10 | 26 | 47 |
| Donor DI | 78 | 75 | 67 | 2 | 6 | 22 |
Neutrophils were incubated with fMLP (1 × 10−6mol/L) in RPMI/5 mmol/L HEPES buffer for 60 minutes at 37°C in a siliconized Eppendorf tube with occasional shaking. As a control, aliquots of cells were incubated without fMLP at 4°C and 37°C for 60 minutes. Then the cells were washed and allowed to form rosettes with EA in ice for 2 h in the presence of medium or Fab fragments of CLBFcgran-1 (final concentration, 2.5 μg/mL). The results are expressed as percentage rosetting. The addition of anti-CD16 (CLBFcgran-1 Fab) and anti-CD32A (IV.3 Fab) inhibited 98% to 100% of EA rosetting by neutrophils from all the donors tested.
EA, antibody-opsonized erythrocytes; fMLP, formyl-methionyl-leucyl-phenylalanine.
Up-regulation of CD32A-dependent soluble immune complex binding in normal neutrophils by fMLP treatment
In the results described so far, we demonstrated the modulation of CD32A ligand-binding function using EA rosette formation. Because the EA rosette formation represents the binding of particulate immune complexes such as IgG-coated SE, we also determined whether such a modulation of the CD32A ligand-binding function could be observed with soluble immune complexes. The CD32A-dependent soluble IC binding was determined by treating neutrophils with Fab fragments of anti-CD16 mAb, CLBFcgran1, followed by 125I-IC binding.
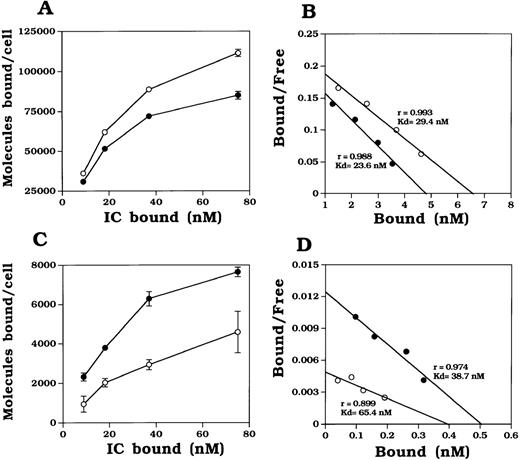
IC was prepared by mixing known protein concentrations of radiolabeled transferrin and rabbit antitransferrin IgG. The molecular size of the IC was determined to be approximately 530 kd by size-exclusion chromatography. The total binding of IC to unactivated neutrophils showed a dose-dependent increase (Figure6A). Total binding of IC to activated neutrophils was decreased to approximately 81% compared with that of unactivated neutrophils (Figure 6A). This decrease in binding may have been caused by the reduced cell surface expression of CD16B as a result of CD16B shedding from activated neutrophils. Under similar conditions, the CD32A-dependent binding of unactivated neutrophils was very low, approximately 3.3% of total binding (Figure 6C). However, after fMLP treatment, the CD32A-dependent binding of IC increased by 2.5-fold after the activation of neutrophils (Figure 6D). Under similar conditions, radiolabeled monomeric IgG, isolated by gel filtration, did not show any detectable binding to activated or unactivated neutrophils, suggesting that CD16B and CD32A cannot bind monomeric IgG stably. This is in agreement with earlier reports from Huizinga et al.38
Effect of neutrophil activation on binding of soluble immune complexes by CD32A.
125I-Transferrin/rabbit antihuman transferrin IgG immune complex (125I-IC) was prepared as described in “Materials and Methods.” The molecular size of the IC was determined by gel filtration. fMLP (1 μmol/L) activation of neutrophils was performed as described in the legend to Figure 5. After washing the cells with RPMI 1640/2% IgG-free fetal bovine serum,125I-IC binding was carried out as described in “Materials and Methods.” Total binding (A) of IC to unactivated (open circle) and activated (closed circle) neutrophils was performed in the presence of a binding buffer. CD32A-dependent binding (C) was performed in the presence of Fab fragments of anti-CD16 mAb, CLBFcgran-1. Scatchard plot analysis of total binding (B) and CD32A-dependent binding (D) was done after converting the specific binding to bound/free IC as functions of the bound IC. Binding in the presence of a 50-fold excess of Fab fragments of mAbs against CD16B and CD32A was taken as nonspecific binding. The IC bound to neutrophils in binding buffer was taken as 100%. Experiments were performed in triplicate.
Effect of neutrophil activation on binding of soluble immune complexes by CD32A.
125I-Transferrin/rabbit antihuman transferrin IgG immune complex (125I-IC) was prepared as described in “Materials and Methods.” The molecular size of the IC was determined by gel filtration. fMLP (1 μmol/L) activation of neutrophils was performed as described in the legend to Figure 5. After washing the cells with RPMI 1640/2% IgG-free fetal bovine serum,125I-IC binding was carried out as described in “Materials and Methods.” Total binding (A) of IC to unactivated (open circle) and activated (closed circle) neutrophils was performed in the presence of a binding buffer. CD32A-dependent binding (C) was performed in the presence of Fab fragments of anti-CD16 mAb, CLBFcgran-1. Scatchard plot analysis of total binding (B) and CD32A-dependent binding (D) was done after converting the specific binding to bound/free IC as functions of the bound IC. Binding in the presence of a 50-fold excess of Fab fragments of mAbs against CD16B and CD32A was taken as nonspecific binding. The IC bound to neutrophils in binding buffer was taken as 100%. Experiments were performed in triplicate.
Scatchard plot analysis of IC binding to unactivated and activated neutrophils was performed to determine the binding affinity of CD32A. The relative affinity of CD32A to IC was increased (Kd 65.4 nmol/L versus 38.7 nmol/L for unactivated and activated neutrophils, respectively) after activation (Figure 6D). The amount of CD32A-dependent binding also increased. Because the expression of CD32A on neutrophils was not increased on activation, the increase in the total number of CD32A-dependent IC binding could have been caused by the increase in the number of active CD32A on activated neutrophils or the increase in the affinity of CD32A to IC. These results demonstrate that the activation of neutrophils up-regulated the binding of CD32A to soluble IC and to particulate ICs such as EA.
Recombinant CD32A expressed on Chinese hamster ovary cells binds ligand-coated SRBC
Leukocyte integrins such as LFA-1 are functionally inactive in resting peripheral blood lymphocytes but functionally active when expressed on COS cells.32 33 A platelet integrin, IIb/IIIa, is expressed as low-avidity state and can be activated to bind ligand regardless of whether it is expressed on platelet or CHO cells. This suggests that not all receptors exhibit similar cell-specific differences in their affinity modulation. Therefore, we tested whether the ligand-binding function of CD32A expressed on CHO cells could be modulated. CHO cell transfectants expressing CD32A were allowed to form rosettes with EA. As shown in Table 2, nearly 53% of CHO cell transfectants formed rosettes. The rosettes were blocked by an anti-CD32A mAb, IV.3. Incubation of CHO cells with fMLP or PMA before rosetting did not influence ligand binding, suggesting that the CD32A ligand-binding function was not modulated in CHO cells. Untransfected CHO cells did not form rosettes with IgG-coated SRBC (data not shown).
Effect of fMLP and PMA on EA rosetting by CD32A expressing CHO cell transfectants
| Treatment . | % Rosetting (mean ± SD) . |
|---|---|
| 4°C | 55.3 ± 4.04 |
| 4°C + IV.3 | 1.33 ± 1.26 |
| 37°C | 42.2 ± 9.38 |
| 37°C + IV.3 | 1.17 ± 1.26 |
| fMLP | 46.8 ± 4.54 |
| fMLP + IV.3 | 2.5 ± 1.5 |
| PMA | 52.0 ± 11.3 |
| PMA + IV.3 | 1.33 ± 1.53 |
| Treatment . | % Rosetting (mean ± SD) . |
|---|---|
| 4°C | 55.3 ± 4.04 |
| 4°C + IV.3 | 1.33 ± 1.26 |
| 37°C | 42.2 ± 9.38 |
| 37°C + IV.3 | 1.17 ± 1.26 |
| fMLP | 46.8 ± 4.54 |
| fMLP + IV.3 | 2.5 ± 1.5 |
| PMA | 52.0 ± 11.3 |
| PMA + IV.3 | 1.33 ± 1.53 |
CHO cell transfectants expressing CD32A were incubated with or without fMLP (10−6 M) or PMA (50 ng/mL) for 1 h at 37°C and allowed to form rosettes with EA in ice for 2 h. Fab fragments of IV.3 (10 μg/mL) was included in some experiments. Results are expressed as mean of three experiments. Untransfected CHO cells did not form EA rosettes.
CHO, Chinese hamster ovary cells; fMLP, formyl-methionyl-leucyl-phenylalanine.
Discussion
Fcγ receptor ligation in neutrophils triggers the release of granule contents and inflammatory mediators. The release of neutrophil granule contents during normal physiologic function, ie, secondary to binding and clearance of IC, could result in severe pathologic reactions. Therefore, it is important that in normal circumstances neutrophils perform IC clearing functions without releasing unwanted inflammatory mediators. The CD16B-deficient neutrophils provide an opportunity to address the mechanisms by which neutrophils use 2 low-affinity FcγRs to carry out their physiologic functions. Using CD16B-deficient neutrophils, we showed that in normal neutrophils, CD32A existed as a low-affinity state that was converted to a high-affinity state on neutrophil activation.
Analysis of neutrophil FcγR' ligand-binding functions showed that CD16B-negative D17 neutrophils did not bind rabbit or human IgG-coated cells efficiently. A similar deficiency in ligand binding in another CD16B-deficient person was reported by Clark et al.23 CD32A exists in 2 allotypes, low responders (CD32ALR) and high responders (CD32AHR), defined by the allotype affinity to human IgG2. CD32ALR binds human IgG2, whereas CD32AHR does not bind human IgG2; however, both allotypes can efficiently bind human IgG1 and IgG3.39 Our rosetting studies with human IgG subtypes showed that the deficiency in rosetting was not caused by CD32A polymorphism.39 Neutrophils from D17 also did not phagocytose EA. Efficient binding and phagocytosis was observed, however, when erythrocytes were coated with high-affinity ligands such as IV.3, an anti-CD32A mAb, indicating that CD32A is competent in signaling for phagocytosis if stable binding of EA is achieved on neutrophils. Therefore, the lack of phagocytosis of EA by D17 neutrophils was a consequence of inefficient ligand binding by CD32A expressed on D17 neutrophils. This also suggested that in normal neutrophils, CD16B is the FcγR that stabilizes IC binding.
In contrast to our observations, Stroncek et al40 reported that 38% of neutrophils from a CD16B-deficient donor can form rosettes. This discrepancy might have resulted from differences in neutrophil preparation and rosetting procedures. In their report, it should be noted, neutrophils were prepared and stored in ice, and the rosetting was carried out at 37°C. We and other investigators41-43 have observed that neutrophils are activated by handling procedures such as changes in temperature. It is clear from our study that neutrophil activation could increase rosetting mediated by CD32A (Table 1), which may explain the observation of Stroncek et al.40 Therefore, it is important that neutrophils be prepared without subjecting them to an activation process. This can be achieved by preparing and storing neutrophils at room temperature and using them as quickly as possible.
The molecular mechanism that converted the CD32A from an inactive to an active state during neutrophil activation was unclear. Possible mechanisms include partial cleavage of CD32A by granule proteases, phosphorylation, receptor clustering, or cytoskeletal attachment of the cytoplasmic domain of CD32A during neutrophil activation. Previous studies have shown that fMLP stimulation results in alteration of the cytoskeletal network of neutrophils.44 CD32A has been shown to be activated by serine proteases and elastase on cultured myeloid cells and neutrophils.45-48 It is also possible that the increased receptor mobility49 or association with other receptors50-53 could cause a change in CD32A affinity. It is possible that various activation signaling mechanisms may differentially regulate CD32A function. Examples of affinity modulation of cell surface receptors by cell activation have been reported by others.32 33
It is also possible that affinity modulation may be cell specific. Such cell specificity has been observed with the LFA-1 molecule.54,55 LFA-1 expressed on unactivated PBL does not bind its ligand ICAM-1, whereas LFA-1 on activated PBL, Jurkat cells, transfected COS cells, or purified LFA-1 can bind ICAM-1, suggesting that affinity modulation by cell activation is a cell-specific phenomenon. Our results with CD32A-transfected CHO cells also showed such a cell-type specific affinity modulation. Affinity modulation has also been reported for mouse CD32B expressed on B lymphocytes.56 Interestingly, the affinity of this receptor has been shown to decrease after the 48-hour incubation of cells with activators such as IL- 4 or PMA.
Various studies have demonstrated that in normal neutrophils, CD16B is the dominant FcγR in binding IC.57,58 These reports are in agreement with our findings that more than 80% to 95% of IC binding to neutrophils was inhibited by anti-CD16 mAb. The high density of CD16B may be responsible for this dominance. However, our observations with D17 neutrophils suggested that CD32A did not contribute to IC binding in normal neutrophils because it was in an inactive or low-affinity state. The results presented here and elsewhere30 31 with anti-CD16 mAb-coated E suggest that CD16B alone is unable to deliver the signal for phagocytosis of EA and that CD32A is required for phagocytosis in normal neutrophils.
It is unclear how CD32A that is unable to bind ligands in resting neutrophils can deliver signals for events such as phagocytosis. One possibility is that CD32A, in its low-affinity state, is able to interact with Fc domains available on IC captured by CD16B. The signal produced by this weak interaction of CD32A, in synergy with the signal delivered by CD16B, may be enough for the phagocytic event. Alternatively, the engagement of CD16B with IgG may be responsible for the up-regulation of CD32A function. CD16B cross-linking promotes actin assembly and enhances the phagocytosis by CD32A in neutrophils.59 It has been shown that CD16B cross-linking induces phosphorylation of CD32A in neutrophils.31 CD16B interaction with IC could also affect the extracellular domain of CD32A at the IC binding site on neutrophil membranes. For example, GPI-anchored CD16B is capable of delivering a signal for lysosomal enzyme release in response to IC binding.17 Therefore, on binding to IC, CD16B can induce the release of lysosomal proteases in the vicinity of the IC-binding site. Consequently, neutrophil lysosomal proteases such as elastase can convert the low-affinity form of CD32A to a high-affinity form that could then efficiently interact with the IC captured by the CD16B and signal for events such as phagocytosis. Indeed, a recent report by Salmon et al60 demonstrated that the cross-linking of neutrophil CD16B could enhance the phagocytosis mediated by CD32A in an oxidant-dependent manner, suggesting that the signal delivered by CD16B influences CD32A function.
The absence of CD16B expression on D17 neutrophils reported here appears to have been caused by partial or complete deletion of the CD16B gene. The D17 blood donor is apparently healthy except for frequent sinusitis. Five persons previously reported are healthy without any complications,29,61 whereas 2 others are reported to have systemic lupus erythematosus.23 62 The pathophysiologic conditions associated with CD16B deficiency are not yet clear. The relatively normal function of these persons probably resulted from the conversion of CD32A to a high-affinity state which is capable of binding IgG-coated particles, suggesting that CD32A can carry out FcγR-dependent neutrophil functions efficiently in the absence of CD16B.
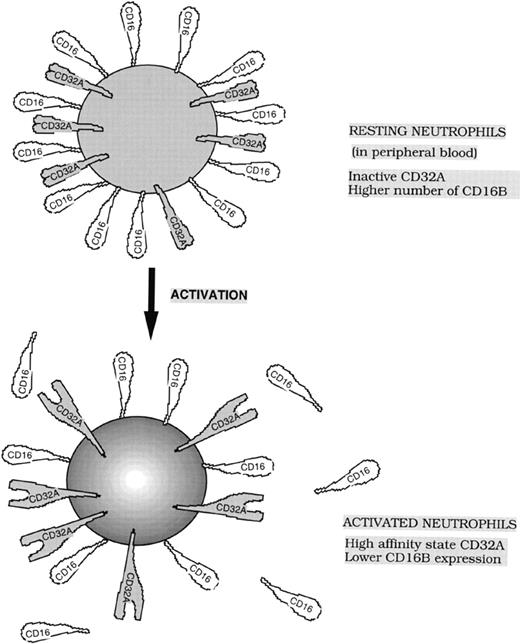
Results from this study and earlier reports57,58 indicate that CD16B is the dominant FcγR for IC binding to neutrophils. The CD32A-dependent particulate IC binding was poor in CD16B-negative neutrophils; however, this binding was increased on activation. Binding studies with soluble IC have shown that the affinity of neutrophil CD32A increased nearly 2-fold on activation with fMLP. However, our experiments with particulate immune complexes such as EA demonstrated that this modest increase in affinity resulted in the efficient binding of EA, suggesting that in addition to moderate affinity changes, other factors such as increased avidity caused by receptor clustering may also contribute to efficient EA binding. Alternatively, the 2-fold increase in CD32A affinity may be sufficient to reach the threshold for efficient CD32A-mediated binding of neutrophils to EA. In summary, our study demonstrated the existence of a novel mechanism of regulation of FcγR-dependent functions of neutrophils. This mechanism, which retained the strong signaling CD32A in a low-affinity state in normal neutrophils and in a high-affinity state in activated neutrophils, is likely to be of physiologic importance. Because CD16B is the dominant FcγR for binding to IC, the IC binding function of neutrophils may be primarily carried out by weak-signaling CD16B. Subsequent to IC binding, it is possible that CD16B delivers a signal that converts only a limited number of CD32A localized at IC contact sites on the neutrophils to a high-affinity state. Thus, controlled activation of CD32A could be achieved. Such a mechanism of engaging a high number of weak-signaling CD16B and low number of strong-signaling CD32A during IC binding may result in the reduced release of inflammatory mediators during normal physiologic states. Neutrophil activation also resulted in the release of CD16B from cell surfaces.37 63 Therefore, activated neutrophils express a lower number of weak-signaling CD16B while maintaining the strong-signaling high-affinity form of CD32A on their cell surfaces (Figure 7). Combinations of these changes in receptor expression and affinity would enable neutrophils to mediate Fcγ-dependent functions when neutrophils encounter bacterial peptides such as fMLP.
Model showing the functional states and expression levels of low-affinity Fcγ receptors, CD32A and CD16B, on resting and activated neutrophils.
Model showing the functional states and expression levels of low-affinity Fcγ receptors, CD32A and CD16B, on resting and activated neutrophils.
Acknowledgments
The authors thank Drs V. Udhayakumar, Peter Jensen, Aron E. Lukacher, and Charles Parkos for their critical comments on the manuscript and Nawaz Ahmed and Terry Vales for their valuable technical assistance.
Supported by grants AI38282 and AI30631 from the National Institutes of Health.
Reprints:Periasamy Selvaraj, Department of Pathology and Laboratory Medicine, 7307 WMB, 1639 Pierce Drive, Emory University, Atlanta GA 30322; e-mail: pselvar@emory.edu.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.


![Fig. 2. Analysis of CD16A and CD16B genes expressed in donor 17 by PCR. / (A) Restriction map of CD16B (neutrophils) and CD16A (natural killer [NK] cells). Gene-specific restriction endonuclease sites Dra I (D) and Taq I (T) are indicated in a box. Also shown are the positions of the primers used for PCR amplification of the segment of the exon 5 (corresponding to 611-825 bp of cDNA). (thick bars) Coding regions of cDNA. (thin bars) Noncoding regions of cDNA. (B) Restriction endonuclease analysis of PCR products recovered from donor 17 and control. The exon 5 segment was amplified with primers 1 (forward, gtttggcagtgtcaa) and 2 (reverse, gctcttattactcccatggga) using genomic DNA from control (left panel) and D17 (right panel). The PCR product was treated without (lanes 1 and 3) or with Dra I (lanes 2 and 4) or with Taq I (lanes 3 and 6), and then it was analyzed by 2% NuSieve agarose gel.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/95/3/10.1182_blood.v95.3.1069.003k14_1069_1077/6/m_bloo00314002w.jpeg?Expires=1763466145&Signature=UtZGdnv9H6TEfK9osTCQQrVfCe2blssC5XrEe5woeZMqq5rQQv3GKk51AT07vNbb1Up7VqHG56mG2CvRRBUcD7rb2F4nWW9Q7SMDJ70dFbEY97lNZzN35dKzvGX357ULZBAnoGwBhyaMRwNBfV1xIvtEzG4ZK6DleH0RXr83HLdmJ6imlWCE8RtuK5tBEXFAuJzG-Q6zIHZFd6C8qLwUTGdKsOOBzNmXpOJ9i2ddRUSfFbIPbJhh5OWM6ZE6QmjNmvSeefxVXfUCbprBWn0GUs0HN61zMvO~cVqxl-Bz8z3nzsDzQiejQnMilpHoK3JwAKYZctB7ovKwFtpHA1GVjw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal