Occlusive thrombosis depends on the net balance between platelets, coagulation, and fibrinolytic factors. Epidemiologic information suggests that plasminogen activator inhibitor-1 (PAI-1), a central regulator of the fibrinolytic system, plays an important role in determining the overall risk for clinically significant vascular thrombosis. Vitronectin (VN), an abundant plasma and matrix glycoprotein, binds PAI-1 and stabilizes its active conformation. This study assessed the role of PAI-1 and VN expression in the formation of occlusive vascular thrombosis following arterial or venous injury. The common carotid arteries of 17 wild-type (WT) mice and 8 mice deficient in PAI-1 were injured photochemically while blood flow was continuously monitored. WT mice developed occlusive thrombi at 52.0 ± 3.8 minutes (mean ± SEM) following injury; mice deficient in PAI-1 developed occlusive thrombosis at 127 ± 15 minutes (P < .0001). Mice deficient in VN (n = 12) developed vascular occlusion 77 ± 11 minutes after injury, intermediate between the values observed for WT mice (P < .03) and mice deficient in PAI-1 (P < .01). PAI-1 and VN also affected the time to occlusion after injury to the jugular vein. Three WT mice developed occlusive venous thrombosis an average of 39.7 ± 1 minutes following the onset of injury, whereas the jugular veins of 4 mice deficient in PAI-1 and 4 deficient in VN occluded 56.7 ± 5 and 58.7 ± 2 minutes, respectively, following injury (P < .04 andP < .01 compared to WT mice). These results suggest that endogenous fibrinolysis and its regulation by PAI-1 and VN have important roles in the development of occlusive vascular thrombosis after vascular injury.
Vascular thrombosis after injury is the critical event leading to acute vascular syndromes including myocardial infarction, unstable angina pectoris, and stroke.1 The fate of a forming thrombus is determined by the balance between coagulation factors and platelets favoring occlusive clot formation and the fibrinolytic system favoring clot dissolution. The relative contributions of the individual factors involved in occlusive thrombus formation following vascular injury are poorly understood.
Intravascular fibrinolysis is mediated primarily by the fibrinolytic protease, plasmin, which is derived from its inactive precursor plasminogen through the action of the plasminogen activators, tissue-type plasminogen activator (tPA) and urokinase plasminogen activator (uPA).2 Plasminogen activator inhibitor-1 (PAI-1) is a member of the SERPIN gene family and regulates the plasminogen activation system by forming irreversible inhibitory complexes with uPA and tPA.3 Vitronectin (VN), an abundant plasma and matrix glycoprotein, binds PAI-1 and may regulate its activity by stabilizing the active PAI-1 conformation4 as well as potentially regulating PAI-1 clearance.5 In addition, VN may play an important role in cellular migration.6 States of PAI-1 deficiency in humans are associated with abnormal bleeding, indicating an important role of PAI-1 in stabilization of the hemostatic clot.7-9 In contrast, inhibition of endogenous fibrinolysis by elevated plasma PAI-1 levels may constitute an important risk factor for myocardial infarction10 and deep venous thrombosis.11
Genetically engineered mice carrying targeted deletions of genes encoding fibrinolytic system components have provided a powerful model for the in vivo characterization of vascular thrombosis.12Mice deficient in PAI-1 exhibit enhanced fibrinolysis13-15and animals deficient in VN demonstrate reduced plasma PAI-1 levels following endotoxin administration, consistent with an important role of VN in ensuring the stability of PAI-1 in vivo.5
Endogenous fibrinolysis and its regulation by PAI-1 and VN may be important in the development of occlusive vascular thrombosis after vascular injury. To test this hypothesis we analyzed wild-type (WT) mice, mice deficient in PAI-1, and mice deficient in VN using a photochemically induced arterial and venous thrombosis model.
Materials and methods
Mice
Mice deficient in PAI-1 were a gift of P. Carmeliet and D. Collen.16 Control C57BL/6J mice were purchased from Jackson Labs, Bar Harbor, ME. Mice deficient in PAI-1 and VN were back-crossed to C57BL/6J mice for at least 8 generations. All mice were maintained on standard chow. Mice were genotyped as previously described.14 All animal care and experimental procedures complied with the Principles of Laboratory and Animal Care established by the National Society for Medical Research and were approved by the University of Michigan Committee on Use and Care of Animals.
Induction of carotid artery and jugular vein thrombosis
Control C57BL/6, PAI-1-deficient and VN-deficient male mice (6-8 weeks old) weighing an average of 23 g were anesthetized with 1.3 mg intraperitoneal sodium pentobarbital (Butler, Columbus, OH). Mice were then secured in the supine position and placed under a dissecting microscope (Nikon SMZ-2T, Mager Scientific, Inc., Dexter, MI). Following a midline cervical incision, the right common carotid artery was isolated and a Doppler flow probe (Model 0.5 VB, Transonic Systems, Ithaca, NY) was applied. The probe was connected to a flowmeter (Transonic Model T106) and interpreted with a computerized data acquisition program (Windaq, DATAQ Instruments, Akron, OH). Rose bengal (Fisher Scientific, Fair Lawn, NJ) was diluted to 10 mg/mL in phosphate-buffered saline and then injected into the tail vein in a volume of 0.12 mL at a concentration of 50 mg/kg (arterial protocol) or 25 mg/kg (venous protocol) using a 27-gauge Precision Glide needle with a 1-mL latex free syringe (Becton Dickinson and Co., Franklin Lakes, NJ). Just before injection, a 1.5-mW green light laser (540 nm) (Melles Griot, Carlsbad, CA) was applied to the desired site of injury from a distance of 6 cm for 60 minutes or until thrombosis occurred. Flow in the vessel was monitored for 150 minutes from the onset of injury, at which time the experiment was terminated. A similar protocol was used for studies of the right internal jugular vein.
Histology
To confirm occlusive thrombosis, carotid arterial segments subjected to injury were excised and embedded in paraffin. Sections were then stained with hematoxylin and eosin.
Statistical analysis
The significance of differences in time to occlusion between groups was determined using the Student 2-tailed ttest. A P value of < 0.05 was considered significant.
Results
A murine model for arterial and venous thrombosis
To establish a photochemically induced thrombosis model in mice, multiple experiments were performed to determine appropriate conditions for reproducible induction of vascular occlusion. The right common carotid artery and right internal jugular vein were selected because of their accessibility and ease of monitoring flow. Control experiments in WT mice revealed that rose bengal at a dose of 50 mg/kg with the laser light source 6 cm away from the carotid artery injury site produced reproducible occlusive thrombosis that was confirmed with hematoxylin and eosin staining of tissue sections. These conditions also produced jugular venous occlusion when applied to the right internal jugular vein in control mice with a mean time to occlusion of 26.5 ± 9 minutes (n = 8). However, to increase the sensitivity of the venous model for detecting changes in fibrinolysis, the dose of rose bengal was decreased to 25 mg/kg, which resulted in a prolongation of the time to occlusion to 39.7 ± 1 minutes in WT animals (n = 3). Visual inspection through a dissecting microscope consistently revealed an intraluminal plug in the carotid artery or jugular vein that corresponded with flow cessation.
Effect of PAI-1 or VN deficiency on development of occlusive arterial thrombosis
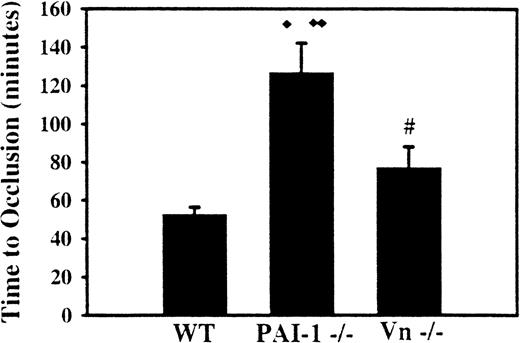
To determine if the development of carotid artery occlusion was affected by the level of PAI-1 expression, mice deficient in PAI-1 and WT mice were subjected to photochemical injury at the mid common carotid artery. Carotid flow was continuously monitored for 150 minutes. Mice that failed to form occlusive thrombosis during the observation period were assigned a value of 150 minutes for statistical analysis. Seventeen WT control mice formed occlusive thrombus at the site of light application an average of 52 ± 3.8 minutes (mean ± SEM) following rose bengal injection, whereas 8 mice deficient in PAI-1 developed occlusive thrombosis with a mean time of 127 ± 15 minutes (6 mice failed to occlude during the observation period) following injury (P < .0001).
Twelve mice completely deficient in VN were also tested in the carotid thrombosis model. The mean time to occlusion for VN null mice was 77 ± 11 minutes (2 mice failed to occlude during the observation period), which was longer than WT mice (P < .03) but shorter than mice deficient in PAI-1 (P < .01) (Figure1).
Effect of PAI-1 and VN deficiency on arterial thrombosis.
The carotid arteries of WT mice, mice deficient in PAI-1, and mice deficient in VN were continuously monitored for flow following photochemical injury to the mid common carotid artery. The average time to occlusive thrombosis was longer in mice deficient in PAI-1 and in mice deficient in VN compared with WT mice. The time to clot in PAI-1-deficient mice was also longer than that of VN-deficient mice. ♦, P < .03 compared with VN -/-mice; ♦♦,P < .0001 compared with WT mice; #, P < .01 compared with WT mice.
Effect of PAI-1 and VN deficiency on arterial thrombosis.
The carotid arteries of WT mice, mice deficient in PAI-1, and mice deficient in VN were continuously monitored for flow following photochemical injury to the mid common carotid artery. The average time to occlusive thrombosis was longer in mice deficient in PAI-1 and in mice deficient in VN compared with WT mice. The time to clot in PAI-1-deficient mice was also longer than that of VN-deficient mice. ♦, P < .03 compared with VN -/-mice; ♦♦,P < .0001 compared with WT mice; #, P < .01 compared with WT mice.
Effect of PAI-1 or VN deficiency on development of occlusive venous thrombosis
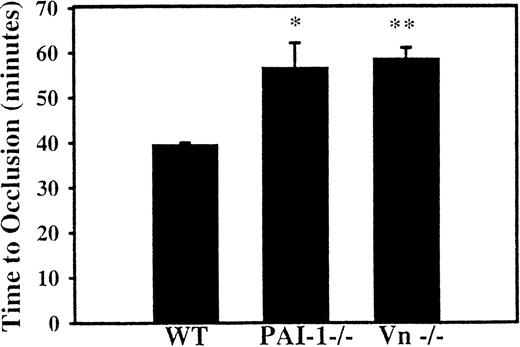
To assess the effect of PAI-1 deficiency on the formation of a venous thrombus, a jugular vein injury model was established. After injection of rose bengal in a dose of 25 mg/kg, WT mice formed occlusive thrombosis at an average of 39.7 ± 1 minutes (n = 3). Compared with WT mice, the time to occlusion was prolonged to 56.7 ± 4 minutes (n = 4, P < .04) in the mice deficient in PAI-1 and to 58.7 ± 2 minutes (n = 4, P < .01) in the VN-deficient mice (Figure 2). The difference in the time to clot between mice deficient in PAI-1 and VN is not statistically significant.
Effect of PAI-1 and VN deficiency on venous thrombosis.
The internal jugular veins of WT, PAI-1-deficient, and VN-deficient mice were continuously monitored for flow following photochemical injury to the internal jugular vein. The average time to occlusive thrombosis was longer in mice deficient in PAI-1 and in mice deficient in VN compared with WT mice. *P < .04 compared with WT; **P < .01 compared with WT mice.
Effect of PAI-1 and VN deficiency on venous thrombosis.
The internal jugular veins of WT, PAI-1-deficient, and VN-deficient mice were continuously monitored for flow following photochemical injury to the internal jugular vein. The average time to occlusive thrombosis was longer in mice deficient in PAI-1 and in mice deficient in VN compared with WT mice. *P < .04 compared with WT; **P < .01 compared with WT mice.
Discussion
Plasminogen activator inhibitor-1, the primary inhibitor of tPA and uPA, is a central regulator of the blood fibrinolytic system.3 Epidemiologic studies of myocardial infarction and deep venous thrombosis,10,11 as well as studies from humans deficient in PAI-1,7-9 suggest that plasma levels of PAI-1 contribute to the maintenance of occlusive thrombosis at sites of vascular injury, although these studies are controversial. Animal studies have demonstrated enhanced lysis of formed thrombi in the setting of PAI-1 deficiency.15,17 In a recent report, deficiency of PAI-1 did not affect the time to thrombosis in the murine carotid artery following ferric chloride injury, although subsequent lysis of the thrombus was enhanced.15 In a murine pulmonary embolism model, lysis of a preformed thrombus was also accelerated in mice with PAI-1 deficiency16 and administration of an inhibitory PAI-1 antibody in a canine coronary thrombosis model resulted in a reduced reperfusion time when administered with tPA.18 In vitro studies also support the role of PAI-1 as an important inhibitor for the lysis of platelet-rich clots, because accelerated lysis is observed when the platelets are derived from a patient deficient in PAI-119 or in the presence of a PAI-1 inhibitor.20
The present study used a model in which flow is continuously monitored during arterial injury, enabling the accurate assessment of time to occlusive thrombus formation. Intravenous rose bengal activated by a green laser light was used to elicit thrombosis because this method produces endothelial damage21 and does not require intra-arterial invasion or full thickness injury. In rats and guinea pigs, reproducible occlusive thrombus formation results.21-23 This method of injury has also been applied in the mouse femoral artery to elicit formation of neointima24 and in the mouse carotid artery to cause thrombosis.25 The subtle endothelial injury induced by rose bengal as compared to other vascular injury models15,26 may provide a sensitive measure of altered fibrinolysis. Our observation of a markedly prolonged time to occlusion in PAI-1 null mice suggests that simultaneous fibrin formation and fibrinolysis are occurring during clot formation, with the net balance determining time to occlusion. During the preparation of this manuscript Matsuno et al.27independently demonstrated an effect of PAI-1 on time to occlusion using a similar model of photochemical injury. However, these workers observed a much shorter baseline time to occlusion, likely due to the greater intensity of the light source.
The high concentration of VN in plasma and its wide distribution in subcellular matrices as well as its interactions with PAI-14 and other cell adhesion molecules suggest a complex role in the formation and stabilization of an occlusive thrombus. Mice deficient in VN exhibit decreased levels of plasma PAI-1 that should presumably lead to enhanced fibrinolysis. However, in a ferric chloride-induced carotid thrombosis model, mice lacking VN exhibited enhanced thrombus formation compared to WT mice, suggesting that VN may exert a previously unappreciated anticoagulant function.28In contrast, the photochemical injury reported here results in a prolonged time to occlusion in the absence of VN. Taken together, these results suggest that VN may have competing positive and negative effects on thrombus formation with the former predominant in the more rapid ferric chloride model (17 minutes)28 and the latter the primary determinant in the more subtle rose bengal injury (52 minutes). Of note, ferric chloride applied to the vessel adventitia causes a full thickness (outside-in) injury, whereas the photochemical injury damages the vessel wall from the inside out. Thus, the depth of vessel wall injury is likely greater with ferric chloride and the contribution of VN to thrombosis may vary significantly depending on the severity of vascular injury. In addition, other differences between the 2 protocols such as depth of anesthesia, the mechanics and timing of carotid artery isolation, and application of the flow probe may exert varying effects on thrombosis in these models.
Similar to our observations in the carotid artery, rose bengal-induced thrombosis in the jugular vein was also delayed in both PAI-1 and VN null mice. The greater sensitivity of the jugular vein to a lower dose of rose bengal may be secondary to the thinner vessel wall, perhaps allowing a more intense light exposure to the endothelium. Other factors such as hemodynamic differences between the arterial and venous circulations might also influence the clotting times. The similar prolongations of venous clotting times in the PAI-1 and VN null animals, in contrast to the more modest effect of VN deficiency in the arterial model, could be the result of varying contributions from PAI-1 in endothelial, plasma, platelet, or vascular smooth muscle cell compartments. For example, the time to clot in the thin-walled vein may be more dependent on circulating plasma PAI-1 and its stabilization by VN, with the absence of VN reproducing the state of PAI-1 deficiency. In the carotid artery, local release of PAI-1 from medial smooth muscle cells or other components of the vessel wall, or a greater contribution of platelet PAI-1, may result in less dependence on stabilization by VN.
Our results indicate that endogenous fibrinolysis and its regulation by PAI-1 and VN are important in the development of occlusive vascular arterial and venous thrombosis after vascular injury in mice. These results also suggest that alterations of endogenous fibrinolytic balance through modulation of PAI-1 and VN might be a useful therapeutic target for the prevention of acute vascular thrombosis in humans.
Acknowledgments
We gratefully acknowledge D. Siemieniak for technical assistance with the photochemical injury model, J. Tyson for assistance with histology, and the University of Michigan Center for Statistical Consultation and Research for statistical assistance.
Supported by National Institutes of Health grants HL03695-02 and HL57345.
D. Ginsburg is a Howard Hughes Medical Institute Investigator.
Reprints:Daniel T. Eitzman, University of Michigan Medical Center, MSRB III Room 7301, 1150 Medical Center Dr., Ann Arbor, MI 49109-0644; e-mail: deitzman@umich.edu.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal