Abstract
β2 integrins are involved in the recruitment of leukocytes to inflammatory sites and in cellular activation. We demonstrate that ligation of CD11b (Mac-1, CR3) or CD11c (p150, CR4) alpha chains of β2 integrins by mAbs or soluble chimeric CD23 (sCD23) on human freshly isolated monocytes rapidly stimulates high levels of interleukin-1β production. This induction takes place at the transcriptional level and is regulated by members of the mitogen-activated protein kinase (MAPK) family. Indeed, stimulation of monocytes through engagement of CD11b or CD11c results in the phosphorylation and activation of ERK1, ERK2, and p38/SAPK2 MAP kinases. U0126, a potent inhibitor of the upstream activator of ERK1/2, ie, MEK1/2, suppresses IL-1β messenger RNA (mRNA) expression in a dose-dependent fashion, showing the implication of this pathway in the transcriptional control of IL-1β production. On the other hand, inhibition of p38 by SB203580 indicates that this MAPK is involved in the control of IL-1β production at both transcriptional and translational levels. Together these data demonstrate that ligation of CD11b and CD11c β2 integrins by mAbs or sCD23 fusion proteins triggers the activation of 2 distinct MAPK signaling pathways that cooperate in controlling IL-1β synthesis at different levels.
The integrin family consists of heterodimeric membrane-bound glycoproteins that mediate homotypic and heterotypic cell-cell adhesion, as well as cell-matrix interactions in a broad range of biologic functions.1 The leukocyte-specific β2 integrin subfamily includes LFA-1, Mac-1 (CR3), and p150,95 (CR4), each consisting of an association of an α chain (CD11a, CD11b, or CD11c) and a common β chain (CD18).2 3
Lack of β2 (CD18) integrin expression, as in the leukocyte adhesion deficiency syndrome, results in an impairment of a variety of immune functions, of which neutrophil transendothelial migration, macrophage oxidative burst and phagocytosis, and lymphocyte proliferation are a few. This results in recurrent bacterial infections, presumably because of impaired chemotaxis and bacterial phagocytosis.4
A variety of counterreceptors and soluble ligands for β2 integrins has already been identified. CD11a/CD18 is mainly expressed on mononuclear leukocytes and binds to ICAM-1 (CD54),5 ICAM-2 (CD102),6,7 and ICAM-3 (CD50),8,9 members of the immunoglobulin superfamily. CD11b/CD18 is expressed on mature neutrophils, monocytes, and natural killer (NK) cells. It also binds to ICAM-1,10 as well as to several soluble ligands, including the complement fragment iC3b,11 fibrinogen,12coagulation factor X,13 and LPS.14 CD11c/CD18 is found on a variety of cells such as monocytes, macrophages, granulocytes, some T and B lymphocytes, and dendritic cells. In contrast with LFA-1 and Mac-1, the ligands and the functional role of p150,95 have not been well defined, but appear to be similar to those of Mac-1. Indeed, CD11c/CD18 has been shown to bind iC3b,15fibrinogen,16 LPS,17 and like the other leukocyte integrins, it is involved in the adhesion of monocytes to endothelium.18 Furthermore, it has recently been reported that CD11b/CD18 and CD11c/CD18 can exhibit an additional adhesive function because of their CD23-binding ability.19-21
In addition to the crucial function of integrins in a variety of cell-adhesion reactions during immune-inflammatory mechanisms, it has been established that engagement of β2 integrins by natural ligands (notably, soluble CD23) and certain mAbs also generates outside-in cellular signaling, leading to cell activation. In monocytes, this activation includes the induction of procoagulant activity, production of inflammatory cytokines (tumor necrosis factor α [TNFα], IL-1β, and IL-6), generation of nitric oxide, and up-regulation of cell-surface molecule expression.19-24
However, most of the studies investigating signaling pathways and, particularly, protein kinases induced by triggering of β2 integrins have been performed on polymorphonuclear neutrophils (PMN).25-27 Conversely, the intracellular events implicated in β2 integrin-mediated human monocyte activation and, particularly, those leading to inflammatory cytokine synthesis are less characterized.
Interleukin-1β is mainly produced by monocytes/macrophages and plays a pivotal role in immuno-inflammatory processes that lead to tissue destruction in chronic diseases, such as rheumatoid arthritis (RA).28 Furthermore, soluble CD23, a multifunctional cytokine whose production is increased in RA,29 plays a role in inflammatory mechanisms and has been shown to induce IL-1β production through activation of macrophages via its interaction with CD11b and CD11c.19 30-32
The signaling pathways leading to IL-1β production have mainly been studied on monocytes stimulated by LPS, antigen-antibody complexes, phorbol esters, or cytokines. In this study, we have investigated the molecular events involved in the regulation of IL-1β production induced by triggering of CD11b/CD18 and CD11c/CD18 β2 integrins at the surface of human monocytes using mAbs or human recombinant fusion proteins for soluble CD23. We have particularly examined the role of mitogen-activated protein kinases (MAPKs), a family of serine/threonine kinases activated by various extracellular stimuli, playing an important role in the regulation of the expression of IL-1β in monocytes and macrophages.33-36
Material and methods
Reagents
RPMI-1640 medium, phosphate-buffered saline (PBS), penicillin, streptomycin, and L-glutamine were supplied by Life Technologies (Paisley, UK). Low-endotoxin fetal calf serum (FCS) was purchased from Seromed (Biochrom KG, Berlin, Germany). Ficoll-Paque was from Pharmacia (Uppsala, Sweden). Polymyxin B sulfate, neuraminidase, 5,6-dichlorobenzimidazoleriboside (DRB), and all other chemicals were purchased from Sigma Chemical (St Louis, MO). [α-32P] dCTP (3000 Ci/mmol) and [α-32P] UTP (3000 Ci/mmol) were from Hartmann Analytic Gmbh (Braunschweig, Germany). U0126 was from Promega (Madison, WI). SB203580 was purchased from Calbiochem (La Jolla, CA). Culture media were shown to contain less than 0.15 U/mL endotoxin as measured by the chromogenic Limulus amebocyte lysate assay.
Monoclonal antibodies and recombinant chimeric proteins
Anti-CD11a antibodies were from The Binding Site (Birmingham, UK) (IgG1, clone BU17) and Pharmingen (San Diego, CA) (IgG2b, clone G43-25B), anti-CD11b antibodies were obtained from R & D Systems (Minneapolis, MN) (IgG1, clone 44) and Serotec (Oxford, UK) (IgG1, clone ICRF44), and anti-CD11c antibodies were from The Binding Site (IgG1, clone BU15) and R & D Systems (IgG1, clone 3.9). The isotype mAb controls were purchased from Pharmingen.
Human recombinant fusion proteins for soluble CD23 were the kind gift of Dr M. Bird (Glaxo Wellcome, Stevenage, UK). hZZ-CD23 is a fusion protein consisting of the lectin domain of human CD23 linked to the protein A IgG binding domain (ZZ) that was produced in insect cells as previously described.37 ZZ-CD23 can form oligomers in solution. For our studies, we used polymeric ZZ-CD23 material purified by gel filtration as previously described for mouse ZZ-CD23.20 ZZ-Pselectin and ZZ-Eselectin fusion proteins were used as negative controls in all experiments. MBP-CD23 chimeric protein consists in maltose binding protein fused to the C-terminal 25-kd form of human CD23. It was expressed in soluble form inEscherichia coli, purified by affinity chromatography on amylose resin and processed to remove endotoxin by repeated passage through Pierce Detoxi-gel. MBP-CD23 mainly consists in oligomers in solution, but conversely to ZZ-CD23, this material was not purified to remove monomeric MBP-CD23.
Isolation of human monocytes
Monocytes from fresh peripheral blood of normal healthy volunteers were prepared as previously described.38 Briefly, peripheral blood mononuclear cells (PBMCs) isolated over a Ficoll density gradient were incubated at 50 × 106cells/mL in RPMI 1640 medium containing 10% heat-inactivated FCS for 40 minutes at 4°C under rotation, leading to monocyte aggregation, followed by 10 minutes of incubation on ice. Pellets of aggregated monocytes were separated by a gradient of FCS and further depleted of T and NK cells by rosetting with neuraminidase-treated sheep red blood cells. Monocyte purity routinely consisted of more than 90% CD14+ cells, less than 1% CD3+ cells, and less than 1% CD19+ cells. Cellular viability was shown to be more than 90% using trypan blue exclusion. Polymyxin B (1 μg/mL) was present throughout the whole isolation procedure and during the activation experiments to rule out any response due to contaminations by low-endotoxin levels.39 Furthermore, to prevent activation on adhesion, monocytes were cultured and stimulated in polypropylene tubes, unless indicated otherwise.
Monocyte activation and measurement of IL-1β
Freshly isolated monocytes were cultured in flat-bottom 96-well tissue culture trays at 100 to 150 × 103 cells per well in complete RPMI medium in the presence of polymyxin B. In some experiments, monocytes were preincubated with specific cell permeable inhibitors for various times and then cultured in the presence of anti-β2 integrin mAbs or recombinant sCD23 fusion proteins for an additional 14 to 16 hours. Then, cells were lysed by the addition of 0.1 volume of 10% NP40, and IL-1β production was measured in total cell lysates by enzyme-linked immunosorbent assay (ELISA) (EIA IL-1β kit; Immunotech, Marseille, France) as described previously.40 The limit of detection of this assay was 10 pg/mL. In some experiments, IL-1β secretion was determined by measuring the cytokine concentration in culture supernatants.
RNA extraction and Northern blot analysis
Human monocytes (5-10 × 106 cells) were starved for 14 hours in medium supplemented with 1% FCS in polypropylene tubes (Falcon). Cells were harvested, resuspended in 500 μL RPMI/HEPES containing 1% FCS and incubated in 2-mL tubes (Eppendorf) with or without effectors at 37°C. Total RNA was isolated by lysing the cells with TRIzol reagent (Life Technologies) according to the manufacturer's instructions. RNAs (4 μg) were separated, transferred, hybridized to 32P-labeled cDNA probes specific for IL-1β (pAT153-hIL-1β gift from Glaxo) or glyceraldehyde-3-phosphate dehydrogenase (GAPDH),41 and autoradiographs quantified as previously described.42
Nuclei isolation and run-on transcription assay
Freshly isolated human monocytes (3 × 107 cells) were starved and then stimulated in polypropylene tubes as described for RNA extraction. Preparation of nuclei, transcription assay, and hybridization were performed as previously described.43Biosynthetically radiolabeled mRNAs (5 × 106 cpm) were hybridized onto slot-blotted complementary DNA (cDNA) (10 μg per slot of linearized pAT153-hIL-1β or pBSGAPDH) for 48 hours at 65°C, then washed and treated with RNAse A. Filters were dried and exposed to Amersham Hyperfilms MP at −80°C.
Western blot analysis and immune complex kinase assay
Nonadherent monocytes were starved as indicated above and stimulated in 2-mL polypropylene tubes at a concentration of 107cells/mL. Cells (5-10 × 106) were treated with or without the effectors for the indicated times at 37°C. After incubations, monocytes were washed twice with 1 mL ice-cold PBS and total cell lysates were prepared as previously described.36For Western analysis, 50 to 100 μg proteins were separated by SDS-PAGE and transferred to Hybond-ECL membrane (Amersham). The blots were probed with antiphospho-p44/42 (Santa Cruz Biotech, CA, and New England Biolabs [NEB], Beverly, MA), antiphospho-p38 (NEB), anti-ERK2, or anti-p38 (Santa Cruz). Secondary horseradish peroxidase-conjugated rabbit antimouse or goat antirabbit antibodies were from DAKO (Copenhagen, Denmark). Antibody-bound proteins were detected by the Amersham ECL system. For immune complex kinase assay, active ERK1/2 was immunoprecipitated from total cell lysates (1-1.5 × 107 cells) with polyclonal rabbit antiphospho-ERK1/2, followed by incubation with protein A-Sepharose (Pharmacia). Immune complexes were suspended in a kinase buffer containing 30 mmol/L HEPES, pH 7.5, 30 mmol/L NaCl, 30 mmol/L MgAcetate, 10% glycerol, 0.1% NP40, 2 mmol/L DTT, 1 mmol/L glycerophosphate, 200 μmol/L Na3VO4, and 200 μmol/L ATP to which 1.5 μg Elk1 fusion protein (NEB) was added for a 30-minute incubation at 30°C. Samples were analyzed by SDS-PAGE and Western blot, probed with phospho-specific Elk1 antibody (NEB).
Results
Anti-CD11b and anti-CD11c mAbs as well as sCD23 fusion proteins trigger IL-1β release on human monocytes
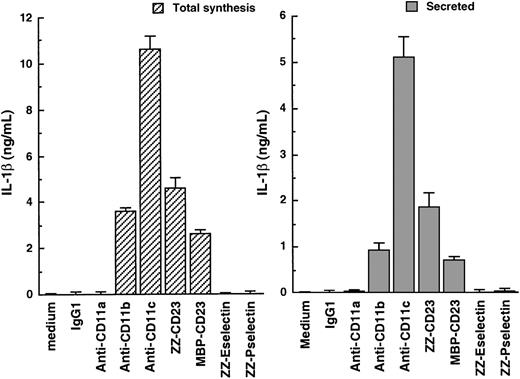
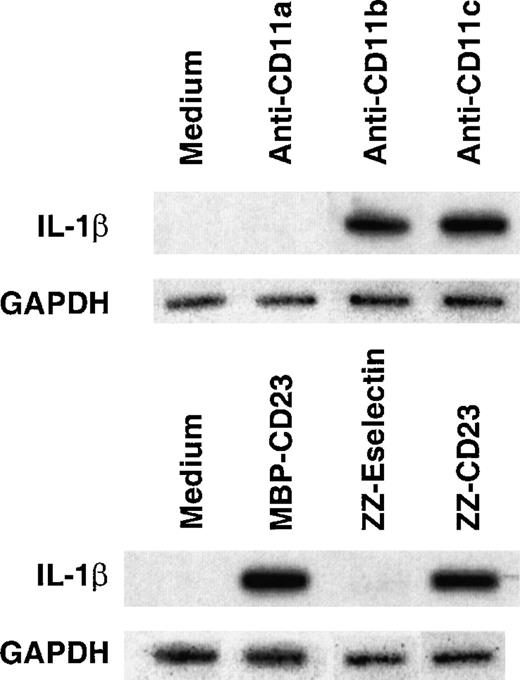
Monocytes are an important source of proinflammatory cytokines. As previously reported by others,19 31 we show that incubation of enriched human monocytes (more than 90% CD14+ cells) with soluble anti-CD11b mAbs markedly stimulated IL-1β production to 3585 ± 149 and with anti-CD11c mAbs to 10 599 ± 522 pg/mL, whereas incubation with anti-CD11a or isotype control antibodies had no effect (Figure 1). This increase in the synthesis of IL-1β was accompanied by an important release of the cytokine in culture supernatants in the amount of 935 ± 113 and 5107 ± 413 pg/mL, respectively. Similar results were obtained using 2 different mAbs specific for each β2 integrin α chain (CD11a, b, c) (not shown). Interestingly, anti-CD11c appears to be more efficient in increasing IL-1β production than anti-CD11b. Indeed, anti-CD11c at the concentration of 5 μg/mL induced a 3-fold higher production of IL-1β than did anti-CD11b at 20 μg/mL. Furthermore, we observed that stimulation of monocytes by 2 distinct sCD23 fusion proteins, ZZ-CD23 and MBP-CD23 (via CD11b and CD11c counterreceptors), were also potent inducers of IL-1β synthesis (4590 ± 431 and 2872 ± 99 pg/mL, respectively) and secretion (1851 ± 279 and 690 ± 57 pg/mL, respectively). This increase in IL-1β synthesis, measured in total cell lysates, was detected as early as 3 hours after β2 integrin engagement, whereas secretion of IL-1β in culture supernatants appeared only after 6 hours.
Induction of IL-1β production by engagement of β2 integrins on the surface of freshly isolated human monocytes.
Human monocytes (150 × 103 cells per well) were incubated for 16 hours with antibodies or recombinant fusion proteins at the following concentrations: isotype control IgG1 (5 μg/mL); anti-CD11a (clone BU17, 5 μg/mL); anti-CD11b (clone ICRF44, 20 μg/mL); anti-CD11c (clone BU15, 5 μg/mL); ZZ-CD23 and MBP-CD23 at 0.5 μg/mL; and ZZ-Eselectin and ZZ-Pselectin at 5 μg/mL. Secreted IL-1β was detected by ELISA in the culture supernatants, total production of the cytokine being determined in total cell lysates after addition of 0.1 volume of 10% NP40 to monocytes. Data are mean ± SD from triplicates of 1 experiment representative of 4 others. Similar results were obtained with anti-CD11a (clone G43-25B), anti-CD11b (clone 44), and anti-CD11c (clone 3.9) mAbs (not shown).
Induction of IL-1β production by engagement of β2 integrins on the surface of freshly isolated human monocytes.
Human monocytes (150 × 103 cells per well) were incubated for 16 hours with antibodies or recombinant fusion proteins at the following concentrations: isotype control IgG1 (5 μg/mL); anti-CD11a (clone BU17, 5 μg/mL); anti-CD11b (clone ICRF44, 20 μg/mL); anti-CD11c (clone BU15, 5 μg/mL); ZZ-CD23 and MBP-CD23 at 0.5 μg/mL; and ZZ-Eselectin and ZZ-Pselectin at 5 μg/mL. Secreted IL-1β was detected by ELISA in the culture supernatants, total production of the cytokine being determined in total cell lysates after addition of 0.1 volume of 10% NP40 to monocytes. Data are mean ± SD from triplicates of 1 experiment representative of 4 others. Similar results were obtained with anti-CD11a (clone G43-25B), anti-CD11b (clone 44), and anti-CD11c (clone 3.9) mAbs (not shown).
Thus, we confirm that triggering of monocyte CD11b or CD11c β2 integrins either by mAbs or sCD23 fusion proteins is a potent way of activating the release of large amounts of IL-1β.
Anti-CD11b and anti-CD11c mAbs as well as sCD23 chimeras stimulate pro–IL-1β gene transcription
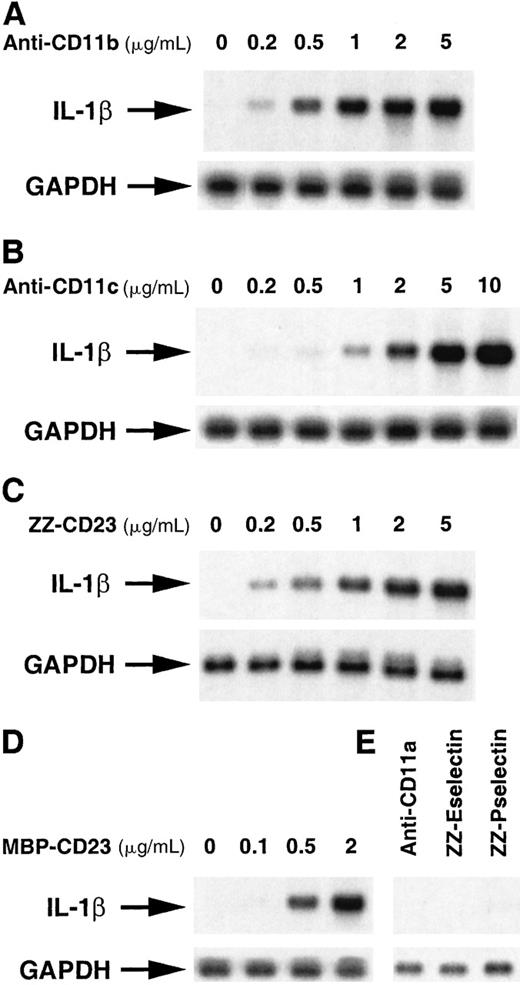
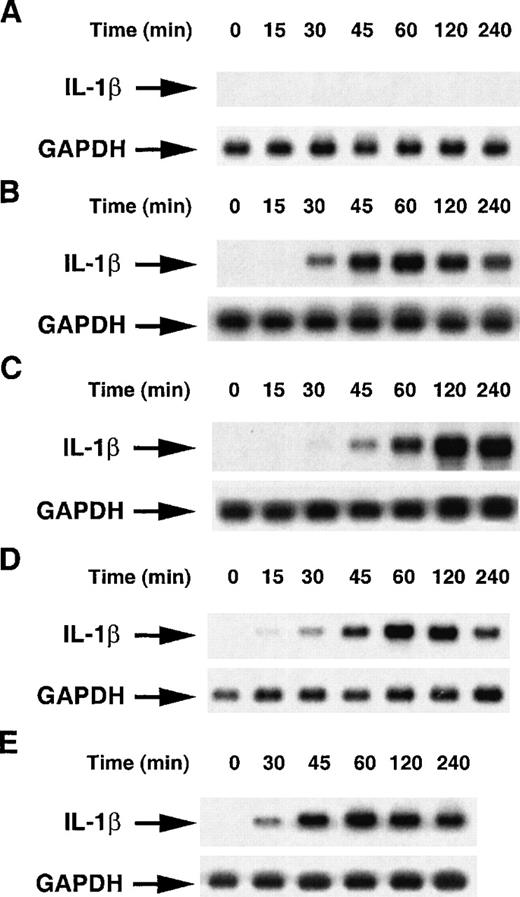
To further investigate the molecular mechanisms underlying the stimulation of IL-1β production, our attention focused on the regulation of pro–IL-1β mRNA expression. To this end, human monocytes starved for 14 hours in medium, supplemented with 1% FCS, were cultured in nonadherent conditions in polypropylene tubes to reduce activation due to adhesion. Cells were then incubated for 1 hour with increasing concentrations of anti-CD11b, anti-CD11c, ZZ-CD23, MBP-CD23, or a high concentration of anti-CD11a, ZZ-Eselectin and ZZ-Pselectin as negative controls, and expression of pro–IL-1β mRNA was determined by Northern blot hybridization with specific probes. As shown in Figure 2, the steady-state level of pro–IL-1β mRNA was up-regulated in a dose-dependent manner by incubation with anti-CD11b or anti-CD11c mAbs, reaching a maximal level with 5 to 10 μg/mL mAbs (panels A and B). At variance with their effect on the IL-1β protein (Figure 1), anti-CD11b and anti-CD11c mAbs stimulated pro–IL-1β mRNA to the same level. ZZ-CD23 and MBP-CD23 increased pro–IL-1β mRNA level in a similar manner, with a maximal effect at 2 μg/mL (panels C and D). Conversely, ZZ-selectin fusion proteins and anti-CD11a mAbs did not modulate IL-1β mRNA level, indicating that up-regulation was not due to nonspecific activation mediated by the ZZ-domain of chimeras or through interaction of mAbs with Fc receptors expressed on monocytes. Time-course experiments were performed in which nonadherent monocytes were incubated in the presence of anti-CD11a, anti-CD11b, anti-CD11c, ZZ-CD23, or MBP-CD23 for different times ranging between 15 minutes and 4 hours (Figure 3). Pro–IL-1β mRNA was detected after 30 minutes, reached a maximal level at 1 to 2 hours, and was maintained after 4 hours of activation by all the effectors except anti-CD11a mAb, which had no effect. Small variations in the kinetics of appearance of pro–IL-1β mRNA were observed in monocytes from one donor to another. However, these time-course experiments indicate thatpro–IL-1β gene is induced early after β2 integrin engagement.
Dose-dependent stimulatory effect of anti-CD11b/c mAbs and soluble CD23 chimeric proteins on the steady-state level of pro–IL-1β mRNA.
Nonadherent human monocytes (7 × 106 cells) were untreated or incubated for 1 hour with various concentrations of the following effectors: A, anti-CD11b (clone 44), B, anti-CD11c (BU15), C, ZZ-CD23, D, MBP-CD23 and E, anti-CD11a (BU17, 5 μg/mL), ZZ-Eselectin (2 μg/mL), and ZZ-Pselectin (2 μg/mL). Then cells were harvested and RNA isolated and analyzed by Northern blot hybridization with pro–IL-1β and GAPDH cDNA probes as described in “Materials and methods.”
Dose-dependent stimulatory effect of anti-CD11b/c mAbs and soluble CD23 chimeric proteins on the steady-state level of pro–IL-1β mRNA.
Nonadherent human monocytes (7 × 106 cells) were untreated or incubated for 1 hour with various concentrations of the following effectors: A, anti-CD11b (clone 44), B, anti-CD11c (BU15), C, ZZ-CD23, D, MBP-CD23 and E, anti-CD11a (BU17, 5 μg/mL), ZZ-Eselectin (2 μg/mL), and ZZ-Pselectin (2 μg/mL). Then cells were harvested and RNA isolated and analyzed by Northern blot hybridization with pro–IL-1β and GAPDH cDNA probes as described in “Materials and methods.”
Time course of pro–IL-1β mRNA induction by β2 integrin engagement.
Monocytes (6-8 × 106 cells) were untreated or stimulated for various times with the following effectors: A, anti-CD11a (BU17, 2 μg/mL), B, anti-CD11b (44, 2 μg/mL), C, anti-CD11c (BU15, 2 μg/mL), D, ZZ-CD23 (1 μg/mL), and E, MBP-CD23 (1 μg/mL). RNA were isolated and analyzed for pro–IL-1β and GAPDH mRNAs expression as previously described. Results are representative of 3 distinct experiments.
Time course of pro–IL-1β mRNA induction by β2 integrin engagement.
Monocytes (6-8 × 106 cells) were untreated or stimulated for various times with the following effectors: A, anti-CD11a (BU17, 2 μg/mL), B, anti-CD11b (44, 2 μg/mL), C, anti-CD11c (BU15, 2 μg/mL), D, ZZ-CD23 (1 μg/mL), and E, MBP-CD23 (1 μg/mL). RNA were isolated and analyzed for pro–IL-1β and GAPDH mRNAs expression as previously described. Results are representative of 3 distinct experiments.
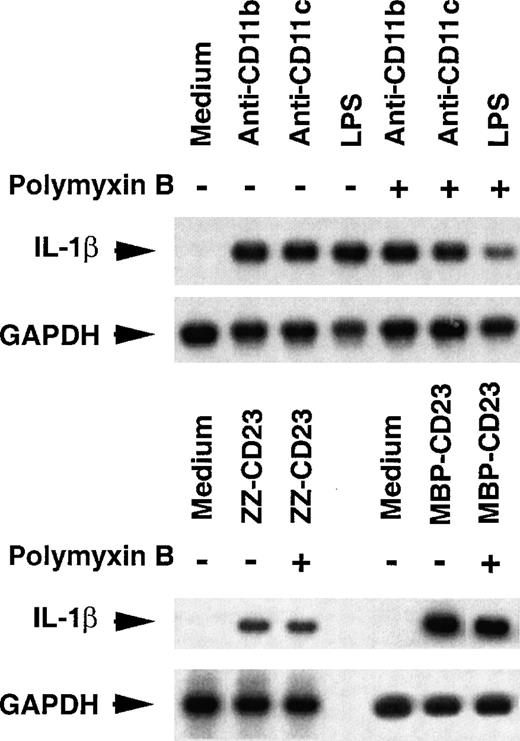
We next ascertained that the effect we observed on IL-1β production would not be due to any endotoxin contamination (Figure4). We found that (1) heated mAbs did not induce pro–IL-1β mRNA expression (not shown), and (2) polymyxin B (10 μg/mL) did not reverse the up-regulatory effect of anti-CD11b and anti-CD11c mAbs or sCD23 fusion proteins on the steady-state level of pro–IL-1β mRNA, whereas it efficiently blocked (60%-70% of inhibition) the expression of pro–IL-1β mRNA induced by a high concentration of LPS (200 ng/mL) (Figure 4). Thus, it is unlikely that monocyte activation should be due to endotoxin contamination.
Effect of polymyxin B on the induction of pro–IL-1β mRNA level.
Nonadherent human monocytes (8 × 106 cells) were untreated or stimulated for 1 hour at 37°C with either anti-CD11b (5 μg/mL), anti-CD11c (5 μg/mL), LPS (200 ng/mL), ZZ-CD23 (1 μg/mL), or MBP-CD23 (1 μg/mL), in the presence or absence of polymyxin B (10 μg/mL).
Effect of polymyxin B on the induction of pro–IL-1β mRNA level.
Nonadherent human monocytes (8 × 106 cells) were untreated or stimulated for 1 hour at 37°C with either anti-CD11b (5 μg/mL), anti-CD11c (5 μg/mL), LPS (200 ng/mL), ZZ-CD23 (1 μg/mL), or MBP-CD23 (1 μg/mL), in the presence or absence of polymyxin B (10 μg/mL).
To gain insight into the mechanism by which β2 integrin triggering elicits the induction of IL-1β in monocytes, we first carried out run-on experiments to determine whether anti-CD11b and anti-CD11c mAbs or sCD23 fusion proteins controlled the transcription of thepro–IL-1β gene. Nascent nuclear RNA chains, biosynthetically labeled with [α-32P] UTP, were isolated from human monocytes previously stimulated for 1 hour with anti-β2 integrin mAbs or sCD23 chimeras, and hybridized to nitrocellulose filters previously spotted with plasmids harboring either the IL-1β or the GAPDH coding sequence. The results demonstrate that anti-CD11b and anti-CD11c mAbs, as well as MBP-CD23 and ZZ-CD23, markedly increased the pro–IL-1βgene transcription rate (Figure 5). Under the same conditions, anti-CD11a mAb and ZZ-Eselectin had no effect whatsoever.
Determination of the transcriptional activity ofpro–IL-1β gene in isolated nuclei from human monocytes stimulated by β2 integrin engagement.
Nonadherent human monocytes (30 × 106 cells) were starved overnight in RPMI 1640 medium supplemented with 1% FCS and then stimulated for 1 hour with anti-CD11a (2 μg/mL), anti-CD11b (2 μg/mL), anti-CD11c (2 μg/mL), MBP-CD23 (1 μg/mL), ZZ-Eselectin (1 μg/mL), or ZZ-CD23 (1 μg/mL). Then cells were lysed, nuclei isolated, and in vitro transcription performed as described in “Materials and methods.” Results are representative of 3 distinct experiments.
Determination of the transcriptional activity ofpro–IL-1β gene in isolated nuclei from human monocytes stimulated by β2 integrin engagement.
Nonadherent human monocytes (30 × 106 cells) were starved overnight in RPMI 1640 medium supplemented with 1% FCS and then stimulated for 1 hour with anti-CD11a (2 μg/mL), anti-CD11b (2 μg/mL), anti-CD11c (2 μg/mL), MBP-CD23 (1 μg/mL), ZZ-Eselectin (1 μg/mL), or ZZ-CD23 (1 μg/mL). Then cells were lysed, nuclei isolated, and in vitro transcription performed as described in “Materials and methods.” Results are representative of 3 distinct experiments.
These data suggest that the activation of human monocytes via the engagement of CD11b and CD11c β2 integrins increasedpro–IL-1β gene expression by a transcriptional mechanism. Furthermore, they indicate that the variations in the synthesis of IL-1β protein mediated by anti-CD11b and anti-CD11c mAbs (Figure 1) cannot be explained by differences in the rate of transcription of the pro–IL-1β gene.
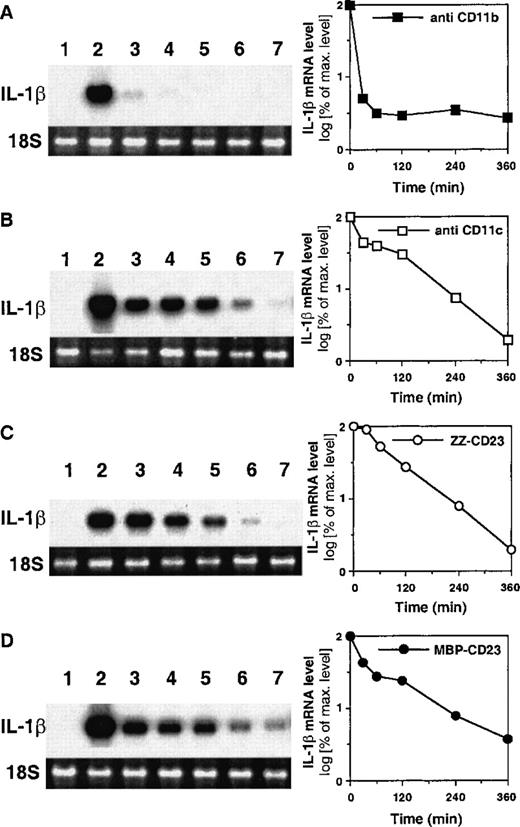
Differential effect of anti-CD11b and anti-CD11c mAbs on pro–IL-1β mRNA stability
To examine the effect of CD11b or CD11c engagement on the stability of pro–IL-1β mRNA, monocytes were incubated for 1 hour in the presence of either anti-CD11b or anti-CD11c mAbs, then the transcription inhibitor DRB was added and the decay of pro–IL-1β mRNA level was monitored as a function of time by quantitative Northern blot hybridization analyses (Figure 6). When monocytes were activated with anti-CD11c mAb (panel B), the steady-state level of pro–IL-1β mRNA decreased rapidly to reach 50% of the initial value 30 minutes after DRB addition, then persisted at a significant level (30% of maximal level) for 2 additional hours, and finally shut off after 4 to 6 hours. When cells were stimulated with ZZ-CD23 or MBP-CD23 (panels C and D, respectively), the profile of pro–IL-1β mRNA decay was quite identical to that observed in the presence of anti-CD11c mAb. Conversely, on monocytes treated with anti-CD11b mAb (panel A), the level of pro–IL-1β mRNA after 1 hour of activation totally disappeared within 30 minutes of blocking transcription. Interestingly, these data demonstrate that the half-life of pro–IL-1β mRNA is significantly shorter in monocytes activated by anti-CD11b mAb than in cells treated with anti-CD11c or sCD23. Thus, the differences in expression of IL-1β protein under CD11b or CD11c stimulation (Figure 1) could result from distinct posttranscriptional mechanisms underlying pro–IL-1β mRNA destabilization.
Analysis of pro–IL-1β mRNA stability in monocytes activated through CD11b or CD11c engagement.
Northern blot analysis of the decay of pro–IL-1β mRNA in human monocytes (7 × 106) stimulated with anti-CD11b (5 μg/mL, panel A), anti-CD11c (5 μg/mL, panel B), ZZ-CD23 (1 μg/mL, panel C), or MBP-CD23 (1 μg/mL, panel D). Cells were untreated (lane 1) or activated for 1 hour (lane 2) with the above effectors. Then DRB (60 μmol/L) was added and cells were incubated for a further 0.5, 1, 2, 4, and 6 hours (lanes 3 to 7, respectively). The level of ribosomal 18S RNA visualized by ethidium bromide was used as a control of the total RNA level. Right-hand side of the figure shows the densitometric scanning quantification of pro–IL-1β mRNA level.
Analysis of pro–IL-1β mRNA stability in monocytes activated through CD11b or CD11c engagement.
Northern blot analysis of the decay of pro–IL-1β mRNA in human monocytes (7 × 106) stimulated with anti-CD11b (5 μg/mL, panel A), anti-CD11c (5 μg/mL, panel B), ZZ-CD23 (1 μg/mL, panel C), or MBP-CD23 (1 μg/mL, panel D). Cells were untreated (lane 1) or activated for 1 hour (lane 2) with the above effectors. Then DRB (60 μmol/L) was added and cells were incubated for a further 0.5, 1, 2, 4, and 6 hours (lanes 3 to 7, respectively). The level of ribosomal 18S RNA visualized by ethidium bromide was used as a control of the total RNA level. Right-hand side of the figure shows the densitometric scanning quantification of pro–IL-1β mRNA level.
Activation of monocytes through CD11b or CD11c engagement results in activation of ERK1/2
MAPKs are commonly activated after cell adhesion or integrin cross-linking.44-48 Furthermore, these kinases are involved in the signaling pathways induced by a variety of stimuli (LPS, cell-contact, FcγR aggregation, microtubule disruption), leading to IL-1β production in monocytes.33-36
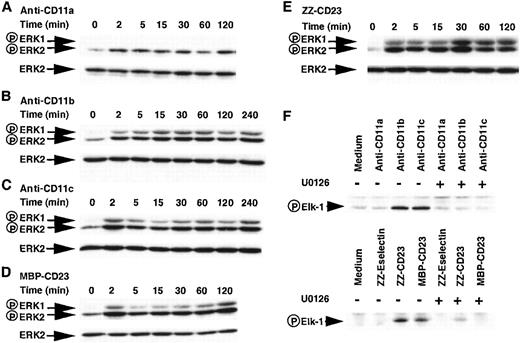
To gain insight into the molecular events responsible for IL-1β induction in our system, we focused our attention on the possible role of ERK1 (p44) and ERK2 (p42) MAPKs. Monocytes were stimulated for increasing periods with either anti-β2 integrin mAbs or sCD23 fusion proteins. Total cell lysates were analyzed for MAPK activation by Western blot using antibodies specific for the dually phosphorylated (Thr202/Tyr204) active forms of both ERK1 and ERK2 (Figure 7, panels A-E). Incubation of monocytes with anti-CD11a mAb resulted in a slight increase in the basal phosphorylation status of ERK2 but did not affect ERK1 phosphorylation (panel A). In contrast, in cells activated by anti-CD11b, anti-CD11c, MBP-CD23, and ZZ-CD23 (panels B, C, D, and E, respectively), we observed a rapid, significant (4- to 8-fold), and persistent up-regulation in the level of phosphorylation of both ERK1 and ERK2 starting at 2 minutes and persisting for 4 more hours. Stripping of the blots and reprobing with an anti-ERK2 antibody showed that the modulation observed was not due to loading variations.
Effect of β2 integrin engagement on the phosphorylation status and activation of ERK1/2 kinase activity.
Panels A to E: Time course of stimulation of the phosphorylation status of ERK1 and ERK2 MAP kinases by β2 integrin engagement. Monocytes (5 × 106 cells) were stimulated for the indicated times with anti-CD11a (5 μg/mL), anti-CD11b (5 μg/mL), anti-CD11c (5 μg/mL), MBP-CD23 (1 μg/mL), and ZZ-CD23 (5 μg/mL), respectively. Cell lysates were analyzed on SDS-PAGE, followed by Western blot using a polyclonal antibody raised against the dually phosphorylated ERK1 (44 kd) and ERK2 (42 kd). Western blots were stripped and reprobed with anti-ERK2 rabbit polyclonal antibody as a loading control. Results are the most representative of 4 distinct experiments. Panel F: Effect of β2 integrin engagement on the activation of ERK1/2 kinase activity. Nonadherent human monocytes (15 × 106 cells) were untreated or stimulated with anti-CD11a (5 μg/mL), anti-CD11b (5 μg/mL), anti-CD11c (5 μg/mL), ZZ-Eselectin (5 μg/mL), ZZ-CD23 (5 μg/mL), or MBP-CD23 (2 μg/mL) in the presence or absence of U0126 (20 μmol/L). After 15 minutes of incubation, cell lysates were prepared and immunoprecipitated with antiphospho-ERK1/2 antibody. The pelleted immunoprecipitates were incubated with Elk1-GST fusion protein as a substrate and phosphorylation of Elk1 was visualized by Western blot using an antibody specific for phosphorylated Elk1.
Effect of β2 integrin engagement on the phosphorylation status and activation of ERK1/2 kinase activity.
Panels A to E: Time course of stimulation of the phosphorylation status of ERK1 and ERK2 MAP kinases by β2 integrin engagement. Monocytes (5 × 106 cells) were stimulated for the indicated times with anti-CD11a (5 μg/mL), anti-CD11b (5 μg/mL), anti-CD11c (5 μg/mL), MBP-CD23 (1 μg/mL), and ZZ-CD23 (5 μg/mL), respectively. Cell lysates were analyzed on SDS-PAGE, followed by Western blot using a polyclonal antibody raised against the dually phosphorylated ERK1 (44 kd) and ERK2 (42 kd). Western blots were stripped and reprobed with anti-ERK2 rabbit polyclonal antibody as a loading control. Results are the most representative of 4 distinct experiments. Panel F: Effect of β2 integrin engagement on the activation of ERK1/2 kinase activity. Nonadherent human monocytes (15 × 106 cells) were untreated or stimulated with anti-CD11a (5 μg/mL), anti-CD11b (5 μg/mL), anti-CD11c (5 μg/mL), ZZ-Eselectin (5 μg/mL), ZZ-CD23 (5 μg/mL), or MBP-CD23 (2 μg/mL) in the presence or absence of U0126 (20 μmol/L). After 15 minutes of incubation, cell lysates were prepared and immunoprecipitated with antiphospho-ERK1/2 antibody. The pelleted immunoprecipitates were incubated with Elk1-GST fusion protein as a substrate and phosphorylation of Elk1 was visualized by Western blot using an antibody specific for phosphorylated Elk1.
To ascertain whether this increase in ERK1/2 phosphorylation would correlate with an increased kinase activity, we performed immune complex kinase assays (Figure 7F). Monocytes were activated for 15 minutes, cell lysates were immunoprecipitated using anti–phospho-ERK1/2 antibodies, and ERK activity was measured by means of the phosphorylation of a recombinant Elk1-GST (glutathione S-transferase) fusion protein. As demonstrated in Figure 7F, Elk1 phosphorylation was increased by anti-CD11b, anti-CD11c, ZZ-CD23, and MBP-CD23 but not by anti-CD11a and ZZ-Eselectin. Furthermore, pretreatment of monocytes with U0126 (20 μmol/L), a specific inhibitor of MEK-1 and MEK-2, 2 upstream activators of ERK1/2,49 50 totally abolished the activation of ERK1/2 mediated by mAbs and sCD23 fusion proteins.
ERK1/2 activity, a prerequisite of β2 integrin signaling of IL-1β synthesis in monocytes
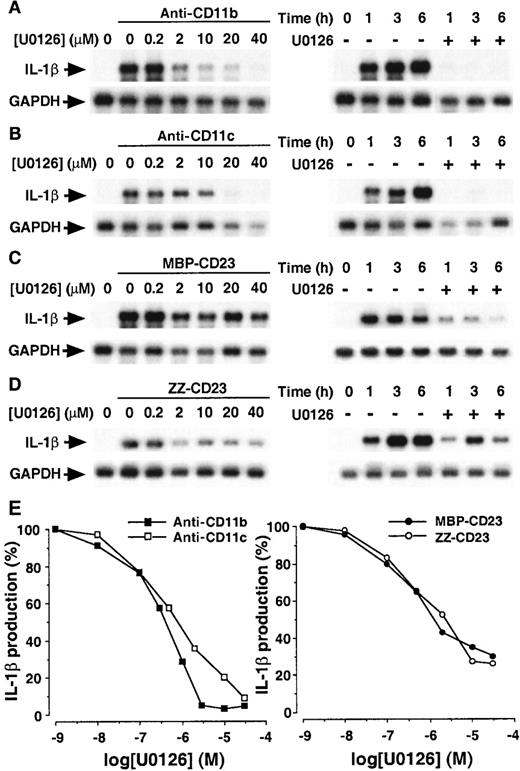
To determine the role of the ERK1/2 pathway in the up-regulation of IL-1β synthesis induced by CD11b and CD11c triggering, we next assessed the effects of U0126 on the expression of pro–IL-1β mRNA (Figure 8, panels A-D) and on IL-1β production (Figure 8E). Nonadherent monocytes were preincubated for 30 minutes with increasing concentrations of U0126 (0.2 to 40 μmol/L), then stimulated for 1 hour with anti-CD11b, anti-CD11c, MBP-CD23, and ZZ-CD23 for 1 hour (Figure 8, left part of panels A to D, respectively). Expression of pro–IL-1β mRNA induced by anti-CD11b and anti-CD11c mAbs was inhibited by U0126 in a dose-dependent manner with a complete inhibition at 20 to 40 μmol/L. In contrast, U0126 inhibited only 60% to 80% of the level of IL-1β messenger induced by MBP-CD23 and ZZ-CD23. Because the expression of pro–IL-1β mRNA and activation of ERK1/2 are long-lasting events, we analyzed the effect of U0126 (40 μmol/L) on the level of pro–IL-1β mRNA 1, 3, and 6 hours, respectively, after monocyte activation by anti-CD11b, anti-CD11c, MBP-CD23, and ZZ-CD23 (right part of panels A to D, respectively). Pro–IL-1β mRNA level induced by anti-CD11b and anti-CD11c mAbs was totally abolished by U0126, whatever the length of activation. This experiment confirmed that 20% to 40% of the signal remained resistant to U0126 in monocytes stimulated by sCD23 fusion proteins. In addition to the effect of U0126 on pro–IL-1β mRNA, we also analyzed its action on IL-1β production (Figure 8E). U0126 inhibited IL-1β production stimulated by anti-CD11b or anti-CD11c mAbs in a dose-dependent fashion with an IC50 of 0.5 to 1 μmol/L. Similar results were obtained with PD98059, another MEK1/2-specific inhibitor51 (not shown). The same experiment was performed on monocytes activated with MBP-CD23 or ZZ-CD23. Results indicate that U0126 also inhibited the production of IL-1β with an IC50 of 0.5 μmol/L. However, in accordance with our previous observations at the IL-1β mRNA level, inhibition was not complete because at 30 μmol/L of U0126, approximately 30% of the maximal production remained.
Effect of U0126 on the production of IL-1β induced by β2 integrin engagement.
Panels A-D: Effects of U0126 on the level of pro–IL-1β mRNA induced by ligation of β2 integrins. Left-hand side: Monocytes were preincubated for 30 minutes with increasing concentrations of U0126 and then stimulated with anti-CD11b (5 μg/mL, A), anti-CD11c (5 μg/mL, B), MBP-CD23 (1 μg/mL, C) or ZZ-CD23 (1 μg/mL, D) for 1 hour at 37°C. Right-hand side: Cells were untreated or preincubated for 30 minutes with U0126 (40 μmol/L) and then stimulated for the indicated times with anti-CD11b (5 μg/mL, A), anti-CD11c (5 μg/mL, B), MBP-CD23 (1 μg/mL, C) or ZZ-CD23 (1 μg/mL, D). Panel E: Dose-dependent inhibitory effect of U0126 on the production of IL-1β. Human monocytes (150 × 103 cells per well) were pretreated for 30 minutes with various concentrations of U0126 and then incubated for an additional 16 hours with anti-CD11b (20 μg/mL), anti-CD11c (5 μg/mL), ZZ-CD23 (2 μg/mL), or MBP-CD23 (1 μg/mL). IL-1β production was determined in total cell lysates by ELISA. Data are means from triplicates of 1 experiment representative of 2 others (in each condition the SD was less than 5%).
Effect of U0126 on the production of IL-1β induced by β2 integrin engagement.
Panels A-D: Effects of U0126 on the level of pro–IL-1β mRNA induced by ligation of β2 integrins. Left-hand side: Monocytes were preincubated for 30 minutes with increasing concentrations of U0126 and then stimulated with anti-CD11b (5 μg/mL, A), anti-CD11c (5 μg/mL, B), MBP-CD23 (1 μg/mL, C) or ZZ-CD23 (1 μg/mL, D) for 1 hour at 37°C. Right-hand side: Cells were untreated or preincubated for 30 minutes with U0126 (40 μmol/L) and then stimulated for the indicated times with anti-CD11b (5 μg/mL, A), anti-CD11c (5 μg/mL, B), MBP-CD23 (1 μg/mL, C) or ZZ-CD23 (1 μg/mL, D). Panel E: Dose-dependent inhibitory effect of U0126 on the production of IL-1β. Human monocytes (150 × 103 cells per well) were pretreated for 30 minutes with various concentrations of U0126 and then incubated for an additional 16 hours with anti-CD11b (20 μg/mL), anti-CD11c (5 μg/mL), ZZ-CD23 (2 μg/mL), or MBP-CD23 (1 μg/mL). IL-1β production was determined in total cell lysates by ELISA. Data are means from triplicates of 1 experiment representative of 2 others (in each condition the SD was less than 5%).
Taken together, these results indicate that the activation of ERK1 and ERK2 seems to be mandatory for mediating the effect of anti-CD11b and anti-CD11c mAbs on pro–IL-1β mRNA levels and on IL-1β production. Regarding IL-1β production induced by sCD23 fusion proteins, the partial inhibitory effect of U0126 suggests that, in this case, IL-1β transcription and synthesis are controlled mainly by an ERK1/2-dependent pathway and to a lesser extent by another, ERK1/2-independent, pathway.
Activation and role of p38/SAPK2 in monocytes triggered by anti-CD11b and anti-CD11c mAbs or sCD23 fusion proteins.
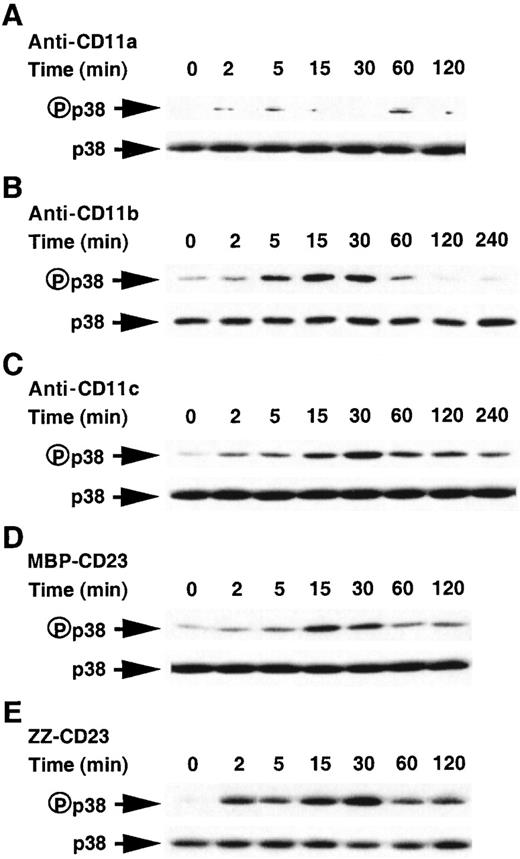
We hypothesize that other congeners of the MAPK family could participate in the β2 integrin-mediated induction of IL-1β synthesis. To this end, we tested the possible activation of p38/SAPK2, which was reported to regulate the production of IL-1β induced by LPS stimulation.33 52 p38 activity was assessed by Western blot using an antibody raised against its dually phosphorylated active form. Results shown in Figure 9A indicate that the weak basal activation of p38 in untreated monocytes was not affected when cells were incubated in the presence of anti-CD11a mAb. In contrast, stimulation of monocytes with anti-CD11b mAb, anti-CD11c mAb, MBP-CD23, or ZZ-CD23 (Figure 9 panels B to E, respectively) for times ranging between 2 minutes and 2 to 4 hours resulted in a marked and transient activation of p38 MAPK (8- to 20-fold induction) with a maximal effect at 15 to 30 minutes. ZZ-Eselectin treatment was without any effect (not shown).
Time course of stimulation of the phosphorylation of p38/SAPK2 kinase in human monocytes activated by anti-CD11b mAb, anti-CD11c mAb, or sCD23 fusion proteins.
Monocytes (5 × 106 cells) were stimulated as depicted in the legend of Figure 7, ie, anti-CD11a (5 μg/mL), anti-CD11b (5 μg/mL), anti-CD11c (5 μg/mL), MBP-CD23 (1 μg/mL), and ZZ-CD23 (5 μg/mL) in panels A to E, respectively. Cell lysates were analyzed by Western blot using a specific antiphospho-p38 antibody. Western blots were stripped and reprobed with anti-p38 rabbit polyclonal antibody as a loading control. Results are the most representative of 4 distinct experiments.
Time course of stimulation of the phosphorylation of p38/SAPK2 kinase in human monocytes activated by anti-CD11b mAb, anti-CD11c mAb, or sCD23 fusion proteins.
Monocytes (5 × 106 cells) were stimulated as depicted in the legend of Figure 7, ie, anti-CD11a (5 μg/mL), anti-CD11b (5 μg/mL), anti-CD11c (5 μg/mL), MBP-CD23 (1 μg/mL), and ZZ-CD23 (5 μg/mL) in panels A to E, respectively. Cell lysates were analyzed by Western blot using a specific antiphospho-p38 antibody. Western blots were stripped and reprobed with anti-p38 rabbit polyclonal antibody as a loading control. Results are the most representative of 4 distinct experiments.
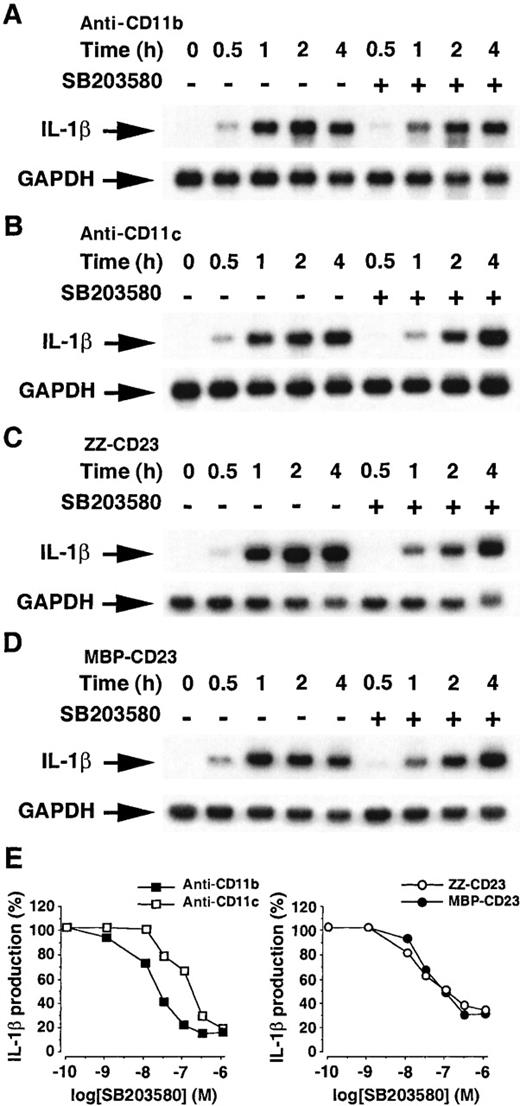
Because the pyridinyl imidazole compound SB203580 is largely reported to be a specific inhibitor of p38 kinase activity, both in vitro and in vivo,53-56 we used this inhibitor to determine whether the activation of p38 MAPK plays a role in the control of pro–IL-1β mRNA expression and IL-1β production (Figure10). Monocytes were used untreated or preincubated for 90 minutes with 10 μmol/L SB203580, a concentration which proved to abolish p38 kinase activity.53 Cells were activated for various times ranging from 30 minutes to 4 hours with anti-CD11b, anti-CD11c mAbs, ZZ-CD23, or MBP-CD23, and pro–IL-1β mRNA level was determined by Northern blot hybridization (Figure 10, panels A-D, respectively). SB203580 inhibited 70% to 80% of the pro–IL-1β mRNA level after short periods (0.5-1 hours) of monocyte activation, but it had no inhibitory effect at later times of cell activation (2-4 hours). Consistent with recent reports, p38 kinase activity seems to be involved in the control of the immediate early induction of pro–IL-1β mRNA transcription.57
Effects of SB203580 on the production of IL-1β induced by β2 integrin engagement.
Panels A-D: Northern blot analysis of the effects of SB203580 on the level of pro–IL-1β mRNA. Monocytes were untreated or preincubated for 90 minutes with SB203580 (10 μmol/L) and then stimulated with anti-CD11b (5 μg/mL, A), anti-CD11c (5 μg/mL, B), ZZ-CD23 (1 μg/mL, C), or MBP-CD23 (1 μg/mL, D) for the indicated times. Panel E: Dose-dependent inhibitory effect of SB203580 on the production of IL-1β. Human monocytes (150 × 103 cells per well) were pretreated for 90 minutes with SB203580 at various concentrations and then incubated for an additional 16 hours with anti-CD11b (20 μg/mL), anti-CD11c (5 μg/mL), ZZ-CD23 (2 μg/mL), or MBP-CD23 (1 μg/mL). IL-1β production was determined in total cell lysates by ELISA. Data are means from triplicates of 1 experiment representative of 2 others (in each condition the SD was less than 10%).
Effects of SB203580 on the production of IL-1β induced by β2 integrin engagement.
Panels A-D: Northern blot analysis of the effects of SB203580 on the level of pro–IL-1β mRNA. Monocytes were untreated or preincubated for 90 minutes with SB203580 (10 μmol/L) and then stimulated with anti-CD11b (5 μg/mL, A), anti-CD11c (5 μg/mL, B), ZZ-CD23 (1 μg/mL, C), or MBP-CD23 (1 μg/mL, D) for the indicated times. Panel E: Dose-dependent inhibitory effect of SB203580 on the production of IL-1β. Human monocytes (150 × 103 cells per well) were pretreated for 90 minutes with SB203580 at various concentrations and then incubated for an additional 16 hours with anti-CD11b (20 μg/mL), anti-CD11c (5 μg/mL), ZZ-CD23 (2 μg/mL), or MBP-CD23 (1 μg/mL). IL-1β production was determined in total cell lysates by ELISA. Data are means from triplicates of 1 experiment representative of 2 others (in each condition the SD was less than 10%).
Because the commonly described mechanism of SB203580-mediated inhibition of cytokine synthesis takes place at the translational level,54 we also tested the effect of this inhibitor on the production of IL-1β by monocytes (Figure 10E). SB203580 inhibited in a dose-dependent manner the production of IL-1β induced by engagement of anti-CD11b, anti-CD11c mAbs, or sCD23 fusion proteins with IC50 of 20, 100, and 30 nmol/L, respectively. However, the inhibition of p38/SAPK2 was not sufficient to abrogate IL-1β synthesis, because 20% to 30% of the maximal cytokine production persisted at 1 μmol/L of SB203580. Similar results have been obtained with SB202190,54 another highly specific blocker of p38 kinase activity (not shown).
Altogether, these data suggest that, in our system, a p38 MAPK-dependent pathway is involved in the control of IL-1β production both at the transcriptional and translational levels.
Discussion
We and others have previously reported that β2 integrins (CD11a, CD11b, and CD11c) play an important role in the production of IL-1β on human monocytes stimulated by direct contact with activated T lymphocytes.58-60 However, because these molecules are expressed on the surface of both monocytes and T lymphocytes, it was difficult to clearly define the role that β2 integrins play on each cell type in this process.
In this study, we investigated the molecular events involved in the control of IL-1β production on freshly isolated human monocytes activated through β2 integrin engagement. In accordance with previous data,19 we confirm that engagement of CD11b and CD11c by mAbs provides a potent signal of activation, resulting in a marked production of IL-1β. Interestingly, anti-CD11c mAbs were more effective in inducing IL-1β synthesis than were anti-CD11b mAbs. However, these results contradict other reports that indicate that ligation of β2 integrins was inefficient in mediating signal transduction, leading to early gene expression and particularly to IL-1β expression.61,62 Taken together, these observations indicate that some, but not all, anti-CD11b and anti-CD11c mAbs are capable of stimulating both IL-1β production and secretion in human monocytes. Similarly, certain anti-CD11b mAbs induced the respiratory burst, whereas other mAbs did not.63 In contrast, the anti-CD11a mAbs we used (BU17 and G43-25B) were inefficient in inducing IL-1β production. This is in agreement with a recent study in which 2 other anti-CD11a IgG (25.3 and B-B15 from Immunotech) did not stimulate cytokine synthesis by human monocytes.19 Altogether, these results rule out the possibility that the stimulatory effects of anti-CD11b and anti-CD11c mAbs were mediated via their binding to Fc receptors.
Interestingly, 2 distinct recombinant fusion proteins of soluble CD23, a natural ligand of CD11b and CD11c, produced effects similar to those triggered by anti-CD11b and anti-CD11c mAbs, suggesting that the stimulation of IL-1β production through β2 integrin ligation was physiologically relevant. The specificity of action of sCD23 by binding to CD11b and CD11c has been clearly demonstrated in that Fab fragments of anti-CD23, anti-CD11b, and anti-CD11c mAbs inhibited IL-1β production induced by sCD23 in monocytes.19 Furthermore, production of other proinflammatory mediators such as nitric oxide, oxidative burst, and other proinflammatory cytokines (TNFα, IL-6), induced by sCD23 and anti-CD11b or anti-CD11c IgG were also specifically blocked by Fab fragments of anti-CD23, anti-CD11b, and anti-CD11c mAbs.19-21 These blocking effects of anti-CD11b and anti-CD11c Fab fragments and the ability of soluble CD23 to oligomerize,64 suggest that cross-linking of CD11b or CD11c could be necessary to mediate monocyte activation.
Production of IL-1β by monocytic cells is a complex and finely regulated process.28 Our study brings new insights in the molecular mechanisms controlling IL-1β synthesis induced by ligation of β2 integrins. Engagement of CD11b or CD11c on monocytes by mAbs or sCD23 fusion proteins markedly induced both steady-state level of proIL-1β mRNA and transcription rate of thepro–IL-1β gene. However, some differences exist between the mechanisms of each effector. Indeed, we notably observed that pro–IL-1β transcripts have a much shorter half-life in monocytes incubated with anti-CD11b mAbs than in cells activated by anti-CD11c mAbs or sCD23 fusion proteins. Furthermore, the half-life of pro–IL-1β mRNA in all our conditions appears much lower than that observed in response to LPS.65 Therefore, the presence of such unstable pro–IL-1β transcripts suggests that the transcription rate must be sufficiently sustained in monocytes activated through β2 integrins to allow translation, whereas this is not necessary on LPS activation. Moreover, the 3′ untranslated region of pro–IL-1β mRNA contains an AU-rich sequence that has been implicated in rapid message turnover.66-68 Thus, the distinct posttranscriptional regulation of pro–IL-1β mRNA degradation induced by ligation of CD11b or CD11c could account for differences in the efficiency of translation and consequently in the production of IL-1β.
MAPK family members are known to play a key role in the control of pro–IL-1β expression induced by a variety of stimuli.33-36 According to a recent report, cross-linking of CD11a and CD11b by mAbs results in the activation of ERK1/2 and the induction of procoagulant activity in the THP-1 monocytic cell line.69 The work presented herein was designed to evaluate the role of MAPKs in the control of IL-1β production in response to ligation of β2 integrins on primary human monocytes. We demonstrated for the first time that engagement of CD11b or CD11c by mAbs on nonadherent monocytes activated both the p42/44 MAPK and p38/SAPK2 pathways, whereas anti-CD11a mAbs had no effect on these kinases. Our results indicate that the stimulation of these MAPK pathways by anti-CD11b and anti-CD11c mAbs is probably physiologically relevant because it can also be activated by sCD23. To our knowledge, this is the first report showing that stimulation of human monocytes by sCD23 leads to the activation of ERK1/2 and p38 MAPKs.
Whereas LPS induces a rapid and transient activation of ERK1/2,50,70 the ligation of CD11b or CD11c by mAbs or sCD23 fusion proteins results in a long-lasting activation of these MAP kinases, which suggests that this pathway plays a major role in monocyte activation. In keeping with this notion, we demonstrated that the inhibition of the ERK1/2 pathway by the specific U0126 compound abolished IL-1β mRNA expression and IL-1β production induced by anti-CD11b and anti-CD11c mAbs. These results clearly indicate that activation of ERK1/2 is a mandatory condition for the transcriptional induction of IL-1β expression mediated by β2 integrin triggering. This observation is consistent with the fact that transactivation of the nuclear factor NF-IL6 essential forpro–IL-1β gene induction, is dramatically up-regulated by MAPK.71-73 Moreover, the lack of effect of anti-CD11a mAbs on IL-1β production could be explained by the fact that these antibodies do not stimulate MAPK congeners. It is noticeable that, in monocytes activated by sCD23 fusion proteins, the inhibition of IL-1β production by U0126 was not total (70%). This partial inhibition could be explained by a recent report suggesting that sCD23 could mediate part of its effects on monocytes and notably the induction of IL-1β synthesis through interaction with the vitronectin receptor and its associated CD47 molecule.74 Thus, U0126 could block the ERK1/2-dependent production of IL-1β induced by the interaction of sCD23 with CD11b/CD18 and CD11c/CD18, whereas signaling mediated by ligation of sCD23 on CD47 could be ERK1/2 independent. Moreover, it has clearly been established that β2 integrins, and notably CD11b/CD18 and CD11c/CD18, can form molecular complexes with other leukocyte receptors such as FcγRI, FcγRII, FcγRIIIB, uPAR (CD87), and CD14.75 Such molecular associations have been implicated in the mediation of the respiratory burst induced by some anti-CD11b mAbs.63 Consequently, such molecular complexes could also participate in the control of IL-1β expression induced by anti-CD11b and anti-CD11c mAbs.
The p38 MAPK is a key component in stress-induced signal transduction pathways, leading notably to inflammatory cytokines production.76,77 Experiments using SB20358056demonstrate that p38 also actively participates in the signaling pathways responsible for IL-1β production induced by β2 integrin ligation. Indeed, inhibition of p38 activity results in a marked decrease (70%-80%) in pro–IL-1β mRNA level at early times (0.5-1 hours) of monocyte activation by anti-CD11b, anti-CD11c, or sCD23 fusion proteins. This is consistent with the transient activation of p38 by β2 integrin ligation and is in total accordance with a recent report demonstrating that p38 MAPK can control the transcriptional activation of the IL-1β gene induced by LPS through interaction with the C/EBPβ (NF-IL6) and C/EBPδ transcription factors.57 However, SB203580 failed to inhibit the expression of the pro–IL-1β transcript at later times of monocyte activation by β2 integrins. Thus, the early inhibition of pro–IL-1β mRNA expression cannot reflect by itself the inhibitory effect of SB203580 observed on IL-1β protein production. This implies that SB203580 also mediates its effects via a more classical translational mechanism.54 78
In conclusion, the above results clearly demonstrate that ERK1/2 and p38/SAPK2 pathways are essential for the stimulation of IL-1β production induced by the direct engagement of the β2 integrin alpha chains CD11b and CD11c either with mAbs or their functional ligand sCD23 in monocytes. This activation of monocytes through β2 integrins may be relevant to physiologic and pathologic conditions involving cell-cell interactions such as adherence of activated monocytes to endothelial cells allowing transmigration, macrophage/T lymphocyte interaction at the inflammatory site, or other pathologic situations in which sCD23 level is increased, such as in rheumatoid arthritis.29 32 Thus, further understanding of the molecular mechanisms controlling the production of proinflammatory cytokines would be helpful in the design of new anti-inflammatory therapies.
Acknowledgments
We are indebted to Dr M. Bird for kindly providing ZZ-CD23, MBP-CD23, and ZZ-selectin fusion proteins. We are also grateful to M. T. Kauffmann for technical assistance and to Dr G. Ponzio, Dr B. Rossi, Dr N. Rochet, and Mrs R. Rehm for critical reading of the manuscript.
Supported by the Swiss National Science Foundation grant no. 31.50930.97 and by the Hans Wilsdorf Foundation.
Reprints:Roger Rezzonico, INSERM U364-Facultè de Mèdecine, Avenue de Valombrose, 06107 Nice cedex 02-France; e-mail: rezzonic@unice.fr.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal