The ability of the 4 integrin counterligands vascular cell adhesion molecule (VCAM)-1 or mucosal addressin (MAd)CAM-1 to support eosinophil rolling or firm adhesion under conditions of physiologic flow has not been delineated. Using a parallel plate flow chamber in vitro and intravital microscopy in vivo, we demonstrate that eosinophil rolling and adhesion on VCAM-1 is mediated by both 4β1 and 4β7 integrins. Eosinophils rolled equally efficiently on both VCAM-1 2 domain and VCAM-1 7 domain, suggesting that the N-terminal 2 domains of VCAM-1 are sufficient to support eosinophil rolling under conditions of flow. Furthermore, activation of the eosinophil β1 integrin with monoclonal antibody (mAb) 8A2 resulted in both resistance to shear stress–induced detachment from VCAM-1 in vitro and in stable arrest of rolling eosinophils on interleukin (IL)-1β–stimulated venules in vivo. Eosinophils rolled less efficiently on MAdCAM-1– than on VCAM-1–coated coverslips under conditions of flow. However, eosinophils firmly adhered as efficiently to MAdCAM-1 as to VCAM-1. Overall, these results demonstrate that both VCAM-1 and MAdCAM-1 can support eosinophil firm adhesion under conditions of flow. In contrast, VCAM-1 is significantly more efficient than MAdCAM-1 in supporting eosinophil rolling under conditions of flow.
The recruitment of eosinophils to extravascular tissue sites during episodes of asthma and allergic inflammation is mediated by the adhesive interactions between circulating eosinophils and vascular endothelial cells.1-4 In vitro, the adherence of eosinophils to cytokine-stimulated cultured endothelial cells is mediated by several endothelial-expressed adhesion molecules, including E-selectin (CD62E),5 P-selectin (CD62P),6 intercellular adhesion molecule (ICAM)-1 (CD54),7 and vascular cell adhesion molecule (VCAM)-1 (CD106).8 The interaction of eosinophils with these vascular adhesion molecules is mediated by eosinophil cell surface receptors, including L-selectin (CD62L),9 P-selectin glycoprotein ligand (PSGL)-1,10 and β2 (CD18)11 and α4 (CD49d) integrins.12Eosinophils express both α4β1 and α4β7 integrins,13,14 both of which bind to VCAM-1.15In contrast, only α4β7 integrins mediate eosinophil adhesion to mucosal addressin MAdCAM-1.16 The importance of α4 integrins to eosinophil recruitment has been supported by several studies demonstrating that anti-α4 monoclonal antibodies (mAbs) block the accumulation of eosinophils at sites of allergic inflammation in vivo.17-19
We have recently demonstrated that the sequential events of eosinophil adhesion to vascular endothelial cells in vivo (ie, rolling, adhesion, and transmigration) are mediated by distinct adhesion receptors.3,20,62 The initial interaction between eosinophils and vascular endothelial cells is weak and transient under conditions of flow and is characterized by rolling in postcapillary venules. Eosinophil rolling in inflamed postcapillary venules is mediated by α4β1 integrins and L-selectin20 as well as by vascular P-selectin21,22 but not E-selectin.23,24 The presence of integrin activating factors released at inflamed tissue sites could then trigger stable adhesion of rolling eosinophils followed by their emigration into extravascular tissue sites.62 Although the relative contribution of individual adhesion molecules and cytokines to the sequential steps of eosinophil adhesion to endothelium is only partially understood, it is clear that the regulation of eosinophil adhesion at any of these sequential steps could be critical to the sequestration of eosinophils at sites of allergic inflammation.
Recent studies have revealed that α4 integrins expressed by eosinophils are capable of existing in different activation states characterized by either low or high affinity binding to counterligands such as VCAM-1.25,26 In a single-cell adhesion assay, activation of eosinophils with granulocyte-macrophage colony-stimulating factor (GM-CSF) resulted in enhancement of the binding strength of eosinophil-expressed α4 integrins for VCAM-1–coated surfaces.25 Similarly, the ability of activating agents such as phorbol myristate acetate (PMA), manganese (Mn)2+, and β1 integrin–activating mAbs to alter the activation state of α4 integrins on lymphocytes has also been demonstrated.27-29 Activation of the lymphocyte α4β1 or α4β7 integrin results in transformation of either of these lymphocyte integrin rolling receptors into firm adhesion receptors in vitro.27 28 However, the regulation of α4 integrin activation in vivo (as opposed to flow chamber studies in vitro) and the relative contribution of the two α4 integrins, α4β1 and α4β7, in mediating the initial events of eosinophil adhesion in vivo (ie, rolling in comparison to firm adhesion) during inflammation has not been clearly delineated. In the present investigation, we have made the novel observation that (a) eosinophils roll more efficiently on VCAM-1 than on MAdCAM-1 and (b) the eosinophil β1 integrin activation state can rapidly upregulate eosinophil firm adhesion and resistance to detachment from VCAM-1 in vitro and in vivo. In addition, we demonstrate that eosinophils roll equally efficiently on both VCAM-1 2d (VCAM-1 2 domain) as well as on VCAM-1 7d (VCAM-1 7 domain), suggesting that the N-terminal 2 domains of VCAM-1 are sufficient to support eosinophil rolling under conditions of flow.
Materials and methods
Isolation and labeling of eosinophils
Eosinophils were purified from the peripheral blood of patients suffering from mild asthma/allergic rhinitis as previously described in this laboratory20 in a protocol approved by the University of California, San Diego, Human Subjects Committee. Eosinophils with more than 95% purity and more than 95% viability were recovered by negative selection using a Magnetic Assembly Cell Separator (Miltenyi Biotec, Burlingame, CA) and magnetized anti-CD16 Ab.30 These eosinophils were used in the in vitro laminar flow chamber adhesion assay and in the in vivo adhesion experiments. For the in vivo experiments, eosinophils were fluorescently labeled with carboxy fluorescein diacetate (CFDA; Molecular Probes, Eugene, OR) as previously described.20Eosinophils were resuspended in Dulbecco's phosphate buffered saline (DPBS) containing 0.01% glucose at a concentration of 1 × 107 cells/mL and kept at room temperature in the dark until use.
Antibodies
Adhesion-blocking mAbs directed against human α4 (mAb P4G9 and mAb P4C2),31 anti-human β1 (mAb P4C10),32anti-human β2 mAb IB4,33 and anti-rabbit VCAM-1 (mAb Rb 1/9)34 were used. A β1 integrin–activating antibody, mAb 8A2,35 was obtained from Dr John Harlan (University of Washington, Seattle, WA), and a rat anti-mouse β7 mAb (F1B504) with cross-reactivity to human β736 was obtained from Dr Eugene Butcher (Stanford University, Stanford, CA) and used in the adhesion experiments in vitro and in vivo.
Recombinant VCAM-1
The soluble VCAM-1 used in the in vitro flow chamber studies is a truncated form of VCAM-1 containing all 7 extracellular immunoglobulin (Ig) domains, including the VLA-4 binding sites on domains 1 and 4. The soluble VCAM-1 was produced by Dr Carl Perez (Cytel Corporation, La Jolla, CA) in a mammalian expression vector and purified by immunoaffinity chromatography on the anti-VLA-4 mAb P3H12 as previously described.25
Recombinant MAdCAM-1
Soluble MAdCAM-1 used in these studies contains the entire extracellular domain of MAdCAM-1.16 The soluble MAdCAM-1 was expressed using a baculovirus expression vector containing the human Fc sequence and Sf9 cells grown in SF90011 serum-free media (Life Technologies, Gaithersburg, MD). Recombinant MAdCAM-1 protein was purified from the supernatants using protein A affinity purification membranes using the procedures recommended by the manufacturer (Nygene, Golden Bridges, NY).
In vitro laminar flow eosinophil VCAM-1 and MAdCAM-1 adhesion assay
The ability of eosinophils to roll on soluble VCAM-1 or MAdCAM-1 was assessed using an in vitro parallel plate laminar flow chamber as previously described in this laboratory.23 Briefly, glass coverslips were coated with soluble VCAM-1 or MAdCAM-1 (200 μL at 10 μg/mL) for 1 hour at 37°C. Unbound sites on the coverslips were then blocked with BSA for 15 minutes. The glass coverslip with immobilized VCAM-1 or MAdCAM-1 was then positioned in the bottom of a parallel plate flow chamber (100 μm thickness), where the coverslip was exposed to different flow conditions. Defined levels of flow were applied to the coverslips in the flow chamber by perfusing warm media (RPMI containing 0.75 mM calcium (Ca)2+ and magnesium (Mg)2+ and 0.2% HSA) through a syringe pump (Harvard Apparatus, South Natick, MA). Different wall shear stress levels were achieved by varying the flow rates as previously described.23 Care was taken to eliminate air bubbles in the channel during loading of the coverslip and assembly of the flow chamber. The flow apparatus with the immobilized VCAM-1 or MAdCAM-1 was mounted onto the stage of an inverted phase contrast microscope (Nikkon Inc, Garden City, NY). The flow chamber was then perfused with eosinophils (2 × 105 cells) for 2 minutes, and interaction of the injected cells with VCAM-1 or MAdCAM-1 was observed and videorecorded. Rolling or adherent eosinophils were identified by qualitative assessment of their interaction with VCAM-1 or MAdCAM-1. Rolling eosinophils in contact with VCAM-1 or MAdCAM-1 demonstrated multiple discrete interruptions and flowed slowly, while adherent eosinophils remained stationary at a given point for extended times (more than 30 seconds). Recorded images were subjected to offline video analysis to manually enumerate the number of interacting eosinophils. All results are expressed as the number of rolling or adherent eosinophils per field (average of 4 fields; 20× field) per 2 × 105 eosinophils during a 2-minute observation period. Data represent mean ± SEM. In some experiments, eosinophils were preincubated with a function-blocking anti-α4, anti-β1, or anti-β7 integrin mAbs (50 μg/mL), or isotype-matched control antibody, for 20 minutes at room temperature prior to infusion of eosinophils into the flow chamber.
Influence of a β1 integrin–activating mAb on the resistance of eosinophils to detachment from VCAM-1
To evaluate the influence of a β1 integrin–activating antibody on the resistance of eosinophils to detachment from VCAM-1, stepwise increases in shear stress were applied for 15 seconds at each shear force (2-20 dyn/cm2) to eosinophils adherent to VCAM-1 in the flow chamber. The number of eosinophils firmly adherent to VCAM-1 per field was recorded before and after each stepwise increase in shear stress. In these experiments, eosinophils were incubated with 2 μg/mL of a β1 integrin–activating mAb 8A2, or control antibody, for 15 minutes prior to infusion of eosinophils into the flow chamber.
Eosinophil static adhesion and resistance to detachment from MAdCAM-1
Because few eosinophils that were perfused into the flow chamber rolled or adhered to MAdCAM-1, additional experiments were performed to demonstrate that eosinophils were able to adhere to MAdCAM-1 in a static adhesion assay as previously reported.14 In these experiments 2 × 105 eosinophils were directly placed on the MAdCAM-1–coated coverslip used in the flow chamber experiments and allowed to adhere for 15 to 30 minutes at room temperature in a static adhesion assay. At the end of the incubation period, the coverslip was washed with media to remove nonadherent eosinophils. The coverslip was then positioned in the bottom of the parallel plate flow chamber and mounted on the stage of the inverted microscope as described above. The number of eosinophils firmly adherent to MAdCAM-1 was videorecorded in the absence of flow conditions. To evaluate the resistance of eosinophils to detachment from MAdCAM-1, stepwise increases in shear stress were then applied for 15 seconds at each shear force (2-20 dyn/cm2) to eosinophils adherent to MAdCAM-1 in the flow chamber.
In selected experiments, the specificity of the eosinophil-expressed α4β7 interaction with MAdCAM-1 was determined by preincubating the eosinophils with an anti-β7 mAb prior to adding eosinophils to MAdCAM-1–coated coverslips used in the static adhesion assay.
VCAM-1 2d and VCAM-1 7d eosinophil flow chamber adhesion assay
To explore the relative importance of the N-terminal 2 domains of VCAM-1 (VCAM-1 2d) as compared to the 7-domain form of VCAM-1 (VCAM-1 7d) in subserving an eosinophil rolling or adhesive interaction under conditions of flow, we used varying concentrations (0.5-10 μg/mL) of VCAM-1 2d and VCAM-1 7d in our flow chamber adhesion assay as described above. VCAM-1 2d and VCAM-1 7d (expressed using a baculovirus expression vector), were kindly provided by Tanabe Research Laboratories, San Diego, CA, and have been used in static eosinophil adhesion assays as previously described.37
Rabbit preparation for local mesenteric vascular bed instillation of an anti-VCAM-1 mAb and infusion of fluorescently labeled eosinophils in vivo
Rolling of human eosinophils in the postcapillary venules of the interleukin [IL]-1β–stimulated mesenteric circulation of New Zealand White rabbits in vivo was visualized by intravital microscopy as previously described.20,23 The ability of local mesenteric vascular bed instillation of an anti-rabbit VCAM-1 mAb Rb 1/9 to block eosinophil adhesion in vivo was assessed using a balloon catheter method previously described to administer anti-E-selectin mAbs.23,38 In brief, the rabbit mesentery was exteriorized and a side branch of the superior mesenteric artery cannulated with PE-10 tubing.23 The collateral mesenteric circulation to the ileum was occluded using occluder clamps. A balloon catheter was then gently wrapped and tied around the main stem of the superior mesenteric artery, upstream of the cannulated side branch, to allow temporary but complete occlusion of the blood flow through the mesenteric artery. Initially, video recordings were obtained to determine the baseline rolling of fluorescently labeled human eosinophils in the mesenteric venules. Subsequently, the mesenteric blood flow was briefly interrupted by inflation of the balloon catheter with an air-filled syringe. Immediately after complete cessation of blood flow, 3 mL of a neutralizing anti-rabbit VCAM-1 (Rb 1/9), or an isotype-matched antibody, at a concentration of 50 μg/mL was slowly infused through the cannulated tubing as previously described.23 The injected mAb Rb 1/9 or isotype-matched control antibodies were allowed to interact with the rabbit endothelium for a maximum of 10 minutes. Thereafter, the balloon catheter was deflated, and previous levels of blood flow resumed immediately. CFDA-labeled eosinophils were then injected and the interaction of eosinophils with antibody-treated vessel walls was determined over a 15-minute observation period.
In some experiments, eosinophils were incubated ex vivo with either function-blocking anti-α4, anti-β1, anti-β7, or control mAbs (50 μg/mL) for 20 minutes at room temperature and then injected into the rabbit mesentery as previously described.20 In other experiments, eosinophils were incubated with 20 μg/mL of a β1 integrin–activating mAb 8A2 for 5 to 15 minutes prior to their injection into the mesenteric circulation.
Intravital microscopy and image analysis
CFDA-labeled eosinophils (0.2 × 107 to 0.5 × 107) were infused through the cannulated artery approximately 6 to 10 hours after IL-1β stimulation. The passage of eosinophils in the inflamed venules was made visible by stroboscopic epi-illumination as previously described20 23 and recorded using an SVHS video recorder. The video recordings were analyzed offline by manually counting the total number of CFDA-labeled eosinophils passing through a venular segment (total flux). The tapes were rewound, and only those cells found to be visibly rolling along the venular wall were counted (rolling flux). The rolling fraction (Rf ) was calculated as the percentage of rolling cells in the total flux of eosinophils passing through a venule during a given injection. Eosinophils were considered adherent if they remained stationary for more than 30 seconds in the mesenteric vessels.
Analysis of eosinophil rolling velocities
The mean rolling velocity of injected eosinophils in IL-1β–stimulated mesenteric venules before and after mAb treatment was manually determined by frame-by-frame analysis of recorded video images as previously described.39 The mean rolling velocity of eosinophils are expressed as mm/sec ± SD.
Statistics
The interaction between eosinophils and venular endothelium in vivo before and after mAb treatment was analyzed by the Student t test using a statistical software package (SigmaStat, Jandel Scientific, San Rafael, CA). The rolling or adhesion of eosinophils to VCAM-1 or MAdCAM-1 in vitro in the laminar flow chamber was compared by the Student t test using a statistical software package (In Stat, San Diego, CA). Results are given as mean ± SD (unless otherwise indicated) and P values of <.05 were considered statistically significant.
Results
Eosinophils roll and adhere on VCAM-1 under conditions of physiologic shear stress in vitro: comparison with rolling and adhesion on MAdCAM-1
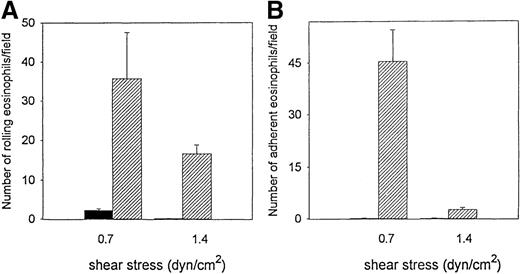
The ability of eosinophils to interact with VCAM-1 at physiologic conditions of shear force was examined using a parallel plate flow chamber in vitro. Significant number of eosinophils rolled on VCAM-1 (35.7 ± 6.8) but not on control BSA-coated cover slips (2.3 ± 1.5) (P = .01) (n = 3) (shear stress 0.7 dyn/cm2) (Figure 1A). There was significant firm adhesion of flowing eosinophils to VCAM-1 (45.4 ± 9.1) but not to control BSA-coated cover slips (0.3 ± 0.1) (P = .01) (n = 3) (shear stress 0.7 dyn/cm2) (Figure 1B). The increase in level of shear stress from 0.7 to 1.4 dyn/cm2 was associated with a decrease in the number of eosinophils rolling on, or adhering to, VCAM-1 (Figure 1).
Eosinophil rolling on VCAM-1 in vitro.
Eosinophils were infused at a flow rate of 0.7 and 1.4 dyn/cm2 into a parallel plate flow chamber containing VCAM-1– or BSA-coated coverslips. The number of rolling eosinophils (A) and adherent eosinophils (B) during continuous flow periods of 2 minutes were recorded and subjected to offline analysis. Results of experiments performed are presented as the mean ± SEM (n = 4 experiments). At flow rates of 0.7 dyn/cm2, significant numbers of eosinophils rolled on VCAM-1 (P = .001 vs BSA) and adhered to VCAM-1 (P = .001 vs BSA). At flow rates of 1.4 dyn/cm2, significant numbers of eosinophils rolled on VCAM-1 (P = .001 vs BSA), but the number of eosinophils adherent to VCAM-1 was significantly reduced. Solid bar, BSA; hatched bar, VCAM-1.
Eosinophil rolling on VCAM-1 in vitro.
Eosinophils were infused at a flow rate of 0.7 and 1.4 dyn/cm2 into a parallel plate flow chamber containing VCAM-1– or BSA-coated coverslips. The number of rolling eosinophils (A) and adherent eosinophils (B) during continuous flow periods of 2 minutes were recorded and subjected to offline analysis. Results of experiments performed are presented as the mean ± SEM (n = 4 experiments). At flow rates of 0.7 dyn/cm2, significant numbers of eosinophils rolled on VCAM-1 (P = .001 vs BSA) and adhered to VCAM-1 (P = .001 vs BSA). At flow rates of 1.4 dyn/cm2, significant numbers of eosinophils rolled on VCAM-1 (P = .001 vs BSA), but the number of eosinophils adherent to VCAM-1 was significantly reduced. Solid bar, BSA; hatched bar, VCAM-1.
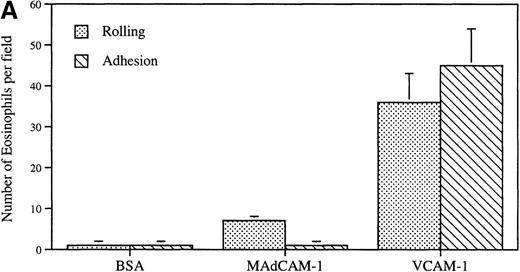
Eosinophils also rolled on MAdCAM-1 (7.1 ± 0.9) (shear stress 0.7 dyn/cm2), but this was less efficient than on VCAM-1 (35.7 ± 6.8) (P = .001) (n = 3) (Figure2A). The eosinophil rolling on MAdCAM-1 (7.1 ± 0.9) did not lead to sustained eosinophil firm adhesion to MAdCAM-1 of the rolling eosinophils. In contrast, eosinophil rolling on VCAM-1 (35.7 ± 6.8) frequently led to subsequent eosinophil firm adhesion to VCAM-1 (45.4 ± 9.1) (Figure 2A).
Eosinophil flow and static adhesion to MAdCAM-1.
(A) Flow chamber study. Eosinophils were infused at a flow rate of 0.7 dyn/cm2 into a parallel plate flow chamber containing MAdCAM-1–, VCAM-1–, or BSA-coated coverslips. The number of rolling eosinophils and adherent eosinophils during continuous flow periods of 2 minutes was recorded and subjected to offline analysis. Results of experiments performed are presented as the mean ± SEM (n = 4 experiments). At flow rates of 0.7 dyn/cm2, significant numbers of eosinophils rolled on VCAM-1 (P = .001 vs BSA) and adhered to VCAM-1 (P = .001 vs BSA). At the same flow rate, significantly fewer eosinophils rolled on MAdCAM-1 compared with VCAM-1 (P = .001) with few rolling eosinophils remaining firmly adherent to MAdCAM-1. (B) Static adhesion assay. Eosinophils were allowed to adhere for 30 minutes to a MAdCAM-1– or BSA-coated coverslip that had been subjected either to no shear stress (preflow panel) or to 1.4 dyn/cm2 shear stress (to simulate whether flow would strip MAdCAM-1 from the coverslip) (postflow panel). Nonadherent cells were then washed from the coverslip, and the coverslip was placed in the flow chamber. The number of adherent eosinophils was recorded on videotape. In the detachment panel, the eosinophils that had adhered to MAdCAM-1 (preflow panel) were subjected to shear stress (20 dyn/cm2) to determine whether the firmly adherent eosinophils were resistant to detachment from MAdCAM-1. There was no significant difference in the number of eosinophils adherent to MAdCAM-1 before and after application of shear force to the coverslip (preflow vs postflow) or after application of shear force to eosinophils adherent to MAdCAM-1 (P = not significant) (n = 3).
Eosinophil flow and static adhesion to MAdCAM-1.
(A) Flow chamber study. Eosinophils were infused at a flow rate of 0.7 dyn/cm2 into a parallel plate flow chamber containing MAdCAM-1–, VCAM-1–, or BSA-coated coverslips. The number of rolling eosinophils and adherent eosinophils during continuous flow periods of 2 minutes was recorded and subjected to offline analysis. Results of experiments performed are presented as the mean ± SEM (n = 4 experiments). At flow rates of 0.7 dyn/cm2, significant numbers of eosinophils rolled on VCAM-1 (P = .001 vs BSA) and adhered to VCAM-1 (P = .001 vs BSA). At the same flow rate, significantly fewer eosinophils rolled on MAdCAM-1 compared with VCAM-1 (P = .001) with few rolling eosinophils remaining firmly adherent to MAdCAM-1. (B) Static adhesion assay. Eosinophils were allowed to adhere for 30 minutes to a MAdCAM-1– or BSA-coated coverslip that had been subjected either to no shear stress (preflow panel) or to 1.4 dyn/cm2 shear stress (to simulate whether flow would strip MAdCAM-1 from the coverslip) (postflow panel). Nonadherent cells were then washed from the coverslip, and the coverslip was placed in the flow chamber. The number of adherent eosinophils was recorded on videotape. In the detachment panel, the eosinophils that had adhered to MAdCAM-1 (preflow panel) were subjected to shear stress (20 dyn/cm2) to determine whether the firmly adherent eosinophils were resistant to detachment from MAdCAM-1. There was no significant difference in the number of eosinophils adherent to MAdCAM-1 before and after application of shear force to the coverslip (preflow vs postflow) or after application of shear force to eosinophils adherent to MAdCAM-1 (P = not significant) (n = 3).
The reduced rolling and adhesion of eosinophils to MAdCAM-1 was not due to an inability of eosinophils to bind in sufficient numbers to MAdCAM-1–coated coverslips as evidenced by the following static adhesion experiments. In a static adhesion assay, using the same MAdCAM-1–coated coverslips as in the flow chamber experiments, significant numbers of eosinophils firmly adhered to MAdCAM-1 (103.3 ± 17.1) (n = 3) as compared to BSA-coated coverslips (2.1 ± 1.3) (P = .001). These firmly adherent eosinophils were as resistant to detachment from MAdCAM-1 as from VCAM-1–coated coverslips (less than 1% of eosinophils detached from either MAdCAM-1 or VCAM-1 when shear stress was increased from 2 to 20 dyn/cm2). Preincubation of eosinophils with a neutralizing antibody to β7 integrins significantly inhibited eosinophil binding to MAdCAM-1 by 94.2 ± 5.7% in the static adhesion assay, whereas a control anti-β1 antibody inhibited eosinophil binding by less than 5%. To determine whether the reduced ability of eosinophils to adhere to MAdCAM-1 in the flow chamber experiments could be due to stripping of MAdCAM-1 off the coverslip during the flow chamber experiments, we performed experiments in which we subjected the MAdCAM-1–coated coverslip to the maximal shear stress (1.4 dyn/cm2) used in the eosinophil MAdCAM-1 flow adhesion studies. After the MAdCAM-1–coated coverslip was subjected to 1.4 dyn/cm2 shear stress, eosinophils were added to the coverslip. The number of eosinophils adherent to MAdCAM-1–coated coverslips that had been subjected to shear stress (105.5 ± 13.9 eosinophils) determined under static adhesion assay conditions did not differ significantly from the number of eosinophils adherent to MAdCAM-1–coated coverslips before they were subjected to 1.4 dyn/cm2 shear stress (103.3 ± 17.1 eosinophils) (Figure2B).
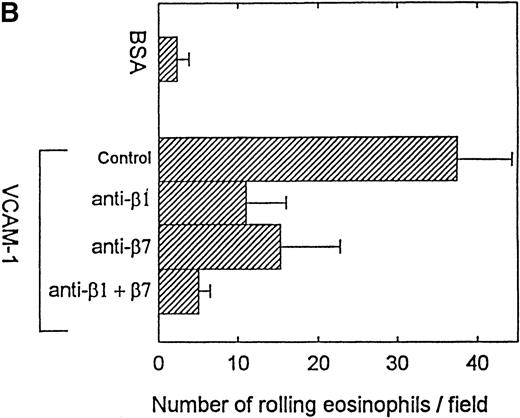
Effect of anti-4, anti-β1, and anti-β7 mAbs on eosinophil rolling and adhesion to VCAM-1 in vitro
Eosinophils preincubated with an anti-α4 mAb P4C2 did not exhibit significant rolling on VCAM-1 (4.3 ± 1.6 eosinophils treated with an anti-α4 mAb rolling on VCAM-1 vs 23.5 ± 9.0 eosinophils treated with an isotype-matched mAb rolling on VCAM-1) (P = .01) (Figure 3A). To determine whether α4β1 or α4β7 integrins subserved the eosinophil rolling function on VCAM-1, eosinophils were preincubated with neutralizing antibod ies to individual β integrins (β1 or β7) or to both integrins. The combination of anti-β1 (P4C10) and anti-β7 (FIB504) integrin mAbs almost completely inhibited eosinophil rolling on VCAM-1 (P = .01) (n = 3 experiments) (Figure3B). When used individually, the anti-β1 Ab and the anti-β7 Ab were each able to inhibit eosinophil rolling on VCAM-1 but did so less efficiently than the combination of the anti-β1 and anti-β7 Abs (Figure 3B).
Effect of anti-4, anti-β1, and anti-β7 integrin mAbs on eosinophil rolling on VCAM-1 in vitro.
Eosinophils (preincubated with either an anti-α4, anti-β1, anti-β7, anti-β1 and anti-β7 in combination, or control mAb) were infused at a flow rate of 0.7 dyn/cm2 into a parallel plate flow chamber containing VCAM-1– or BSA-coated coverslip. The number of rolling eosinophils during continuous flow periods of 2 minutes was recorded and subjected to offline analysis. Results of experiments performed are presented as the mean ± SEM (n = 3 experiments). (A) The anti-α4 mAb significantly inhibited eosinophil rolling on VCAM-1 (P = .01 vs control). (B) The anti-β1 and anti-β7 mAbs in combination (P = .01 vs control) as well as the individual anti-β1 mAb (P = .05 vs control) and anti-β7 mAb (P = .05 vs control) inhibited eosinophil rolling on VCAM-1.
Effect of anti-4, anti-β1, and anti-β7 integrin mAbs on eosinophil rolling on VCAM-1 in vitro.
Eosinophils (preincubated with either an anti-α4, anti-β1, anti-β7, anti-β1 and anti-β7 in combination, or control mAb) were infused at a flow rate of 0.7 dyn/cm2 into a parallel plate flow chamber containing VCAM-1– or BSA-coated coverslip. The number of rolling eosinophils during continuous flow periods of 2 minutes was recorded and subjected to offline analysis. Results of experiments performed are presented as the mean ± SEM (n = 3 experiments). (A) The anti-α4 mAb significantly inhibited eosinophil rolling on VCAM-1 (P = .01 vs control). (B) The anti-β1 and anti-β7 mAbs in combination (P = .01 vs control) as well as the individual anti-β1 mAb (P = .05 vs control) and anti-β7 mAb (P = .05 vs control) inhibited eosinophil rolling on VCAM-1.
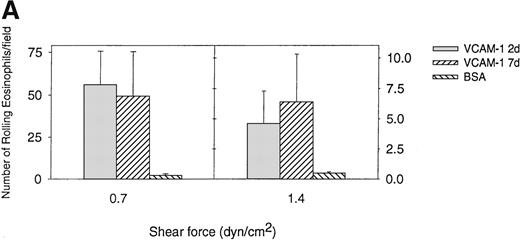
Comparison of eosinophil rolling and adhesion on VCAM-1 2d and VCAM-1 7d under conditions of flow
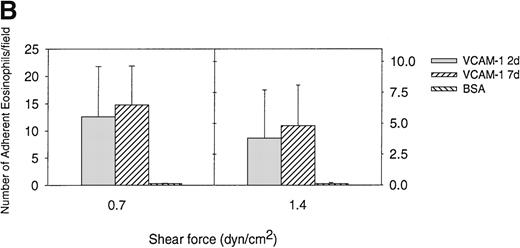
Previous studies have demonstrated the importance of an IDSPL sequence in domains 1 and 4 of VCAM-1 to the binding of α4 integrins to VCAM-1 in static adhesion assays40-42 but have not investigated their role in eosinophil/VCAM-1 interactions under conditions of flow. We have compared the ability of eosinophils to roll and adhere to a 2-domain VCAM-1 (VCAM-1 2d containing α4 integrin binding domain 1 but not domain 4) and a 7-domain VCAM-1 (VCAM-1 7d containing α4 integrin binding domain 1 and domain 4) under conditions of flow. In these studies, we noted that the VCAM-1 2d was as efficient as the VCAM-1 7d in mediating eosinophil rolling under conditions of flow (Figure 4A). Although there was a trend for eosinophils to firmly adhere in greater numbers to VCAM-1 7d compared with VCAM-1 2d at shear stress of 0.7 dyn/cm2 (14.8 ± 7.1 adherent eosinophils vs 12.6 ± 9.2 adherent eosinophils) (n = 4) (P = .59) and at shear stress of 1.4 dyn/cm2 (4.8 ± 3.3 adherent eosinophils vs 3.8 ± 3.9 adherent eosinophils) (n = 4) (P = .39), this was not statistically significant (Figure4B). These studies suggest that the N-terminal 2 domains of VCAM-1 (containing the IDSPL sequence recognized by α4 integrins in domain 1 of VCAM-1)40-42 are sufficient to subserve an eosinophil rolling function under conditions of flow. These studies do not exclude a role for VCAM-1 domain 4 (or other VCAM-1 domains 3-7) in strengthening the adhesive interaction of eosinophils that have rolled or adhered on the N-terminal 2 domains of VCAM-1.
Eosinophil rolling and firm adhesion on VCAM-1 2d and VCAM-1 7d.
Eosinophils were infused at a flow rate of 0.7 and 1.4 dyn/cm2 into a parallel plate flow chamber containing VCAM-1 2d–, VCAM-1 7d–, or BSA-coated coverslip. The number of rolling eosinophils (A) and adherent eosinophils (B) during continuous flow periods of 2 minutes was recorded and subjected to offline analysis. Results of experiments performed are presented as the mean ± SEM (n = 4 experiments). At flow rates of 0.7 dyn/cm2, significant numbers of eosinophils rolled on VCAM-1 2d (P = .01 vs BSA) and VCAM-1 7d (P = .01 vs BSA) as well as adhered to VCAM-1 2d (P = .01 vs BSA) and VCAM-7d (P = .001 vs BSA). There was no significant difference in the number of eosinophils rolling or adhering to VCAM-1 2d vs VCAM-1 7d (P = not significant).
Eosinophil rolling and firm adhesion on VCAM-1 2d and VCAM-1 7d.
Eosinophils were infused at a flow rate of 0.7 and 1.4 dyn/cm2 into a parallel plate flow chamber containing VCAM-1 2d–, VCAM-1 7d–, or BSA-coated coverslip. The number of rolling eosinophils (A) and adherent eosinophils (B) during continuous flow periods of 2 minutes was recorded and subjected to offline analysis. Results of experiments performed are presented as the mean ± SEM (n = 4 experiments). At flow rates of 0.7 dyn/cm2, significant numbers of eosinophils rolled on VCAM-1 2d (P = .01 vs BSA) and VCAM-1 7d (P = .01 vs BSA) as well as adhered to VCAM-1 2d (P = .01 vs BSA) and VCAM-7d (P = .001 vs BSA). There was no significant difference in the number of eosinophils rolling or adhering to VCAM-1 2d vs VCAM-1 7d (P = not significant).
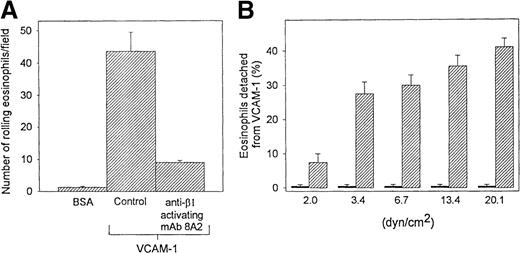
Effect of β1 integrin–activating mAb on eosinophil rolling and detachment from VCAM-1 in vitro
The β1 integrin–activating mAb 8A2 decreased the number of eosinophils rolling on VCAM-1 (Figure 5A) and resulted in firmly adherent eosinophils that were resistant to detachment from VCAM-1 when exposed to stepwise increases in shear stress from 2 to 20 dyn/cm2 (Figure 5B). To investigate the specificity of the eosinophil β1 integrin interaction with VCAM-1, we examined the ability of the anti-β1 integrin mAb P4C10 to block the β1 integrin–activating mAb 8A2–induced adhesion of eosinophils to VCAM-1–coated coverslips. These studies demonstrated that the anti-β1 mAb P4C10 inhibited the β1-activating mAb 8A2–induced adhesion of eosinophils to VCAM-1 by 92 ± 7%. These experiments demonstrate the specificity of the β1 integrin/VCAM-1 interaction studied and exclude significant integrin cross talk as being responsible for the results observed.
Effect of β1 integrin activation on eosinophil rolling and detachment from VCAM-1 in vitro.
Eosinophils (preincubated with a β1 integrin–activating Ab or control Ab) were infused into a parallel plate flow chamber containing VCAM-1– or BSA-coated coverslip. The number of (A) rolling eosinophils during continuous flow periods of 2 minutes was recorded and subjected to offline analysis. To evaluate the influence of the β1-activating antibody on the resistance of eosinophils to detachment from VCAM-1 (B), stepwise increases in shear stress were applied for 15 seconds at each shear force (2 to 20 dyn/cm2) to eosinophils adherent to VCAM-1 in the flow chamber. The number of eosinophils firmly adherent to VCAM-1 per field was recorded before and after each stepwise increase in shear stress. The number of detached eosinophils is expressed as a percentage of the total number of eosinophils adherent to VCAM-1 before stepwise increases in shear stress were applied to the coverslip in the flow chamber. Results of experiments performed at a flow rate of 0.7 dyn/cm2 are presented as the mean ± SEM (n = 3 experiments). Solid bar, mAb 8A2 treated; hatched bar, control.
Effect of β1 integrin activation on eosinophil rolling and detachment from VCAM-1 in vitro.
Eosinophils (preincubated with a β1 integrin–activating Ab or control Ab) were infused into a parallel plate flow chamber containing VCAM-1– or BSA-coated coverslip. The number of (A) rolling eosinophils during continuous flow periods of 2 minutes was recorded and subjected to offline analysis. To evaluate the influence of the β1-activating antibody on the resistance of eosinophils to detachment from VCAM-1 (B), stepwise increases in shear stress were applied for 15 seconds at each shear force (2 to 20 dyn/cm2) to eosinophils adherent to VCAM-1 in the flow chamber. The number of eosinophils firmly adherent to VCAM-1 per field was recorded before and after each stepwise increase in shear stress. The number of detached eosinophils is expressed as a percentage of the total number of eosinophils adherent to VCAM-1 before stepwise increases in shear stress were applied to the coverslip in the flow chamber. Results of experiments performed at a flow rate of 0.7 dyn/cm2 are presented as the mean ± SEM (n = 3 experiments). Solid bar, mAb 8A2 treated; hatched bar, control.
Demonstration of 4β1- and 4β7-dependent eosinophil rolling in inflamed mesenteric venules in vivo
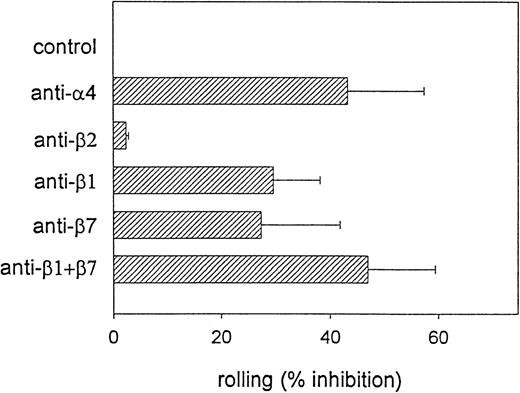
To determine the relative in vivo contribution of α4β1 and α4β7 integrins to eosinophil rolling, we visualized the interaction of CFDA-labeled eosinophils in the IL-1β–stimulated mesenteric circulation by intravital microscopy. Although donor-to-donor variation was observed, eosinophils were found to roll avidly (Rf: 42.2 ± 22.2%) in postcapillary venules (Figure6) as previously reported.20,23Because we previously demonstrated α4-dependent eosinophil rolling in mesenteric venules,20 we next compared the relative ability of (α4)β1 versus (α4)β7 integrins to mediate the α4 component of eosinophil rolling in vivo. CFDA-labeled eosinophils were first preincubated with 50 μg/mL of either the anti-β1 mAb P4C10, the anti-β7 mAb FIB504, a combination of the anti-β1 and anti-β7 mAbs, or anti-β2 mAb IB4 for 20 minutes prior to intravascular administration of eosinophils in vivo. Pretreatment of eosinophils with either the anti-β1 or the anti-β7 mAbs resulted in a significant inhibition of eosinophil rolling (anti-β1: 29.6 ± 8.5% inhibition, P = .001 vs control; anti-β7: 21.5 ± 15.9% inhibition, P = .004 vs control) (Figure 6). Preincubation of eosinophils with the anti-β1 and anti-β7 mAb in combination resulted in a greater reduction in eosinophil rolling than that induced by the individual mAbs alone (46.9 ± 12.5% inhibition,P = .0001 vs control) and was comparable to the effect of the anti-α4 mAb treatment (43.2 ± 14.1% inhibition,P = .0001 vs control) (Figure 6). These results suggest that eosinophil rolling in cytokine-stimulated mesenteric venules is mediated by both α4β1 and α4β7 integrins.
4β1 and 4β7 integrins support eosinophil rolling in postcapillary venules in vivo.
CFDA-labeled eosinophils were preincubated with either function-blocking anti-α4, anti-β1, anti-β7, or anti-β1 plus anti-β7 mAb in combination prior to the administration of eosinophils into the mesenteric microcirculation. The fraction of rolling eosinophils (Rf ) was determined in IL-1β–stimulated rabbit mesenteric venules (n = 5 to 12 rabbits). The ability of the different mAbs to block eosinophil rolling (% inhibition) was determined. Data represent mean ± SD. There was significant inhibition of eosinophil rolling induced by the anti-α4 mAb (P = .0001 vs control), the anti-β1 mAb (P = .001 vs control), the anti-β7 mAb (P = .004 vs control), and the combination of anti-β1 and anti-β7 mAbs (P = .0001 vs control) but not by the anti-β2 mAb (P = not significant vs control).
4β1 and 4β7 integrins support eosinophil rolling in postcapillary venules in vivo.
CFDA-labeled eosinophils were preincubated with either function-blocking anti-α4, anti-β1, anti-β7, or anti-β1 plus anti-β7 mAb in combination prior to the administration of eosinophils into the mesenteric microcirculation. The fraction of rolling eosinophils (Rf ) was determined in IL-1β–stimulated rabbit mesenteric venules (n = 5 to 12 rabbits). The ability of the different mAbs to block eosinophil rolling (% inhibition) was determined. Data represent mean ± SD. There was significant inhibition of eosinophil rolling induced by the anti-α4 mAb (P = .0001 vs control), the anti-β1 mAb (P = .001 vs control), the anti-β7 mAb (P = .004 vs control), and the combination of anti-β1 and anti-β7 mAbs (P = .0001 vs control) but not by the anti-β2 mAb (P = not significant vs control).
Anti-rabbit VCAM-1 mAbs blocks eosinophil rolling in vivo
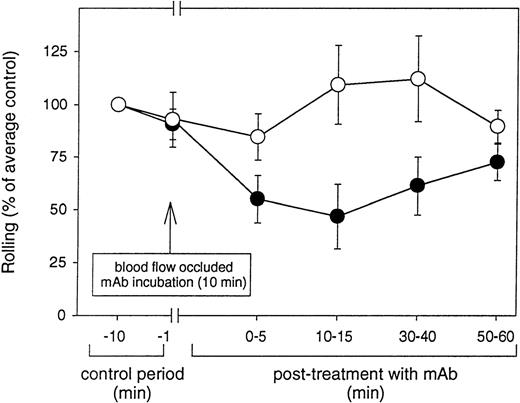
Because VCAM-1 is one of the endothelial-expressed ligands for both α4β1 and α4β7, we investigated whether inducible rolling of human eosinophils in the rabbit mesentery was mediated by VCAM-1. Blood vessels pretreated with an anti-VCAM-1 mAb Rb 1/9 exhibited a 27% to 53% inhibition in rolling at the different time points studied as compared to rolling observed in the control period (ie, prior to mAb administration) (Figure 7). Similar treatment of blood vessels with an isotype-matched control antibody (IgG1) had minimal effect on the flux of rolling eosinophils (Figure 7). As previously reported,23,38 the local intravascular administration of anti-adhesion molecule mAbs in the rabbit mesentery allows for the analysis of the function of the mAb for a brief time until the mAb is presumably removed from the endothelial cell surface by blood flow or other mechanisms. The effect of the anti-VCAM-1 mAb blockade induced by local instillation of the mAb was observed to last up to about 30 minutes, after which time eosinophils rolled at the same flux as observed during the control period (Figure 7). As described previously,23 temporary occlusion of the mesenteric artery neither altered the rolling flux of injected eosinophils nor induced any spontaneous adhesion of injected eosinophils.
Anti-VCAM-1 mAb Rb 1/9 blocks eosinophil rolling in venular endothelium in vivo.
Fluorescently labeled eosinophils were injected into the superior mesenteric artery and their baseline rolling on IL-1β–stimulated venular endothelium determined. The flow of the mesenteric circulation was temporarily occluded and an anti-VCAM-1 (Rb 1/9) or control (mouse IgG1) mAb infused. After a 10-minute incubation of the mAb with the endothelial surface, the blood flow was restored and the CFDA-labeled eosinophils injected. The flux of rolling eosinophils was determined at different time points (up to 1 hour postinfusion of eosinophils into the antibody treated mesenteric venules) by frame-by-frame analysis of recorded video images. The effect of mAb blockade lasted for up to about 30 minutes after resumption of flow. The results are expressed as percent rolling of eosinophils compared with rolling observed before mAb treatment (% of average control). The values represent mean ± SD. There was significant inhibition of eosinophil rolling 10 to 15 minutes posttreatment with mAb Rb 1/9 (P = .05 vs control) but not with mouse IgG (P = not significant vs control). •, mAb Rb 1/9; ○, mouse IgG1.
Anti-VCAM-1 mAb Rb 1/9 blocks eosinophil rolling in venular endothelium in vivo.
Fluorescently labeled eosinophils were injected into the superior mesenteric artery and their baseline rolling on IL-1β–stimulated venular endothelium determined. The flow of the mesenteric circulation was temporarily occluded and an anti-VCAM-1 (Rb 1/9) or control (mouse IgG1) mAb infused. After a 10-minute incubation of the mAb with the endothelial surface, the blood flow was restored and the CFDA-labeled eosinophils injected. The flux of rolling eosinophils was determined at different time points (up to 1 hour postinfusion of eosinophils into the antibody treated mesenteric venules) by frame-by-frame analysis of recorded video images. The effect of mAb blockade lasted for up to about 30 minutes after resumption of flow. The results are expressed as percent rolling of eosinophils compared with rolling observed before mAb treatment (% of average control). The values represent mean ± SD. There was significant inhibition of eosinophil rolling 10 to 15 minutes posttreatment with mAb Rb 1/9 (P = .05 vs control) but not with mouse IgG (P = not significant vs control). •, mAb Rb 1/9; ○, mouse IgG1.
Effect of 4β1 integrin activation on eosinophil firm adhesion in vivo
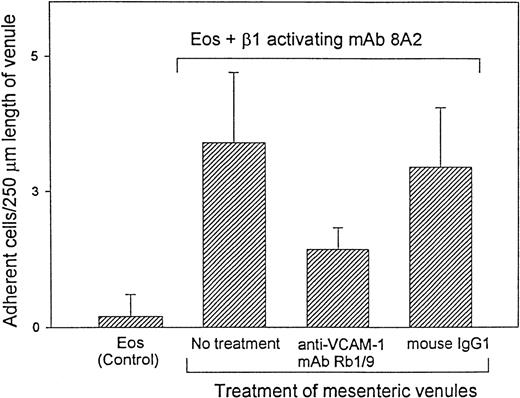
Because we25 and others26 have demonstrated that α4β1 integrins on eosinophils can exist in different functional states in vitro, we examined whether altering the functional state of α4β1 in vivo would convert it from an eosinophil rolling receptor to a firm adhesion receptor. Spontaneous adhesion of eosinophils infused into mesenteric venules is not frequently encountered and has been observed in less than 10% of the donors we have tested thus far. CFDA-labeled eosinophils were therefore activated with mAb 8A2 (β1 integrin–activating mAb) or a control function-blocking mAb P4C10 for 3 to 5 minutes prior to administration of eosinophils into the IL-1β–stimulated mesenteric circulation. Similar to our in vitro observations demonstrating a resistance to detachment from VCAM-1 of β1 integrin activated eosinophils (Figure5B), activation of the eosinophil β1 integrin resulted in an increase in the number of firmly adherent eosinophils (2 to 6 adherent eosinophils/250 μm venular length) in the mesenteric venules in vivo (P = .02 vs control) (Figure 8). In contrast to the β1-activating mAb 8A2, treatment of eosinophils with a control mAb P4C10 did not result in the increased adhesion of rolling eosinophils. The β1-activated adherent eosinophils were observed to be stationary for up to 15 to 30 minutes, after which time some of the adherent eosinophils (about 25%) were observed to detach from the endothelial surface. No firm adhesion of eosinophils was observed in the arterioles.
Stimulation with β1-activating mAb 8A2 results in stable arrest of rolling eosinophils in IL-1β–stimulated mesenteric venules.
Eosinophils were incubated ex vivo with anti-β1 integrin–activating mAb 8A2 (20 μg/mL) for 3 to 5 minutes prior to administration of eosinophils into the rabbit mesentery. The ability of the rolling eosinophils to adhere firmly in postcapillary venules (treated with anti-VCAM-1 mAb Rb 1/9 or control antibody (IgG1) (as described in Figure 7) was determined. The results represent the number of adherent eosinophils per 250 μm length of venule (mean ± SD) during the 5 minutes of eosinophil infusion after resumption of blood flow. Eosinophil control vs eosinophil and β1-activating mAb 8A2 (P = .02); eosinophil and β1-activating mAb 8A2 vs anti-VCAM-1 mAb Rb 1/9 (P = .04): anti-VCAM-1 vs mouse IgG1 control (P = .05).
Stimulation with β1-activating mAb 8A2 results in stable arrest of rolling eosinophils in IL-1β–stimulated mesenteric venules.
Eosinophils were incubated ex vivo with anti-β1 integrin–activating mAb 8A2 (20 μg/mL) for 3 to 5 minutes prior to administration of eosinophils into the rabbit mesentery. The ability of the rolling eosinophils to adhere firmly in postcapillary venules (treated with anti-VCAM-1 mAb Rb 1/9 or control antibody (IgG1) (as described in Figure 7) was determined. The results represent the number of adherent eosinophils per 250 μm length of venule (mean ± SD) during the 5 minutes of eosinophil infusion after resumption of blood flow. Eosinophil control vs eosinophil and β1-activating mAb 8A2 (P = .02); eosinophil and β1-activating mAb 8A2 vs anti-VCAM-1 mAb Rb 1/9 (P = .04): anti-VCAM-1 vs mouse IgG1 control (P = .05).
The activation-dependent stable adhesion of eosinophils induced by the β1 integrin–activating mAb 8A2 was significantly inhibited (58.8 ± 8.9% inhibition) (P = .04) when the mesenteric venules were pretreated with an anti-VCAM-1 mAb Rb 1/9 (Figure 8). In contrast, mouse IgG1 (Figure 8) or adhesion blocking anti-β1, anti-β7, or anti-α4 mAbs (data not shown) failed to stimulate the adhesion of rolling eosinophils. These studies suggest that the α4β1 integrin can subserve multiple functions of eosinophil-endothelial cell interactions in vivo, ie, rolling as well as activation-dependent firm adhesion in vivo.
Effect of antibody blockade on the velocity distribution profiles of rolling eosinophils
In addition to evaluating the influence of the anti-α4, anti-β1, and anti-β7 mAbs on eosinophil rolling in vivo, we also examined the effect of these mAbs on the velocity distribution profiles of rolling eosinophils in venular segments in vivo. The mean velocities (mm/sec) of rolling eosinophils prior to and after ex vivo mAb treatment were determined in each of the representative venules (n = 7 rabbits, 2 to 3 venules/rabbit) (Figure 9). Fluorescently labeled eosinophils were pretreated with 50 μg/mL of either anti-β1, anti-β7, anti-β1 and anti-β7, or anti-β2 integrin mAbs prior to their injection into the cannulated mesentery artery. The mean velocity of each eosinophil in a representative venule was determined by frame-by-frame analysis. The mean rolling velocity of control eosinophils was observed to be 0.26 ± 0.07 mm/sec. In contrast, eosinophils rolled at significantly higher velocities when pretreated with either the anti-α4 mAb P4G9 (0.44 ± 0.09 mm/sec;P = .003 vs control), the anti-β1 mAb P4C10 (0.43 ± 0.11 mm/sec; P = .004 vs control), or the anti-β7 mAb FIB504 (0.42 ± 0.09 mm/sec; P = .005 vs control). In contrast, treatment of eosinophils with a control anti-β2 integrin mAb IB4 (which does not block eosinophil rolling)20 had no significant effect on mean rolling velocity (0.28 ± 0.06 mm/sec).
Effect of anti-integrin mAb treatment on the velocity distribution profiles of rolling eosinophil in mesenteric venules.
The passage of rolling eosinophils in IL-1β–stimulated mesenteric venules was recorded. The velocity of consecutive rolling eosinophils was determined before and after eosinophil treatment with either anti-β1, anti-β7, anti-β1 plus anti-β7, anti-α4, or anti-β2 mAbs (n = 7 rabbits; 2 to 3 representative venules per rabbit). The velocity of rolling eosinophils (mm/sec) was manually determined by frame-by-frame analysis of recorded video images and represented as mean ± SD. The rolling velocity of eosinophils was increased by pretreatment of eosinophils with either anti-β1 (P = .004 vs control), anti-β7 (P = .005 vs control), anti-β1 plus anti-β7 (P = .003 vs control), and anti-α4 mAbs (P = .003 vs control) but not by pretreatment with anti-β2 mAbs.
Effect of anti-integrin mAb treatment on the velocity distribution profiles of rolling eosinophil in mesenteric venules.
The passage of rolling eosinophils in IL-1β–stimulated mesenteric venules was recorded. The velocity of consecutive rolling eosinophils was determined before and after eosinophil treatment with either anti-β1, anti-β7, anti-β1 plus anti-β7, anti-α4, or anti-β2 mAbs (n = 7 rabbits; 2 to 3 representative venules per rabbit). The velocity of rolling eosinophils (mm/sec) was manually determined by frame-by-frame analysis of recorded video images and represented as mean ± SD. The rolling velocity of eosinophils was increased by pretreatment of eosinophils with either anti-β1 (P = .004 vs control), anti-β7 (P = .005 vs control), anti-β1 plus anti-β7 (P = .003 vs control), and anti-α4 mAbs (P = .003 vs control) but not by pretreatment with anti-β2 mAbs.
Discussion
In contrast to the well-established role of selectins43,44 in mediating the initial rolling of leukocytes on vascular endothelium, the potential importance of α4 integrins to this rolling interaction has only recently been appreciated.20,28,45 Our initial identification of α4-integrin dependent rolling of eosinophils on endothelium in vivo20 did not explore the relative contribution of α4β1 compared with α4β7 integrins to eosinophil rolling; nor did it characterize whether α4 integrin counterligands VCAM-1 and MAdCAM-1 could both support eosinophil rolling and firm adhesion under conditions of flow. The novel observations in this study include the observations that (a) eosinophils roll more efficiently on VCAM-1 than on MAdCAM-1, (b) both VCAM-1 and MAdCAM-1 contribute to eosinophil firm adhesion under conditions of flow, (c) eosinophils roll equally efficiently on both VCAM-1 2d and VCAM-1 7d, suggesting that the N-terminal 2 domains of VCAM-1 are sufficient to support eosinophil rolling under conditions of flow, and (d) the functional status of the eosinophil β1 integrin determines the number of eosinophils firmly adherent to endothelium in vivo.
Prior studies have demonstrated by fluorescence-activated cell sorting, immunostaining, and immunoprecipitation that eosinophils from mild atopics, a similar study population to that used in our study, express both α4β1 and α4β7.13,14 Under static conditions, eosinophils are able to use both α4β1 or α4β7 integrins to bind to VCAM-1,14 while only α4β7 mediates eosinophil binding MAdCAM-1.46 The reduced rolling of eosinophils on MAdCAM-1 as compared to VCAM-1 noted in our flow chamber studies may be due to the weaker single receptor interaction of eosinophils with MAdCAM-1 (ie, only α4β7 interacts with MAdCAM-1) compared with the stronger dual eosinophil receptor interaction with VCAM-1 (ie, α4β7 and α4β1 both interact with VCAM-1). The reduced ability of eosinophils to roll on MAdCAM-1 was not due to technical factors related to eosinophil adhesion to MAdCAM-1 because eosinophils bound firmly to MAdCAM-1 and VCAM-1 in static adhesion assays and resisted detachment from both of these ligands. In addition, stripping of MAdCAM-1 from the coverslip during the flow chamber experiment is unlikely because eosinophils adhered in static adhesion assays to MAdCAM-1–coated coverslips that had previously been subjected to flow. Based on these observations, MAdCAM-1 expression by endothelial cells is more likely to contribute to eosinophil firm adhesion as opposed to eosinophil rolling on endothelial cells. In the context of eosinophils and asthma, VCAM-1 is more likely than MAdCAM-1 to play a significant role in eosinophil recruitment to the lung because VCAM-1 is expressed in the lung47 but MAdCAM-1 is predominantly expressed in the gastrointestinal tract and is absent or expressed at very low levels in nongastrointestinal sites including the lung.48Nevertheless, MAdCAM-1 may play a more significant role in eosinophil recruitment to the gastrointestinal tract.
Because MAdCAM-1 binds only to α4β7 but VCAM-1 binds to both α4β7 and α4β1, research has focused on understanding the different integrin binding sites in VCAM-1 and MAdCAM-1. Domain swapping and construction of chimeric soluble forms of MAdCAM-1 have shown that the N-terminal 2 domains of MAdCAM-1 are both required and sufficient for efficient α4β7 binding.49,50 A GLTDSL amino acid sequence in domain 1 of MAdCAM-1 is essential for binding of MAdCAM-1 to α4β7.49,50 Another unique feature of MAdCAM-1 is present in domain 2, which contains a negatively charged β ribbon loop of 11 amino acids.49,50 This negatively charged “antenna,” extending outward from domain 2 and reaching close to the GLTDSL motif in domain 1, may contribute to integrin binding. The interaction of α4β7 with MAdCAM-1 or VCAM-1 is likely to involve both unique as well as overlapping adhesion contact sites based on studies demonstrating differential inhibition of adhesion induced by mAb directed against different epitopes of α4β7.51
Our intravital microscopy studies extend our understanding of the adhesion receptors that subserve a rolling function on the eosinophil cell surface in vivo to include α4β1 and α4β7 as well as previously described L-selectin.20 In addition to α4β1, α4β7, and L-selectin, flow chamber studies in vitro have demonstrated that eosinophils also use PSGL-1 to roll on the endothelial ligand P-selectin.10 Thus, eosinophils express at least four receptors (α4β1, α4β7, L-selectin, and PSGL-1) capable of mediating eosinophil rolling on endothelial cells expressing the appropriate counterligand at sites of allergic inflammation.
This study also extends our knowledge of the endothelial cell surface adhesion counterreceptors that subserve a rolling function for eosinophils in vivo to include VCAM-1 as well as previously reported P-selectin.21,22 The importance of extending results obtained with eosinophils in static adhesion assays to in vivo physiologic evaluation is underscored by studies of eosinophil adhesion to E-selectin. While E-selectin is readily able to support eosinophil adhesion under static conditions in vitro,5 studies performed with eosinophils in flow chambers and eosinophils in vivo23,24 demonstrate that E-selectin preferentially supports neutrophil but not eosinophil rolling. As tissue infiltration by eosinophils but not neutrophils is a hallmark of allergic inflammation, whereas tissue infiltration with neutrophils but not eosinophils is a hallmark of bacterial infection, the eosinophil α4β1/α4β7 rolling interaction with endothelial-expressed VCAM-1, and the neutrophil rolling interaction with endothelial expressed E-selectin, may allow for preferential eosinophil or neutrophil recruitment pathways. In contrast, both eosinophils and neutrophils use L-selectin and P-selectin as rolling receptors in vivo, suggesting that neither of these pathways would account for selective eosinophil or neutrophil tissue recruitment. The selective recruitment of eosinophils as opposed to neutrophils to sites of allergic inflammation will not only be influenced by the above-mentioned adhesion receptors but also by the tissue expression of chemokines that preferentially attract eosinophils (ie, eotaxin, RANTES, MIP-1α, MCP-4)52 53 as opposed to neutrophils.
Recent studies have assisted in defining the ligand binding sites on α4 integrins and its counterreceptor VCAM-1.40-42,54 The N-terminal portion of integrin α subunits (about 440 amino acids) contains 7 sequence repeats.54 The regions of α4 integrin critical for ligand binding contained in this N-terminal portion are not adjacent in the primary α4 integrin structure.54Rather, the 7 N-terminal sequence repeats of α4 integrins are proposed to fold into a β-propeller domain with the integrin binding site for VCAM-1 on the upper face of the β propeller.55The predominant form of VCAM-1 in vivo has an amino-terminal extracellular region comprising 7 immunoglobulin-like domains.54 Functional studies of VCAM-1 have identified a conserved integrin-binding motif in VCAM-1 domains 1 and 4.40-42 The crystal structure of the first 2 domains of VCAM-1 has been elucidated56 and demonstrates that the integrin binding motif (IDSPL) of VCAM-1 domain 1 is highly exposed and available for integrin binding.56 At present, the crystal structure of VCAM-1 domains 3 through 7 has not been reported. Our studies demonstrate that eosinophils roll equally efficiently on both VCAM-1 2d as well as on VCAM-1 7d, suggesting that the N-terminal 2 domains of VCAM-1 are sufficient to support eosinophil rolling under conditions of flow. These studies do not exclude a role for VCAM-1 domain 4 (containing a known integrin binding site) or other sequences in VCAM-1 domains 3-7 in strengthening the adhesive interaction of eosinophils that have rolled or adhered on the N-terminal 2 domains of VCAM-1. Interestingly, VCAM-1 domain 1 would project furthest from the endothelial cell surface into the lumen of the blood vessel, allowing for ease of initial eosinophil α4-integrin rolling interaction with this VCAM-1 domain as opposed to other VCAM-1 domains not projecting as far into the blood vessel lumen. Studies using Ramos cells (a Burkitt's lymphoma cell line), cytokine-stimulated human umbilical vein endothelial cells, and Abs against domain 1 and domain 4 of VCAM-1 also demonstrate that VCAM-1 domain 1 is solely responsible for α4-integrin–dependent primary capture of Ramos cells under conditions of flow, whereas both VCAM-1 domains 1 and 4 are used in α4-integrin–dependent Ramos cell adhesion under static conditions.57
Several laboratories,27-29 including ours,25have demonstrated that integrins such as α4β1 (VLA-4) expressed by eosinophils can change their affinity for counterligands such as VCAM-1 without changing their level of integrin expression, presumably by changing the conformation of the integrin from a low- to a high-affinity state. This change in integrin affinity can be induced by several stimuli including cytokines,25 extracellular divalent cations such as manganese,26 and a β1 integrin–activating Ab.26 We were interested to determine how upregulating the functional state of α4β1 on eosinophils with a β1 integrin–activating Ab might influence the ability of α4β1 to function either as a rolling or firm adhesion receptor under conditions of blood flow in vivo. Our studies of eosinophils activated with the β1-activating Ab suggest that upregulating β1 integrin function increases the number of eosinophils adherent to VCAM-1 in vivo. This β1 integrin effect on eosinophil accumulation on VCAM-1 in vivo is probably mediated by increasing the strength of eosinophil adhesion to VCAM-1 in vivo with resultant fewer eosinophils detaching from VCAM-1 once adherent. This conclusion is also supported by our in vitro studies in which β1 integrin–activated eosinophils were resistant to detachment from VCAM-1 in a flow chamber at levels of shear stress considerably higher than those observed in the microcirculation in vivo. Thus, locally released cytokines such as GM-CSF,25chemokines such as eotaxin,58 or other integrin-activating mediators, released at sites of allergic inflammation in vivo, have the potential to upregulate α4β1 function as well as the number of endothelial adherent eosinophils by promoting their resistance to detachment.
The importance of α4 integrins to eosinophil recruitment has been suggested from several studies of animal models of allergic inflammation, in which pretreatment with anti-α4 mAbs resulted in the reduction in the accumulation of eosinophils in the airways as well as reduced bronchial hyperreactivity.17-19 However, not all studies have demonstrated a correlation between the anti-α4–Ab-mediated inhibition of eosinophil recruitment, and the anti-α4–mediated inhibition of bronchial hyperreactivity,59 suggesting that the anti-α4 Ab may not only be influencing eosinophil recruitment but also eosinophil activation in tissue sites. In this regard, it is of interest that eosinophil α4β1 receptors can be activated with either the CS-1 region of fibronectin (induces eosinophils to release GM-CSF),60 or with VCAM-1 (induces eosinophils to release superoxide).61 While targeting the α4/VCAM-1 eosinophil endothelial cell adhesion pathway in allergic inflammation is attractive in terms of its selective effect on eosinophil but not neutrophil rolling and adhesion, eosinophils can bypass this pathway using alternate endothelial-expressed rolling receptors (P-selectin) and firm adhesion receptors (ICAM-1). Indeed, our studies of ragweed-induced peritoneal eosinophil recruitment in P-selectin/ICAM-1–deficient mice treated with an anti-VCAM-1 mAb (near complete inhibition of eosinophil recruitment) suggest that the VCAM-1 pathway contributes approximately 25% to 38% to eosinophil peritoneal recruitment.21 However, the degree of inhibition of eosinophil recruitment may vary with the vascular bed studied, the animal species studied, or the route of administration of the anti-α4 Ab.59
Overall, this study demonstrates that eosinophils roll more efficiently on VCAM-1 versus MAdCAM-1, whereas eosinophils firmly adhere as efficiently to MAdCAM-1 as to VCAM-1. The α4 integrin VCAM-1 pathway may therefore be able to support both of the first 2 sequential steps of eosinophil adhesion to endothelium (ie, rolling and firm adhesion) independent of the requirement for alternate adhesion receptors (ie, selectins, β2 integrin/ICAM-1). In contrast, the α4 integrin MAdCAM-1 pathway would be more efficient in subserving eosinophil firm adhesion to endothelium as opposed to eosinophil rolling on endothelium, especially in the gastrointestinal tract where MAdCAM-1 is expressed. In addition, because α4β1 can rapidly modulate its function in vivo to resist eosinophil detachment from endothelium under conditions of flow in vivo, this modulation of receptor function may promote eosinophil accumulation at sites of inflammation.
Acknowledgments
We thank Greg Hughes and Tim Gifford for technical assistance and Lanesha Hill for assistance in the preparation of the manuscript. This study complies with National Institutes of Health guidelines for the care and use of laboratory animals.
Supported by the UCSD General Clinical Research Center grant M01 RR0827 from the National Institutes of Health and by National Institutes of Health grants AI 35796, AI 29974, AI 33977, AI 38425, and AI 22415.
Reprints:Pragada Sriramarao, Laboratory of Immunology and Vascular Biology, La Jolla Institute of Experimental Medicine, 505 Coast Blvd South, La Jolla, CA 92037; email: rao@ljiem.org.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal