Thrombomodulin is an endothelial cell receptor for thrombin. It functions as a natural anticoagulant by greatly accelerating activation of protein C by thrombin. Using a direct gene screening strategy we identified a frameshift insertion mutation, insT 1689, in the thrombomodulin gene of a patient with myocardial infarction. The mutation predicts an elongated gene product because of substitution of the 12 C-terminal amino acids by 61 abnormal residues. Pedigree analysis showed that the mutation was also likely to have been present in a sibling who had had fatal myocardial infarction. Carriers of the mutant allele express significantly lower amounts of thrombomodulin on the surface of their monocytes detected by flow cytometry and have lower levels of soluble thrombomodulin in plasma. Wild type and the mutant thrombomodulin were expressed in COS-7 cells. Cellular distribution of the expressed proteins was evaluated by immunofluorescence microscopy, which showed reduced cell surface expression and intense juxtanuclear localization of the abnormal protein. This suggests impaired translocation through the endoplasmic reticulum/Golgi apparatus. Cells expressing abnormal thrombomodulin had reduced ability (∼2.5-fold) to accelerate the thrombin mediated activation of protein C. This is the first demonstration of reduced expression arising from a natural thrombomodulin gene mutation. The results provide support for the suggestion that gene mutation of thrombomodulin may be important in the pathogenesis of some cases of occlusive thrombotic disease.
The pathogenetic process leading to myocardial infarction (MI) involves the formation of the atherosclerotic plaque followed by its rupture, with subsequent thrombosis and coronary occlusion. It is now considered that thrombin is involved both in the atherosclerotic process and in arterial thrombus formation.1 Thrombin has mitogenic effects on smooth muscle cells2 and fibroblasts3 and facilitates leukocyte activation and adhesion.4 Thrombin is also the key enzyme in blood coagulation. It promotes clot formation, platelet activation and, by activating factors V and VIII, the positive feed-back mechanism that amplifies its generation.5
Under physiologic conditions, procoagulant and natural anticoagulant mechanisms ensure that the production and inhibition of thrombin are in equilibrium. Two major anticoagulant mechanisms are involved in the suppression of excessive thrombin generation.6 Antithrombin forms an inactive complex with thrombin, which is then rapidly removed from the circulation. Activated protein C (APC), together with its cofactor protein S inactivates factors Va and VIIIa, thereby suppressing the major positive feedback mechanism in coagulation. Efficient protein C activation requires formation of a complex between thrombin and its endothelial cell receptor thrombomodulin (TM).7
TM is a transmembrane glycoprotein expressed mainly on the endothelial surface of blood vessels, but also on several other cells such as platelets,8 monocytes,9 and certain tumor cells.10 TM is a modular protein composed of 5 structural domains.11 The 3 extracellular domains consist of a large hydrophobic region, 6 EGF-like repeats and a Ser/Thr rich region. A transmembrane region is followed by a short cytoplasmic domain. The function of the last 3 EGF-like repeats and of the Ser/Thr rich domain is to mediate thrombin binding and protein C activation.12On binding to TM, thrombin undergoes conformational change and loses its ability to activate platelets and to cleave fibrinogen.13 The rate of protein C activation by the thrombin-TM complex is a 20 000-fold increase compared with thrombin alone.
The TM-APC pathway appears to be the main mechanism by which excessive thrombin formation is counteracted in the small vessels, including the coronary arteries. Impaired activity of this natural anticoagulant mechanism leads to an increased predisposition toward thrombosis. It has been shown that genetic defects of protein C, protein S, factor V (1691G to A), and prothrombin (20 210G to A) predispose to venous thrombosis.14,15 There is increasing evidence that the latter 2 defects can contribute to the development of arterial thrombosis, although the relative risk associated with a mutation is less than that in venous thrombosis.16-18
Inherited deficiency of TM in thrombotic disease has remained largely unexplored because of the difficulty of examining the phenotype of this transmembrane protein. Recently, 2 systematic programs of study involving a direct gene screening strategy have been conducted.19 20 These studies have provided highly suggestive evidence for a role of TM mutations in both venous and arterial thrombosis. To date, however, there has been no experimental evidence defining the effects of naturally occurring TM mutations on TM expression and function in human disease. We report here the identification and functional characterization of a TM gene mutation in a kindred with a history of MI.
Materials and methods
The propositus in the kindred under investigation was part of a study of TM mutations in patients with MI. The investigation has been subject of prior reports.20 21 Briefly, the patient group consisted of 104 unselected patients admitted to a west London hospital who had confirmed MI using WHO criteria. Controls matched on a 1:1 basis for sex, age, and race were patients attending the Outpatient Department of Charing Cross Hospital, London, for blood tests. The only criterion used for exclusion of controls was that they had had venous or arterial thrombosis, which was ascertained by questionnaire.
Clinical data
The propositus is a male patient who suffered acute MI at the age of 52 years. Twelve members of his family were available for investigation. The clinical data of interest for this study are presented in Table 1. Smoking and a history of high blood pressure were ascertained by questionnaire. Serum triglycerides and cholesterol levels were measured enzymatically on an Olympus clinical chemistry analyzer (Olympus Diagnostica GmbH, Hamburg, Germany).
Clinical data from the family members included in the study
| Individual . | Relation to the Proband . | Age (y) . | MI . | Smoker . | History of High Blood Pressure . | Serum Triglycerides Level* . | Serum Cholesterol Level† . | Plasma TM Level1-153 . |
|---|---|---|---|---|---|---|---|---|
| II/1 | Brother | 78 | No | Yes | Yes | Normal | Normal | 4.38 |
| II/2 | Brother | 70 | No | Yes | Yes | High | High | 4.31 |
| II/3 | Sister | 64 | No | Yes | No | Normal | Normal | 3.62 |
| II/4 | Brother | 63 | No | No | Yes | Normal | Normal | 3.44 |
| II/5 | Brother | 60 | No | Yes | Unknown | Normal | Normal | 3.38 |
| II/6 | Propositus | 58 | Yes | Yes | No | Normal | High | 3.54 |
| II/7 | Wife | 55 | No | Yes | No | High | Normal | 3.75 |
| II/8 | Brother | 54‡ | Yes | Unknown | Unknown | Unknown | Unknown | n.t. |
| III/1 | Daughter | 34 | No | No | No | Normal | Normal | 3.22 |
| III/2 | Son | 33 | No | Yes | Yes | High | Normal | 4.23 |
| III/3 | Daughter | 27 | No | No | No | Unknown | Unknown | 3.75 |
| III/4 | Nephew | 33 | No | No | Unknown | Unknown | Unknown | n.t. |
| III/5 | Niece | 30 | No | No | Unknown | Unknown | Unknown | n.t. |
| Individual . | Relation to the Proband . | Age (y) . | MI . | Smoker . | History of High Blood Pressure . | Serum Triglycerides Level* . | Serum Cholesterol Level† . | Plasma TM Level1-153 . |
|---|---|---|---|---|---|---|---|---|
| II/1 | Brother | 78 | No | Yes | Yes | Normal | Normal | 4.38 |
| II/2 | Brother | 70 | No | Yes | Yes | High | High | 4.31 |
| II/3 | Sister | 64 | No | Yes | No | Normal | Normal | 3.62 |
| II/4 | Brother | 63 | No | No | Yes | Normal | Normal | 3.44 |
| II/5 | Brother | 60 | No | Yes | Unknown | Normal | Normal | 3.38 |
| II/6 | Propositus | 58 | Yes | Yes | No | Normal | High | 3.54 |
| II/7 | Wife | 55 | No | Yes | No | High | Normal | 3.75 |
| II/8 | Brother | 54‡ | Yes | Unknown | Unknown | Unknown | Unknown | n.t. |
| III/1 | Daughter | 34 | No | No | No | Normal | Normal | 3.22 |
| III/2 | Son | 33 | No | Yes | Yes | High | Normal | 4.23 |
| III/3 | Daughter | 27 | No | No | No | Unknown | Unknown | 3.75 |
| III/4 | Nephew | 33 | No | No | Unknown | Unknown | Unknown | n.t. |
| III/5 | Niece | 30 | No | No | Unknown | Unknown | Unknown | n.t. |
Normal values: 0.5-2.2 mmol/L.
Normal values: 3.5-6.5 mmol/L.
Deceased at the age of 54 y.
Normal values: 4-5.35 ng/mL in men, 2.73 ng/mL in women aged 21-30 y and 4.79 in women aged 61-70 y (according to the manufacturer of the ELISA kit).
n.t. = not tested.
Blood collection
Written consent was obtained from all relatives of the propositus after the study had been fully explained to them. The Riverside Research Ethics Committee gave clearance for all the reported studies. Blood was collected from the antecubital vein in vacutainers containing tripotassium EDTA to a final concentration of 1.5 mg/mL or lithium heparin to a final concentration of 12 IU/mL.
DNA extraction
DNA was extracted from the tripotassium EDTA anticoagulated whole blood samples using the Nucleon II kit (Scotlab Ltd, Coatbridge, Scotland).
Single strand conformation polymorphism (SSCP) analysis
The TM fragment spanning nucleotides 1577 (numbering from the translation start site) to 1760 was amplified by polymerase chain reaction (PCR) using primers 21A (sense): 5′-TGTGCCTGGTGGTGGCGCTT-3′ and 21B (antisense): 5′-TGGACGGAGCCAGGCTCCT-3′. The fragment was denatured for 4 minutes at 96°C, strands were separated by electrophoresis for 650 Vhs on a 20% precast polyacrylamide gel and silver-stained using the Phast System (Pharmacia, Uppsala, Sweden).
Direct sequencing
A fragment spanning nucleotides 1376 to 1789 was amplified with primers 18A (sense): 5′-CGACGGTTTCATCTGCACGG-3′ and 22B (antisense): 5′-CAAA GCTGGGGGTGAGG-3′ and then both strands were sequenced with primers 18A and 22B, respectively. The sequencing was performed with ABI Prism Big Dye Terminator Cycle sequencing kit on an automated sequencer type 373 XL (Perkin-Elmer Applied Biosystems, Warrington, Cheshire, UK), using the services of the Advanced Biotechnology Centre, Charing Cross Hospital Site. The PCR product amplified from the genomic DNA of the propositus was cloned into the pCR® 2.1-TOPO vector (Invitrogen, Leek, The Netherlands). After propagation in Escherichia coli, 10 clones containing either of the amplified alleles were sequenced.
Flow cytometry
Packed cells were separated from heparinized whole blood by centrifugation at 300 × g for 8 minutes. They were then diluted in 15 mL of RPMI medium (Sigma-Aldrich, England) and layered above 5mL Lymphoprep (Nycomed Pharma, Oslo, Norway). After centrifugation at 500 × g for 25 minutes, the white cells were collected from the interphase and washed once with RPMI. The cells were fixed with 3% formaldehyde in phosphate buffered saline (2.7 mmol/L potassium chloride, 137 mmol/L sodium chloride, pH 7.4, PBS), washed, and stored in PBS with 0.2% sodium azide at 4°C until staining. Indirect fluorescent staining of the monocytes was performed in duplicate. Each sample was incubated on ice for 1 hour with 1.25 μg of mouse monoclonal antihuman TM antibody (Dako Corp, Carpinteria, CA), washed with PBS, then incubated on ice for 1 hour with 4 μg of FITC conjugated goat antimouse antibody [F (ab′)2 specific, SIGMA-Aldrich Co, Ltd, England, UK], and washed once more to remove excess antibody. Cells were blocked for 30 minutes in 20% normal mouse serum and, after removal of the blocking solution, were incubated for 1 hour with CD33 PE antibody (Becton Dickinson, Oxford, UK) to tag the monocytes. This was followed by a final washing step with PBS. Unstained cells and cells stained with the FITC conjugated secondary antibody or with the CD33 PE antibody only, were included as reference for analysis. The samples were analyzed on a Becton Dickinson FACScan flow cytometer (Becton-Dickinson Immunocytometry Systems, San Jose, CA). Data acquisition and analysis were carried out using CELLQuest software. Monocytes identified by positive staining with CD33PE were live gated at acquisition. Calculation was performed for each sample, by subtracting the nonspecific fluorescence (measured in the absence of the specific antibody) from the TM associated fluorescence (measured in the presence of the specific antibody).
Mutagenesis
The expression vector pRSVSVOTM22 was a kind gift from Prof. E. Sadler (Howard Hughes Medical Institute, Washington University School of Medicine, St. Louis, MO). This vector contains the full length TM cDNA and the SV40 origin, therefore is suitable for high-level transient TM expression in COS cells. It will be termed below as pRSVSVOTMwt. Plasmid pRSVSVOTMmut, which contained the identified mutations, 1686G to C and ins T 1689 was constructed by oligonucleotide-directed mutagenesis using the PCR strategy.23 The sequence of the in vitro amplified insert was directly confirmed.
Cell culture
COS-7 cells were purchased from the European Cell Culture Collection and propagated in DME containing 4.5 mg/mL glucose, 3.7 mg/mL sodium bicarbonate supplemented with 10% FCS, 100 U/mL penicillin, and 100 μg/mL streptomycin (all from Gibco BRL, Glasgow, UK).
Transfections
COS-7 cells were transfected using 6 × 104exponentially growing cells seeded in 35 mm well plates 24 hours before transfection. Cells (50% confluent) in each well were cotransfected by the standard calcium phosphate method24 with 2 μg of plasmid pSEAP2-Control (Clontech Laboratories, Inc, Palo Alto, CA) (for assessment of transfection efficiency) and 3 μg of either pRSVSVOTMwt or pRSVSVOTMmut. Transfections were performed in triplicate on 3 separate occasions. Transfection efficiency was determined by measuring the concentration of secreted alkaline phosphatase in the culture medium 56 hourss after transfection by means of chemiluminiscence assay (Great Escape SEAP Reporter System, Clontech Laboratories) with a Fluroscan FL plate luminometer (Labsystems, Finland).
Indirect immunofluorescence staining
For these studies, autoclaved thin coverslips were laid in the bottom of the cell culture wells before seeding. Fifty-six hours after transfection, cells were washed 3 times with PBS, fixed with ice cold 3.7% paraformaldehyde in PBS for 20 minutes at 4°C, washed with PBS, and permeabilized with 0.1% Triton-X in PBS for 10 minutes. After 3 further washes, nonspecific binding sites were blocked with 5% normal goat serum and 1% BSA in PBS for 1 hour at room temperature. After removal of the blocking solution, the cells were incubated with 2 μg of monoclonal anti-TM antibody per well for 1 hour at 37°C. The cells were washed in PBS 3 times, for 10 minutes, with gentle rocking to remove any unbound antibody and incubated for 1 hour at 37°C with 7 μg of FITC conjugated goat antimouse antibody per well. Cells were washed in PBS 3 times with rocking, the coverslips were removed from the well, drained, and mounted in Vectashield (Vector Labs Inc, Peterborough, UK) onto microscope slides. The slides were examined with epi-fluorescence optics on an Olympus PROVIS microscope (Olympus Optical Co, UK) and captured onto 35 mm transparency film. In addition, cells were imaged using a Color Coolview CCD camera (Photonic Science, Robertsbridge, UK) mounted onto a BioRad DVC250 (Bio-Rad Laboratories, UK) confocal microscope. In this case, single confocal images (1 μm in Z-axis) were captured through individual cells, at a level where the optical section cut through the maximum diameter of the nucleus.
Western blotting
Fifty-six hours after transfection, the cells transfected with pRSVSVOTM or pRSVSVOTMmut or vector without TM cDNA, were washed twice with ice cold PBS; 1 mL of lysis buffer containing 50 mmol/L Tris-HCl, pH 7.5, 150 mmol/L NaCl, 1% NP 40, 0.5% sodium deoxycholate, with a cocktail of protease inhibitors (Boehringer Mannheim, UK) was added. The lysing cells were scraped and collected into 1.5 mL tubes on ice. The lysate was centrifuged at maximum speed for 15 minutes at 4°C to remove debris; 10 μL of the cell lysate or 1 μL of protein molecular weight marker (ECL protein molecular weight markers code RPN 2107) or 250 ng recombinant truncated TM lacking the transmembrane and cytoplasmic domain (Recombinant TM, American Diagnostica, Greenwich, CT), respectively, were mixed each with 10 μL of sample buffer (50 mmol/L Tris-HCl, pH 6.8, 2% SDS, 0.1% BPB, 10% glycerol, 200 mmol/L DTT). For nonreducing conditions, the DTT was omitted from the sample buffer used for truncated TM and cell lysates. The mixtures were boiled for 3 minutes, and electrophoresed in SDS-polyacrylamide gel. Proteins were electroblotted onto Hybond P (PVDF) membrane (Amersham, Little Chalfont, UK). The membrane was incubated in blocking solution (5% BSA in TBS-T [100 mmol/L Tris-HCl pH 7.5, 150 mmol/L NaCl, 0.1% Tween 20]), overnight at 4°C. Immunodetection was performed by incubating the membrane for 1 hour at room temperature with a 50 ng/mL dilution of polyclonal sheep anti-TM antibody (American Diagnostica Inc, Greenwich, CT) in TBS-T, followed by a 1 hour incubation with a 1:1000 dilution of HRP conjugated antigoat antibody in TBS-T (Dako Corp, Carpinteria, CA). After electroblotting and blocking, the lane with the molecular weight marker was separately incubated with 1:1500 dilution of streptavidin-HRP and realigned for final detection. The final detection step was peformed using Enhanced Chemiluminiscence (ECL) Western blotting detection system and exposure of the membrane to Hyperfilm ECL (both Amersham, Little Chalfont, UK).
Thrombomodulin cofactor activity assay
Cell surface TM cofactor activity was assayed essentially as previously described.25 Transfected cells were washed 3 times with assay buffer (50 mmol/L Tris-HCl, pH 8.0, 2 mmol/L CaCl2, 100 mmol/L sodium chloride, 1% BSA), scraped into 1 mL of the same buffer and pelleted by centrifugation at 3000 × g for 3 minutes. The cells were gently resuspended in 180 μL of assay buffer and assayed in 2 dilutions, each at a final volume of 180 μL. The cells were incubated with 0.89 μmol human protein C and 14.9 nmol human thrombin for 30 minutes at 37°C. The reaction was stopped by addition of 0.4 μmol antithrombin and 13 IU /mL heparin. The amidolytic activity of APC was assayed with the chromogenic substrate S 2366 at a concentration of 200 μmol. A reference curve was constructed with known dilutions of purified APC. Cofactor activity is expressed in nanomoles (nmol) APC generated in 30 minutes per 106 transfected cells. Human thrombin, protein C, antithrombin, and APC were purchased from Enzyme Research Laboratories (Swansea, UK) and S 2366 was from Chromogenix (Molndal, Sweden). The cofactor activity was normalized for transfection efficiency. In negative controls, either protein C or thrombin were omitted.
Measurement of TM antigen
Plasma TM was measured in 10 family members, 5 carriers of the mutation and 5 noncarriers (see Table 1). For each of the carriers, plasmas from 2 nonfamily normal subjects matched for age and sex (n = 10 controls in all) were also assayed. Soluble TM in plasma and TM antigen in the cell lysates prepared as above from transfected cells, was quantified with a commercially available ELISA (Immunbind TM, American Diagnostica Inc, Greenwich, CT). For the latter, measurements were performed on triplicate samples from 2 separate transfections (n = 6).
Quantitation of activation markers
D-dimer in plasma was measured using the Asserachrom D-Di commercial ELISA from Diagnostica Stago (Asnieres, France). Measurement of F1 + 2 activation fragment was performed with the ELISA method previously developed in our laboratory.26 All the measurements were done using citrate anticoagulated plasma stored at −70°C.
Statistical methods
Means, variance, and standard error were calculated for data sets and all results are expressed in the text as means ± SE (standard error). Significance was calculated after logarithmic transformation of the data, with the t test for sample means with unequal variance. All operations were performed in Microsoft Excel (Microsoft Corp, Redmond, WA).
Results
The propositus who was identified by an abnormal SSCP pattern of TM fragment 1577-1760, had suffered MI at the age of 52 years. Sequence analysis identified a complex mutation consisting of a base substitution, 1686G to C and a base insertion, ins T 1689. No other mutation was detected within the entire coding sequence. Cloning and direct sequencing of both alleles identified 1 normal allele and 1 allele with both mutations (Figure1), confirming that the 2 sequence alterations are present on the same allele. The G to C substitution has no effect on the predicted amino acid sequence of the protein, whereas the insertion creates a shift in the reading frame. In the protein predicted by the abnormal nucleotide sequence, the last 12 normal amino acids of the cytoplasmic domain are replaced by 61 different residues. There is no predicted change to the transmembrane domain. Figure2 illustrates the effect of the nucleotide insertion on the deduced amino acid sequence of the carboxy-terminal region of TM. The propositus was normal at the factor V Leiden polymorphic site (F V 1691) and the prothrombin 20 210 polymorphic site.
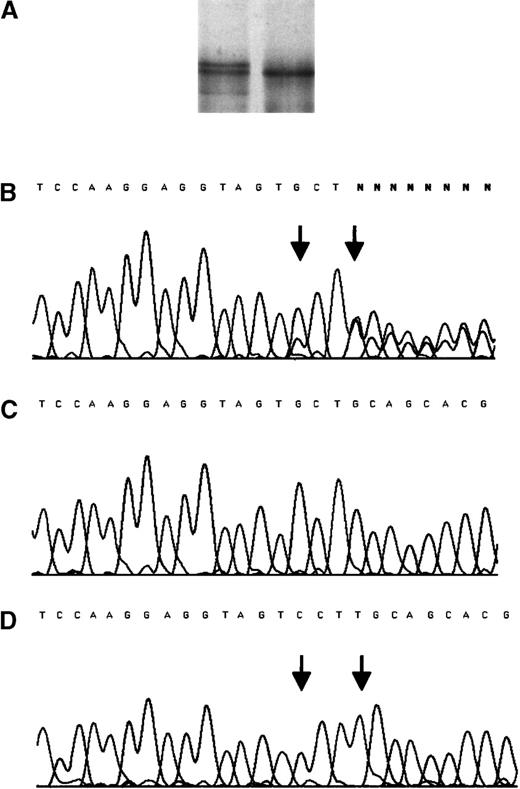
SSCP analysis and sequencing chromatogram for the mutation insT 1689 after amplification from genomic DNA of the propositus.
(A) SSCP analysis. (B) Sequencing chromatogram for the mutation. The PCR product amplified from the genomic DNA of the propositus was cloned into the pCR® 2.1-TOPO vector (Invitrogen, Leek, The Netherlands). After propagation in E coli, 10 clones containing either of the amplified alleles were sequenced. (C) The normal allele. (D) The mutant allele. Arrows indicate the position of mutations.

SSCP analysis and sequencing chromatogram for the mutation insT 1689 after amplification from genomic DNA of the propositus.
(A) SSCP analysis. (B) Sequencing chromatogram for the mutation. The PCR product amplified from the genomic DNA of the propositus was cloned into the pCR® 2.1-TOPO vector (Invitrogen, Leek, The Netherlands). After propagation in E coli, 10 clones containing either of the amplified alleles were sequenced. (C) The normal allele. (D) The mutant allele. Arrows indicate the position of mutations.
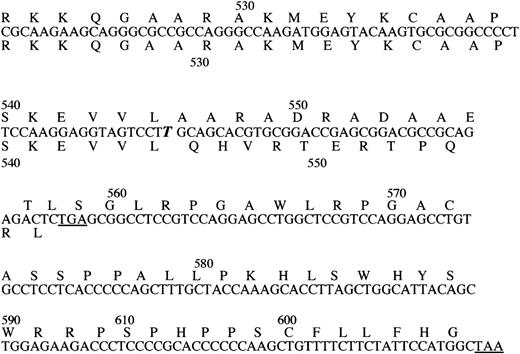
The effect of the nucleotide insertion on the deduced amino acid sequence of the carboxy-terminal region of TM.
The inserted nucleotide is identified by bold italic letter. The deduced amino acid sequence of the mutant protein is written above, the normal below the nucleotide sequence. The termination codons are underlined (numbering as in the mature protein).

The effect of the nucleotide insertion on the deduced amino acid sequence of the carboxy-terminal region of TM.
The inserted nucleotide is identified by bold italic letter. The deduced amino acid sequence of the mutant protein is written above, the normal below the nucleotide sequence. The termination codons are underlined (numbering as in the mature protein).
Pedigree analysis
As shown in Figure 3, the frameshift mutation identified by SSCP analysis was also confirmed by sequence analysis in 2 living brothers aged 63 years (II /4) and 60 years (II /5), in 2 daughters aged 34 years (III /1) and 27 years (III /3) and in the niece aged 30 years (III /5). Identification of the niece as a carrier of the mutation (III/5) provides indirect evidence that the deceased brother of the propositus (II/8), who had suffered fatal MI, had also been a carrier. The father of the propositus was reported by the family to have died of MI at 74 years; his carrier status was unknown. The propositus and his family members have known risk factors for MI, including smoking habit, increased blood pressure, elevated triglycerides, and elevated cholesterol, see Table 1.
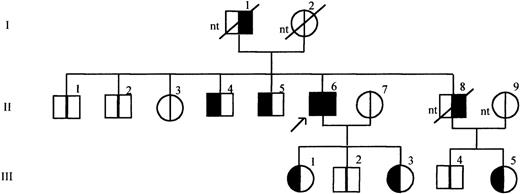
Pedigree of the propositus with mutation insT 1689 in the TM gene.
The propositus is indicated by the arrow. Generations are indicated on the left, by roman numerals, individuals in each generation are numbered at the top of the symbols. Clinical data referring to each family member are summarized in Table 1. For each individual the left half of the symbol in black indicates presence of the mutation and the right half of the symbol in black indicates MI in history. nt: not tested.

Pedigree of the propositus with mutation insT 1689 in the TM gene.
The propositus is indicated by the arrow. Generations are indicated on the left, by roman numerals, individuals in each generation are numbered at the top of the symbols. Clinical data referring to each family member are summarized in Table 1. For each individual the left half of the symbol in black indicates presence of the mutation and the right half of the symbol in black indicates MI in history. nt: not tested.
TM expression on the monocyte surface
TM antigen on the monocyte surface was detected by flow cytometry using anti-TM mouse monoclonal antibody and a secondary, FITC conjugated antimouse antibody. Ten blood samples were blind tested, 5 from carriers of the mutation ins T 1689 and 5 from normal family members. The results are shown in Table 2. Although fluorescence is low because of weak expression of TM on the surface of monocytes, the results show clearly a significantly lower cell surface expression of TM resulting from the mutation (carriers vs non carriers, 8.0 ± 1.1 vs 16.3 ± 2.2 arbitrary units of fluorecsence, P = .01). Representative histograms are shown in Figure 4.
Plasma markers and TM expressed on the surface of monocytes in carriers and non-carriers of mutation insT1689
| . | Carriers of Mutation (n = 5) means ± SE . | Non-carriers (n = 5) means ± SE . | Significance . |
|---|---|---|---|
| TM on monocytes* | 8.0 ± 1.1 | 16.3 ± 2.2 | P = .006 |
| TM in plasma† | 3.46 ± 0.082-154 | 4.06 ± 0.15 | P = .01 |
| D-dimer‡ | 419 ± 982-155 | 293 ± 44.2 | P = .31 |
| F1 + 22-153 | 43.4 ± 15.52-155 | 26.6 ± 4.42 | P = .56 |
| . | Carriers of Mutation (n = 5) means ± SE . | Non-carriers (n = 5) means ± SE . | Significance . |
|---|---|---|---|
| TM on monocytes* | 8.0 ± 1.1 | 16.3 ± 2.2 | P = .006 |
| TM in plasma† | 3.46 ± 0.082-154 | 4.06 ± 0.15 | P = .01 |
| D-dimer‡ | 419 ± 982-155 | 293 ± 44.2 | P = .31 |
| F1 + 22-153 | 43.4 ± 15.52-155 | 26.6 ± 4.42 | P = .56 |
Results expressed in ng/mL, except for TM on monocytes.
Values expressed in arbitrary units of fluorescence.
Normal values: 4-5.35 ng/mL in men, 2.73 ng/mL in women aged 21-30 y and 4.79 in women aged 61-70 y (according to the manufacturer of the ELISA kit).
Normal values: <400 ng/mL.
Normal mean value: 31.0 ± 3.7 ng/mL.
n = 6.
Levels also significantly lower (P = .02) than those of normal controls (n = 10), matched for age and sex (see text).
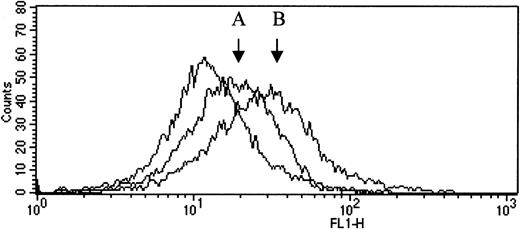
Histogram plot of flow cytometric analysis.
TM antigen on the monocyte surface was detected by flow cytometry using unlabeled anti-TM mouse monoclonal antibody and a secondary, FITC conjugated antimouse antibody. The fluorescence intensity in the absence of the specific antibody (plot on the left) was set as a reference. Changes in fluorescence intensity on the horizontal axis reflect the specific TM labeling on the surface of the cells. The increase in fluorescence intensity associated with TM antigen expression was more pronounced in a normal sample (B) than in a sample from a carrier of the mutation (A).

Histogram plot of flow cytometric analysis.
TM antigen on the monocyte surface was detected by flow cytometry using unlabeled anti-TM mouse monoclonal antibody and a secondary, FITC conjugated antimouse antibody. The fluorescence intensity in the absence of the specific antibody (plot on the left) was set as a reference. Changes in fluorescence intensity on the horizontal axis reflect the specific TM labeling on the surface of the cells. The increase in fluorescence intensity associated with TM antigen expression was more pronounced in a normal sample (B) than in a sample from a carrier of the mutation (A).
Transient expression of wild type and mutant TM in COS-7 cells
After transient expression with wild type and mutant expression vectors, Western blotting revealed bands of appropriate mobility (∼100 kd, under reducing conditions), with the mutant TM having slightly lower mobility than the wild-type TM and the truncated TM, Figure 5, panel B. The molecular weight (mol wt) of all TM molecules (truncated, wild type, and mutant) appeared anomalous (low) under nonreducing conditions with respect to the mol wt standards used, but the differences between them were consistent (Figure 5). There is also evidence of higher molecular forms of TM, that may represent either membrane fragments or TM polymers. A lower mol wt band, 60 kd, is also present under reducing conditions. As it also appears in the lysates from mock transfected cells (Figure 5), we conclude that it represents nonspecific protein crossreacting with the polyclonal antibody. The discrete mobility difference between the wild type and the mutant bands presumably represents the predicted difference in mol wt caused by the C-terminal extension. This difference between wild type and mutant expressed proteins, was also observed by immunoprecipitation of labeled expressed proteins (results not illustrated).
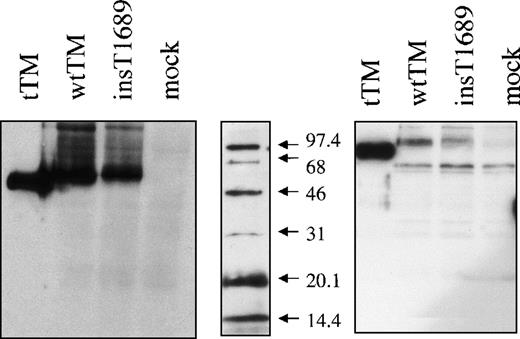
Western blot of the expressed proteins.
SDP-PAGE and immunoblot of a truncated TM form (tTM) and of cell lysates from cells expressing wild type (wtTM), insertional mutant (ins T 1689) TM or no TM (mock). Immunodetection was performed with sheep polyclonal anti-TM antibody, followed by HRP-conjugated antigoat antibody and ECL detection. Panels represents proteins under nonreducing (left) and reducing (right) conditions as shown. The mol wt marker (middle) was always reduced, and following electroblotting was detected separately with streptavidin-HRP conjugate.

Western blot of the expressed proteins.
SDP-PAGE and immunoblot of a truncated TM form (tTM) and of cell lysates from cells expressing wild type (wtTM), insertional mutant (ins T 1689) TM or no TM (mock). Immunodetection was performed with sheep polyclonal anti-TM antibody, followed by HRP-conjugated antigoat antibody and ECL detection. Panels represents proteins under nonreducing (left) and reducing (right) conditions as shown. The mol wt marker (middle) was always reduced, and following electroblotting was detected separately with streptavidin-HRP conjugate.
The amount of mutant TM antigen determined by “sandwich” ELISA in cell lysates was consistently lower than the amount of wild-type protein: 25.8 ± 2.6 versus 51.5 ± 7.1 ng/106transfected cells (n = 6). No TM antigen could be detected in the lysates of untransfected cells, or of cells transfected with the vector lacking the TM insert. To verify that the difference in levels was not due to differences in transfection efficiency, we used a control reporter plasmid encoding the secretable form of placental alkaline phosphatase (SEAP). This showed that transfection efficiency was similar for wild type and mutant plasmids in all experiments: 2.02 ± 0.1 versus 2.43 ± 0.01 (n = 9) μg alkaline phosphatase secreted by 106 cells. SEAP values were used to correct the measured antigen levels (see above) and cofactor activity (see below) on the cell surface.
Cellular distribution of the expressed proteins
Visualization of TM expression by COS-7 cells was accomplished with confocal microscopy after indirect immunofluorescent staining with monoclonal anti-TM antibody and secondary FITC labeled antibody. Cells transfected with plasmid pRSVSVOTMwt, showed localization of TM in the plasma membrane (Figure 6A, B). In contrast, in the cells transfected with plasmid pRSVSVOTMmut, a much reduced membrane incorporation of the TM antigen and a high juxtanuclear staining was observed (Figure 6C, D, and E). Untransfected cells and cells transfected with plasmid without TM insert showed no staining.
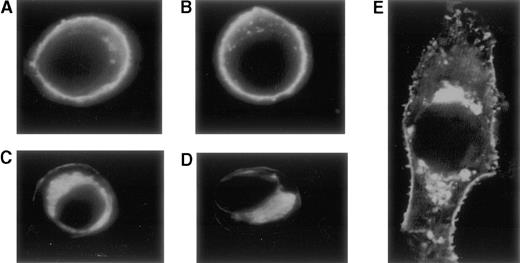
Confocal microscopy after indirect immunofluorescent staining with monoclonal anti-TM antibody and secondary FITC-labeled antibody.
Cells transfected with plasmid pRSVSVOTMwt, show a high concentration of the TM-anti-TM complexes in the plasma membrane (A, B). In contrast, cells transfected with plasmid pRSVSVOTMmut show a much reduced membrane incorporation of the TM antigen and a high juxtanuclear staining (C-E). Panel E represents an enlarged photograph of a cell expressing the mutant insT 1689.

Confocal microscopy after indirect immunofluorescent staining with monoclonal anti-TM antibody and secondary FITC-labeled antibody.
Cells transfected with plasmid pRSVSVOTMwt, show a high concentration of the TM-anti-TM complexes in the plasma membrane (A, B). In contrast, cells transfected with plasmid pRSVSVOTMmut show a much reduced membrane incorporation of the TM antigen and a high juxtanuclear staining (C-E). Panel E represents an enlarged photograph of a cell expressing the mutant insT 1689.
Thrombomodulin cofactor activity
To assess the functional impact of the lowered membrane incorporation of the mutant compared with the wild type TM, a 2-stage TM cofactor activity assay was performed on the surface of transfected cells. Protein C activation was significantly reduced on the surface of cells expressing mutant TM, 214 ± 25 nmol APC/106transfected cells compared with cells expressing the normal TM, 553 ± 117 nmol APC/106 transfected cells, n = 9,P = .0001. Untransfected cells and cells transfected with the vector without TM insert had no detectable activity.
Plasma levels of activation markers
As shown in Table 2, a significantly lower concentration of soluble TM was found in the plasma from carriers of the mutation ins T 1689, 3.46 ± 0.08 versus 4.06 ± 0.15 ng/mL in normal individuals (P = .01). The difference was also significant when the levels in carriers were compared with those measured in the normal age and sex matched control group, 4.69 ± 0.52 versus 3.46 ± 0.08, P = .02. The concentrations of coagulation activation markers F1 + 2 and D-dimer were higher in the mutation carriers compared with noncarriers of the mutation, 43.3 ± 15.7 versus 26.6 ± 4.42 ng/mL and 419 ± 98 versus 293 ± 44.2 ng/mL, respectively, but the increases were not statistically significant (P = .56 andP = .31, respectively).
Discussion
We report a mutation in the TM gene of a patient with MI, consisting of a silent base substitution, 1686G to C, and a base insertion, insT 1689, the latter predicted to cause a shift in the reading frame and an elongated gene product. The predicted mutant protein has normal sequences for its extracellular and transmembrane domains, but an elongated intracellular C terminal tail. The consequences of the mutation are decreased surface expression of TM on the monocytes of carriers and decreased levels of soluble TM in plasma.
In vitro expression showed that TM with slightly increased mol wt is synthesized by cells transfected with mutant TM cDNA. Reduced incorporation of this protein in the plasma membrane and its increased juxtanuclear concentration may reflect a trafficking impediment in the endoplasmic-reticulum (ER)/Golgi apparatus. Altered secondary structure of the mutant TM at its C terminus is assumed to cause altered folding of this domain. Misfolded proteins are often associated with ER-specific chaperones and removed from the cell by degradation.27 Measurement of TM antigen in cell lysates prepared after transfection showed a ∼50% reduction in the expression of the mutant protein. This may be due either to sequestration of the protein in the Golgi apparatus, the membrane of which is not broken by the lysis method used, or to modified antigenicity of the sequestrated/degraded protein. The ∼50% decrease in protein C activation, measured in the TM cofactor assay, is compatible with the antigen measurement. This indicates that loss of anticoagulant function on the cell surface is probably due solely to reduced surface expression of the elongated TM which, as noted previously, has predicted normal extracellular structure. The reduction in anticoagulant function is less pronounced than the more dramatic decrease in TM incorporated in the plasma membrane seen in the immunocytochemistry studies. This may be due to the nonquantitative nature of the confocal microscopy. The above in vitro results, therefore support the suggestion of lowered in vivo TM expression in family members with mutation ins T 1689. Taken together, the results of the present study clearly establish a relationship between the ins T 1689 mutation and its in vitro and in vivo effects on TM expression.
TM is an essential component of the anticoagulant pathway regulating thrombin activity. Soluble plasma TM has been implicated in the regulation of activity of thrombin activatable fibrinolysis inhibitor. To address the question of whether increased thrombin generation and fibrinolysis, a hypercoagulable state, exists in the carriers of the mutation, we determined the levels of 2 activation markers, prothrombin activation fragment F1 + 2 and D-dimer. The data from this small family study do not conclusively support a procoagulant imbalance. Previous studies failed to detect a hypercoagulable state in individuals with asymptomatic antithrombin deficiency28 and detected such a state only in one quarter of a large number of patients with inherited thrombophilia (antithrombin, protein C, and protein S defficiencies).29 The hypothesis of a diffuse thrombotic diathesis in inherited thrombophilia now appears very unlikely. A more considered view is that the endothelium integrates different extracellular signals and cellular responses in a vascular-bed specific manner.30 The effect of reduced endothelial expression of TM may be selectively pronounced in the coronary arteries, where the receptor density is relatively high. Although the reduction of endothelium TM expression in vivo in carriers of the mutation ins T 1689 could not be directly determined here, the analysis of monocytes from carriers of mutation, suggest that it causes almost complete loss of cell surface expression arising from the mutant allele (note that heterozygous carriers have ∼50% monocyte surface TM). However, TM expression on monocytes is 3 orders of magnitude lower than that on the endothelium, making exact measurements on the monocyte surface and extrapolation of the results to the endothelium difficult. The extent of the reduction and the resulting effect on hypercoagulability both remain uncertain, but reduced in vivo expression of the mutant TM can be predicted.
The association between the insT 1689 mutation of TM and myocardial infarction is intriguing. On the one hand, the mutation is associated with MI in this family, with 2 carriers suffering the disease, 1 having a fatal event. On the other hand, the evidence linking changes in TM expression to arterial thrombosis is still tentative, but growing. It is of interest to note the known acquired risk factors (smoking, hypertension, and hyperlipidaemia) in the presently reported family. A recent study of plasma TM levels measured in the Atherosclerosis Risk in Communities (ARIC) study found a strong, graded, and inverse relation between plasma TM and incident coronary heart disease (CHD).31 In the current study, the plasma TM was significantly lower in carriers of mutation insT 1689 than in noncarriers. Our data constitute the first direct indication that soluble TM levels may reflect TM expression on the endothelium, and may be, in part, genetically determined.
The screening program from which the present case originated identified a number of mutations in the cohort of patients with arterial thrombosis. The results of this screening program are summarized in Table 3. The mutations identified in the 5′ region of the TM gene lie adjacent to gene regulatory elements and conceivably may decrease TM expression. Mutation-33G to A has also been identified in patients with venous thrombosis. Reporter gene analysis showed a slightly reduced transcriptional activity of the variant promoter.32 The mutation 127G to A coding for Ala25 to Thr substitution, occurred in 2 patients with MI, but not in the control group. We assessed the risk of MI associated with the mutation in an additional investigation, “Study of Myocardial Infarctions Leiden” (SMILE), a large population-based case-control study. Although not conclusive, this investigation strongly suggested that the mutation is a risk (∼2-fold) for MI and interacts with acquired risk factors to further enhance the risk.21 An independent study conducted in the context of familial venous thrombosis identified this mutation also in a Swedish family.33 Furthermore, the same investigators have identified several missense mutations in families or individuals with deep venous thrombosis.19 Because these mutations are rare events and the families involved have not been large, firm evidence relating them causally to venous thrombosis is not yet available. The dimorphism,34 Ala455 to Val, has been investigated with respect to MI. One recent study has shown that among 97 Swedish patients with premature acute MI, the allele coding for Ala455 was slightly over-represented, suggesting it as a risk factor for MI.35 These results were not confirmed by our study,20 see Table 3.
Summary of mutations identified in TM gene in patients with myocardial infarction
| Nucleotide Change . | Amino Acid Change . | MI (n = 104) . | Controls (n = 104) . |
|---|---|---|---|
| −133C to A3-150 | None | 1 | 0 |
| −33G to A3-150 | None | 1 | 1 |
| −9/−10GG to AT3-150 | None | 3 (1 homozygous) | 0 |
| 127G to A3-151 | Ala25 to Thr | 2 | 0 |
| 682G to A | (Pro 209) None | 1 | 0 |
| 1418C to T3-152 | Ala455 to Val | 18 (3 homozygous) | 18 (3 homozygous) |
| insT 16893-153 | Sequence changes from Ala 546 | 1 | 0 |
| Nucleotide Change . | Amino Acid Change . | MI (n = 104) . | Controls (n = 104) . |
|---|---|---|---|
| −133C to A3-150 | None | 1 | 0 |
| −33G to A3-150 | None | 1 | 1 |
| −9/−10GG to AT3-150 | None | 3 (1 homozygous) | 0 |
| 127G to A3-151 | Ala25 to Thr | 2 | 0 |
| 682G to A | (Pro 209) None | 1 | 0 |
| 1418C to T3-152 | Ala455 to Val | 18 (3 homozygous) | 18 (3 homozygous) |
| insT 16893-153 | Sequence changes from Ala 546 | 1 | 0 |
Decisively establishing causality between TM gene mutation and occlusive thrombotic events may not be a facile task, in view of the known requirement for genetic and acquired risk factors to interact to precipitate an obstructive thrombosis.14 36-38 In venous thrombotic disease, the risk conferred by mutation of other genes involved in the TM-APC anticoagulant pathway can be modest or high. In MI, however, the risk appears much less, and may not be readily manifest without recourse to very large study groups. This is made more difficult by the low prevalence of the mutations identified to date.
Experimental evidence is emerging to support a role of TM gene mutation in thrombotic occlusion. A transgenic mouse carrying an amino acid substitution in the thrombin binding region of TM has been generated. Protein C activation in homozygous animals is seriously impaired and the fibrin deposition after a thrombotic challenge is more severe than in normal animals.39,40 It has also been demonstrated that, in a porcine model, protein C activation is essential for maintaining microvascular patency and left ventricular function after transient vascular occlusion.41 These data support the sugestion of a possible role of TM in protection against myocardial injury. Such protective effect is also suggested by the findings of the ARIC study cited above. Endothelium exhibiting reduced TM expression on its surface will have a reduced thrombin binding capacity and reduced ability to activate protein C. Reduced binding of thrombin to TM implies more free thrombin will be available to activate platelets and clot fibrin. Consequently, reduced TM expression on the coronary endothelium could facilitate thrombus formation at the site of plaque injury, therefore leading to early MI or to extended myocardial damage. It is tempting to speculate that such mechanisms may indeed have contributed to coronary occlusion in the currently studied propositus and his deceased brother.
Acknowledgments
We are grateful to Dr Philip Mason and Dr Blandine Mille (Imperial College School of Medicine) for their help with mutagenesis.
Supported by grants from the British Heart Foundation (PG/98152 and PG/96065), the Graham Dixon Trust, the Coronary Thrombosis Trust and the Special Trustees of Charing Cross Hospital.
Reprints:Gabriella Kunz, Department of Hematology, Imperial College School of Medicine, Charing Cross Campus, Hammersmith, London W6 8RP, UK.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal