Factor VIII (fVIII) circulates as a heavy chain/light chain (A1-A2-B/ap-A3-C1-C2) heterodimer. The 41-residue light chain activation peptide, ap, is cleaved from fVIII during proteolytic activation by thrombin or factor Xa. We constructed 7 active recombinant hybrid B-domainless human/porcine fVIII molecules that contained combinations of porcine sequence replacements within the A2, ap-A3, and C2 domains. The cross-reactivity of 23 high-titer inhibitory antibodies between human fVIII and the hybrids was inversely related to the degree of porcine substitution. In all plasmas, the substitution of all 3 regions yielded cross-reactivities that were not significantly different from those of porcine fVIII. To differentiate between inhibitor binding to the ap region and the A3 domain, we constructed 2 additional hybrids that contained porcine A2 and C2 domain substitutions and either porcine A3 or porcineap substitutions. The porcine ap segment was less antigenic than the human ap segment in several plasmas that had activity against the ap-A3 region. This indicates that some inhibitor plasmas contain antibodies directed against the fVIIIap segment in addition to A2, A3, and C2 domain epitopes identified in previous studies. Substitution of porcine sequences within the A2, A3, C2, and ap regions of human fVIII is necessary and sufficient to achieve a maximal reduction in antigenicity relative to porcine fVIII with respect to most inhibitory antibody plasmas.
Inhibitory antibodies (inhibitors) to factor VIII (fVIII) arise as alloantibodies in hemophiliacs who have undergone transfusion and as autoantibodies in persons without hemophilia.1-4 As a rule, they are polyclonal IgG populations directed against multiple epitopes. FVIII contains a sequence of domains designated NH2-A1-A2-B-ap-A3-C1-C2-COOH.5 It is cleaved intracellularly and circulates as an A1-A2-B/ap-A3-C1-C2 heavy chain/light chain heterodimer.6 During proteolytic activation by thrombin, the B domain and the 41-residue light chain activation peptide,ap, are released, and cleavage occurs between the A1 and A2 domains. The final product is a 160-kd A1/A2/A3-C1-C2 fVIIIa heterotrimer.7 8
Most domain-specific inhibitors interfere with fVIIIa function rather than with the activation or the circulatory lifetime of fVIII. Anti-A2 inhibitors bind to an epitope bounded by amino acids 484–508.9 They are noncompetitive inhibitors of the fVIIIa/factor IXa complex.10 Anti-C2 inhibitors bind to a discontinuous epitope derived from the NH2-terminal and COOH-terminal ends of the domain11,12 and block the binding of fVIIIa to phospholipid.13 Anti-A3 inhibitors bind to an epitope that includes amino acids 1811–1818 and block the binding of fVIIIa to factor IXa.14,15 Inhibitor neutralization studies indicate that the effects of anti-A2, anti-A3, and anti-C2 inhibitors are additive.16 Thus, inhibitors to a given domain appear to act independently of inhibitors to other domains. Despite the different immunologic settings in which they arise, alloantibody and autoantibody epitopes appear to be the same, though autoantibody plasmas are more likely to be specific for single domains.16
Clinically significant inhibitors usually cross-react poorly with porcine fVIII,17-19 forming the basis for the therapeutic use of porcine fVIII concentrate. We have used recombinant hybrid human/porcine fVIII molecules to map epitopes in the A2 and C2 domains.9,11 Epitopes are located by identifying porcine amino acid substitutions that result in a decrease in antibody cross-reactivity. These studies have taken advantage of exceptional patient plasmas monospecific for the A2 or C2 domains. Antibodies that are monospecific for domains outside A2 and C2 are rare,16which makes analysis of epitope(s) in the ap-A3 region using hybrid human/porcine fVIII molecules more difficult. To approach this problem, we made a series of constructs that contained combinations of porcine substitutions in the A2, ap, A3, and C2 domains, and we studied the cross-reactivity of 23 high-titer, primarily polyspecific, inhibitory antibody plasmas against them. Our results indicated that multiply-substituted hybrid fVIII molecules are useful for identifying inhibitor domain specificity. Using this method, we found that some inhibitors have activity directed against the ap region, which previously had not been recognized.
Materials and methods
Materials
Citrated hemophilia A and normal pooled human plasma were purchased from George King Biomedical (Overland Park, KA). pBlueScript II KS− was purchased from Stratagene (La Jolla, CA). Synthetic oligonucleotides were purchased from Life Technologies (Gaithersburg, MD) or Cruachem (Sterling, VA). Restriction enzymes were purchased from New England Biolabs (Beverly, MA) or Promega (Madison, WI). Polymerase chain reaction (PCR) products and restriction fragments were gel purified, precipitated with ethanol, and ligated to plasmid DNA using T4 DNA ligase (Rapid DNA ligation kit; Boehringer Mannheim). Insert-containing plasmids were used to transformEscherichia coli Epicurean XL1-Blue cells (Stratagene). Novel fVIII DNA sequences generated by PCR were confirmed by dideoxy sequencing using an Applied Biosystems 373a automated DNA sequencer and the PRISM dye terminator kit (Perkin Elmer, Norwalk, CT). Murine monoclonal antibody CLB-CAg A was a generous gift of Dr Jan van Mourik (Central Laboratory, Netherlands Red Cross).
Construction and expression of hybrid human/porcine fVIII molecules
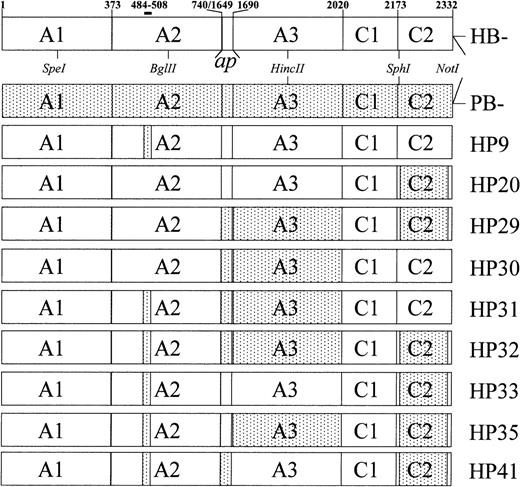
The B-domainless hybrid fVIII molecules used in this study are shown in Figure 1 along with B-domainless human fVIII (HB−) and porcine fVIII (PB−). These molecules lack the entire B domain, defined as the thrombin cleavage fragment corresponding to amino acids 741–1648. HB−, PB−, HP9, and HP20 have been described previously.9 20
Domain structure of recombinant fVIII constructs.
Amino acid numbering refers to mature, full-length human fVIII. The A3, C1, and C2 domains are defined as amino acids 1690-2019, 2020-2172, and 2173-2332, respectively.36 The light chain activation peptide, ap, corresponds to amino acids 1649-1689. The constructs lack the B domain, which is defined as amino acids 741-1648. Stippled regions indicate areas of porcine substitution. Boundaries are defined by amino acids in which porcine and human fVIII differ. Porcine A2, ap, A3, and C2 substitutions correspond to amino acids 484-508, 1649-1687, 1694-2019, and 2181-2321, respectively.
Domain structure of recombinant fVIII constructs.
Amino acid numbering refers to mature, full-length human fVIII. The A3, C1, and C2 domains are defined as amino acids 1690-2019, 2020-2172, and 2173-2332, respectively.36 The light chain activation peptide, ap, corresponds to amino acids 1649-1689. The constructs lack the B domain, which is defined as amino acids 741-1648. Stippled regions indicate areas of porcine substitution. Boundaries are defined by amino acids in which porcine and human fVIII differ. Porcine A2, ap, A3, and C2 substitutions correspond to amino acids 484-508, 1649-1687, 1694-2019, and 2181-2321, respectively.
Initially, HP29, HP30, HP31, HP32, HP33, HP35, and HP41 were constructed in pBluescript. HP29 was made by splicing-by-overlap extension (SOE) mutagenesis 21 using procedures described previously.9,11 HB− and HP18, a human heavy chain/porcine light chain hybrid,11 were used as PCR templates. The SOE product, a porcine ap-A3/human C1 fragment, was ligated to HincII/SphI–digested HP18 to produce HP29 in pBluescript.
HP30 was made using SphI/NotI digestion and ligation, with HP29 and HB− as vector and insert, respectively. HP31 was made using SpeI/BglII digestion and ligation, with HP30 and HP9 as vector and insert, respectively. HP32 was made usingSpeI/BglII digestion and ligation, with HP29 and HP9 as vector and insert, respectively. HP33 was made usingSpeI/BglII digestion and ligation using HP20 and HP9 as vector and insert, respectively. Similarly, HP35 and HP41, which are complementary constructs, were made by SOE mutagenesis using HB− and HP30 as PCR templates.
The constructions were moved into ReNeo, a mammalian expression vector,20 using SpeI/NotI digestions and ligations. cDNA in ReNeo initially was transfected into COS-7 cells to confirm that active protein could be expressed. Then it was stably transfected into baby hamster kidney (BHK) cells, partially purified, and concentrated as described previously.11 20
FVIII assays
The activity of recombinant fVIII proteins was measured by one-stage clotting assay1 22 using an ST4 BIO Coagulation Instrument (Diagnostica Stago). One unit of fVIII is defined as the activity in 1 mL pooled normal citrated human plasma.
FVIII inhibitor titers were measured by the Bethesda assay.23 Inhibitor samples are identified by patient initials. Inhibitors were measured either in dilutions of patient plasma or IgG preparations (samples KB, MS, MU, RJ, and SCN). To measure the inhibitor activity against fVIII, constructs were added to hemophilia-A plasma to a final concentration of 0.8 to 1.2 U/mL. One Bethesda unit (BU) is defined as the amount of inhibitory activity that produces 50% inhibition of fVIII activity in the one-stage assay. The 50% inhibition point was identified by interpolation23 using only data points falling within a range of 40% to 60% inhibition. At least 3 data points were used for each determination, from which the mean and sample SD were calculated. The cross-reactivity of inhibitors with hybrid fVIII molecules or porcine fVIII was defined as the percentage Bethesda titer relative to human-B domainless fVIII.
Results
Initially, we constructed 7 B-domainless hybrid human/porcine fVIII cDNA that contained combinations of substitutions of porcine sequence for human sequences in the A2, ap-A3, and C2 domains (Figure1). HP9 contains a substitution encoding porcine replacement of human amino acids 484-508, a region that appears to contain most, if not all, of the epitope recognized by anti-A2 human inhibitors.9 HP30 and HP20 contain porcine replacement of most of the ap-A3 and C2 regions of fVIII, respectively. HP31, HP33, and HP29 contain the corresponding combinations of these mutants in the A2/ap/A3, A2/C2, and ap/A3/C2 domains, respectively. Finally, HP32 contains the quadruply-substituted A2/ap/A3/C2 hybrid.
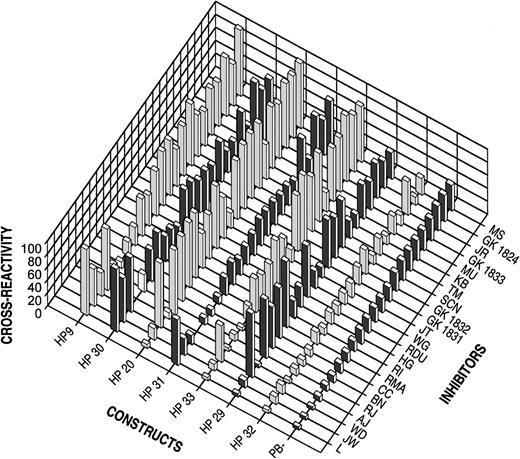
The hybrids were stably expressed in BHK cells and partially purified as described in “Materials and Methods.” The cross-reactivity of 23 human inhibitors between human B-domainless fVIII and the hybrids was measured using the Bethesda assay and was compared to porcine B-domainless fVIII. In Figure 2, the hybrids are ranked from left to right in terms of increasing degree of porcine substitution. Inhibitor plasmas are ranked from left to right according to their cross-reactivity with porcine B-domainless fVIII. The results show that the cross-reactivity of the hybrids decreased as the degree of porcine substitution increased.
Inhibitor cross-reactivity with human fVIII and hybrid fVIII constructs or porcine fVIII.
Inhibitor activity was measured by the Bethesda assay as described in “Materials and Methods.” Cross-reactivity is expressed as the percentage Bethesda titer relative to human B-domainless fVIII.
Inhibitor cross-reactivity with human fVIII and hybrid fVIII constructs or porcine fVIII.
Inhibitor activity was measured by the Bethesda assay as described in “Materials and Methods.” Cross-reactivity is expressed as the percentage Bethesda titer relative to human B-domainless fVIII.
Within the calculated coefficient of variation of 0.2, the cross-reactivity of HP32 was not greater than porcine fVIII in any of the plasmas (Table 1). The difference between HP32 and porcine fVIII was not statistically significant by either the paired Student t test or the Wilcoxon signed-rank test. Thus, the decrease in cross-reactivity toward porcine fVIII appeared to be completely conferred by the porcine substitutions in HP32, indicating that the epitopes recognized by the plasmas were confined to A2 amino acids 484-508, ap amino acids 1649-1687, A3 amino acids 1694-2017, and C2 amino acids 2181-2321.
Cross-reactivity of human FVIII inhibitors with hybrid and porcine FVIII
| . | Construct . | HP9 . | HP30 . | HP20 . | HP31 . | HP33 . | HP29 . | HP32 . | PB− . |
|---|---|---|---|---|---|---|---|---|---|
| . | Porcine Substitution . | A2 . | ap, A3 . | C2 . | A2, ap,A3 . | A2, C2 . | ap, A3, C2 . | A2, ap, A3, C2 . | ALL . |
| Plasma . | Bethesda titer versus HB− . | . | . | . | . | . | . | . | . |
| MS | 177 | 138 | 48 | 118 | 66 | 83 | 36 | 23 | 39 |
| GK1824 | 350 | 63 | 48 | 95 | 52 | 83 | 39 | 9 | 39 |
| JR | 63 | 86 | 73 | 106 | 73 | 102 | 51 | 49 | 37 |
| GK1833 | 222 | 65 | 23 | 82 | 22 | 80 | 38 | 12 | 32 |
| MU | 15 790 | 70 | 59 | 93 | 69 | 79 | 49 | 26 | 30 |
| KB | 462 | 51 | 27 | 50 | 20 | 27 | 19 | 11 | 30 |
| TM | 241 | 61 | 29 | 131 | 27 | 113 | 24 | 22 | 29 |
| SCN | 17 | 94 | 59 | 112 | 29 | 82 | 71 | 12 | 29 |
| GK1832 | 1 566 | 74 | 39 | 97 | 33 | 114 | 60 | 35 | 26 |
| GK1831 | 2 212 | 54 | 46 | 87 | 35 | 84 | 72 | 24 | 19 |
| JT | 127 | 83 | 47 | 22 | 37 | 16 | 14 | 16 | 18 |
| WG | 971 | 81 | 59 | 60 | 30 | 48 | 24 | 16 | 18 |
| RDU | 72 | 66 | 63 | 94 | 26 | 61 | 78 | 12 | 17 |
| HG | 192 | 44 | 21 | 66 | 21 | 40 | 30 | 16 | 15 |
| RI | 1 054 | 55 | 34 | 52 | 32 | 39 | 23 | 8 | 13 |
| RMA | 46 | 26 | 39 | 143 | 24 | 48 | 111 | 17 | 10 |
| CC | 10 642 | 19 | 58 | 128 | 10 | 20 | 75 | 5 | 9 |
| BN | 30 | 4 | 47 | 93 | 5 | 2 | 60 | 5 | 6 |
| RJ | 16 | 14 | 25 | 88 | 8 | 8 | 88 | <6 | <6 |
| AJ | 163 | 85 | ND* | 7 | ND | 2 | 2 | 3 | 4 |
| WD | 189 | 34 | 81 | 122 | 12 | 53 | 97 | 12 | 3 |
| JW | 386 | 54 | 64 | 14 | 23 | 8 | 9 | 9 | 3 |
| L | 39 | 108 | 95 | <3 | 67 | <3 | <3 | <3 | 3 |
| . | Construct . | HP9 . | HP30 . | HP20 . | HP31 . | HP33 . | HP29 . | HP32 . | PB− . |
|---|---|---|---|---|---|---|---|---|---|
| . | Porcine Substitution . | A2 . | ap, A3 . | C2 . | A2, ap,A3 . | A2, C2 . | ap, A3, C2 . | A2, ap, A3, C2 . | ALL . |
| Plasma . | Bethesda titer versus HB− . | . | . | . | . | . | . | . | . |
| MS | 177 | 138 | 48 | 118 | 66 | 83 | 36 | 23 | 39 |
| GK1824 | 350 | 63 | 48 | 95 | 52 | 83 | 39 | 9 | 39 |
| JR | 63 | 86 | 73 | 106 | 73 | 102 | 51 | 49 | 37 |
| GK1833 | 222 | 65 | 23 | 82 | 22 | 80 | 38 | 12 | 32 |
| MU | 15 790 | 70 | 59 | 93 | 69 | 79 | 49 | 26 | 30 |
| KB | 462 | 51 | 27 | 50 | 20 | 27 | 19 | 11 | 30 |
| TM | 241 | 61 | 29 | 131 | 27 | 113 | 24 | 22 | 29 |
| SCN | 17 | 94 | 59 | 112 | 29 | 82 | 71 | 12 | 29 |
| GK1832 | 1 566 | 74 | 39 | 97 | 33 | 114 | 60 | 35 | 26 |
| GK1831 | 2 212 | 54 | 46 | 87 | 35 | 84 | 72 | 24 | 19 |
| JT | 127 | 83 | 47 | 22 | 37 | 16 | 14 | 16 | 18 |
| WG | 971 | 81 | 59 | 60 | 30 | 48 | 24 | 16 | 18 |
| RDU | 72 | 66 | 63 | 94 | 26 | 61 | 78 | 12 | 17 |
| HG | 192 | 44 | 21 | 66 | 21 | 40 | 30 | 16 | 15 |
| RI | 1 054 | 55 | 34 | 52 | 32 | 39 | 23 | 8 | 13 |
| RMA | 46 | 26 | 39 | 143 | 24 | 48 | 111 | 17 | 10 |
| CC | 10 642 | 19 | 58 | 128 | 10 | 20 | 75 | 5 | 9 |
| BN | 30 | 4 | 47 | 93 | 5 | 2 | 60 | 5 | 6 |
| RJ | 16 | 14 | 25 | 88 | 8 | 8 | 88 | <6 | <6 |
| AJ | 163 | 85 | ND* | 7 | ND | 2 | 2 | 3 | 4 |
| WD | 189 | 34 | 81 | 122 | 12 | 53 | 97 | 12 | 3 |
| JW | 386 | 54 | 64 | 14 | 23 | 8 | 9 | 9 | 3 |
| L | 39 | 108 | 95 | <3 | 67 | <3 | <3 | <3 | 3 |
Cross-reactivity is defined as the percentage Bethesda titer relative to human B-domainless fVIII. Data are expressed as the mean of at least three determinations as described in “Materials and Methods.” The average coefficient of variation of the determinations was 0.2.
ND, not determined.
Some inhibitor plasmas contain antibodies whose specificity is restricted to the A2 or C2 domains.16,24 These antibodies have been useful for mapping epitopes in the A2 and C2 domains using hybrid human/porcine fVIII molecules.9,11,20,25 In the current study, BN contained predominantly anti-A2 activity because substitution of the porcine 484-508 sequence in the HP9 hybrid was sufficient to decrease antibody cross-reactivity by >90%. We have reported similar results with HP9 and patient plasmas SC, JM, NS, and RC,9 which have been shown by neutralization studies to be A2-specific.24 Similarly, plasma from patient L was C2-specific because substitution of the porcine C2 sequence in HP20 was sufficient to decrease antibody cross-reactivity by >90%. This result was consistent with neutralization of patient L's plasma with recombinant C2.16 The agreement between results using HP20 and antibody neutralization experiments for 5 other C2-specific inhibitors, HR, LK, AA, YA, and RvR, has been reported.11
In contrast, plasmas with inhibitors specific for the ap-A3 region have been more difficult to identify. Table 1 shows that several plasmas had a substantial amount of activity against ap-A3. Substitution of the porcine sequence within the ap-A3 region in HP30 decreased antibody cross-reactivity to <40% and produced at least a 15% difference between HP32 and HP33 cross-reactivity in 7 of the 23 plasmas (GK1832, GK1833, HG, KB, RI, RMA, and TM). The bulk of the inhibitor activity of KB, GK1833, and TM appeared directed against the ap-A3 region. This was consistent with antibody neutralization experiments on KB and GK1833, which requiredap-A3-C1-C2 to achieve most of the neutralization.16
To determine whether antibodies to the ap-A3 region are directed against the ap peptide, the A3 domain, or both, we constructed 2 additional hybrids, HP35 and HP41 (Figure 1). HP35 was identical to HP32 except that it contained the human ap peptide instead of the corresponding porcine peptide. Similarly, HP41 differs from HP32 by containing the human A3 domain. The cross-reactivity of HP41 and HB− toward mAb CLB-CAg A, which binds the epitope recognized by human anti-A3 inhibitors,14 26 was not significantly reduced (data not shown). This demonstrated that the A3 inhibitory epitope in HP41 was intact.
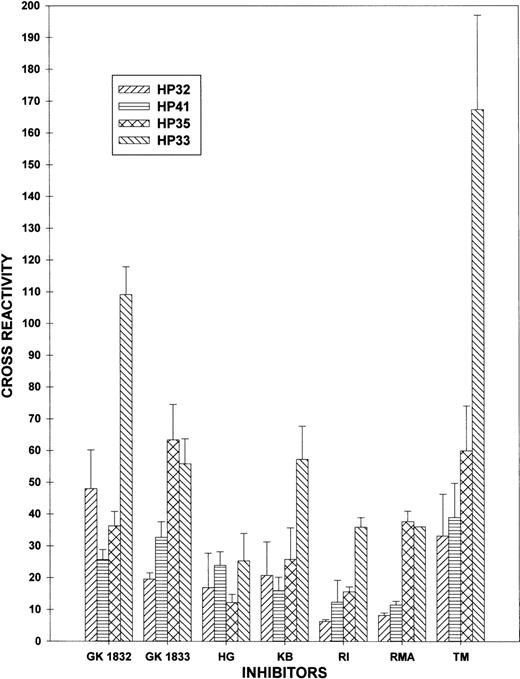
The cross-reactivity of GK1832, GK1833, HG, KB, RI, RMA, and TM against HP32, HP33, HP35, and HP41 was compared (Figure3). In 4 of the 7 plasmas (GK1833, RI, RMA, and TM), HP35 was significantly more cross-reactive than HP32. Conversely, HP41 was less cross-reactive than HP33 in all but 1 of the plasmas (HG). These results are consistent with the presence of an epitope in the human ap segment that is recognized by some inhibitor plasmas.
Identification of ap-directed inhibitory activity.
Plasma from 7 patients with inhibitor activity against the apdomain, the A3 domain, or both was defined by a decrease in HP30 cross-reactivity to <40% and at least a 15% difference in cross-reactivity between HP32 and HP33 using the data in Table 1. Data are expressed as mean cross-reactivity and SD, as described in “Materials and Methods.”
Identification of ap-directed inhibitory activity.
Plasma from 7 patients with inhibitor activity against the apdomain, the A3 domain, or both was defined by a decrease in HP30 cross-reactivity to <40% and at least a 15% difference in cross-reactivity between HP32 and HP33 using the data in Table 1. Data are expressed as mean cross-reactivity and SD, as described in “Materials and Methods.”
Discussion
In previous studies, we constructed novel hybrid human/porcine fVIII molecules to map inhibitor epitopes.9,11 20 These studies were restricted to the use of hybrids with substitutions of porcine sequence within single domains of fVIII. Thus, it has been necessary to use monospecific inhibitor plasma to avoid the confounding effects of activity against epitopes outside the region of porcine substitution.
In this study, we made combinations of substitutions of porcine sequence in the A2, ap, A3, and C2 domains of fVIII (Figure 1). The antigenicity of the quadruply-substituted (A2/ap/A3/C2) hybrid, HP32, was not significantly different from B-domainless porcine fVIII (Figure 2, Table 1). This indicates that clinically significant antibodies recognize epitopes restricted to A2 amino acids 484-508,ap amino acids 1649-1687, A3 amino acids 1694-2017, and C2 amino acids 2181-2321, and it specifically excludes significant effects of anti-A1 and anti-C1 inhibitors.
There is considerable evidence for the presence of an inhibitor epitope in the A3 domain. An anti-A3 inhibitor was identified in a patient with hemophilia A by deletion mapping, which placed the epitope within an A3 segment bounded by amino acids 1778-1823.14 The antibody also inhibited the binding of factor IXa to the factor VIII light chain, which is necessary for assembly of the intrinsic factor X activation complex.27The fVIII light chain binding site for factor IXa has been localized to amino acids 1811-1818 in the A3 domain.26 Consistent with those observations, 3 inhibitor IgGs have been identified that prevented the binding of factor IXa to the fVIII light chain.15 In that study, the binding of these inhibitors to the fVIII light chain was completed by a synthetic peptide corresponding to A3 amino acids 1804-1819 competed for the binding of those inhibitors to the fVIII light chain.
The results shown in Figure 2 and Table 1 are consistent with the presence of an inhibitor epitope in the A3 domain, but they do not exclude the possibility of an anti-ap inhibitor epitope. An anti-ap murine monoclonal antibody has been identified that inhibits fVIII in the Bethesda assay,28 but human anti-ap inhibitory antibodies have not been clearly demonstrated. To differentiate between inhibitor binding to theap region versus the A3 domain, we constructed 2 additional triply-substituted hybrids, HP35 and HP41, that differed from HP32 by containing the human ap or human A3 domain, respectively (Figure 1). In most plasmas with antibodies against the ap-A3 region, the cross-reactivity of HP35 was greater than HP32 and the cross-reactivity of HP41 was less than HP33 (Figure 3), indicating that these inhibitors recognize the ap peptide, as well as the A3 domain epitope identified in earlier studies.
During the activation of fVIII by thrombin, the ap peptide is released.29 This step is necessary for fVIII to dissociate from von Willebrand factor and to participate in blood coagulation.30 31 Thus, antibodies to the apsegment may block the activation of the fVIII-von Willebrand factor complex.
Porcine fVIII concentrate (Hyate:C) is useful in inhibitor patients who do not have significant cross-reactivity with porcine fVIII.32-34 After exposure to porcine fVIII, anti-porcine fVIII antibodies develop in some of these patients. The epitopes recognized by these antibodies have not been characterized in detail. Western blotting has identified antiporcine antibodies specific for the A1 domain,35 which is rarely targeted by antihuman fVIII antibodies. Whether anti-A1 antibodies are inhibitory is unknown. A hybrid human/porcine fVIII molecule could be superior to porcine fVIII therapeutically if it lacks immunogenic porcine epitopes. The cross-reactivity of HP32 was as low as or slightly lower than porcine B-domainless in the series of high-titer inhibitor plasmas in this study (Table 1). HP32 or a more humanized derivative thereof that lacks the potentially immunogenic porcine A1 domain is a potential alternative to porcine fVIII.
Supported by grants R01-HL46215 and RO1-HL55273 from the National Institutes of Health.
Reprints:Pete Lollar, Emory University, Room 1003 Woodruff Memorial Building, 1639 Pierce Dr, Atlanta, GA 30322; e-mail: jlollar@emory.edu.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal