Soluble vascular cell adhesion molecule-1 (sVCAM-1) is generated during inflammation and can alter lymphocyte functions. The authors report that the binding of sVCAM-1 to 4 integrin-bearing cells is a dynamically regulated, active cellular process. Binding of recombinant sVCAM-1 to 4 integrins on peripheral blood mononuclear cells was cell-type specific. Circulating CD16+ NK cells constitutively bound sVCAM-1 with high affinity, whereas a subpopulation of T-lymphocytes, primarily CD45RO+ (memory), bound sVCAM-1 only after phorbol ester stimulation. sVCAM-1 binding to homogenous stable cell lines was also cell-type specific, and required active cellular processes because it was blocked by the inhibition of ATP synthesis and by Fas-induced apoptosis. Indeed, the loss of high-affinity VCAM-1 binding was an early event in apoptosis. Furthermore, an H-Ras/Raf-initiated signaling pathway also suppressed sVCAM-1 binding to 4β1 integrins. Collectively, these results showed that the capacity of 4 integrins to bind VCAM-1 is actively regulated and that this regulation may control 4 integrin-dependent cellular functions.
The α4 integrins are adhesion receptors widely expressed on mononuclear leukocytes and eosinophils.1 A major ligand for α4 integrins is vascular cell adhesion molecule-1 (VCAM-1).2 VCAM-1 is a member of the immunoglobulin superfamily and is expressed on vascular endothelium at sites of inflammation and on selected other sites, such as thymic cortical epithelium and bone marrow stroma.2,3 Cells expressing α4 integrins can interact with VCAM-1 to mediate events such as the recruitment of leukocytes to sites of inflammation and the development and activation of lymphocytes.4 5
In addition to its cell surface expression, VCAM-1 has been found in a soluble form in the culture supernatant of cytokine-treated endothelial cells, in human serum, and in the synovial fluid of patients with rheumatoid arthritis.6,7 The origin of this soluble VCAM-1 (sVCAM-1) is not entirely known, but release from the cell surface by proteolytic cleavage is possible.8 sVCAM-1 may act to suppress further leukocyte migration to tissues by removing the ligand for α4 integrins. The soluble form of VCAM-1 could also act as a competitive inhibitor of ligand binding. In addition, the interaction of the sVCAM-1 with α4 integrins could provide a signal to alter cell function. Indeed, in T cells, sVCAM-1 acts as a chemotractant,9 and sVCAM-1 binding inhibits T-cell activation.10
Integrin function can be dynamically regulated without changes in integrin expression, a process termed “inside-out signaling.”11,12 Two general mechanisms account for such regulation. One involves a change in the affinity of ligand binding, operationally defined as “activation.”11Alternatively, affinity-independent mechanisms, such as changes in cell shape, spreading, and lateral mobility/clustering of integrins, also modulate cell adhesion.13-15 Many integrins, such as αIIbβ3, α5β1, and αMβ2, manifest active cellular regulation of the affinity of ligand binding.16,17 In sharp contrast, active cellular regulation of α4β1 affinity for soluble ligand is controversial, and a recent review emphasized that unlike other integrins, the affinity of α4β1 may not be physiologically regulated.13 It is clear that soluble ligand binding to α4β1 can be increased by exogenous agents such as Mn++ or activating antibodies.18 A report has demonstrated increased soluble fibronectin (FN) binding to α4β1 on T cells stimulated by L-selectin engagement, but its significance is unclear.19 Furthermore, several studies have implicated α4β1 activation indirectly with reported antibodies such as monoclonal antibody (mAb) 15/7, but whether these antibodies truly recognize high-affinity integrin conformations has been questioned.9,13,20 21
In the current study, we investigated the regulation of sVCAM-1 binding to α4β1 integrin using a sVCAM-1-immunoglobulin fusion protein as ligand. We report that sVCAM-1 binding to α4 integrins was cell-type–specific and energy dependent and that it was disrupted during apoptosis. Specific intracellular signaling pathways also regulated sVCAM-1 binding to α4β1. Thus, the function of the α4β1 integrin can be physiologically regulated by changes in soluble ligand binding.
Materials and methods
Antibodies and reagents
The antihuman β1 mAb, 8A2, was the generous gift of Drs N. Kovach and J. Harlan (University of Washington, Seattle, WA). Antihuman α4 (9F10), antihuman β1 (9EG7), and fluorescein isothiocyanate (FITC)-conjugated antihuman CD3 (HIT3a) mAbs were purchased from PharMingen (San Diego, CA). Function-blocking antihuman α4 (HP2/1) and the anti-Fas (CH11) IgM were purchased from Immunotech (Westbrook, ME). The antihuman CD3 (OKT3) hybridoma was obtained from American Type Culture Collection (ATCC; Rockville, MD) and IgG purified as previously described.17 Phorbol 12-myristate 13-acetate (PMA) and sodium azide were purchased from Sigma Chemical (St. Louis, MO), and 2-deoxyglucose was purchased from Calbiochem (La Jolla, CA). Anti-Tac antibody, 7G7B6, was obtained from ATCC, and biotinylated with biotin-N-hydroxy-succinimide was obtained from Sigma Chemical. Phycoerythrin (PE)-conjugated annexin V was purchased from R&D Systems (Minneapolis, MN). PE-conjugated streptavidin was purchased from Molecular Probes (Eugene, OR). A VCAM–Ig fusion protein containing the 2 N-terminal domains of human VCAM-1 fused to the human IgG1 heavy chain was a generous gift of Dr Roy Lobb (Biogen, Cambridge, MA) and was prepared as previously described.18 A VCAM-Cκ fusion protein containing the 7 Ig domains of human VCAM-1 fused to the murine Cκ segment was a gift from Dr. Richard M. Wright (Novartis Pharmaceuticals, Summit, NJ). Purified human plasma FN was purchased from Life Technologies (Grand Island, NY) and fluoresced as follows: FN was resuspended in phosphate-buffered saline at 5 mg/mL and was added to a reaction buffer containing 0.1 mol/L NaHCO3, pH 9, and 2 mg/mL FITC-celite (Sigma Chemical). The solution was incubated for 60 minutes at room temperature in the dark. The FITC-FN was purified on a PD-10 Sephadex column from AmershamPharmacia Biotech (Wikstroms, Sweden) and analyzed by spectrophotometry.
Cells
The T-leukemic cell lines Jurkat and HUT-78, the monocytic cell lines THP-1 and U937, and the rat basophilic leukemia (RBL) cell line were purchased from ATCC and cultured in RPMI-1640 medium (Biowhittaker, Walkersville, MD) supplemented with 10% fetal calf serum (Biowhittaker), 1% glutamine, 1% penicillin, and 1% streptomycin (Sigma Chemical). Human lymphocytes were purified from peripheral blood from normal donors by centrifugation through a Ficoll-Paque gradient (Pharmacia Biotech, Uppsala, Sweden), panning for monocytes on tissue culture plastic, and passaging over a nylon wool column.17 Chinese hamster ovary (CHO) cells expressing both human α4 and β1 integrin subunits were a generous gift from Dr Yoshikazu Takada (The Scripps Research Institute, La Jolla, CA) and have been described elsewhere.22
cDNA constructs and transfections
The chimeric Tac-α5 and Tac-β1 constructs in CDM8 vector have been previously described.23 The pDCR-H-Ras (G12V) was a gift from Dr M. H. Wigler (Cold Spring Harbor Laboratory, Cold Spring Harbor, NY). The vector encoding RafBxB has been previously described.24 CHO-α4β1 cells were transiently transfected using lipofectamine (Gibco BRL, Grand Island, NY) according to the manufacturer's instructions, as previously described.25
Construction and expression of VCAM-1-immunoglobulin fusion protein
Total RNA was isolated from HUVECs stimulated with IL-1β using Ultraspec (BioTecX, Houston, TX) following the instructions of the manufacturer. First-strand cDNA was generated using the GeneAmp kit of Perkin/Elmer (Norwalk, CT) and oligo-dT as a primer following the manufacturer's instructions. The entire coding region of the VCAM-1 cDNA was cloned to generate pRmHaVCAM. The entire coding sequence of the extracellular domain (7 Ig-like domains) of VCAM-1 was polymerase chain reaction-amplified from pRmHaVCAM using the following primers: 5′-GCTAGCGTCGACATGCCTGGGAAGATGGTC-3′; 5′-AGCGTCAGCCTCAGGAGAAAAATAGTC-3′. The resultant fragment was then subcloned into the NheI site of pBB4Ig, which contains the human Fc coding sequences at the carboxy terminus of the recombinant protein. Recombinant protein was expressed in Sf9 cells grown in SF900 II SFM (Life Technologies, Gaithersburg, MD). Recombinant protein was purified using Protein A filters (Nygene, Goldens Bridge, NY). Analysis of recombinant protein by sodium dodecyl sulphate–polyacrylamide gel electrophoresis revealed a major molecular species of ∼240 kd under nonreducing conditions and ∼115 kd under reducing conditions. These are the predicted weights of the dimer and the monomer, respectively.
Soluble ligand-binding assay
Cells (5 × 105) were resuspended in Dulbecco's minimum essential medium (DMEM; Biowhittaker) containing 0.1% bovine serum albumin (BSA; Sigma Chemical) or in a modified Tyrode's buffer (150 mmol/L NaCl, 2.5 mmol/L KCl, 12 mmol/L NaHCO3, 1 mg/mL glucose, and 1 mg/mL BSA) with 1 mmol/L MnCl2. A modified Tyrode's buffer was used with MnCl2 because this divalent cation precipitates in phosphate-containing solutions. The VCAM-Ig fusion protein, or FITC-FN, was added to the mixture and incubated for 30 minutes at room temperature (preliminary studies indicated no significant differences in ligand binding at room temperature and 37°C). Afterward, cells were washed twice with 0.5 mL of their respective buffers. If cells were also being analyzed for surface antigen expression, they were incubated with primary antibody for 30 minutes on ice and again washed twice. Cells were then resuspended in the same buffer containing either PE- or FITC-conjugated donkey antihuman IgG (Jackson Immunoresearch, West Grove, PA) at a 1:100 dilution (if they were dual labeled, FITC-conjugated antimouse IgG was also added) and incubated for 30 minutes. Cells were washed twice and fluorescence analyzed on a FACSCalibur flow cytometer (Becton Dickinson, Mountain View, CA) using CellQuest software.
Analysis of apoptosis
The assessment of apoptosis was measured by the binding of PE-conjugated annexin V to externalized phosphatidylserine by flow cytometry according to the manufacturer's instructions (R&D Systems).
Results
sVCAM-1 binding to isolated human peripheral blood cells
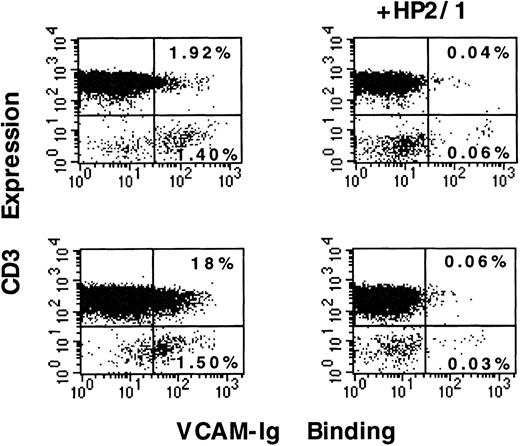
Because the interaction of α4 integrins with VCAM-1 modulates the functions of mononuclear leukocytes, we examined the regulation of sVCAM-1 binding to human peripheral blood lymphoid cells. These cells were analyzed for both CD3 expression and sVCAM-1 (7 Ig-domain form) binding by 2-color flow cytometry. A population of CD3-negative cells bound sVCAM-1, whereas few (typically less than 2%) of the CD3-positive T-lymphocytes manifested such constitutive binding (Figure1) The α4 integrins were functional on these CD3+ T-lymphocytes because 60% of these cells bound sVCAM-1 in the presence of an exogenous integrin activator, MnCl2 (data not shown). Stimulation with PMA (50 ng/mL) for 30 minutes resulted in increased sVCAM-1 binding in a subgroup representing ∼15% of the CD3-positive T-lymphocytes (Figure 1). Binding in each case was α4 integrin specific because it was completely blocked by an anti-α4 antibody, HP2/1. Furthermore, binding was not simply ascribable to the dimeric nature of the VCAM-Ig construct. A monomeric VCAM-1 construct containing the 7 extracellular Ig domains fused to murine κ light chain showed similar binding characteristics (data not shown). These results indicated that a subgroup of peripheral T-lymphocytes responds to PMA stimulation with increased sVCAM-1 binding to α4 integrins.
sVCAM-1 binding to isolated peripheral blood lymphocytes.
Human peripheral blood lymphoid cells were isolated as described in “Materials and Methods.” Cells were resuspended in Tyrode's buffer containing 1 mmol/L CaCl2, 1 mmol/L MgCl2, and 0.1% BSA and incubated for 30 minutes at room temperature in the absence of stimulation (upper panels) or in the presence of PMA (50 ng/mL) (lower panels). In the presence or absence of the function blocking anti-α4 mAb HP2/1, 7 Ig-domain VCAM-Ig (20 μg/mL) was added to the cells, and the mixture was incubated for 30 minutes at room temperature. After washing, the cells were stained with FITC-anti-CD3 mAb (HIT3a). At the same time, they were stained with PE-conjugated donkey antihuman IgG to detect the VCAM-Ig fusion protein as described in “Materials and Methods.” The percentage of cells in quadrants is indicated. Results are representative of 3 separate experiments.
sVCAM-1 binding to isolated peripheral blood lymphocytes.
Human peripheral blood lymphoid cells were isolated as described in “Materials and Methods.” Cells were resuspended in Tyrode's buffer containing 1 mmol/L CaCl2, 1 mmol/L MgCl2, and 0.1% BSA and incubated for 30 minutes at room temperature in the absence of stimulation (upper panels) or in the presence of PMA (50 ng/mL) (lower panels). In the presence or absence of the function blocking anti-α4 mAb HP2/1, 7 Ig-domain VCAM-Ig (20 μg/mL) was added to the cells, and the mixture was incubated for 30 minutes at room temperature. After washing, the cells were stained with FITC-anti-CD3 mAb (HIT3a). At the same time, they were stained with PE-conjugated donkey antihuman IgG to detect the VCAM-Ig fusion protein as described in “Materials and Methods.” The percentage of cells in quadrants is indicated. Results are representative of 3 separate experiments.
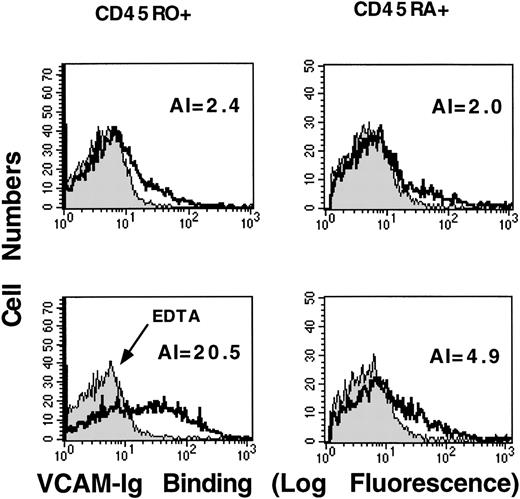
We next used surface markers to characterize the subpopulation of CD3-positive T-lymphocytes that bound sVCAM-1 in response to PMA stimulation. Resting CD45RO+ (memory) and CD45RA+ (naive) T-lymphocytes showed little difference in sVCAM-1 binding (Figure2). However, after PMA stimulation, CD45RO+ cells bound much more sVCAM-1 than CD45RA+ cells (Figure 2). This CD45RO+ cell response was not simply a response to the differences in α4-integrin expression because sVCAM-1 binding could be induced on both CD45RO+ and CD45RA+ cells after exogenous activation with MnCl2 (data not shown). These results showed that CD45RO+ memory T-lymphocytes responded to phorbol ester stimulation by increasing soluble VCAM-1 binding.
PMA stimulates sVCAM-1 binding to CD45RO-positive peripheral T-lymphocytes.
Human peripheral blood T-lymphocytes were isolated as described in “Materials and Methods”. Cells were resuspended in Tyrode's buffer containing 1 mmol/L CaCl2, 1 mmol/L MgCl2, and 0.1% BSA (solid line) or 5 mmol/L EDTA (shaded line). They were incubated for 30 minutes at room temperature in the absence of stimulation (upper panels) or in the presence of PMA (50 ng/ml) (lower panels). 7 Ig-domain VCAM-Ig (20 μg/mL) was added, and binding was allowed to proceed for 30 minutes at room temperature. After they were washed, cells were incubated with anti-CD45RA mAb (HI100) or anti-CD45RO mAb (UCHL1). Binding of VCAM-Ig and anti-CD45 mAbs was detected with PE-conjugated donkey antihuman IgG and FITC-conjugated donkey antimouse IgG, respectively, as described in “Materials and Methods.” Histograms depict sVCAM-1 binding to cells gated for CD45 expression. To obtain a quantitative estimate of sVCAM-1 binding, we calculated a numeric activation index (AI) defined as 100 × (Fo−Fr)/Fmax−Fr), where Fo is the mean fluorescence intensity of sVCAM-1 binding, Fr is background fluorescence in the presence of 5 mmol/L EDTA, and Fmax is the fluorescence intensity in the presence of 1 mmol/L MnCl2 (not shown). Results are representative of 3 separate experiments.
PMA stimulates sVCAM-1 binding to CD45RO-positive peripheral T-lymphocytes.
Human peripheral blood T-lymphocytes were isolated as described in “Materials and Methods”. Cells were resuspended in Tyrode's buffer containing 1 mmol/L CaCl2, 1 mmol/L MgCl2, and 0.1% BSA (solid line) or 5 mmol/L EDTA (shaded line). They were incubated for 30 minutes at room temperature in the absence of stimulation (upper panels) or in the presence of PMA (50 ng/ml) (lower panels). 7 Ig-domain VCAM-Ig (20 μg/mL) was added, and binding was allowed to proceed for 30 minutes at room temperature. After they were washed, cells were incubated with anti-CD45RA mAb (HI100) or anti-CD45RO mAb (UCHL1). Binding of VCAM-Ig and anti-CD45 mAbs was detected with PE-conjugated donkey antihuman IgG and FITC-conjugated donkey antimouse IgG, respectively, as described in “Materials and Methods.” Histograms depict sVCAM-1 binding to cells gated for CD45 expression. To obtain a quantitative estimate of sVCAM-1 binding, we calculated a numeric activation index (AI) defined as 100 × (Fo−Fr)/Fmax−Fr), where Fo is the mean fluorescence intensity of sVCAM-1 binding, Fr is background fluorescence in the presence of 5 mmol/L EDTA, and Fmax is the fluorescence intensity in the presence of 1 mmol/L MnCl2 (not shown). Results are representative of 3 separate experiments.
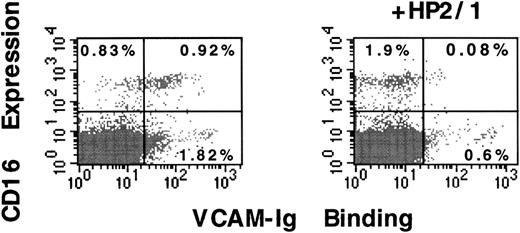
We also characterized the CD3-negative cells that manifested constitutive VCAM-1 binding. This population of cells had the size and granularity of lymphoid cells in flow cytometry. They were not, however, depleted by passage through a nylon wool column, suggesting that they were not B-lymphocytes. Using CD16 as a surface marker of natural killer (NK) cells, we found that more than 50% of circulating CD16+ NK cells constitutively bound sVCAM-1 and that this binding was inhibited by the function-blocking anti-α4 antibody, HP2/1 (Figure 3). Thus, most circulating CD16+ NK cells express α4 integrins that constitutively bind soluble VCAM-1. Analysis of sVCAM-1 binding to circulating B cells was not possible because of the interaction of our secondary detection antibody with surface-bound IgG or Fc-receptors (data not shown).
CD16-positive human peripheral blood natural killer cells constitutively bind sVCAM-1.
Human peripheral blood lymphoid cells were isolated as described in “Materials and Methods”. Cells were resuspended in Tyrode's buffer containing 1 mmol/L, CaCl2, 1 mmol/L MgCl2, and 0.1% BSA. In the presence or absence of the function blocking anti-α4 mAb HP2/1 (10 μg/mL), 7 Ig-domainVCAM-Ig (20 μg/mL) was added, and cells were incubated for 30 minutes at room temperature. After they were washed, cells were incubated with FITC-anti-CD16 mAb.3G8 Binding of VCAM-Ig was detected with PE-conjugated donkey antihuman IgG as described in “Materials and Methods.” The percentage of cells in quadrants is indicated. Results are representative of 3 separate experiments.
CD16-positive human peripheral blood natural killer cells constitutively bind sVCAM-1.
Human peripheral blood lymphoid cells were isolated as described in “Materials and Methods”. Cells were resuspended in Tyrode's buffer containing 1 mmol/L, CaCl2, 1 mmol/L MgCl2, and 0.1% BSA. In the presence or absence of the function blocking anti-α4 mAb HP2/1 (10 μg/mL), 7 Ig-domainVCAM-Ig (20 μg/mL) was added, and cells were incubated for 30 minutes at room temperature. After they were washed, cells were incubated with FITC-anti-CD16 mAb.3G8 Binding of VCAM-Ig was detected with PE-conjugated donkey antihuman IgG as described in “Materials and Methods.” The percentage of cells in quadrants is indicated. Results are representative of 3 separate experiments.
Cell-type–specific binding of sVCAM-1 to 4β1 integrin
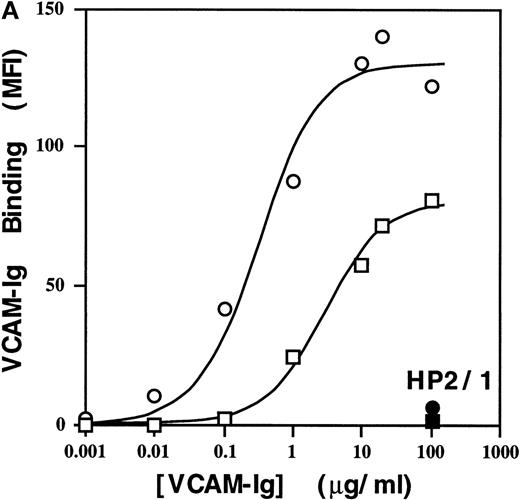
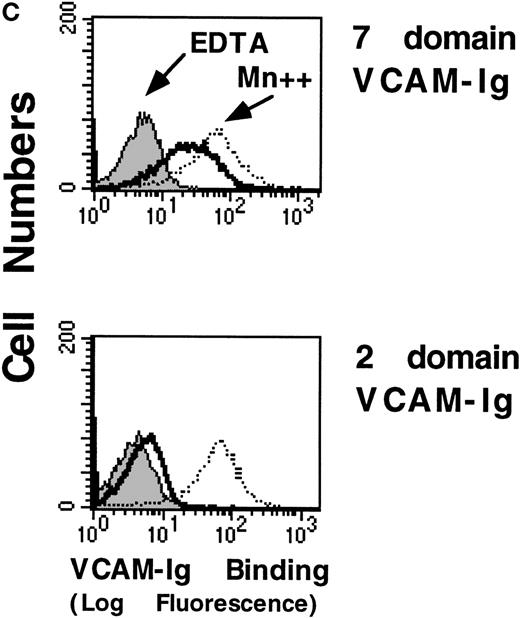
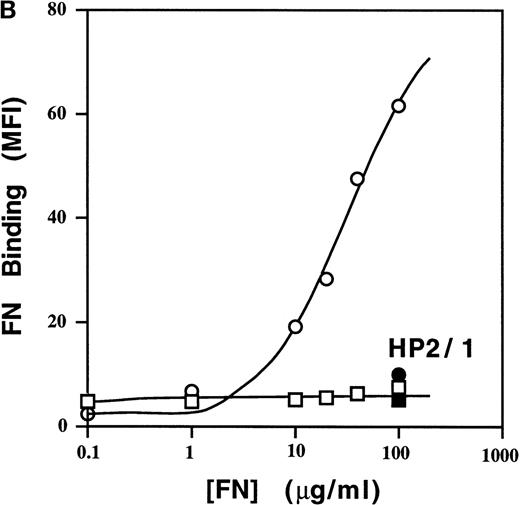
To assess sVCAM-1 binding to homogenous cell populations, we examined binding to stable cell lines. In the Jurkat T-leukemia cell line, sVCAM-1 (7 Ig domain) bound tightly (EC50 ∼50 nmol/L), and binding was completely blocked by the anti-α4 antibody HP2/1 (Figure 4a) and by the anti-VCAM-1 antibody P8B1(data not shown). The addition of 1 mmol/L MnCl2, to activate α4β1 integrins exogenously resulted in ∼2-fold greater maximal sVCAM-1 binding at saturation. Thus, ∼50% of the functionally expressed α4β1 integrins constitutively bound sVCAM-1. Similar results were obtained with α4β1-transfected CHO cells (CHO-α4β1) (Figure 5). In contrast to sVCAM-1 binding, soluble FN showed no detectable binding to Jurkat cells unless integrins were exogenously activated with MnCl2 (Figure 4B). More than 80% of this binding was inhibited by HP2/1, reflecting the 5-fold greater abundance of α4β1 than other FN binding integrins on these cells (data not shown). Furthermore, the Mn++-induced soluble FN binding was inhibited by antibodies hfn7.1 and FN1-8 (data not shown), which recognize the ninth and tenth type 2I homology segments of FN.26,27 These results suggest that this binding occurs through the central cell-binding region of FN, as has been reported.28 In addition, minimal sVCAM-1 binding to α4β1 was seen with a VCAM-Ig construct containing only the 2 N-terminal Ig-like domains unless cells were exogenously activated with 1 mmol/L MnCl2 (Figure 4c).
High affinity binding of a sVCAM-Ig fusion protein and soluble FN to 4β1 integrins on Jurkat cells.
Cells were resuspended in DMEM containing 0.1% BSA or in a Tyrode's buffer containing 1 mmol/L MnCl2 and 0.1% BSA (Mn++). The indicated concentrations of VCAM-Ig (A) or FITC-FN (B) were added, and the mixtures were incubated for 30 minutes at room temperature. ○, Mn++ added; □, no addition. Where indicated by • and ▪, function-blocking anti-α4 mAb HP2/1 was added before the soluble ligand. VCAM-Ig binding was evaluated with FITC-conjugated donkey antihuman IgG using flow cytometry as described in “Materials and Methods” and expressed as geometric mean fluorescence intensity (MFI). (C) Jurkat cells were resuspended in either DMEM containing 0.1% BSA (solid line), DMEM with the addition of 5 mmol/L EDTA (shaded line), or Tyrode's buffer containing 1 mmol/L MnCl2(dotted line). VCAM-Ig containing only the first 2 N-terminal or all 7 Ig domains (20 μg/mL) was added to the cells, and the mixtures were incubated at room temperature for 30 minutes. Results are representative of 3 separate experiments.
High affinity binding of a sVCAM-Ig fusion protein and soluble FN to 4β1 integrins on Jurkat cells.
Cells were resuspended in DMEM containing 0.1% BSA or in a Tyrode's buffer containing 1 mmol/L MnCl2 and 0.1% BSA (Mn++). The indicated concentrations of VCAM-Ig (A) or FITC-FN (B) were added, and the mixtures were incubated for 30 minutes at room temperature. ○, Mn++ added; □, no addition. Where indicated by • and ▪, function-blocking anti-α4 mAb HP2/1 was added before the soluble ligand. VCAM-Ig binding was evaluated with FITC-conjugated donkey antihuman IgG using flow cytometry as described in “Materials and Methods” and expressed as geometric mean fluorescence intensity (MFI). (C) Jurkat cells were resuspended in either DMEM containing 0.1% BSA (solid line), DMEM with the addition of 5 mmol/L EDTA (shaded line), or Tyrode's buffer containing 1 mmol/L MnCl2(dotted line). VCAM-Ig containing only the first 2 N-terminal or all 7 Ig domains (20 μg/mL) was added to the cells, and the mixtures were incubated at room temperature for 30 minutes. Results are representative of 3 separate experiments.
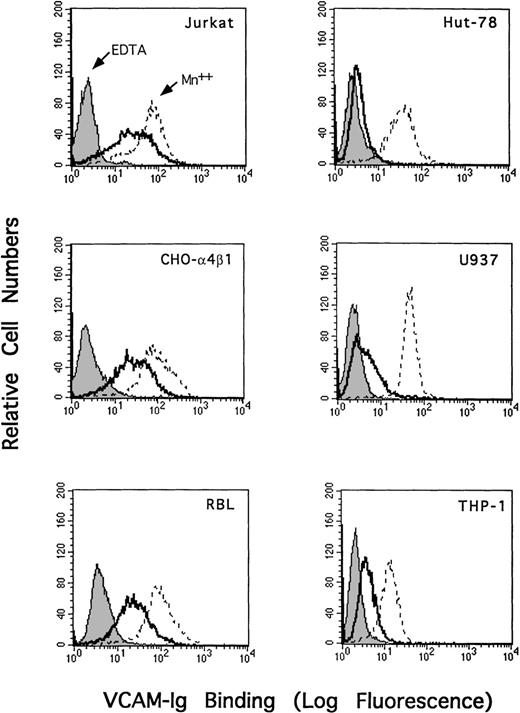
Cell-type–specific binding of sVCAM-1 to 4β1 integrin.
Jurkat, CHO-α4β1, RBL, THP-1, U937, or HUT-78 was resuspended in either DMEM containing 0.1% BSA (solid line), DMEM with the addition of 5 mmol/L EDTA (shaded line), or Tyrode's buffer containing 1 mmol/L MnCl2 (dotted line). Seven Ig-domain VCAM-Ig (20 μg/mL) was added, and the mixture was incubated at room temperature for 30 minutes. VCAM-Ig binding was detected with FITC-conjugated donkey antihuman IgG as described in “Materials and Methods.” Results are representative of 3 separate experiments.
Cell-type–specific binding of sVCAM-1 to 4β1 integrin.
Jurkat, CHO-α4β1, RBL, THP-1, U937, or HUT-78 was resuspended in either DMEM containing 0.1% BSA (solid line), DMEM with the addition of 5 mmol/L EDTA (shaded line), or Tyrode's buffer containing 1 mmol/L MnCl2 (dotted line). Seven Ig-domain VCAM-Ig (20 μg/mL) was added, and the mixture was incubated at room temperature for 30 minutes. VCAM-Ig binding was detected with FITC-conjugated donkey antihuman IgG as described in “Materials and Methods.” Results are representative of 3 separate experiments.
To assess further the cell-type specificity of sVCAM-1 binding (the 7 Ig domain form), we assayed other α4 expressing cell lines. In contrast to Jurkat cells, the human T-leukemic line HUT-78 bound sVCAM-1 minimally (Figure 5). However, the α4β1 on these cells was functional because soluble VCAM-Ig binding could be induced either by treatment with MnCl2 or by the β1 integrin activating antibodies 8A2 and 9EG7 (data not shown). Differences in sVCAM-1 binding were not attributable to differences in α4β1 expression because they express similar integrin levels, reflected by the similar sVCAM-1 binding in the presence of MnCl2. The human monocytic lines THP-1 and U937 also bound little sVCAM-1 without exogenous activation, whereas RBL bound sVCAM-1 in a manner similar to Jurkat and CHO-α4β1 cells (Figure 5). These results indicated that the capacity of α4β1 integrin to bind sVCAM-1 is dependent on the cellular context.
sVCAM-1 binding to 4β1 integrin is dependent on active cellular processes
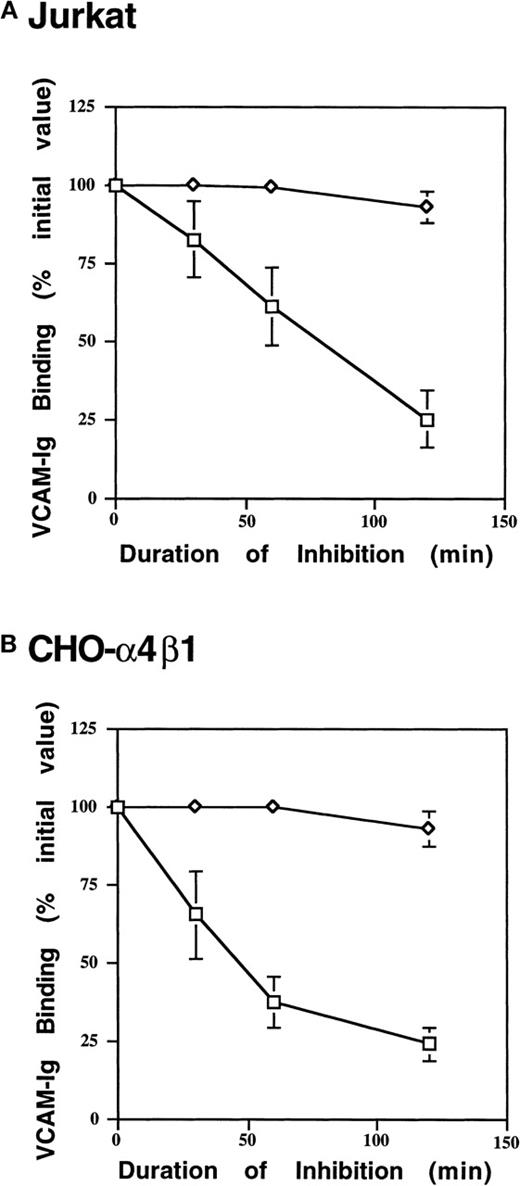
We next questioned whether binding of sVCAM-1 to α4β1 is a passive or an active cellular event. Treating Jurkat and CHO-α4β1 cells with the metabolic inhibitors sodium azide and 2-deoxyglucose resulted in a time-dependent suppression of sVCAM-1 binding (Figure6). This decrease in sVCAM-1 binding was not caused by a loss of α4β1 integrin expression because the binding could be recovered by exogenous activation with Mn++. Thus, metabolic energy is required to maintain sVCAM-Ig binding to α4β1 integrin.
Cellular-energy depletion suppresses sVCAM-Ig binding.
Jurkat (A) or CHO-α4β1 (B) cells were placed in glucose-free Tyrode's buffer containing 2-deoxyglucose (2 mg/mL) and sodium azide (0.1% wt/vol) and were incubated at 37°C for 0, 30, 60, or 120 minutes. Subsequently, cells were resuspended in DMEM or Tyrode's buffer containing 1 mmol/L MnCl2 (Mn++), and 7 Ig-domain VCAM-Ig binding was assessed as described in “Materials and Methods”. VCAM-Ig binding was expressed as a percentage of initial binding in cells incubated in the absence of 2-deoxyglucose and sodium azide. Results are the mean ± SEM of 3 independent experiments. ◊, Mn++ added; □, no addition.
Cellular-energy depletion suppresses sVCAM-Ig binding.
Jurkat (A) or CHO-α4β1 (B) cells were placed in glucose-free Tyrode's buffer containing 2-deoxyglucose (2 mg/mL) and sodium azide (0.1% wt/vol) and were incubated at 37°C for 0, 30, 60, or 120 minutes. Subsequently, cells were resuspended in DMEM or Tyrode's buffer containing 1 mmol/L MnCl2 (Mn++), and 7 Ig-domain VCAM-Ig binding was assessed as described in “Materials and Methods”. VCAM-Ig binding was expressed as a percentage of initial binding in cells incubated in the absence of 2-deoxyglucose and sodium azide. Results are the mean ± SEM of 3 independent experiments. ◊, Mn++ added; □, no addition.
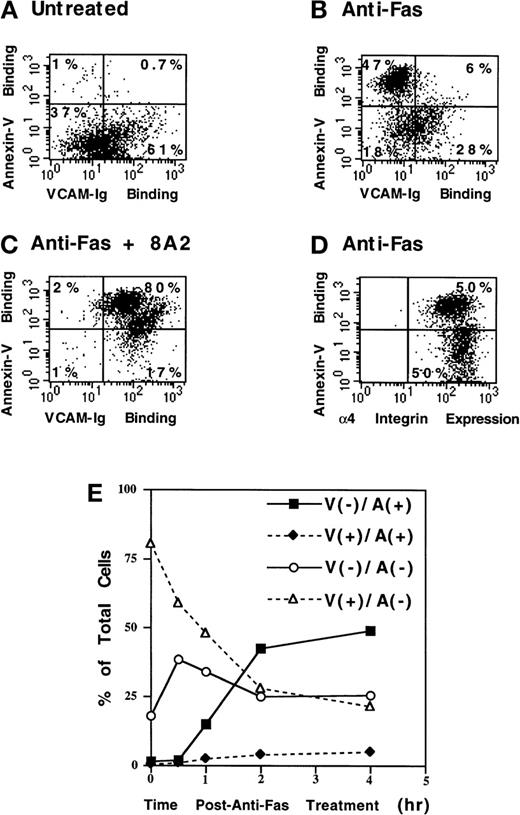
sVCAM-1 binding is disrupted during apoptosis
We next determined whether a physiologic relevant stimulus could modulate the activation of α4β1. An early feature of apoptotic cell death is loss of cell adhesion. Thus, we examined the effect of Fas-induced apoptotic cell death on sVCAM-1 binding to Jurkat cells. Treatment of Jurkat cells with anti-Fas antibody resulted in apoptosis, as measured by annexin-V binding to surface-expressed phosphatidylserine (Figure 7). The annexin-V positive cells (apoptotic) manifested markedly reduced sVCAM-1 binding compared with annexin-V–negative cells (Figure 7B). However, the surface expression of α4 on apoptotic cells was not markedly different from that on nonapoptotic cells (Figure 7D). Furthermore, binding to apoptotic cells could be restored by adding the activating antibody 8A2 (Figure 7C). In addition, 8A2-induced sVCAM-1 binding increased annexin-V binding to the previously negative cells (Figure 7C). This suggests that sVCAM-1 binding promotes Fas-induced apoptosis in these cells. Through kinetic analysis, we found that the reduction in sVCAM-1 binding occurred rapidly after Fas cross-linking. A large proportion of cells lost sVCAM-1 binding while they remained annexin-V negative during the first 30 minutes after Fas ligation (Figure 7E). These results indicated that the disruption of sVCAM-1 binding is an early event in apoptosis.
Fas-induced apoptosis suppresses sVCAM-Ig binding to Jurkat cells.
Jurkat cells were untreated (A) or were treated with anti-Fas antibody CH11 (300 ng/mL) for 4 hours (B-D). 7 Ig-domain VCAM-Ig binding was assayed as described in “Materials and Methods” (A-C). (D) Cells were stained with the anti-α4-integrin mAb 9F10. Outer membrane phosphatidylserine exposure was assayed by PE-conjugated annexin-V binding, as described in “Materials and Methods”. The percentage of cells in each quadrant is indicated. Note the marked reduction in VCAM-Ig binding to annexin-V(+) cells (B) without a major reduction in α4 expression (D). VCAM-Ig binding to annexin-V(+) cells is restored by the activating antibody 8A2 (C). 8A2 also markedly increased annexin-V binding to previously negative cells (C). (E) Kinetics of sVCAM-1 and annexin-V binding to Jurkat cells after anti-Fas antibody treatment was analyzed. Cells that bound VCAM-Ig (V+) or failed to bind VCAM-Ig (V−) are indicated. Similarly, annexin-V binding (A+) and nonbinding (A−) cells are also indicated. Note that the A(−)/V(−) cells initially increased coordinately with the decrease in A(−)/V(+) cells, before any increase in A(+) cells, and note the nearly complete absence of V(+)/A(+) cells, indicating that in apoptotic cells α4-integrin activation is blocked. Results are representative of 3 separate experiments.
Fas-induced apoptosis suppresses sVCAM-Ig binding to Jurkat cells.
Jurkat cells were untreated (A) or were treated with anti-Fas antibody CH11 (300 ng/mL) for 4 hours (B-D). 7 Ig-domain VCAM-Ig binding was assayed as described in “Materials and Methods” (A-C). (D) Cells were stained with the anti-α4-integrin mAb 9F10. Outer membrane phosphatidylserine exposure was assayed by PE-conjugated annexin-V binding, as described in “Materials and Methods”. The percentage of cells in each quadrant is indicated. Note the marked reduction in VCAM-Ig binding to annexin-V(+) cells (B) without a major reduction in α4 expression (D). VCAM-Ig binding to annexin-V(+) cells is restored by the activating antibody 8A2 (C). 8A2 also markedly increased annexin-V binding to previously negative cells (C). (E) Kinetics of sVCAM-1 and annexin-V binding to Jurkat cells after anti-Fas antibody treatment was analyzed. Cells that bound VCAM-Ig (V+) or failed to bind VCAM-Ig (V−) are indicated. Similarly, annexin-V binding (A+) and nonbinding (A−) cells are also indicated. Note that the A(−)/V(−) cells initially increased coordinately with the decrease in A(−)/V(+) cells, before any increase in A(+) cells, and note the nearly complete absence of V(+)/A(+) cells, indicating that in apoptotic cells α4-integrin activation is blocked. Results are representative of 3 separate experiments.
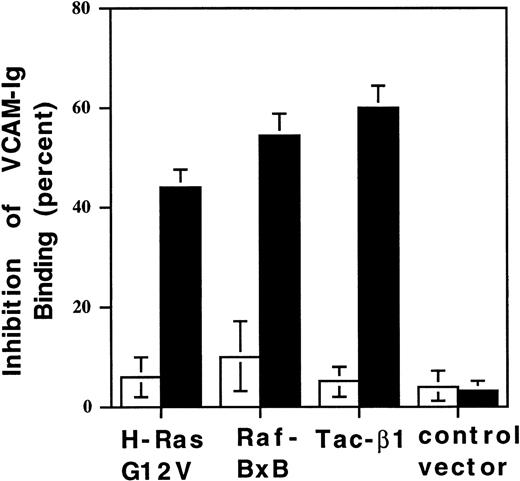
Suppression of 4β1-mediated sVCAM-1 binding by a Ras/Raf-initiated signaling pathway
A Ras/Raf-initiated signaling pathway suppressed the activation of chimeric integrins.25 Thus, we questioned whether such a signaling pathway could regulate α4β1-mediated soluble VCAM-1 binding. CHO-α4β1 cells were transiently cotransfected with cDNA encoding constitutively active H-Ras (H-RasG12V) or Raf-1 (RafBxB) and Tac-α5 (used as a surface marker of transfection). Forty-eight hours after transfection, cells were analyzed for Tac expression and VCAM-Ig binding by flow cytometry. Transfection of Tac-α5 alone, or in combination with vector control DNA, resulted in little change in sVCAM-1 binding (Figure8). Transfection with activated forms of H-Ras or Raf resulted in an approximately 50% reduction in sVCAM-1 binding (Figure 8), similar to the suppression seen by the over-expression of free β1 cytoplasmic domain (Tac-β1) (Figure 8), which has also been described for chimeric integrins.29This suppression was cell-autonomous; only transfected cells showed reduced sVCAM-1 binding. In addition, this reduced binding was not caused by a loss of α4β1 integrins given that activating the integrins with mAb 9EG7 (data not shown) could restore the binding. sVCAM-1 binding was also suppressed by the overexpression of wild-type H-Ras, but dominant negative H-Ras N17 and constitutively active CDC42 and Rac-1 had no effect on soluble ligand binding (data not shown). Thus, as with chimeric integrins, the binding of soluble ligands to α4β1 integrin is regulated by a Ras/Raf-mediated suppression pathway.
Activated H-Ras and Raf-1 suppress 4β1 integrin-mediated VCAM-1 ligand binding.
CHO-α4β1 cells were transiently transfected with cDNA encoding Tac-α5 or Tac-β1 alone or with a combination of Tac-α5 plus vector control DNA, H-Ras G12V, or RafBxB. After 48 hours, cells were analyzed for Tac expression and soluble VCAM-Ig binding by 2-color flow cytometry. 7 Ig-domain sVCAM-Ig binding was evaluated in low Tac-expressing cells, designated Tac(−) (□), and high Tac expressing cells, designated Tac(+) (▪). Percentage inhibition of VCAM-Ig binding was calculated relative to cells transfected with Tac-α5 cDNA alone. Results are the means ± SEM of 3 separate experiments.
Activated H-Ras and Raf-1 suppress 4β1 integrin-mediated VCAM-1 ligand binding.
CHO-α4β1 cells were transiently transfected with cDNA encoding Tac-α5 or Tac-β1 alone or with a combination of Tac-α5 plus vector control DNA, H-Ras G12V, or RafBxB. After 48 hours, cells were analyzed for Tac expression and soluble VCAM-Ig binding by 2-color flow cytometry. 7 Ig-domain sVCAM-Ig binding was evaluated in low Tac-expressing cells, designated Tac(−) (□), and high Tac expressing cells, designated Tac(+) (▪). Percentage inhibition of VCAM-Ig binding was calculated relative to cells transfected with Tac-α5 cDNA alone. Results are the means ± SEM of 3 separate experiments.
Discussion
Soluble VCAM-1 is generated at sites of inflammation and regulates lymphocyte function.9 10 We now report that active cellular processes regulate the binding of sVCAM-1 to the integrin α4β1. Binding of sVCAM-1 to α4β1 is cell-type specific in primary cells and in cell lines. In addition, binding is energy dependent and is disrupted during apoptosis. sVCAM-1 binding is also suppressed by constitutively active variants of Ras and Raf-1. CD45RO+ peripheral blood T cells are stimulated to bind sVCAM-1 by treatment with phorbol ester. Thus, the affinity of α4β1 for its ligand, VCAM-1, is regulated by active cellular events. Inside-out signaling through α4 integrins may regulate events such as leukocyte trafficking, migration, survival, and hematopoiesis.
Activation-dependent binding of soluble ligands to α4β1 integrin is differentially regulated. We found that a soluble recombinant fusion protein containing the complete extracellular 7 Ig domains of VCAM-1 bound spontaneously to α4β1 on Jurkat cells. In contrast, soluble FN failed to bind to these cells unless the integrins were exogenously activated, as has been described.19 Furthermore, a soluble VCAM-Ig protein consisting of the first 2 N-terminal Ig domains was a poor soluble ligand for α4β1 compared with the 7 Ig domain form. Ig domains 1 and 4 of VCAM-1 can mediate α4β1-dependent cell adhesion independently.30 Thus, it is unclear whether domain 4 is solely responsible for the active binding of sVCAM-1 or whether there is cooperation between domains 1 and 4. Mutational analysis of each of these domains is ongoing and will provide insight into this question. The binding observed in this study did not result simply from the dimeric nature of the fusion protein; a monomeric 7 domain VCAM-1 showed similar binding characteristics. However, cooperative interaction between domains 1 and 4 on a single VCAM-1 molecule with 1 or more α4β1 integrins could play a role in soluble ligand binding. Interestingly, a splice variant of VCAM-1 lacking domain 4 has been described.31 It will be of interest to determine whether differential expression of the 2 VCAM-1 variants and their interaction with α4β1 integrin may regulate the migration or development of α4 integrin-expressing cells.
The cellular context in which the α4β1 is expressed controls sVCAM-1 binding. Cell-type differences in adhesion to immobilized VCAM-1 correlated with sVCAM-1 binding. In general, cell types previously reported to adhere avidly to VCAM-1 (ie, Jurkat and CHO-α4β1) also showed the greatest sVCAM-1 binding. In contrast, cell types that adhere poorly to VCAM-1 (ie, THP-1 and HUT-78) bound less soluble ligand.20,32 In addition, the binding of sVCAM-1 to α4β1 integrin is an energy-dependent process because metabolic inhibitors suppressed soluble binding. Other investigators have failed to observe activation-dependent binding of a 7 Ig domain sVCAM-Ig fusion protein to α4 integrin-expressing cells.33,34 However, some of these studies were performed on a single cell type such as the erythroleukemic line K562,33 a cell line previously reported to express β1 integrins in a low-affinity state.35 Others failed to observe active sVCAM-1 binding to α4β1 at 4 °C.34Because we found that VCAM-1 binding is energy dependent, the process may have been blocked in experiments conducted at 4°C. Thus, sVCAM-1 binding is active and cell type-specific.
The differences in sVCAM-1 binding properties of α4 integrins on subgroups of blood mononuclear cells suggest important variations in α4 integrin-dependent functions. Many circulating CD16+ NK cells express α4 integrins in a high-affinity state based on constitutive sVCAM-1 binding. NK cells act as a first line of defense against many pathogens and accumulate rapidly in tissues early in the course of inflammation.36,37 The high-activation state of α4 integrins on NK cells may facilitate the attachment to endothelium early in inflammation, when VCAM-1 may be expressed at relatively low levels. Furthermore, high integrin affinity promotes cell migration at low-substrate densities.38 Consequently, NK cells may preferentially migrate at sites of low VCAM-1 expression. In contrast to NK cells, most circulating CD3-positive T-lymphocytes express α4 integrins that fail to bind sVCAM-1. Cellular activation may be required for the expression of high-affinity α4 integrins on these cells. PMA stimulation activated the α4 integrins, primarily on CD45RO+ memory T-lymphocytes rather than on CD45RA+ naı̈ve T cells. Future studies will determine whether more physiologically relevant stimuli, such as chemokines and T-cell-antigen-receptor engagement, will also activate α4 integrins on this T-cell subset. The activation of α4 integrins on these cells may facilitate their interaction with VCAM-1 and their recruitment to sites of inflammation. This is consistent with a paradigm of lymphocyte recirculation in which memory, but not naı̈ve, T-lymphocytes preferentially recirculate through peripheral blood vessels at inflammatory sites at which VCAM-1 is expressed.39,40 sVCAM-1 exhibits chemotactic activity for CD45RO+ memory T cells.9 This, too, may facilitate the influx of memory T cells to sites of inflammation. Interestingly, Giblin et al19 reported preferential activation of α4β1 and FN binding on naive T cells stimulated by L-selectins. Thus, the differential activation of α4β1 and ligand preference on naive and memory T cells may facilitate the recruitment of each of these T-cell subsets to selective sites.
A soluble form of VCAM-1 is present in the blood and synovial fluid of patients with inflammatory diseases at concentrations of 10 to 20 nmol/L,9 and higher levels could occur at localized regions of inflammation. The ED50 of ∼50 nmol/L for the binding of sVCAM-1 to Jurkat cells indicates that the pathologic levels of sVCAM-1 could occupy a significant fraction of activated α4β1. Perhaps sVCAM-1 acts as a competitive ligand inhibitor blocking adhesion to inflamed vascular endothelium. Furthermore, Kitani et al10 have shown that sVCAM-1 inhibits T-cell proliferation possibly because of the suppression of IL-2 production. Recent studies suggest that sVCAM-1 binding to T cells may induce apoptotic cell death (Ishii JK, et al, manuscript submitted for publication). Hence, though the expression of VCAM-1 on vascular endothelium is involved in the recruitment of α4-expressing cells to sites of inflammation, sVCAM-1 may dampen or downregulate the inflammatory response by altering leukocyte trafficking, activation, and survival.
Specific intracellular signaling pathways regulate the binding of sVCAM-1 to α 4 β 1 integrins. We observed that activated variants of H-Ras or Raf-1 inhibited α4β1 ligand binding. Thus, the activation state of α4β1, like chimeric αIIbβ3,25is subject to downregulation by an integrin-suppression pathway. Suppression of VCAM-1 binding to α4β1 could play a role in the release of mononuclear cells from sites such as bone marrow and thymus. For example, double-positive thymocytes express α4β1 in a constitutively active state that facilitates adhesion to VCAM-1 expressed in the cortex and at the corticomedullary junction of the thymus.3 41 A decrease in α4β1 affinity for VCAM-1 could be important in allowing the release of thymocytes from the cortex, facilitating their migration to the medulla. We are now in a position to study how extracellular stimuli, acting through Ras/Raf signaling, modulate α4β1 affinity.
sVCAM-1 binding to α4β1 on Jurkat cells was disrupted early in Fas-induced apoptosis. This disruption was not associated with a significant loss of α4-integrin expression, suggesting that the affinity of α4β1 for VCAM-1 was reduced. Cell detachment from the substratum and neighboring cells is a hallmark feature of apoptosis.42,43 How this occurs is unknown, but caspase-dependent proteolysis of proteins such as actin, fodrin, β-catenin, and FAK could play a role in this process.44-46 Our results suggest that decreased integrin affinity for ligands may play a role in the loss of cell adhesion associated with apoptosis. Apoptosis could suppress VCAM-1 binding by the induction of a suppressor-signaling pathway or by the proteolytic cleavage of integrin activators or of integrin cytoplasmic domains themselves.47 48 Our results now make it possible to analyze this newly observed early event in T-cell apoptosis.
Integrin-affinity modulation plays several roles in cellular processes, such as adhesion, migration, and extracellular matrix assembly. The prototypic integrin in which this form of regulation has been studied is platelet αIIbβ3. Using recombinant αIIbβ3, a number of novel integrin-regulatory molecules have been studied by genetic and biochemical approaches.25,29 49 However, there are cell-type and integrin-specific differences in integrin regulation. The use of soluble VCAM-1 offers a useful means to study affinity modulation of α4 integrins expressed in their natural cellular environment. Thus, genetic strategies, such as those developed for αIIbβ3, can now be used to dissect the regulation of α4β1 function in mononuclear cells. sVCAM-1 itself appears to be a ligand that regulates mononuclear cell function. Thus, the alteration of VCAM-1 binding through α4-integrin activation is likely to contribute to mononuclear cell-mediated inflammation and immune responses.
Acknowledgments
The authors thank Richard M. Wright (Novartis Pharmaceuticals, Summit, NJ) for the VCAM-Cκ fusion protein and Roy Lobb (Biogen, Cambridge, MA) for the 2-domain VCAM-Ig.
Supported by grants from the National Institutes of Health and by grant 3FB-0164 from the California Breast Cancer Research Program of the University of California.
Reprints:Mark H. Ginsberg, Department of Vascular Biology, The Scripps Research Institute, 10550 N Torrey Pines Rd, VB-2, La Jolla, CA 92037; e-mail: ginsberg@scripps.edu.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal