We have used a murine retrovirus vector containing an enhanced green fluorescent protein complimentary DNA (EGFP cDNA) to dynamically follow vector-expressing cells in the peripheral blood (PB) of transplanted rhesus macaques. Cytokine mobilized CD34+ cells were transduced with an amphotropic vector that expressed EGFP and a dihydrofolate reductase cDNA under control of the murine stem cell virus promoter. The transduction protocol used the CH-296 recombinant human fibronectin fragment and relatively high concentrations of the flt-3 ligand and stem cell factor. Following transplantation of the transduced cells, up to 55% EGFP-expressing granulocytes were obtained in the peripheral circulation during the early posttransplant period. This level of myeloid marking, however, decreased to 0.1% or lower within 2 weeks. In contrast, EGFP expression in PB lymphocytes rose from 2%-5% shortly following transplantation to 10% or greater by week 5. After 10 weeks, the level of expression in PB lymphocytes continued to remain at 3%-5% as measured by both flow cytometry and Southern blot analysis, and EGFP expression was observed in CD4+, CD8+, CD20+, and CD16/56+ lymphocyte subsets. EGFP expression was only transiently detected in red blood cells and platelets soon after transplantation. Such sustained levels of lymphocyte marking may be therapeutic in a number of human gene therapy applications that require targeting of the lymphoid compartment. The transient appearance of EGFP+ myeloid cells suggests that transduction of a lineage-restricted myeloid progenitor capable of short-term engraftment was obtained with this protocol.
The ability to transfer therapeutic genes to autologous hematopoietic stem cells could afford a variety of opportunities for therapeutic intervention of human diseases.1 Viral vectors based on murine retroviruses have been used for this purpose because of their ability to efficiently integrate exogenous genes into the genome of transduced cells. When transplanted into mice,2 these vectors permit gene transfer in >50% of circulating blood cells. Similar levels of gene transfer have not been achieved in either nonhuman primate models or human gene therapy trials.3-6 To increase transduction efficiency, investigators have used cytokine-primed stem cell targets,7 culture of cells with vector in the presence of recombinant fibronectin (FN) fragments,8 incorporation of flt-3 ligand and stroma support during ex vivo culture,9 and pseudotyped retroviral vectors.10 With these strategies, the proportion of transduced circulating leukocytes in nonhuman primates has increased to 5%-10% shortly after transplant but then is followed by a subsequent decline.8 9 Although these results are encouraging, it is clear that further progress will be required for successful gene therapy of many candidate hematopoietic disorders.
In murine models, first-generation retroviral vectors are prone to transcriptional silencing,11,12 while newer vectors, such as murine stem cell virus–based (MSCV-based) vectors,13direct more stable levels of exogenous gene expression in vivo.14 An important advance for evaluation of gene transfer vectors has been the use of the enhanced green fluorescent protein (EGFP) as a reporter molecule. This allows precise measurements of the number of EGFP-expressing, transduced hematopoietic cells both in vitro and in vivo.2,15-17 Using this strategy, the bicistronic MSCV-based retroviral vector, MGirL22Y, has been shown to effectively transduce murine bone marrow (BM) cells that, based on EGFP expression, contribute to the generation of all circulating myeloid and lymphoid cells.2 18 In this study, we used an EGFP-expressing, MSCV-based amphotropic retroviral vector to transduce rhesus monkey CD34+ cells, which were collected from cytokine-mobilized peripheral blood (PB). Here we document the usefulness of this vector system for analyzing stem cell gene transfer in nonhuman primates using high concentrations of early-acting cytokines during viral transduction.
Materials and methods
Animals
Young adult rhesus macaques (Macaca mulatta) were used in these studies. They were serologically negative for simian T-cell lymphotropic virus, simian immunodeficiency virus, simian AIDS-related type D virus, and herpes B virus. Animals of the type B blood group were selected, and indwelling cardiac catheters were established. Experimental animals were quarantined and housed in accordance with the guidelines set by the Committee on Care and Use of Laboratory Animals (DHHS Public #NIH85-23, Revised 1985) and the policies set by the Veterinary Research Program of the National Institutes of Health. The protocols were evaluated and approved by the Animal Care and Use Committee of the National Heart, Lung, and Blood Institute.
Murine retroviral vector producer cells
MGirL22Y-Pre is a bicistronic MSCV-based vector that expresses an EGFP (Clontech Laboratories, Palo Alto, CA) under control of the MSCV long terminal repeat (LTR) and the human L22Y dihydrofolate reductase (DHFR) variant from an internal ribosome entry site. The pre-sequence is a posttranscriptional element derived from the hepatitis B virus that enhances transport of nuclear RNA into the cytoplasm of a cell. The vector producer cell line was grown in supplemented medium (Dulbecco's modified Eagle's medium [DMEM]; Biofluids, Rockville, MD) containing 15% fetal calf serum (FCS) (HyClone, Logan, UT) and cultured at 37°C in a 5% carbon dioxide + 95% air-humidified incubator.
Rhesus leukapheresis procedure
Rhesus macaques received a combination of both 10 μg/kg/d granulocyte colony-stimulating factor (G-CSF) and 200 μg/kg/d stem cell factor (SCF) (provided by Amgen, Thousand Oaks, CA) as a subcutaneous injection for 5 days. All cytokines used for this study were pyrogen-free. Following the 5-day cytokine mobilization, PB mononuclear (PBMN) cells were collected as previously described.19 Prior to cytokine mobilization, approximately 100 mL of autologous PB was collected in citrate and stored at 4°C. This PB was used to prime the cell separator (CS3000 Plus Blood Cell Separator; Baxter Healthcare, Fenwal Division, Deerfield, IL) at the time of leukapheresis. Under general anesthesia, the leukapheresis product of PBMN cells was collected using a single small-volume chamber leukapheresis procedure that was specially adapted to permit leukapheresis procedures to be performed on rhesus macaques weighing <5 kg.19
Immunoselection of nonhuman primate CD34+ cells
CD34+ cells were immunoselected from the PB leukapheresis product (Streptavidin MicroBeads, MACS Separation columns; Miltenyi Biotech, Auburn, CA) according to the manufacturer's instructions. A biotinylated CD34+ antibody (CellPro, Bothell, WA) capable of recognizing rhesus macaque CD34+cells was used in the immunoselection procedure. Following immunoselection, the PB leukapheresis CD34+ cells were analyzed for purity by staining with an anti–CD34-allophycocyanin (APC) conjugated antibody.19 The anti-CD34 monoclonal antibody (mAb) clone 563 (Dr G Gaudernack, Institution of Transplantation Immunology, Oslo, Norway) recognizes a CD34 epitope in a different way from the anti-CD34 mAb clone 12.8 used in the immunoselection procedure. The CD34 clone 563 was directly conjugated to APC (CellPro; Molecular Probes, Eugene, OR). The purity of the immunoselected CD34+ cells was routinely >90%.
Transduction of immunoselected CD34+ cells
Following immunoselection, the selected CD34+ cells were plated at 0.5-1 × 106cells/mL and cultured in DMEM + 15% FCS in the presence of one of two supplements: (1) high dose: IL-6, 50 ng/mL; SCF, 300 ng/mL; and flt-3, 300 ng/mL, or (2) low dose: interleukin-6 (IL-6), 50 ng/mL (Amgen); SCF, 100 ng/mL (Amgen); and flt-3, 100 ng/mL (R&D Systems, Minneapolis, MN). In all cases, the immunoselected CD34+ cells were maintained on nontissue culture-treated 6-well plates (Becton Dickinson Labware, Franklin Lakes, NJ) coated with 25-50 μg/mL of the recombinant human FN fragment CH-296 (RetroNectin7; BioWhittaker, Walkersville, MD) according to the manufacturer's instructions. Cells were prestimulated overnight with hematopoietic growth factors and then transduced twice a day with fresh retroviral supernatant, fresh cytokines, and 8 μg/mL of protamine sulfate (Fujisawa USA, Deerfield, IL) for a total of 3.5 days. On the last day of transduction, the cells were counted, an aliquot of cells was taken for CD34 and EGFP analysis, and the remaining cells were reinfused into the irradiated animal.
Autologous PB transplantation procedure
The transplantation protocol used in these studies has previously been described.4 5 Day 0 is designated as the day the animal was reinfused with the cultured cells. On days -2 and -1, the animal received 500 rads (dose rate: 8.8 rads/min) total body γ-irradiation (TBI). Standard supportive care for the BM transplant recipient was initiated the day following irradiation.
Flow cytometry
Lymphocytes expressing EGFP were phenotypically defined as CD4+ and CD8+ T cells, CD20+ B cells, and CD16/56+ natural killer (NK) cells using mAbs directly conjugated with phycoerythrin (PE) (Becton Dickinson, Mountain View, CA). CD34+ cells were identified as such with an anti–CD34-APC conjugated antibody (clone 563) and were directly conjugated to APC (Molecular Probes, Eugene, OR). PB samples were stained with the appropriate antibodies, and red blood cells were lysed (Coulter Q-Prep; Coulter Electronics, Hialeah, FL). EGFP expression was measured (Coulter Elite flow cytometer, Coulter Electronics) using a standard filter setup for fluorescein (525 nm bandpass filter). Routine analysis was run with 50 000 events for lymphocytes, monocytes, and granulocytes and 200 000 events for platelets and red blood cells.
Southern blot analysis
PB leukocytes were separated into granulocyte and mononuclear fractions (Ficoll-Paque7, Pharmacia Biotech, Uppsala, Sweden), and DNA was isolated by standard techniques. DNA (15 μg) was digested with EcoRV restriction enzyme and separated on a 1% agarose gel. Membranes were probed with a radiolabeled DNA fragment from the EGFP cDNA and then washed to high stringency. Autoradiograms were performed (PhosphoImager; Molecular Dynamics, Sunnyvale, CA).
Analysis for DHFR expression
Transduced CD34+ PB cells were stained with propidium iodide, and viable cells were gated for EGFP analysis. EGFP+ cells were sorted (Turbo FACStar Plus cell sorter; Becton Dickinson, San Jose, CA). Sorted cells were then reanalyzed for EGFP expression, resuspended at 1000 cells/mL in methylcellulose medium that supports myeloid colony formation (MethoCult GF+ H4535; StemCell Technologies, Vancouver, British Columbia, Canada), and plated into 35-mm dishes. The methylcellulose medium was first treated with 3 units/mL of thymidine phosphorylase (Sigma, St. Louis, MO) for 4 hours at 37°C and then supplemented with 3 units/mL of recombinant human erythropoietin (Amgen). To some cultures, 200 nmol/L of trimetrexate was also added (US Bioscience, West Conshohocken, PA).
Lymphocyte cultures from leukapheresis samples were set up by isolating mononuclear cells from density gradients (Ficoll-Paque7, Pharmacia). EGFP-expressing mononuclear cells were isolated by cell sorting and plated in 96-well plates at a density of 50 000 cells per 100 μL/well in triplicate. The gate for EGFP positivity was set based on a negative control sample from an untransplanted monkey. The cells were grown in DMEM + 10% FCS that had been treated with thymidine phosphorylase as described previously. Also added were human IL-2, 25 units, and phytohemagglutinin (PHA) (Sigma), 5 μg/mL of medium.
Results
Producer cell and vector
A bicistronic vector was constructed based on the MSCV vector that contained an upstream EGFP cDNA followed by an IRES-driven human DHFR variant (L22Y) which confers resistance to antifolate drugs.20 Downstream of the L22Y-DHFR cDNA, an RNA processing element from the hepatitis B element21 was inserted to potentially increase the titer and expression of the viral vector. Using a transient ecotropic supernatant generated from 293T cells, PA317 cells were transduced and selected in trimetrexate. The resistant population was sorted for cells expressing high levels of the amphotropic envelope protein and for EGFP. The polyclonal producer cells used for the transplant experiments had a titer of 5 × 106/mLtrimetrexate-resistant colonies on 3T3 cells. Southern blot analysis of targeted 3T3 cells showed no evidence of rearrangements of the proviral DNA (data not shown).
Transduction and transplantation of immunoselected CD34+ cells
G-CSF– and SCF-mobilized, immunoselected PB CD34+ cells were isolated from 5 animals. Cells from 3 of the animals were cultured in DMEM + 15% FCS with high-dose supplements: SCF, 300 ng/mL; flt-3, 300 ng/mL; and IL-6, 50 ng/mL. Cells from the other 2 animals were cultured in DMEM + 15% FCS with low-dose supplements: SCF, 100 ng/mL; flt-3, 100 ng/mL; and IL-6, 50 ng/mL. In all instances the immunoselected cells were cultured in nontissue culture-treated plates coated with recombinant human FN fragment CH-296 (RetroNectin7, Bio Whittaker). Cells were initially cultured overnight without vector and subsequently transduced twice a day with fresh virus supernatant for 3.5 days. The cells were then harvested, counted, and reinfused into the animals after they had received a total of 1000 rads (administered as a split dose of 500 rads over 2 consecutive days) TBI. Prior to transplant, the percentage of CD34+ cells expressing EGFP that were reinfused into the 3 high-dose animals averaged 34% (range, 28.9%-43.6%), and the cells from the 2 low-dose animals ranged from 14.8% to 18.0% (Table 1). The 3 high-dose animals received an average cell dose of 1.6 × 107cells/kg (range, 0.7-2.3 cells × 107/kg), and the 2 low-dose animals were reinfused with 0.3 and 4.0 × 107 cells/kg (Table1). All animals recovered uneventfully; the leukocyte count reached 1000 leukocytes/μl or better by day 14 after transplant, and the platelet count was up to 50 000 platelets/μl by day 27 (Table 1). Those animals receiving 0.7 × 107 cells/kg or greater had rapid platelet reconstitution, either achieving a platelet count of 50 000 platelets/μl by day 7 or never experiencing a platelet count that fell below 50 000 platelets/μl.
Outcome of G-CSF–mobilized and SCF-mobilized PB transplantation using immunoselected CD34+cells
| Animal No.(cytokine dose) . | No. Cultured CD34+Cells Reinfused (×107) . | No. Cultured CD34+ Cells/kg (×107) . | Percentage of Reinfused Cells Transduced . | Day That WBC Count Reached 1000 . | Day That Platelet Count Reached 50 000 . |
|---|---|---|---|---|---|
| 94E027 (low dose) | 16.0 | 4.0 | 18.0 | 7 | N/A |
| RC409 (low dose) | 1.3 | 0.3 | 14.8 | 14 | 27 |
| 95E041 (high dose) | 8.8 | 2.3 | 29.5 | 9 | N/A |
| RC601 (high dose) | 2.5 | 0.7 | 28.9 | 8 | N/A |
| RC502 (high dose) | 9.1 | 1.9 | 43.6 | 7 | 7 |
| Animal No.(cytokine dose) . | No. Cultured CD34+Cells Reinfused (×107) . | No. Cultured CD34+ Cells/kg (×107) . | Percentage of Reinfused Cells Transduced . | Day That WBC Count Reached 1000 . | Day That Platelet Count Reached 50 000 . |
|---|---|---|---|---|---|
| 94E027 (low dose) | 16.0 | 4.0 | 18.0 | 7 | N/A |
| RC409 (low dose) | 1.3 | 0.3 | 14.8 | 14 | 27 |
| 95E041 (high dose) | 8.8 | 2.3 | 29.5 | 9 | N/A |
| RC601 (high dose) | 2.5 | 0.7 | 28.9 | 8 | N/A |
| RC502 (high dose) | 9.1 | 1.9 | 43.6 | 7 | 7 |
WBC, white blood cell.
EGFP expression posttransplant in myeloid cells
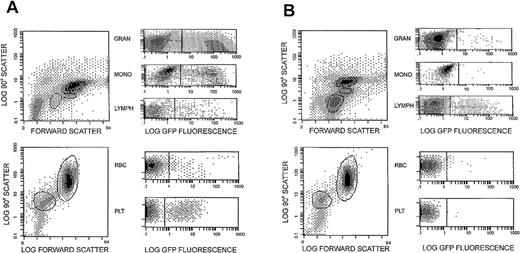
Similar patterns of EGFP expression in PB granulocytes were observed in all 3 high-dose animals. Initially 30%-55% of circulating granulocytes, as defined by forward and side-light scatter characteristics, expressed EGFP within the first 2 weeks following transplant (Figure 1A). These levels subsequently fell to ≤1% by week 10 (Figure 1B), and ongoing studies show that they continue to persist at this low level. In all 3 high-dose animals, EGFP expression was transiently noted in platelets and red blood cells at a lower level (Figure 1A). The kinetics of EGFP-expressing myeloid cells was similar for all 3 high-dose animals and was monitored between 21 and 35 weeks after transplant (Figure 2). In all 3 cases, a rapid decay in the proportion of EGFP-expressing myeloid cells was noted. BM aspirate-derived CD34+ cells also were analyzed for EGFP expression at various time points. For animal 95E041, 3.4% of CD34+ cells were found to be EGFP+ at 6 weeks, and 1.4% were positive 7 weeks after transplant. For animal RC502, 0.1% of the BM-derived CD34+ cells were EGFP+ 4 weeks after transplant.
EGFP expression in circulating leukocyte populations.
Flow cytometric analysis was performed on the circulating leukocytes of a transplanted animal (95E041) at 2 weeks (A) and 10 weeks (B) following transduction and reinfusion of autologous immunoselected CD34+ cells. The immunoselected CD34+ cells were transduced using treated nontissue culture plates and maintained in MGirL22Y-Pre vector containing media supplemented with 300 ng/mL SCF, 300 ng/mL flt-3, and 50 ng/mL IL-6. The circulating leukocyte populations were gated, as shown, into lymphocyte, monocyte, and granulocyte populations based on their forward and 90°C light scatter properties and evaluated for EGFP expression.
EGFP expression in circulating leukocyte populations.
Flow cytometric analysis was performed on the circulating leukocytes of a transplanted animal (95E041) at 2 weeks (A) and 10 weeks (B) following transduction and reinfusion of autologous immunoselected CD34+ cells. The immunoselected CD34+ cells were transduced using treated nontissue culture plates and maintained in MGirL22Y-Pre vector containing media supplemented with 300 ng/mL SCF, 300 ng/mL flt-3, and 50 ng/mL IL-6. The circulating leukocyte populations were gated, as shown, into lymphocyte, monocyte, and granulocyte populations based on their forward and 90°C light scatter properties and evaluated for EGFP expression.
Time course evaluation of EGFP expression in peripheral blood cells.
In the upper panels, the percentage of circulating lymphocytes (•), monocytes (▾), and granulocytes (○) expressing EGFP was evaluated over a course of 35 weeks in animals RC601, 95E041, and RC502 receiving cells transduced with MGirL22Y-Pre vector containing, on treated 6-well plates, media supplemented with SCF, 300 ng/mL; flt-3, 300 ng/mL; and IL-6, 50 ng/mL. The lower panels show comparative analysis of EGFP expression in platelets (□) and red blood cells (▪) over time.
Time course evaluation of EGFP expression in peripheral blood cells.
In the upper panels, the percentage of circulating lymphocytes (•), monocytes (▾), and granulocytes (○) expressing EGFP was evaluated over a course of 35 weeks in animals RC601, 95E041, and RC502 receiving cells transduced with MGirL22Y-Pre vector containing, on treated 6-well plates, media supplemented with SCF, 300 ng/mL; flt-3, 300 ng/mL; and IL-6, 50 ng/mL. The lower panels show comparative analysis of EGFP expression in platelets (□) and red blood cells (▪) over time.
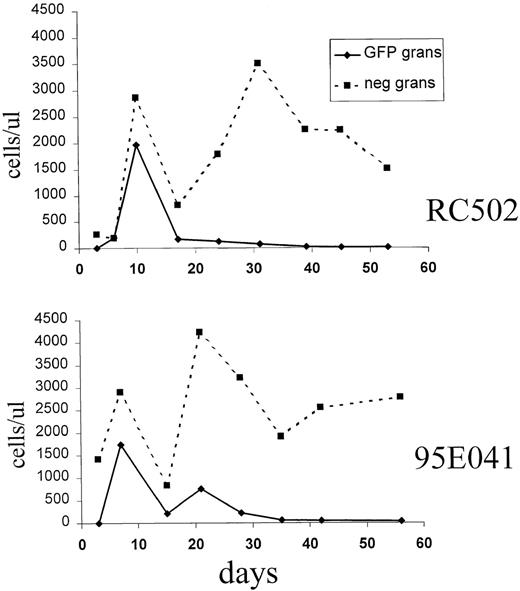
The decrease in the proportion of EGFP-expressing granulocytes was not simply due to a relative delay in the appearance of untransduced granulocytes, but instead it was due to a decrease in the absolute number of EGFP-expressing granulocytes over time (Figure3). To determine if this loss of transduced myeloid cells was due to recovery of endogenous hematopoiesis, a fourth monkey was transplanted after receiving 1300 rads for myeloablation, which can be lethal without stem cell rescue. A transient marking pattern was again observed in granulocytes, red blood cells, and platelets, indicating that the loss of vector-expressing myeloid cells was not due to endogenous stem cell recovery (data not shown).
Absolute number of EGFP+ and EGFP− granulocytes in the early posttransplant period.
The absolute number of EGFP+ granulocytes (solid line) and EGFP− granulocytes (dashed line) is shown for animals 95E041 and RC502. The time after transplant is shown in days on the x-axis.
Absolute number of EGFP+ and EGFP− granulocytes in the early posttransplant period.
The absolute number of EGFP+ granulocytes (solid line) and EGFP− granulocytes (dashed line) is shown for animals 95E041 and RC502. The time after transplant is shown in days on the x-axis.
EGFP expression posttransplant in lymphoid cells
EGFP expression in PB lymphocytes rose to the 10% level by week 10 (Figure 2) in 2 of the 3 animals, and the expression persisted at detectable levels in all 3 animals for up to 35 weeks. Between 10 and 24 weeks after transplant, the absolute number of EGFP lymphocytes in the peripheral circulation peaked at 569, 340, and 217 cells/μl for animals RC601, 95E041, and RC502, respectively. At the latest time points examined, the number of EGFP+ lymphocytes for each animal were: RC601, 24 cells/μl at 35 weeks; 95E041, 167 cells at 42 weeks; and RC502, 141 cells/μl at 21 weeks. Phenotypic analysis of the lymphoid population demonstrated EGFP expression in T cells (CD4+ and CD8+), B cells (CD20+), and NK cells (CD16/56+) at different time points posttransplant (Figure 4).
Subset analysis at 2, 5, 10, and 20 weeks posttransplant (CD2, CD4, CD8, CD16/56, and CD20) in high-dose animals.
T lymphocytes expressing CD2, CD4, CD8, and/or CD16/56, and B-lymphocytes expressing CD20 were evaluated by flow cytometry for coexpression of EGFP. Data are the mean percentage ± SEM (standard error of the mean) of these T and B lymphocytes expressing EGFP.
Subset analysis at 2, 5, 10, and 20 weeks posttransplant (CD2, CD4, CD8, CD16/56, and CD20) in high-dose animals.
T lymphocytes expressing CD2, CD4, CD8, and/or CD16/56, and B-lymphocytes expressing CD20 were evaluated by flow cytometry for coexpression of EGFP. Data are the mean percentage ± SEM (standard error of the mean) of these T and B lymphocytes expressing EGFP.
Marking in animals transduced using lower cytokine concentrations
When the exact same transduction protocol was used in 2 other animals, but with reduced cytokine concentrations of 100 ng/mL SCF and 100 ng/mL flt-3 in the transduction media, much lower levels of circulating EGFP-expressing cells were observed in the peripheral circulation. In these cases, EGFP expression in granulocytes never exceeded 10% early posttransplant; the level of marked lymphocytes never exceeded the 2%-5% level, and it rapidly declined to <1% within 3 weeks of transplant. EGFP expression in platelets and red blood cells was not observed in the low-dose animals (data not shown).
Southern blot analysis
To determine if the decay in EGFP+ myeloid cells was due to vector silencing versus a loss in the number of transduced cells, Southern blot analysis was performed on DNA from PB populations using a vector-specific probe. Using flow cytometry, it was determined that the average copy number was tightly correlated with the proportion of EGFP+ cells. When animal 95E041 was analyzed at 6 weeks after transplant, proviral DNA was barely detectable in PB granulocytes (about 2% average copy number), correlating with 2% EGFP+ cells by flow cytometry (Figure 5A). This was consistent with the absence of transduced myeloid progenitors in the BM of 95E041. PCR analysis for the proviral genome showed no transduced colonies in 44 BM-derived colony-forming unit cells (CFU-C) obtained 12 weeks after transplant (data not shown).
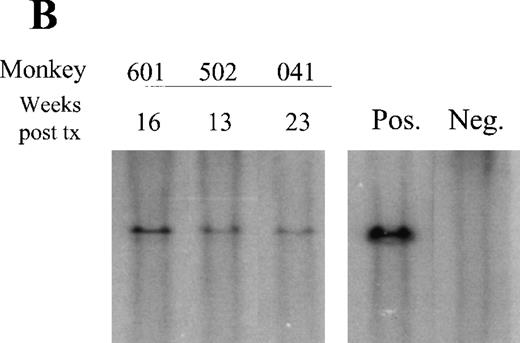
Southern blot analysis.
(A) Circulating leukocytes from animal 94E041 were collected from whole blood at 6 weeks and fractionated into mononuclear (MN) and granulocyte (Gran) fractions using a density centrifugation as previously described. Similarly bone marrow MN cells were also isolated at the same time point. Quantitative Southern blot analysis was performed on the isolated DNA, and the calculated copy number is shown and compared to the proportion of transduced cells as determined by flow cytometric analysis. The flow data are shown for EGFP-expressing lymphocytes (L), monocytes (M), and granulocytes (Gran). (B) Southern blot analysis of DNA obtained from PBMN cells in the 3 high-dose animals. The sample was obtained at the indicated number of weeks after transplant. Also shown is the single copy fibroblast control (Pos.) and DNA from an untransplanted animal (Neg.).
Southern blot analysis.
(A) Circulating leukocytes from animal 94E041 were collected from whole blood at 6 weeks and fractionated into mononuclear (MN) and granulocyte (Gran) fractions using a density centrifugation as previously described. Similarly bone marrow MN cells were also isolated at the same time point. Quantitative Southern blot analysis was performed on the isolated DNA, and the calculated copy number is shown and compared to the proportion of transduced cells as determined by flow cytometric analysis. The flow data are shown for EGFP-expressing lymphocytes (L), monocytes (M), and granulocytes (Gran). (B) Southern blot analysis of DNA obtained from PBMN cells in the 3 high-dose animals. The sample was obtained at the indicated number of weeks after transplant. Also shown is the single copy fibroblast control (Pos.) and DNA from an untransplanted animal (Neg.).
In contrast to the low vector copy number observed in granulocytes, the average copy number in PBMN cells was almost 20% (Figure 5A), correlating with the relatively high level of EGFP-expressing PB lymphocytes noted at this time point. A detectable vector signal by Southern blot has been maintained in the PBMN cells for all 3 high-dose animals at the latest time points examined: 95E041, 23 weeks; 1RC601, 16 weeks; and RC502, 13 weeks (Figure 5B). These data also demonstrate the stability of the vector since only full-length provirus was detectable on these analyses.
Expression of the DHFR gene in transduced hematopoietic cells
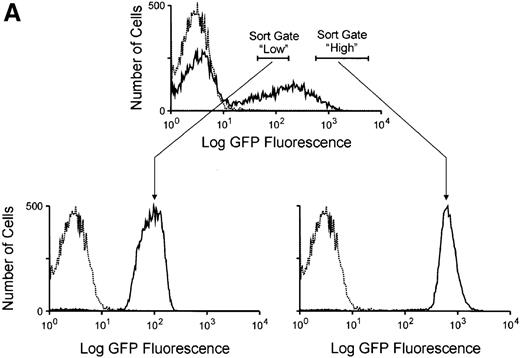
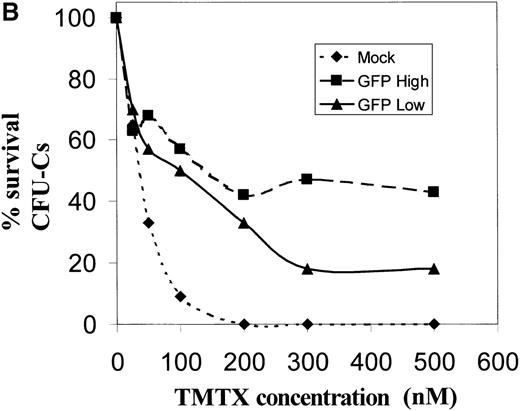
It was noted that the mean EGFP fluorescence in circulating transduced lymphocytes was almost 1 order of magnitude less than that seen in transduced myeloid cells (Figure 1). This raises the question of whether the level of DHFR expression would be functionally significant in cells with these low levels of EGFP expression. To more closely examine the relationship between the magnitude of EGFP expression and the degree of drug resistance conferred from expression of the DHFR variant, CD34+ cells were transduced and sorted into populations expressing either high or low amounts of EGFP (Figure6A). The mean fluorescence in these sorted groups approximated that observed in vivo in transduced lymphocyte and myeloid populations, respectively. These GFP-high and GFP-low populations were then plated in semisolid media containing increasing concentrations of trimetrexate, and myeloid colony formation was scored after 10 days of growth. Significant levels of drug resistance were noted in both populations when compared to mock-transduced cells (Figure 6B). At a trimetrexate concentration of 200 nmol/L, colony growth was completely inhibited in control cultures, while the colony numbers obtained with EGFP high–expressing and EGFP low–expressing cells were 42% and 33% of that seen on drug-free plates, respectively. These data indicate that significant degrees of drug resistance can be obtained in cells expressing relatively low levels of EGFP, presumably due to the potency of the variant DHFR resistance gene.
Resistance to trimetrexate in transduced CD34+ cells expressing high or low amounts of EGFP.
(A) Sorting based on EGFP expression. Peripheral blood CD34+ cells were transduced with the MGirL22Y-Pre vector and sorted based on mean EGFP. Based on EGFP expression levels, 2 populations were isolated and defined as EGFP-high and EGFP-low based. The top panel shows the sorting gates used on the bulk transduced population, and the lower panels show the reanalysis for EGFP expression in the 2 sorted populations. Note the relationship between the mean expression in these 2 sorted populations and the mean EGFP expression seen in granulocytes and lymphocytes (Figure 1). (B) Trimetrexate resistance in CFU-C derived from sorted cells. Myeloid colony survival is shown in cultures containing increasing concentrations of trimetrexate. Survival curves were generated for mock-transduced cells (♦, short-dashed line), sorted EGFP high cells (▪, long-dashed line), and EGFP low cells (▴, solid line). Colony survival was calculated as the % CFU-C on drug-containing plates relative to drug-free plates. The total number of myeloid colonies was scored after 10 days of growth.
Resistance to trimetrexate in transduced CD34+ cells expressing high or low amounts of EGFP.
(A) Sorting based on EGFP expression. Peripheral blood CD34+ cells were transduced with the MGirL22Y-Pre vector and sorted based on mean EGFP. Based on EGFP expression levels, 2 populations were isolated and defined as EGFP-high and EGFP-low based. The top panel shows the sorting gates used on the bulk transduced population, and the lower panels show the reanalysis for EGFP expression in the 2 sorted populations. Note the relationship between the mean expression in these 2 sorted populations and the mean EGFP expression seen in granulocytes and lymphocytes (Figure 1). (B) Trimetrexate resistance in CFU-C derived from sorted cells. Myeloid colony survival is shown in cultures containing increasing concentrations of trimetrexate. Survival curves were generated for mock-transduced cells (♦, short-dashed line), sorted EGFP high cells (▪, long-dashed line), and EGFP low cells (▴, solid line). Colony survival was calculated as the % CFU-C on drug-containing plates relative to drug-free plates. The total number of myeloid colonies was scored after 10 days of growth.
To further assess expression of the DHFR resistance gene in vivo, animal RC601 underwent leukapheresis 55 weeks after transplant, and mononuclear cells were sorted into EGFP-positive and EGFP-negative populations. These cells were then placed in suspension cultures in the presence or absence of 200 nmol/L trimetrexate, together with PHA and IL-2, to induce proliferation of T lymphocytes. After 9 days of culture in trimetrexate, the number of cells in the EGFP-negative cultures had fallen to 15% that seen with drug-free conditions. In contrast, the number of cells in the EGFP-positive cultures containing trimetrexate were 54% that seen in drug-free controls. These data indicate that there were some EGFP+ T lymphocytes in monkey RC601 that were resistant to trimetrexate more than 1 year after transplant.
Discussion
Most prior studies of hematopoietic cell gene transfer in primates have used retroviral vectors expressing the neoR gene as a marker. As a result, the detection of marked cells in the PB has relied on semiquantitative PCR and, more recently, Southern blot analysis to detect proviral DNA sequences. These assays are limited in several important ways. First, it is difficult to determine whether an average vector copy number of 10% indicates that 10% of the cells are marked or whether 1% of the cells have 10 copies. Secondly, these assays are relatively imprecise, particularly with PCR-based methods, so that differences in the signal intensity of <2-fold to 3-fold, while potentially biologically significant, cannot be reliably discriminated. Lastly, the DNA-based assays do not indicate whether a transduced cell is expressing the vector, and they cannot be used to follow marking in mature erythrocytes or platelets. Because silencing of vector expression is known to occur in vivo in murine systems11,12and because vector expression is the relevant end point for therapeutic applications, it is important to determine the number of cells that are actually expressing the transferred vector. Our prior work with transplanted mice has shown that the EGFP reporter system provides a powerful system for tracking vector-expressing cells in vivo,2,18 and we have recently shown the feasibility of using this system in the rhesus monkey gene transfer model.17 We now describe the use of this system to precisely measure the kinetics of reconstitution with vector-expressing cells from multiple hematopoietic lineages in a series of transplanted rhesus macaques.
In an effort to achieve efficient transduction of pluripotent stem cells, we incorporated a number of recent advances in stem cell gene transfer into our protocol. Cytokine-mobilized PB stem cells have been shown to be a good target for murine retroviral vectors. They have been associated with high levels of marking in murine experiments22 and in recent rhesus transplant experiments.9 The MSCV vector was chosen based on its reported ability to sustain gene expression in vivo when exogenous genes are driven from the viral LTR.14,18 The use of recombinant FN has been shown to enhance gene transfer in nonhuman primates.8 This is apparently due to colocalization of stem cells and retroviral particles23 and perhaps by direct effects on stem cells during ex vivo culture.24Transduction cultures using flt-3 ligand in conjunction with other early-acting cytokines have resulted in improved stem cell gene transfer in several systems.9 25 We therefore incorporated these elements into an integrated gene transfer protocol in order to achieve maximally efficient stem cell transduction.
In the first 2 animals that were transplanted using concentrations of 100 ng/mL flt-3 ligand and 100 ng/mL SCF in the transduction cultures, we obtained disappointingly low levels of marked cells as measured by flow cytometry analysis for EGFP expression in PB cells. Recent studies have shown that concentrations of 300 ng/mL flt-3 ligand and SCF, together with lower concentrations of other cytokines, can result in an expansion of human stem cells in culture.26 27 We therefore hypothesized that using these higher concentrations would enhance stem cell transduction by inducing self-renewing stem cell divisions in the presence of vector. Increasing the concentrations of these cytokines did result in much higher levels of EGFP-expressing cells, but the pattern was not consistent with transduction of pluripotent hematopoietic stem cells. We observed transient but high proportions of EGFP-expressing granulocytes, monocytes, red blood cells, and platelets during the first 2 weeks after transplant. More stable marking was seen in PB lymphocytes, with T and B lymphocytes continuing to express the EGFP marker for as long as 35 weeks after transplant. This marking pattern was remarkably consistent among all 3 rhesus macaques transplanted with cells transduced using high concentrations of flt-3 and SCF.
There are several potential explanations for the early disappearance of marked myeloid cells. Silencing of expression from the vector was ruled out by the demonstration that vector DNA levels in granulocyte populations correlated with the proportion of EGFP-expressing granulocytes. Furthermore, the absence of marked CFU-C in the bone marrow at later time points showed that myeloid marking at the DNA level was absent. We considered the possibility that immune responses directed against EGFP could lead to clearance of transduced cells, but we believe that this is unlikely for several reasons. In our experience with EGFP vectors in transplanted mice, levels of EGFP-expressing cells have been stable for as long as 1 year in primary recipients and for over 6 months in secondary transplant recipients.2,17 18This suggests that an immune response does not occur in mice. Secondly, an immune response would be expected to result in clearance of EGFP-expressing lymphocytes, which remained present at relatively high levels for extended periods of time in all 3 rhesus macaques. Another possibility was that the disappearance of EGFP-expressing myeloid cells represented recovery of unmodified endogenous stem cells occurring as a result of sublethal irradiation. To rule this possibility out, a fourth monkey was transplanted using an identical transduction protocol but receiving a higher dose of myeloablative irradiation (1300 rads). Marking in this animal was no better than in the animals receiving 1000 rads.
The most plausible explanation for transient erythroid and myeloid marking following engraftment would be transduction of a myeloid progenitor that was restricted in its proliferative capacity. The higher levels of lymphoid marking could be due either to concurrent transduction of a common lymphocyte progenitor or to transduction of lympho-myeloid stem cells with limited self-renewal capacity. In the latter case, the longer half-life of mature lymphoid cells would allow for the slower decay of transduced lymphoid versus myeloid cells. A relative skewing toward lymphocyte marking has been noted in other primate studies.5,28 More recent nonhuman primate marking studies, however, have documented higher levels of myeloid stem cell transduction as determined by proviral DNA analysis in peripheral blood granulocytes and in bone marrow–derived CFU-C.7,9 The reason for the differences in marking profiles between these studies and our current study is not clear, given multiple differences in the transduction protocols. One possibility is that our transduction conditions resulted in a loss of stem cells with long-term self-renewal capacity. It has been demonstrated that similar culture conditions can lead to stem cell differentiation with loss of SRC which repopulate immunodeficient mice.29 Therefore, the possibility exists that our protocol initially resulted in transduction of pluripotent stem cells, but that these cells lost the capacity to persist for prolonged periods of time in vivo due to the differentiative effects of the ex vivo manipulation. Recent studies have defined conditions that may lead to better preservation of repopulating stem cells during ex vivo transduction, as defined by marking in the NOD/SCID mouse30,31 and in clinical gene therapy trials.32
A second possible explanation for the relatively poor transduction of long-term reconstituting stem cells is the use of an amphotropic retrovector. It has been shown that primitive hematopoietic stem cells express very low levels of the receptor for amphotropic retroviruses.33 Furthermore, a direct comparison of marking using an amphotropic versus a gibbon ape leukemia virus (GALV) pseudotyped vector in transplanted baboons showed better long-term marking with the GALV vector, however it is not clear if this was mostly due to marking of lymphocytes rather than myeloid cells.8,10 Furthermore, relatively high degrees of stable marking of myeloid cells has been obtained in 2 transplanted rhesus monkeys using an amphotropic virus.9 Further studies will be required to determine the optimal vector pseudotype for hematopoietic stem cell transduction in primates.
There may be several applications where the pattern and degree of marking could be potentially useful. The brief appearance of high numbers of marked myeloid cells could be effective in chemoprotection strategies that aim to protect BM cells from cytotoxic drugs. It has been proposed that repeated administration of transduced cells could be used to protect against serial rounds of chemotherapy.34 In this case, chemotherapy would be administered shortly after transplant so that the peak of transduced cells would overlap with exposure to the drug. A second possibility would be to use this approach to achieve transduction of lymphocytes for diseases resulting in immunodeficiency. Although lymphocytes have been directly targeted in patients with adenosine deaminase (ADA) deficiency,35 the persistence of transduced cells was more stable when BM-derived CD34+cells were targeted.36 The in vivo selective advantage for corrected cells seen in ADA deficiency37 and in JAK3 deficiency38 could permit therapeutic levels of corrected cells to persist, even if lymphoid stem cell marking was initially marginal. Ultimately, it will be desirable to obtain efficient transduction of pluripotent stem cells, and the use of EGFP marking vectors in the nonhuman primate models of gene transfer should facilitate accomplishment of this goal.
Acknowledgments
The authors would like to thank Barrington Thompson, Earl West, and the staff of Rowe Inc (Rockville, MD) and the Laboratory of Small Animal Surgery and Medicine for their assistance in caring for the animals. We also acknowledge Christopher Reed for his technical contributions to these studies.
Supported by a grant from the National Heart, Lung, and Blood Institute Program (Project Grant No. P01 HL 53749); a grant from the ASSISI Foundation of Memphis; a Sponsored Research Grant from Systemix, Inc. (Palo Alto, CA); and a grant from the American Lebanese Syrian Associated Charities (ALSAC).
Reprints:Robert E. Donahue, Hematology Branch, National Heart, Lung, and Blood Institute, 5 Research Ct, Rockville, MD 20550; email:donahuer@gwgate.nhlbi.nih.gov.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal