We investigated the in vitro growth inhibitory and apoptotic effects of clinically achievable concentrations of As2O3 (0.5 to 2.0 μmol/L) against human myeloid leukemia cells known to be resistant to a number of apoptotic stimuli. These included chronic myelocytic leukemia (CML) blast crisis K562 and HL-60/Bcr-Abl cells, which contain p210 and p185 Bcr-Abl, respectively, and HL-60 cell types that overexpress Bcl-2 (HL-60/Bcl-2), Bcl-xL(HL-60/Bcl-xL), MDR (HL-60/VCR), or MRP (HL-60/AR) protein. The growth-inhibitory IC50 values for As2O3 treatment for 7 days against all these cell types ranged from 0.8 to 1.5 μmol/L. Exposure to 2 μmol/L As2O3 for 7 days induced apoptosis of all cell types, including HL-60/Bcr-Abl and K562 cells. This was associated with the cytosolic accumulation of cyt c and preapoptotic mitochondrial events, such as the loss of inner membrane potential (▵Ψm) and the increase in reactive oxygen species (ROS). Treatment with As2O3 (2 μmol/L) generated the activities of caspases, which produced the cleavage of the BH3 domain containing proapoptotic Bid protein and poly (ADP-ribose) polymerase. Significantly, As2O3-induced apoptosis of HL-60/Bcr-Abl and K562 cells was associated with a decline in Bcr-Abl protein levels, without any significant alterations in the levels of Bcl-xL, Bax, Apaf-1, Fas, and FasL. Although As2O3 treatment caused a marked increase in the expression of the myeloid differentiation marker CD11b, it did not affect Hb levels in HL-60/Bcr-Abl, K562, or HL-60/neo cells. However, in these cells, As2O3 potently induced hyper-acetylation of the histones H3 and H4. These findings characterize As2O3 as a growth inhibiting and apoptosis-inducing agent against a variety of myeloid leukemia cells resistant to multiple apoptotic stimuli.
Arsenic trioxide (As2O3) is a clinically active agent against acute promyelocytic leukemia (APL).1,2 Treatment with clinically achievable concentrations of As2O3 has been shown to cause apoptosis and down-regulation of the anti-apoptotic Bcl-2 protein in the APL NB4 cells.3 At lower concentrations (0.1 to 0.5 μmol/L), As2O3 was also demonstrated to induce partial differentiation of NB4 cells.4 These dose-dependent dual in vitro effects may explain the clinical activity of As2O3 in APL.4
Recently, As2O3 was shown to inhibit growth, reduce intracellular Bcl-2 levels, and induce apoptosis of several other myeloid leukemia, multiple myeloma, and HTLV-1-transformed T cells.5-7 Additionally, low concentrations of an organic arsenical melarsoprol (0.1 μmol/L), but not As2O3, were demonstrated to down-regulate Bcl-2 and to mediate apoptosis of B-leukemia cell lines.8 Both Bcl-2 and its homologue Bcl-xL confer resistance against apoptosis by inhibiting the preapoptotic mitochondrial permeability transition (ΔΨm), the cytosolic accumulation of cytochrome c (cyt c), and the activation of the executioner caspases of apoptosis.9-12 The mitochondrial effects of Bcl-2 include an antioxidant effect.12,13 In this context, it is noteworthy that the intracellular levels of the antioxidant glutathione (GSH) were shown to modulate the cytotoxic effects of As2O3 against lymphoma cells (ie, lower glutathione levels resulted in enhanced cytotoxicity of As2O3).14 Collectively, these findings suggest that Bcl-2 or Bcl-xL may exert an inhibitory effect on the antileukemic activity of As2O3. In contrast, the effects of other multidrug resistance proteins, including the mdr-1 encoded P-glycoprotein and MRP, on the antileukemic activity of As2O3 have not been investigated.15
Several reports have indicated that in leukemic blasts, the expression of CML-associated Bcr-Abl tyrosine kinase also inhibits anti-leukemia drug-induced mitochondrial ΔΨm and cyt c release, thereby blocking the activation of the downstream caspases and apoptosis.16-18 Bcr-Abl expression is known to increase Bcl-xL levels and the activity of NFkB in myeloid leukemia cells18,19; the latter has also been implicated in conferring resistance to apoptosis.20 However, whether As2O3 can overcome this resistance and trigger the cascade of preapoptotic molecular events in Bcr-Abl-positive cells has not been reported. In the current report, we have demonstrated that As2O3 induced the apoptosis of multidrug-resistant acute myelocytic leukemia cells, regardless of whether they overexpressed Bcl-2, Bcl-xL, P-glycoprotein, or MRP. Significantly, we also presented evidence that clinically achievable concentrations of As2O3induced preapoptotic mitochondrial events, caspase activity, and apoptosis of Bcr-Abl-positive cells. In conjunction with these effects, As2O3 treatment produced a significant decline in the Bcr-Abl protein levels in HL-60/Bcr-Abl and K562 cells. Recently, it has been shown that some agents that promote differentiation, apoptosis, or both of leukemic cells may concomitantly inhibit the activity of the enzyme histone deacetylase.21,22 This results in increased acetylation of histones, which facilitates gene transcription.23 In this study, we also demonstrated that the concentrations of As2O3 that induce apoptosis clearly increase the acetylation of the intracellular histones H3 and H4 in Bcr-Abl-positive leukemic blasts.
Materials and methods
Reagents
As2O3 and trichostatin A were purchased from Sigma (St. Louis, MO). As2O3 was dissolved in 1.65 mol/L sodium hydroxide (NaOH) to make a stock solution of 1 mmol/L, which was serially diluted in RPMI 1640. A monoclonal anti-Bcl-2 antibody was purchased from DAKO (Carpinteria, CA). Polyclonal anti-Bcl-x and anti-Bax antibodies and monoclonal anti-cyt c, anti-cIAP, and anti-Bcr-Abl antibodies were purchased from Pharmingen (San Diego, CA). Rabbit anti-DFF (DNA fragmentation factor),24 anti-Apaf-1,25 and anti-Bid antisera26 were kindly provided by Dr Xiaodong Wang (University of Texas Southwestern Medical Center, Dallas, TX).
Cell culture and cell growth inhibition
Growth inhibitory effects of As2O3
Logarithmically growing cells were exposed to various concentrations and exposure intervals (up to 7 days) of As2O3.After these treatments with As2O3, aliquots of cells were withdrawn and cell numbers were determined using a Coulter particle count and size analyzer (Coulter, Hialeah, FL). Suspension culture growth inhibition and the 50% inhibitory concentration values (IC50) for As2O3 were determined as previously described.29
Flow cytometric analysis of cell-cycle status and apoptosis
The flow cytometric evaluation of the cell-cycle status and apoptosis was performed according to a modification of a previously described method.30 Briefly, untreated or As2O3-treated cells were centrifuged, washed in Hanks' balanced salt solution, and fixed in 70% ethanol. The tubes containing the cell pellets were stored at −20°C for at least 24 hours. After this, the cells were centrifuged at 800g for 15 minutes, and the supernatant was discarded to remove ethanol completely. The pellets were resuspended in 40 μL (for 2-3 × 106 cells) of phosphate-citrate buffer at room temperature for 30 minutes. After this incubation, cells were washed with 4 to 5 mL phosphate-buffered saline (PBS) and stained with propidium iodide (PI) solution (20 μg/mL PI and 20 μg/mL RNAse A in PBS) for 30 minutes. The samples were read on a Coulter Elite flow cytometer using Elite software program 4.0 for 2-color detection. The percentage of cells in the apoptotic sub-G1 and the G1-S phase and G2-M phases were calculated using Multicycle software (Phoenix Flow Systems, San Diego, CA).
Western analyses of proteins
Western analyses of Bcl-2, Bcl-xL, Bax, Bid, Fas receptor (CD95), Fas ligand (Fas L), Bcr-Abl, DFF, cIAP, and β-actin were performed using specific antisera or monoclonal antibodies (see above), as described previously.15 18 Horizontal scanning densitometry was performed on Western blots by using acquisition into Adobe Photo Shop (Apple, Cupertino, CA) and analysis by the NIH Image Program (National Institutes of Health, Bethesda, MD). The expression of β-actin was used as a control.
Histone acetylation analysis
Histones were acid-extracted from whole cells as described previously21 22; 20 μg-isolated histones were subjected to SDS-PAGE as above (15% gel). Ponceau stain (Sigma) visualization was used as a control for the amount of protein loading. Antibodies that specifically recognize the acetylated forms of histone H3 and H4 (Upstate Biotechnology, Lake Placid, NY) were used to detect hyperacetylated histones.
Measurement of mitochondrial membrane potential and ROS
For As2O3-induced changes in mitochondrial membrane potential (ΔΨm) and ROS, 5 × 105HL-60/neo, HL-60/Bcr-Abl, and K562 cells were incubated with 40 nmol/L 3,3 dihexyloxacarbocyanine iodide or 5 μmol/L dichlorodihydrofluorescein diacetate, respectively, and were analyzed by flow cytometry, as described previously.18,31 32
Immunophenotyping for differentiation markers and hemoglobin production
HL-60/neo, HL-60/Bcr-Abl, and K562 cells were treated with various concentrations of As2O3 for up to 7 days. Cells were then washed with PBS and resuspended in 100 μL FACS wash buffer (PBS, 0.2% NaN3, 0.1% bovine serum albumin, 2% human AB-positive serum, filtered by suction at 0.45 μm). After 10 μL PE antihuman CD11b, CD33, CD34, or HLA-DR antibody (Pharmingen, San Diego, CA) was added,33,34 the cells were incubated in the dark at 4°C for 30 minutes. Samples were then analyzed by flow cytometry. Alternatively, untreated or As2O3-treated cells were washed in PBS and intracellular hemoglobin levels were determined by a previously described method.35
Preparation of S-100 fraction and Western blot analysis for cytochrome c
Untreated and As2O3-treated cells were harvested by centrifugation at 1000g for 10 minutes at 4°C. Cell pellets were washed once with ice-cold PBS and resuspended with 5 vol buffer (20 mmol/L HEPES-KOH, pH 7.5, 10 mmol/L KCl, 1.5 mmol/L MgCl2, 1 mmol/L sodium EDTA, 1 mmol/L sodium EGTA, 1 mmol/L dithiothreitol, and 0.1 mmol/L phenylmethylsulfonyl fluoride), containing 250 mmol/L sucrose. Cells were homogenized with a 22-gauge needle, and the homogenates were centrifuged at 100,000g for 30 minutes at 4°C (S-100 fraction).18 Supernatants were collected, and the protein concentrations of S-100 were determined by the Bradford method (Bio-Rad, Hercules, CA). After that, 20 to 30 μg S-100 was used for Western blot analysis of cyt c, as described previously.15 18
Morphology of apoptotic cells
After treatment with or without As2O3, 50 × 103 cells were washed with PBS (pH, 7.3) and resuspended in the same buffer. Cytospin preparations of the cell suspensions were fixed and stained with Wright stain. Cell morphology was determined by light microscopy. In all, 5 different fields were randomly selected for counting of at least 500 cells. The percentage of apoptotic cells was calculated for each experiment, as described previously.36
Apoptosis assessment by annexin-V staining
After drug treatment, 5 × 105 to 1 × 106 cells were washed in PBS and resuspended in 100 μL staining solution (containing annexin-V fluorescein and PI in a HEPES buffer, annexin-V-Fluos Staining Kit; Boehringer-Mannheim, Indianapolis, IN). After 15-minute incubation at room temperature, cells were analyzed by flow cytometry. Annexin-V binds to those cells that express phosphatidylserine on the outer layer of the cell membrane, and PI stains the cellular DNA of those that have a compromised cell membrane. This allows for the discrimination of live cells (unstained with either fluorochrome) from apoptotic cells (stained only with annexin-V) and necrotic cells (stained with both annexin-V and PI).37
Statistical analysis
Significant differences between values obtained in a population of leukemic cells treated with different experimental conditions were determined by paired Student t test analyses. A 1-way analysis of variance was also applied to the results of the various treatment groups, and post hoc analysis was performed using the Bonferroni correction method.
Results
Effects of As2O3 on cell proliferation and apoptosis
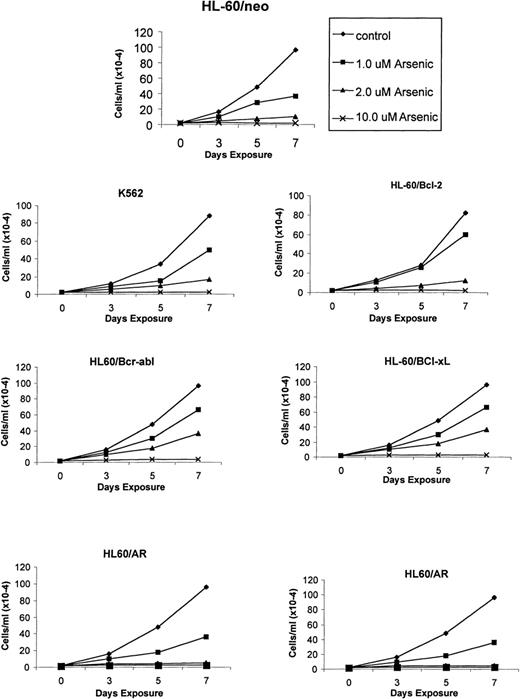
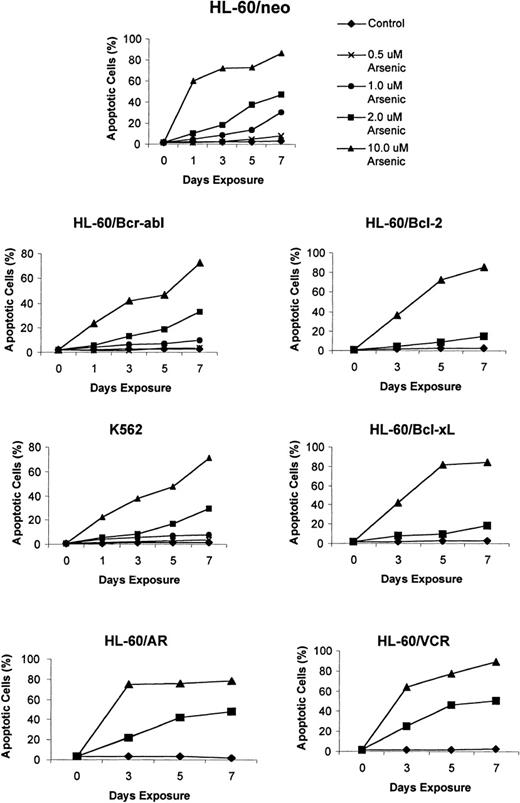
Recent reports indicate a broad spectrum of antileukemic activity for As2O3.5,7 This prompted us to investigate its efficacy against a variety of human myeloid leukemia cell types that display a multidrug-resistant phenotype caused by diverse mechanisms.15,18 27 The growth inhibitory effects of 1, 2, and 10 μmol/L As2O3 were determined after 3-, 5-, and 7-day exposure intervals. Figure 1demonstrates that a dose-dependent growth inhibitory effect of As2O3 was evident against the control HL-60/neo, as well as against HL-60/AR, HL-60/Bcl-2, HL-60/Bcl-xL, HL-60/Bcr-Abl, and K562 cells. After exposure to clinically achievable concentrations of As2O3(1 or 2 μmol/L) for 7 days, the relative degree of growth inhibition in the various cell lines was HL-60/neo > HL-60/VCR > HL-60/AR > HL-60/Bcr-Abl > K 562 > HL-60/Bcl-2 > HL-60/Bcl-xL (Figure 1). In these cell lines, the IC50 values for As2O3 were determined to be between 0.8 and 1.5 μmol/L. Figure2 shows the percentage of apoptotic cells observed in the various cell types after exposure to As2O3 (0.5 to 10 μmol/L) from 1 to 7 days. At the end of a 7-day exposure to 2 μmol/L As2O3, approximately 30% to 50% of cells showed either the morphologic features of apoptosis (data not shown) or the cell-surface phosphatidylserine expression detectable by annexin-V staining and flow cytometry (Table1). After exposure to 2 μmol/L As2O3 for 7 days, there was no significant difference in the apoptotic rate in HL-60/AR or HL-60/VCR versus HL-60/neo cells. However, a lower percentage of apoptotic cells was observed in identically treated HL-60/Bcl-2, HL-60/Bcl-xL, HL-60/Bcr-Abl, and K562 cells.
As2O3 (arsenic)-mediated growth inhibition of K562 and the various HL-60 cell types.
Cells were incubated with the indicated concentrations and exposure intervals of As2O3. After this treatment, total number of cells were counted using a Coulter Z2 particle count and size analyzer. Data represent the mean of 4 independent experiments.
As2O3 (arsenic)-mediated growth inhibition of K562 and the various HL-60 cell types.
Cells were incubated with the indicated concentrations and exposure intervals of As2O3. After this treatment, total number of cells were counted using a Coulter Z2 particle count and size analyzer. Data represent the mean of 4 independent experiments.
Effects of As2O3 (arsenic) on the apoptotic rate of K562 and the various HL-60 cell types.
Cells were treated with the indicated concentrations and exposure intervals of As2O3, and percentage apoptotic cells were characterized as those that stained with annexin-V and excluded PI, using the annexin-V assay kit (see “Materials and Methods”). Data represent the mean of 3 independent experiments.
Effects of As2O3 (arsenic) on the apoptotic rate of K562 and the various HL-60 cell types.
Cells were treated with the indicated concentrations and exposure intervals of As2O3, and percentage apoptotic cells were characterized as those that stained with annexin-V and excluded PI, using the annexin-V assay kit (see “Materials and Methods”). Data represent the mean of 3 independent experiments.
As2O3-induced apoptosis in human myeloid leukemia cells
| . | % Apoptosis . | |||
|---|---|---|---|---|
| Control . | 0.5 μmol/L . | 2.0 μmol/L . | 10.0 μmol/L . | |
| HL-60/neo | 1.9 ± 1.5 | 7.9 ± 2.2 | 47.5 ± 3.6 | 86.1 ± 5.7 |
| HL-60/AR | 2.1 ± 0.5 | 8.5 ± 1.2 | 47.8 ± 2.1 | 78.5 ± 2.5 |
| HL-60/VCR | 2.3 ± 1.1 | 7.9 ± 1.2 | 50.3 ± 1.9 | 88.9 ± 2.3 |
| HL-60/Bcl-2 | 2.5 ± 0.6 | 4.5 ± 0.9 | 32.3 ± 1.1* | 85.5 ± 2.4 |
| HL-60/Bcl-xL | 2.9 ± 0.9 | 5.4 ± 1.3 | 35.6 ± 2.1* | 84.4 ± 2.7 |
| H-60/Bcr-Abl | 1.8 ± 2.3 | 3.6 ± 2.1 | 32.9 ± 2.9* | 72.2 ± 3.3 |
| K562 | 1.5 ± 1.0 | 3.5 ± 1.9 | 29.4 ± 2.2* | 70.9 ± 3.6 |
| . | % Apoptosis . | |||
|---|---|---|---|---|
| Control . | 0.5 μmol/L . | 2.0 μmol/L . | 10.0 μmol/L . | |
| HL-60/neo | 1.9 ± 1.5 | 7.9 ± 2.2 | 47.5 ± 3.6 | 86.1 ± 5.7 |
| HL-60/AR | 2.1 ± 0.5 | 8.5 ± 1.2 | 47.8 ± 2.1 | 78.5 ± 2.5 |
| HL-60/VCR | 2.3 ± 1.1 | 7.9 ± 1.2 | 50.3 ± 1.9 | 88.9 ± 2.3 |
| HL-60/Bcl-2 | 2.5 ± 0.6 | 4.5 ± 0.9 | 32.3 ± 1.1* | 85.5 ± 2.4 |
| HL-60/Bcl-xL | 2.9 ± 0.9 | 5.4 ± 1.3 | 35.6 ± 2.1* | 84.4 ± 2.7 |
| H-60/Bcr-Abl | 1.8 ± 2.3 | 3.6 ± 2.1 | 32.9 ± 2.9* | 72.2 ± 3.3 |
| K562 | 1.5 ± 1.0 | 3.5 ± 1.9 | 29.4 ± 2.2* | 70.9 ± 3.6 |
Various leukemia cell types were incubated with or without 0.5, 2.0, or 10.0 μmol/L As2O3 for 7 days. After this treatment, the percentage of total cells that were apoptotic was determined by annexin-V (excluding PI) staining and flow cytometry, as described in the text. Data represent the mean ± SD of 3 separate experiments.
Values are significantly different than those observed in HL-60/neo cells treated with 2.0 μmol/L As2O3 for 7 days (P < .05).
As2O3-induced preapoptotic mitochondrial events, cytosolic cyt c accumulation, and caspase activities
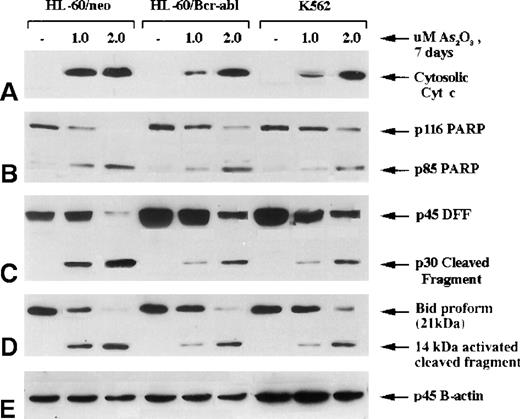
A previous report18 from our laboratory demonstrates that the expression of Bcr-Abl in HL-60/Bcr-Abl (p185) and K562 (p210) produces resistance against antileukemic drug-induced mitochondrial ΔΨm and cyt c release from the mitochondria into cytosol, caspase activation, and apoptosis. In the current study, we compared the effect of As2O3 on the mitochondrial ΔΨm and the release of cyt c and on caspase activation in HL-60/neo versus HL-60/Bcr-Abl or K562 cells. Figure 3demonstrates that a 7-day exposure to 2 μmol/L As2O3 produced similar levels of accumulation of cyt c in the cytosol of HL-60/neo, HL-60/Bcr-Abl, and K562 cells. This was associated with the cleavage of p116 poly (ADP-ribose) polymerase (PARP) into its p85 and p31 fragments and with the degradation of p45 DNA fragmentation factor (DFF) into its p30 and p11 fragments (not shown). As has been reported,24 cleavage of PARP and DFF largely results from the generation of caspase-3 activity. DFF is known to be the inhibitory protein for the endonuclease (caspase-associated DNase), which produces the DNA fragmentation of apoptosis.38,39 Recently, a pathway has been elucidated by which the activity of the upstream caspases can cause the release of cyt c from the mitochondria to the cytosol, resulting in Apaf-1-mediated sequential cleavage of caspase-9 followed by caspase-3.40 Bid (p21), a BH3 domain containing proapoptotic members of the Bcl-2 family, was cleaved directly by caspase-8, and the C-terminal fragment (p14) acted on the mitochondria to trigger cyt c release.26 41 As shown in Figure 3, treatment with 1 or 2 μmol/L As2O3for 7 days produced p21 Bid cleavage into its p14 fragment in HL-60/neo and in HL-60/Bcr-Abl and K562 cells, though more Bid cleavage occurred in HL-60/neo cells treated with 1 μmol/L As2O3. These data indicated that treatment with As2O3 generated the activity of both the upstream (caspase-8) and the effector caspase-3.
Molecular events of apoptosis induced by As2O3 treatment.
HL-60 control (neo) and cells stably transfected with Bcr-Abl, as well as K562 cells, were treated with the indicated concentrations of As2O3 for 7 days; cells were then harvested for the following Western blot analyses: (A) cytosolic levels of cytochrome c; (B) full-length PARP (116 kd) and 1 of its cleaved fragments (85 kd); (C) DNA fragmentation factor (DFF45) and its cleaved intermediate fragment (30 kd); (D) Bid proform (21 kd) and its 14-kd-activated cleaved product.
Molecular events of apoptosis induced by As2O3 treatment.
HL-60 control (neo) and cells stably transfected with Bcr-Abl, as well as K562 cells, were treated with the indicated concentrations of As2O3 for 7 days; cells were then harvested for the following Western blot analyses: (A) cytosolic levels of cytochrome c; (B) full-length PARP (116 kd) and 1 of its cleaved fragments (85 kd); (C) DNA fragmentation factor (DFF45) and its cleaved intermediate fragment (30 kd); (D) Bid proform (21 kd) and its 14-kd-activated cleaved product.
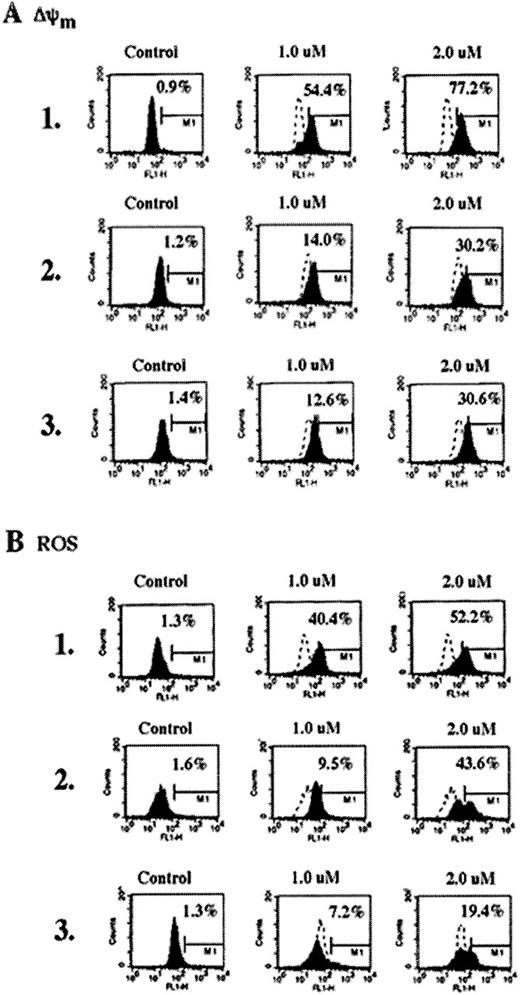
Because the activated Bid acted on the mitochondria, we examined whether As2O3-induced Bid cleavage and the release of cyt c from mitochondria were associated with ΔΨm and the increase in ROS. Figure 4 demonstrates that the treatment with 1 or 2 μmol/L As2O3produced more ΔΨm and the generation of ROS in HL-60/neo versus HL-60/Bcl-Abl and K562 cells, though significant preapoptotic mitochondrial alterations were also produced in HL-60/Bcr-Abl and K562 cells after exposure to 2 μmol/L As2O3(Figure 4, panels A1 and B1 versus panels A2, A3, B2, and B3). Reduced mitochondrial effect of 1 μmol/L As2O3 in HL-60/Bcr-Abl and K562 cells was consistent with decreased Bid cleavage in these cells caused by this dose; however, after treatment with As2O3, the cytosolic accumulation of cyt c was similar in the 3 cell types (Figure 3). Table2 also demonstrates the cell-cycle effects of As2O3 (1 and 2 μmol/L for 7 days) determined by flow cytometry in the control HL-60/neo versus multidrug resistant HL-60/Bcr-Abl and K562 cells. As shown, treatment with 1 or 2 μmol/L As2O3 significantly increased the percentage of HL-60/Bcr-Abl and K562 cells accumulated in the G2/M phase of the cell cycle. Although in HL-60/neo cells this was not obvious by flow cytometry (Table 2), mitotically arrested HL-60/neo cells were observed by Wright staining and light microscopy (data not shown). In comparison with HL-60/Bcr-Abl and K562, a higher percentage of HL-60/neo cells underwent apoptosis after treatment with As2O3. This could also partly explain the low percentage of HL-60/neo cells in the G2/M phase observed after exposure to As2O3. Our data corroborate a recent report that demonstrates that As2O3-induced mitotic arrest in myeloid leukemia cells coincides with loss of viability.34
Reduction of the mitochondrial membrane potential (▵Ψm,) (A) and production of ROS (B) in untreated control or As2O3-treated (7 days) HL-60/neo, HL-60/Bcr-abl, and K562 cells.
As2O3 treatment increased the percentage of HL-60/neo, HL-60/Bcr-abl, and K562 cells, which displayed low ΔΨm and high ROS production. Data are representative of 3 independent experiments.
Reduction of the mitochondrial membrane potential (▵Ψm,) (A) and production of ROS (B) in untreated control or As2O3-treated (7 days) HL-60/neo, HL-60/Bcr-abl, and K562 cells.
As2O3 treatment increased the percentage of HL-60/neo, HL-60/Bcr-abl, and K562 cells, which displayed low ΔΨm and high ROS production. Data are representative of 3 independent experiments.
As2O3-induced cell-cycle effects and apoptosis
| . | % Cells . | |||
|---|---|---|---|---|
| sub-G1 . | G1 . | S . | G2/M . | |
| HL-60/neo | ||||
| Control | 1.4 ± 1.2 | 33.0 ± 3.3 | 16.8 ± 2.5 | 35.5 ± 3.6 |
| 1.0 μmol/L As2O3 | 17.0 ± 2.6 | 40.3 ± 4.5 | 12.6 ± 3.5 | 30.3 ± 5.6 |
| 2.0 μmol/L As2O3 | 24.6 ± 3.5 | 39.3 ± 2.1 | 12.5 ± 2.7 | 27.1 ± 6.3 |
| HL-60/Bcr-abl | ||||
| Control | 1.5 ± 1.8 | 40.8 ± 5.6 | 18.1 ± 2.1 | 30.7 ± 2.5 |
| 1.0 μmol/L As2O3 | 12.0 ± 3.6 | 32.2 ± 4.1 | 8.1 ± 3.4 | 51.4 ± 4.9* |
| 2.0 μmol/L As2O3 | 21.1 ± 5.5 | 20.7 ± 3.6 | 9.4 ± 4.7 | 53.2 ± 5.6* |
| K562 | ||||
| Control | 3.1 ± 1.9 | 38.2 ± 2.9 | 16.7 ± 1.9 | 36.7 ± 3.1 |
| 1.0 μmol/L As2O3 | 10.5 ± 2.5 | 26.1 ± 5.4 | 12.4 ± 1.8 | 46.1 ± 4.5* |
| 2.0 μmol/L As2O3 | 18.6 ± 4.2 | 22.6 ± 3.9 | 12.8 ± 2.6 | 48.1 ± 4.2* |
| . | % Cells . | |||
|---|---|---|---|---|
| sub-G1 . | G1 . | S . | G2/M . | |
| HL-60/neo | ||||
| Control | 1.4 ± 1.2 | 33.0 ± 3.3 | 16.8 ± 2.5 | 35.5 ± 3.6 |
| 1.0 μmol/L As2O3 | 17.0 ± 2.6 | 40.3 ± 4.5 | 12.6 ± 3.5 | 30.3 ± 5.6 |
| 2.0 μmol/L As2O3 | 24.6 ± 3.5 | 39.3 ± 2.1 | 12.5 ± 2.7 | 27.1 ± 6.3 |
| HL-60/Bcr-abl | ||||
| Control | 1.5 ± 1.8 | 40.8 ± 5.6 | 18.1 ± 2.1 | 30.7 ± 2.5 |
| 1.0 μmol/L As2O3 | 12.0 ± 3.6 | 32.2 ± 4.1 | 8.1 ± 3.4 | 51.4 ± 4.9* |
| 2.0 μmol/L As2O3 | 21.1 ± 5.5 | 20.7 ± 3.6 | 9.4 ± 4.7 | 53.2 ± 5.6* |
| K562 | ||||
| Control | 3.1 ± 1.9 | 38.2 ± 2.9 | 16.7 ± 1.9 | 36.7 ± 3.1 |
| 1.0 μmol/L As2O3 | 10.5 ± 2.5 | 26.1 ± 5.4 | 12.4 ± 1.8 | 46.1 ± 4.5* |
| 2.0 μmol/L As2O3 | 18.6 ± 4.2 | 22.6 ± 3.9 | 12.8 ± 2.6 | 48.1 ± 4.2* |
Effects of As2O3 on the cell-cycle status and apoptosis in HL-60/neo, HL-60/Bcr-Abl, and K562 cells. Cells were incubated with the indicated concentrations of As2O3 for 7 days, stained with propidium iodide, and assayed by flow cytometry. The percentages of cells in the various phases of the cell cycle and of cells containing subdiploid DNA content (sub-G1) were determined. Data represent the mean ± SD of 3 independent experiments.
Values are significantly different than those observed in HL-60/neo cells treated with 1 or 2 μmol/L As2O3 for 7 days (P < .05).
Compared with the apoptotic rate in Table 1, detected by annexin-V staining, Table 2 shows a lower rate of As2O3-induced apoptosis, represented by the percentage of sub-G1 cells containing hypodiploid amounts of DNA. This is caused by the differences in the 2 methods used to detect apoptosis. Flow cytometry to detect apoptotic cells with hypodiploid DNA content may miss those apoptotic cells that undergo apoptosis in the G2/M-arrested state and do not lose enough DNA from fragmentation to be detected as hypodiploid. Additional confirmation of the apoptosis data shown in Tables 1 and 2was obtained by performing TUNEL assays on untreated and As2O3-treated cells using the in situ cell death detection kit (Boehringer-Mannheim, Indianapolis, IN). TUNEL assay results (data not shown) were consistent with the flow cytometric findings of the percentage sub-G1 cells containing hypodiploid amounts of DNA (Table 2). As2O3-induced mitochondrial ΔΨm, ROS, and apoptosis (as detected by annexin-V staining) were also determined after exposure to HL-60/neo, HL-60/Bcr-Abl, and K562 cells at time points earlier than 7 days (ie, 24 and 72 hours). As shown in Table3, (and compared with Tables 1 and 2), there was a time-dependent increase in the effects of As2O3 (1 or 2 μmol/L) on mitochondrial ΔΨm, ROS, and apoptosis of the 3 cell types. In general, the total loss of cell viability detected by PI staining occurred after the mitochondrial effects and annexin-V staining, as has been previously reported.30,35 36
As2O3-induced mitochondrial ▵Ψm, ROS, CD11b expression, and apoptosis
| . | % Positive Cells . | |||||||
|---|---|---|---|---|---|---|---|---|
| 24 h . | 72 h . | |||||||
| A-V/PI . | ΔΨm . | ROS . | CD11b . | A-V/PI . | ΔΨm . | ROS . | CD11b . | |
| HL-60/neo control | 1.4/0.0 | 17.8 | 3.7 | 5.8 | 3.1/1.4 | 12.0 | 8.5 | 3.9 |
| HL-60/neo 1.0 μmol/L arsenic | 5.2/2.6 | 26.9 | 4.8 | 2.1 | 11.7/4.6 | 12.3 | 12.0 | 8.8 |
| HL-60/neo 2.0 μmol/L arsenic | 11.6/4.6 | 32.9 | 10.2 | 2.6 | 18.0/6.8 | 27.9 | 54.1 | 12.6 |
| HL-60/Bcr-Abl control | 2.4/1.4 | 16.6 | 1.4 | 2.1 | 4.7/1.1 | 9.0 | 1.1 | 1.6 |
| HL-60/Bcr-Abl 1.0 μmol/L arsenic | 6.8/3.1 | 22.9 | 2.3 | 3.0 | 11.4/6.6 | 11.3 | 2.1 | 8.5 |
| HL-60/Bcr-Abl 2.0 μmol/L arsenic | 8.9/1.3 | 27.4 | 4.4 | 5.8 | 12.4/4.2 | 28.3 | 21.3 | 25.1 |
| K562 control | 2.9/1.9 | 10.7 | 3.7 | 1.4 | 3.8/1.1 | 3.7 | 3.0 | 1.1 |
| K562 1.0 μmol/L arsenic | 7.2/2.2 | 17.5 | 4.4 | 1.4 | 7.2/2.8 | 4.2 | 4.0 | 3.8 |
| K562 2.0 μmol/L arsenic | 8.8/2.9 | 19.2 | 4.4 | 2.2 | 13.2/4.4 | 9.4 | 23.6 | 20.6 |
| . | % Positive Cells . | |||||||
|---|---|---|---|---|---|---|---|---|
| 24 h . | 72 h . | |||||||
| A-V/PI . | ΔΨm . | ROS . | CD11b . | A-V/PI . | ΔΨm . | ROS . | CD11b . | |
| HL-60/neo control | 1.4/0.0 | 17.8 | 3.7 | 5.8 | 3.1/1.4 | 12.0 | 8.5 | 3.9 |
| HL-60/neo 1.0 μmol/L arsenic | 5.2/2.6 | 26.9 | 4.8 | 2.1 | 11.7/4.6 | 12.3 | 12.0 | 8.8 |
| HL-60/neo 2.0 μmol/L arsenic | 11.6/4.6 | 32.9 | 10.2 | 2.6 | 18.0/6.8 | 27.9 | 54.1 | 12.6 |
| HL-60/Bcr-Abl control | 2.4/1.4 | 16.6 | 1.4 | 2.1 | 4.7/1.1 | 9.0 | 1.1 | 1.6 |
| HL-60/Bcr-Abl 1.0 μmol/L arsenic | 6.8/3.1 | 22.9 | 2.3 | 3.0 | 11.4/6.6 | 11.3 | 2.1 | 8.5 |
| HL-60/Bcr-Abl 2.0 μmol/L arsenic | 8.9/1.3 | 27.4 | 4.4 | 5.8 | 12.4/4.2 | 28.3 | 21.3 | 25.1 |
| K562 control | 2.9/1.9 | 10.7 | 3.7 | 1.4 | 3.8/1.1 | 3.7 | 3.0 | 1.1 |
| K562 1.0 μmol/L arsenic | 7.2/2.2 | 17.5 | 4.4 | 1.4 | 7.2/2.8 | 4.2 | 4.0 | 3.8 |
| K562 2.0 μmol/L arsenic | 8.8/2.9 | 19.2 | 4.4 | 2.2 | 13.2/4.4 | 9.4 | 23.6 | 20.6 |
Various cell types were incubated with or without 1 or 2 μmol/L As2O3 for 24 or 72 hours. After this treatment, the percentage of total cells demonstrating an increase in the mitochondrial ΔΨm, ROS, CD11b expression and annexin-V or PI staining was determined by flow cytometry, as described in the text.
Data represent the mean of 2 experiments performed in duplicate.
ROS, reactive oxygen species.
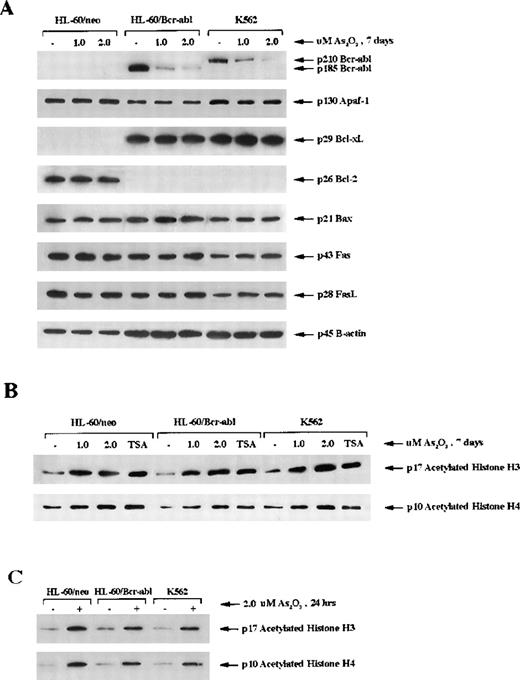
As2O3 treatment down-regulates Bcr-Abl but not Bcl-xL in HL-60/Bcr-Abl or K562 cells
Previous reports18 indicate that ectopic expression of Bcr-Abl in HL-60 cells is associated with a marked down-regulation of Bcl-2 and an increased expression of Bcl-xL in HL-60 cells. Treatment with 1 or 2 μmol/L As2O3 for 7 days did not cause any significant alterations in Bcl-2 in HL-60/neo or Bcl-xL in HL-60/Bcr-Abl or K562 cells (Figure5A). Bax, Apaf-1, cIAP, Fas L, and Fas levels in the 3 cell types were also unaffected by treatment with As2O3 (Figure 5). However, it is important to note that a dose-dependent decline in p185 Bcr-Abl in HL-60/Bcr-Abl and p210 Bcr-Abl in K562 was clearly observed (Figure 5A). At the higher dose levels of As2O3, greater than or equal to 2 μmol/L, this was also observed after exposure to shorter intervals (48 hours) (data not shown). Because Bcr-Abl expression is known to exert resistance against apoptosis, the decline in Bcr-Abl levels may explain why As2O3 treatment induced apoptosis in HL-60/Bcr-Abl and K562 cells.
Intracellular level of protein modulators of apoptosis and acetylation of histones H3 and H4.
(A) Western blot analysis of the levels of Bcr-abl, Apaf-1, Bcl-xL, Bcl-2, Bax, Fas receptor (Fas), and Fas ligand (FasL) in HL-60/neo, HL-60/Bcr-Abl, and K562 cells. β-Actin was used as a control for equal protein loading. (B) Western blot analysis of acetylated histones H3 and H4 in response to treatment with As2O3 (1 or 2 μmol/l for 7 days). (C) Western blot analysis of histone H3 and H4 after treatment with 2 μmol/L As2O3 for 24 hours. Hyperacetylation was detected by the use of antibody against acetylated histone H3 and H4. Histones were acid-extracted from the indicated cell lines after exposure to As2O3. The histone deacetylase inhibitor trichostatin A (150 nmol/L, 24 hours) was used as a positive control.
Intracellular level of protein modulators of apoptosis and acetylation of histones H3 and H4.
(A) Western blot analysis of the levels of Bcr-abl, Apaf-1, Bcl-xL, Bcl-2, Bax, Fas receptor (Fas), and Fas ligand (FasL) in HL-60/neo, HL-60/Bcr-Abl, and K562 cells. β-Actin was used as a control for equal protein loading. (B) Western blot analysis of acetylated histones H3 and H4 in response to treatment with As2O3 (1 or 2 μmol/l for 7 days). (C) Western blot analysis of histone H3 and H4 after treatment with 2 μmol/L As2O3 for 24 hours. Hyperacetylation was detected by the use of antibody against acetylated histone H3 and H4. Histones were acid-extracted from the indicated cell lines after exposure to As2O3. The histone deacetylase inhibitor trichostatin A (150 nmol/L, 24 hours) was used as a positive control.
As2O3 induces hyperacetylation of histones
Recent reports42 indicate that hybrid polar compounds and sodium phenylbutyrate, which induce terminal differentiation or apoptosis of leukemic cells, concomitantly induce hyperacetylation of the histones. Based on this, we determined the effect of As2O3 on the acetylation status of histones H3 and H4 in HL-60/neo, HL-60/Bcr-Abl, and K562 cells. Immunoblot analysis in Figure 5B demonstrates that, after treatment with 1 or 2 μmol/L As2O3 for 7 days, approximately, a 3- to 4-fold increase in the amount of acetylated histones was observed in HL-60/neo, HL-60/Bcr-Abl, and K562 cells. This approximated the amount of histone hyperacetylation seen with treatment of these cells with trichostatin A, a known inhibitor of histone deacetylase (Figure 5B). Figure 5C demonstrates that the hyperacetylation of histones H3 and H4 was evident even after a shorter exposure to As2O3 (2.0 μmol/L for 24 hours).
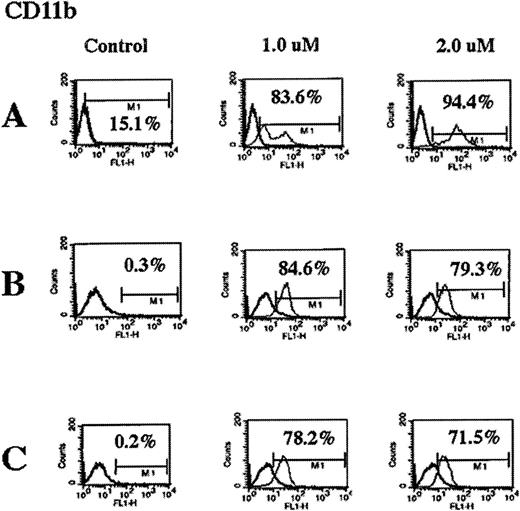
Effect of As2O3 on the immunophenotype of myeloid leukemia cells
After exposure to 1 or 2 μmol/L As2O3, flow cytometric analyses of the cell-surface expression of CD11b, CD33, CD34, and HLA-DR in HL-60/neo, HL-60/Bcr-Abl, and K562 cells were performed. Figure 6 demonstrates that As2O3 markedly increased the percentage of cells expressing of the myeloid differentiation marker CD11b in all cell types. Taken together with the data in Table 3, these data demonstrate that there was a progressive increase in the percentage of cells expressing CD11b in all cell types after exposure to 2 μmol/L As2O3 from 24 hours to 7 days.These data do not exclude the possibility that thereis a CD11b-positive subgroup of leukemic cells that is relatively insensitive to As2O3 and is selectively expanded during treatment with As2O3. However, As2O3treatment did not affect the expression of CD33, CD34, and HLA-DR (data not shown). Because the differentiation of K562 cells was shown in a previous study35 to be associated with increased intracellular levels of hemoglobin, this was determined in the untreated and the As2O3-treated cells. Treatment with As2O3 did not induce hemoglobin production or morphologic differentiation in K562, HL-60/Bcr-Abl, or HL-60/neo cells (data not shown).
As2O3 treatment induces CD11b expression in HL-60/neo (A), HL-60/Bcr-abl (B), and K562 (C) cells.
Cells were treated with the indicated concentrations of As2O3 for 7 days, and the percentage of cells expressing CD11b on the cell surface was determined by fluorescence-activated cytometry. Data are representative of 3 separate experiments.
As2O3 treatment induces CD11b expression in HL-60/neo (A), HL-60/Bcr-abl (B), and K562 (C) cells.
Cells were treated with the indicated concentrations of As2O3 for 7 days, and the percentage of cells expressing CD11b on the cell surface was determined by fluorescence-activated cytometry. Data are representative of 3 separate experiments.
Discussion
Data presented here clearly demonstrate that exposure to clinically achievable concentrations of As2O3 is able to induce growth inhibition and apoptosis of human myeloid leukemia cells resistant to multiple apoptotic stimuli. In these cells, apoptosis and multidrug resistance were shown to be secondary to diverse mechanisms, including the expression of Bcr-Abl or the overexpression of Bcl-2, Bcl-xL, MDR, or MRP.15,18,27,29,34As2O3-induced apoptosis of HL-60/VCR or HL-60/AR cells was not significantly different from HL-60/neo cells (P > .05). This suggests that As2O3is not a substrate for the mdr-1 gene-encoded p-glycoprotein nor is it exported by MRP. Compared with HL-60/neo, however, As2O3-induced apoptosis was partially attenuated in HL-60/Bcl-2, HL-60/Bcl-xL, HL-60/Bcr-Abl, and K562 cells. Consistent with recent reports, our findings demonstrate that As2O3 (2 μmol/L for 7 days) was able to induce mitochondrial ΔΨm and cytosolic accumulation of cyt c in HL-60/neo, HL-60/Bcr-Abl, and K562 cells.43,44As2O3 also generated PARP, DFF, and Bid cleavage activities of caspases in these cells.9,22,24 This suggests that As2O3 is able to induce the cleavage and activity of both the upstream (caspase-8) and the downstream executioner caspase (such as caspase-3) in HL-60/neo, HL-60/Bcr-Abl, and K562 cells.45 A recent report34 indicates that As2O3inhibits the binding of guanosine triphosphate to tubulin, its polymerization, and its microtubule formation, resulting in mitotic arrest of myeloid leukemia cells.34 This led to the apoptosis of leukemic cells. Our results confirmed that As2O3 treatment produces mitotic arrest (Table 2) and apoptosis (Table 1) of Bcr-Abl-positive and Bcr-Abl-negative myeloid leukemia cells. However, as for other antimicrotubule agents such as paclitaxel and vincristine, the precise mechanism by which the mitotic arrest induced by As2O3 is linked to preapoptotic mitochondrial events, cyt c release, and caspase activity remains to be elucidated.46
Bcr-Abl expression mediates resistance to apoptosis.47 we have reported that HL-60/Bcr-Abl and K562 cells are highly resistant to high-dose ara-C and etoposide-induced mitochondrial ΔΨm, cytosolic accumulation of cyt c caspase activation, and apoptosis.18In addition, ara-C and etoposide fail to alter p210 or p185 Bcr-Abl levels in these cells.18 In contrast, treatment with As2O3 significantly down-regulates Bcr-Abl levels in both cell types, which may explain why As2O3 causes mitochondrial ΔΨm, accumulation of cyt c in the cytosol, and apoptosis of HL-60/Bcr-Abl and K562 cells. These findings are consistent with a previous report48 demonstrating that the abrogation of Bcr-Abl expression by antisense oligonucleotides selectively eliminates CML blast cells. In addition, the abrogation of Bcr-Abl activity by a relatively specific tyrosine kinase inhibitor CGP57 148B recently has been shown to cause in vitro and in vivo eradication of human Bcr-Abl positive leukemia cells.49 Although ectopic or endogenous Bcr-Abl expression is associated with the up-regulation of Bcl-xL,18,35,47 our current data show that As2O3-mediated declines in Bcr-Abl levels do not down-regulate Bcl-xL levels. Recently, it has been shown that Bcl-xL is a caspase-3 substrate and that the cleavage of Bcl-xL in the loop region releases a c-terminal product that lacks the BH4 homology domain and induces cell death.50,51 However, As2O3-induced apoptosis of HL-60/Bcr-Abl and K562 cells was not seen to be associated with Bcl-xL cleavage. Furthermore, As2O3also did not alter Bax, Apaf-1, Fas L, Fas R, and cIAP levels. Collectively, these findings point to As2O3-mediated down-regulation of Bcr-Abl as the key perturbation responsible for facilitating the apoptosis of HL-60/Bcr-Abl and K562 cells. Bcl-2 or Bcl-xLoverexpression (as in HL-60/Bcl-2 or HL-60/Bcl-xL cells) also inhibits preapoptotic mitochondrial events.9 However, the relative potency of Bcl-2 or Bcl-xL versus Bcr-Abl for exerting this effect is unknown. As shown in the current study, though As2O3 does not lower Bcl-2 and Bcl-xL levels in HL-60/neo, HL-60/Bcl-2, and HL-60/Bcl-xL, the mitochondrial toxic effects of As2O3 may be potent enough to overcome the inhibitory effects of Bcl-2 and Bcl-xL and may induce apoptosis of HL-60/neo, HL-60/Bcl-2, and HL-60/Bcl-xLcells. In contrast, As2O3-induced down-regulation of Bcr-Abl may be necessary for facilitating apoptosis of HL-60/Bcr-Abl and K562 cells.
Recent studies have firmly established that targeted acetylation of the internal lysine residues in the amino-terminal tails of histones relieves nucleosomal repression, which limits the access of the transcriptional machinery to the DNA template and facilitates transcriptional activation.23,50 Histone acetylation is a reversible process.23,52 Histone acetyltransferases transfer the acetyl moiety from acetyl coenzyme A to the lysine residues neutralizing the positive charge and increasing hydrophobicity, whereas histone deacetylases remove the acetyl groups and reestablish the positive charge in the histones.23,52Recently, a class of hybrid polar compounds and sodium phenylbutyrate, which induce terminal differentiation or apoptosis, were shown to induce concomitantly the hyperacetylation of histones by inhibiting histone deacetylase.21,22 Our data demonstrate, for the first time, that clinically relevant concentrations of As2O3 induce the hyperacetylation of histones H3 and H4. It is unclear whether this is a direct or an indirect effect mediated through the modulation of transcriptional coactivators or corepressors that may have histone acetyltransferase or deacetylase activity, respectively.42,53 The effect of these corepressors and coactivators is selective. Some promoters and transcription factors are blocked, whereas others are not, by this recruitment of histone deacetylase or histone acetyltransferase.42,53 54As2O3-induced histone hyperacetylation may be responsible for altering the transcription of a number of genes, which may collectively mediate As2O3-induced growth inhibition and apoptosis. Our studies do not establish whether the As2O3-induced down-regulation of Bcr-Abl is transcriptionally or posttranscriptionally regulated. If As2O3 affects the transcription of the bcr-abl fusion gene, this may also be mediated directly or indirectly by altered gene-transcription and expression brought about by As2O3-induced histone hyperacetylation. These mechanistic issues would have to be resolved by future studies.
In summary, this article highlights the activity of As2O3 against leukemic cells that are resistant to apoptotic stimuli either because of the expression of Bcr-Abl or the overexpression of Bcl-2, Bcl-xL, MDR, or MRP proteins. These findings, as well as As2O3-induced declines in Bcr-Abl, suggest that As2O3 should be investigated for potential in vivo activity against refractory acute myelocytic leukemia and CML.
Reprints:Kapil Bhalla, Division of Clinical and Translational Research, Sylvester Comprehensive Cancer Center University of Miami School of Medicine (M710), 1550 NW 10th Avenue, Miami, FL 33136; e-mail: kbhalla@med.miami.edu.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal