Abstract
Adenosine deaminase (ADA) deficiency causes severe combined immunodeficiency (SCID) and is accompanied by T-cell depletion and accumulation of both intracellular and extracellular adenosine (extAdo) and deoxyadenosine. To better understand the causes of T-cell depletion in vivo and to discriminate between extracellular and intracellular effects of exogenously added adenosine in vitro, we investigated mechanisms of 2 different effects of adenosine on murine thymocytes. These effects of adenosine include direct induction of apoptosis in about 6% to 15% thymocytes and inhibition of T-cell receptor (TCR)-induced activation of the majority of thymocytes with inhibited ADA. A2A adenosine receptors, but not A2B, A1, or A3 receptors, are shown to be mostly responsible for extAdo-triggered signaling (cyclic adenosine monophosphate [cAMP] accumulation) in murine thymocytes and this prompted studies of the effects of extAdo on thymocytes from A2AR gene-deficient mice. It is found that direct apoptotic effects of extAdo on CD4+CD8+ double positive (DP) thymocytes are completely accounted for by signaling through A2AR, with no contribution of intracellular lymphotoxicity or of compensating A2BRs because only A2AR +/+, but not A2AR −/− thymocytes were susceptible to apoptotic effects of extAdo. Studies of the effects of cAMP-raising agents support observations of extAdo/A2AR/cAMP–triggered apoptosis in DP thymocytes. Unexpectedly, the extAdo strongly inhibited TCR-triggered activation of both A2AR +/+ and A2AR −/− thymocytes in the presence of ADA inhibitors. This was confirmed with thymocytes from ADA gene-deficient mice, suggesting the existence of A2AR-independent effects of extAdo on thymocytes. The presented data raises questions about the identity and functional role of A2AR-expressing thymocytes in T-cell differentiation and of the role of TCR-antagonizing effects of extAdo in conditions of ADA SCID.
Studies of biochemical mechanisms of intracellular and extracellular effects of adenosine are important because of its role in neuromodulation, cardiovascular biology, immune response, and pathogenesis of human diseases. It was understood for a long time that the accumulation of adenosine in the absence of adenosine deaminase activity (ADA) is lymphotoxic and causes severe combined immunodeficiency (SCID), which is characterized by hypoplastic thymus and a decrease in the number of peripheral lymphocytes.1-4
Mostly intracellular mechanisms of ADA SCID were discussed by implicating the formation of intracellular deoxyadenosine, deoxyATP, and/or S-adenosyl homocysteine, as well as pyrimidine starvation2-7 as the direct cause of intracellular lymphotoxicity. The direct demonstration of ability of dATP and cytochrome c to induce apoptotic program was recently described in cell-free extracts.8 The “signaling” mechanism of lymphotoxicity of adenosine and of T-cell depletion during ADA SCID9,10 is based on the ability of exogenously added extracellular adenosine (extAdo) to mediate transmembrane signaling through purinergic receptors on T cells and to cause cell death.9-18
The interest of immunologists to the effects of adenosine is also driven by the unusual properties of adenosine, because extAdo promotes both the thymocyte survival9 and the thymocyte death.10 Only one other class of biologically active compounds, glucocorticoids, has been shown to have similar extracellular adenosine effects on thymocytes, leading to the consideration of the role of steroid hormones in T-cell development.19
The effects of extAdo, which include physiologic regulation of blood supply to the heart,20,21 are considered to be mediated by G protein-coupled receptors. These receptors were classified as A1, A2A, A2B, and A3 on the basis of biochemical, pharmacologic, and molecular biologic characterization.22,23 It was demonstrated earlier that A2A receptors (A2ARs) are mainly responsible for cyclic adenosine monophosphate (cAMP) accumulation in peripheral murine T cells during incubation with extAdo.17
However, despite the importance assigned to the abilities of A2A adenosine receptors to trigger apoptosis in many cell types (reviewed in Jacobson et al24), it has not been conclusively proven that A2A receptor-mediated signaling does indeed cause apoptosis of thymocytes. Indeed, apoptotic effects of extracellular adenosine and adenosine analogs could easily be explained by its transport into cytoplasm, followed by intracellular mechanisms of cytotoxicity.25-28
To discriminate between the intracellular and extracellular mechanisms of adenosine-induced cell death and to explore the possibility of A2AR-transduced signaling being the mechanism of thymocyte death, we tested the effects of extracellular adenosine with T cells that do not express A2A adenosine receptors. Cells from A2A gene-deficient mice were also used to test whether A2ARs are responsible for extAdo-mediated inhibition of T-cell receptor (TCR)-triggered signaling in thymocytes in an in vitro model of ADA deficiency.
The experiments reported here provide the genetic evidence for signaling and the A2AR-mediated mechanism of adenosine-induced apoptosis in thymocytes and for thymocyte subset-specific expression of A2ARs in thymocytes. The observation of TCR-inhibiting effects of extAdo, even in experiments with A2AR-deficient thymocytes, suggest that effects of extAdo on thymocytes in conditions of ADA deficiency involve both A2AR-dependent and A2AR-independent pathways. These results may provide a clue as to whether T-cell activation defects in conditions of ADA deficiency are partially responsible for blocks in thymocyte differentiation, which, in turn, may lead to severe peripheral T-cell depletion that is observed in patients with ADA SCID.
Materials and methods
Animals
Mice were maintained in a pathogen-free environment at NIH animal care facilities. Mice were 6 to 10 weeks old, and 2 to 3 animals were used in each experiment. A2AR gene-deficient mice (−/−) were generated by gene targeting as described.40 Littermates were screened for wild type (+/+), heterozygous (+/−), and homozygous (−/−) mutations using Southern blot procedures. ADA gene-deficient mice were developed using a 2-stage genetic engineering transgenic strategy41to rescue the ADA-deficient fetuses from perinatal lethality. The ADA-deficient mice were bred and maintained in pathogen-free NIH animal facilities and screened by both ADA zymograms of blood samples and Southern blot analysis of tail DNA for genotypes. The measurements of ADA activity in samples of blood from littermates born to ADA (+/−) heterozygous parents were performed by zymogram analysis41 using G493 agarose gel and a temperature-controlled electrophoresis chamber made by Innovative Chemistry (Marshfield, MA). ADA +/+ wild type and ADA (+/−) heterozygous littermates were used as control mice for ADA-deficient animals. Only ADA +/+ and ADA −/− littermates were used for in vitro studies of T-cell activation.
Cells and medium
Thymocytes were isolated from adult thymus ex vivo and incubated in RPMI-1640 (Biofluids, Rockville, MD), supplemented with 5% dialyzed fetal calf serum (FCS) (heat inactivated) and 100 U/mL penicillin, 100 μg/mL streptomycin, 1 mmol/L sodium pyruvate, 1 mmol/L HEPES, nonessential amino acids, and 5 × 10−5 mol/L 2-β mercaptoethanol.
Reagents
Adenosine and adenosine analogs were prepared freshly as 100 mmol/L stock solution with pH adjusted to 7.1 and were purchased either from Sigma ImmunoChemicals (St Louis, MO) or from RBI (Natick, MA). Forskolin and DBMX were purchased from Alexis (San Diego, CA). Dideoxyforskolin and dbcAMP were obtained from Sigma (St Louis, MO). mAbs were purchased from PharMingen (San Diego, CA).
Analysis of thymocytes
A single-cell suspension of murine thymocytes was isolated by standard procedures. Cells were washed and incubated at 37°C in a 5% CO2 incubator. Cells (0.5-1 × 106/mL) were cultured in a total of 0.2 mL of medium in 96-well plates. Control incubation was performed in parallel at 4°C at the same cell concentration. Adenosine and adenosine analogs were added at various concentrations as indicated in figure legends. After incubation for 16 to 18 hours, or as indicated, cells were harvested and analyzed by flow cytometry.
Flow cytometric quantitation of live, apoptotic, and dead cells was performed according to a modified flow cytometry procedure.29 The assay intended to quantitate spontaneous thymocyte death and to determine the proportion of cells that were killed as a result of adenosine-induced cytotoxicity. Briefly, cells from the short-term culture were gently pipetted and transferred from 96-well plates (200 mL) directly into polystyrene tubes (12 × 75 mm; Falcon, Becton Dickinson Labware, Lincoln Park, NJ), and 200 mL of FACS buffer (phosphate-buffered saline [PBS] with 2% FCS and 0.05% sodium azide) was added to each sample. Each sample had equal volume and was analyzed at the same flow rate and for the standard time (20 seconds) or triplicates. Propidium iodide solution (1 μg/mL final concentration) was added to each tube for 10 seconds before FACS analysis. Live, dead, and apoptotic cells were estimated by counting cell numbers in appropriate gates using a forward/side-scatter dot plot in linear scale and propidium iodide (PI) staining in log scale.9 Data are presented as a percentage of surviving cells from total cell input. Statistical analysis of triplicate sample measurements was performed as described earlier.9 Standard deviations of triplicate measurements within the same experiment usually were lower than 1%.
Fluorimetric measurements of apopotosis in cell culture were also performed using the Annexin V binding assay as described.30Briefly, 0.6 to 1 × 106 cells from 96-well plates were resuspended in 100 mL of buffer containing 10 mmol/L HEPES pH 7.3, 150 mmol/L NaCl, 5 mmol/L KCL, 1 mmol/L MgCl2, 1.8 mmol/L CaCl2, and incubated with 0.3 μg/mL of fluorescein isothiocyanate (FITC)-conjugated Annexin V and with 5 μg/mL propidium iodide for 15 minutes. After incubation, samples were diluted 4 times with buffer containing 1.8 mmol/L CaC12 and analyzed by flow cytometry. Annexin V-FITC was purchased from Trevingen (Gaithersburg, MD). Flow cytometry data acquisition and analysis were performed on FACScan using FACScan research software and CellQuest programs (Becton Dickinson, San Jose, CA).
Measurements of cyclic adenosine monophosphate
DBA-2 thymuses were harvested, and thymocytes were isolated and resuspended at 4 × 106 cells/mL in the culture media (RPMI-1640) at 4°C. Incubations of cells (4 × 105 thymocytes per assay) with various agents were performed in 1.5 mL Eppendorf tubes in a final volume of 200 μL, containing media alone, or adenosine (0 to 125 μmol/L), CGS21680 (0 to 10 μmol/L), and/or ZM241385 (0 to 1 μmol/L). Controls, such as the complete reaction mix at time zero or no cell mixture, were used with every experimental set. The reactions were initiated by the addition of adenosine or adenosine analogs and incubation lasted as indicated at 37°C in an Eppendorf Thermomixer Model 5436 (Brinkman Instruments, Westbury, NY). Thymocyte reaction mixtures were gently resuspended every 5 minutes, and reactions were terminated by the addition of 25 μL of 1N HCL, followed by freezing the samples on dry ice. The cAMP levels were determined using a cAMP enzyme immunoassay (EIA) kit from Amersham (Buckinghamshire, England), according to manufacturer's instructions. The lower limit of detection of cAMP detection using the nonacetylation EIA system by Amersham Pharmacia Biotech is 12.5 fmol. Cell incubations and extractions were performed in the absence of cAMP phosphodiesterase inhibitors to avoid complications with interpretation of results, due to the possibility of their effects on adenosine receptors.
Northern blot studies
Northern blot studies were performed with thymocyte clones that were derived from p53 gene-deficient thymoma, spontaneously formed in p53 gene-deficient mice. These cells were used to provide a clean source of cells without contaminating thymic epithelial or other cells.
Results
A2A (not A1, A3, or A2B) receptors responsible for extAdo-induced cAMP accumulation in murine thymocytes
In our earlier studies9,10 and preliminary experiments (data not shown), we observed the thymocyte death after exposure to extracellular adenosine both in the presence or absence of adenosine deaminase inhibitors. The induction of death of thymocytes by exogeneous adenosine could be explained by both intracellular lymphotoxicity3 and by signaling through adenosine receptors.9-18 The exposure of thymocytes to adenosine leads to accumulation of cAMP (Figure 1A), thereby suggesting the possible signaling mechanism of apoptotic effects of extAdo. Indeed, the accumulation of intracellular cAMP was shown to be apoptotic for thymocytes.31
Adenosine-induced accumulation of cAMP is not observed in mouse thymocytes with inactivated A2AR gene.
Ex vivo thymocytes from wild type mice (A, +/+) or from A2AR deficient (B,−/−) mice were incubated for 30 minutes with extracellular adenosine in the presence or absence of A2AR antagonist ZM241385 and cAMP accumulation was measured as described in “Materials and methods.” Insert in panel B demonstrates the typical result of Southern blot DNA screening for +/+, +/−, and −/− A2AR mice.
Adenosine-induced accumulation of cAMP is not observed in mouse thymocytes with inactivated A2AR gene.
Ex vivo thymocytes from wild type mice (A, +/+) or from A2AR deficient (B,−/−) mice were incubated for 30 minutes with extracellular adenosine in the presence or absence of A2AR antagonist ZM241385 and cAMP accumulation was measured as described in “Materials and methods.” Insert in panel B demonstrates the typical result of Southern blot DNA screening for +/+, +/−, and −/− A2AR mice.
Our earlier biochemical studies pointed to A2A receptors as being mostly responsible for cAMP-accumulation in mouse peripheral T cells,17,32 33 but it remained to be conclusively proven whether A2A receptors are also responsible for cAMP accumulation in thymocytes. Experiments in Figures 1 and2 provide both pharmacologic and genetic evidence that extAdo-induced cAMP accumulation in murine thymocytes is accounted for by signaling through A2A receptors. It is shown that, not only extAdo, but also the selective agonist of A2A receptors CGS21680 are able to trigger cAMP accumulation in thymocytes (Figure 2A).
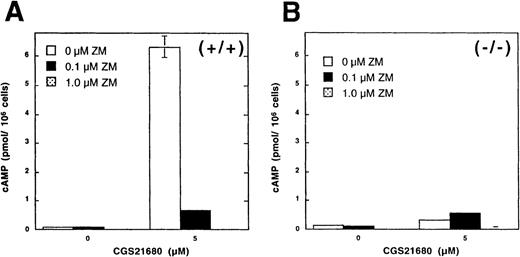
A2AR are responsible for adenosine-induced accumulation of cAMP in murine thymocytes.
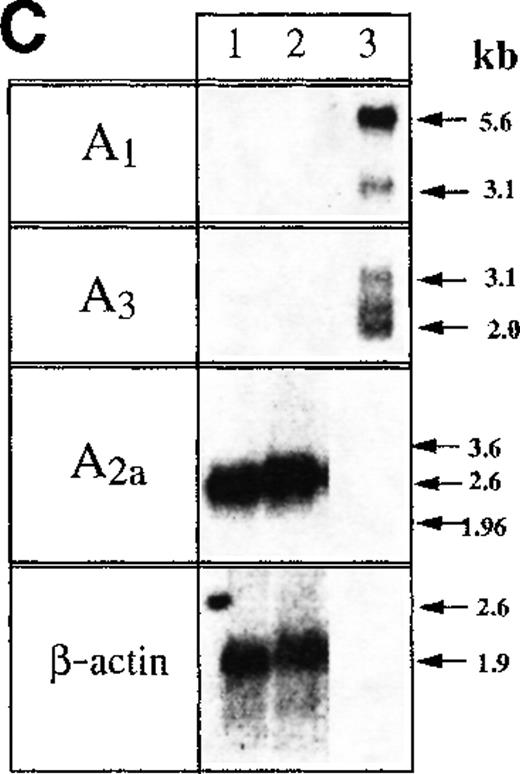
(A,B) Thymocytes from wild type (+/+) but not from A2AR gene deficient mice (−/−) respond to exposure to A2AR agonist CGS21680 by cAMP accumulation. Ex vivo thymocytes from wild type mice (A, +/+) or from A2AR deficient (B,−/−) mice were incubated 30 minutes with A2AR agonist CGS21680 in the presence of A2AR antagonist ZM241385, and cAMP accumulation was measured as described in “Materials and methods.” (C) Northern blot analysis of mRNA expression for A1, A2A, and A3receptors in mouse thymocytes (lane 1) and thymocyte cell line established from p53 −/− mice (lane 2). Samples in lane 3 represent mRNA from brain tissues (positive control for A1receptor mRNA) and the mastocytoma cell line P815 (positive control for A3 receptor mRNA).
A2AR are responsible for adenosine-induced accumulation of cAMP in murine thymocytes.
(A,B) Thymocytes from wild type (+/+) but not from A2AR gene deficient mice (−/−) respond to exposure to A2AR agonist CGS21680 by cAMP accumulation. Ex vivo thymocytes from wild type mice (A, +/+) or from A2AR deficient (B,−/−) mice were incubated 30 minutes with A2AR agonist CGS21680 in the presence of A2AR antagonist ZM241385, and cAMP accumulation was measured as described in “Materials and methods.” (C) Northern blot analysis of mRNA expression for A1, A2A, and A3receptors in mouse thymocytes (lane 1) and thymocyte cell line established from p53 −/− mice (lane 2). Samples in lane 3 represent mRNA from brain tissues (positive control for A1receptor mRNA) and the mastocytoma cell line P815 (positive control for A3 receptor mRNA).
Because both A2A and A2B receptors for extracellular adenosine could be responsible for cAMP accumulation as observed here, we attempted to discriminate between these receptors by using selective antagonist of A2A receptor ZM241385. It is shown in Figures 1A and 2A that ZM241385 does completely block adenosine-induced accumulation of cAMP, suggesting the involvement of A2A receptors in the observed effects of adenosine. Further pharmacologic evidence for the predominant expression of A2AR was provided by the demonstration of cAMP increases in thymocytes after incubation with selective agonist of A2AR adenosine agonist CGS21680 (Figure 2A, B).
The inability of thymocytes to respond to extAdo by cAMP accumulation in the presence of A2AR antagonists provided strong pharmacologic evidence against the functioning of A2Breceptor, which is the only other known cAMP-inducing receptor for extAdo (Figures 1A and 2A). Importantly, even much higher concentrations of adenosine, NECA or CGS21680 (up to 50 μmol/L, data not shown), were not sufficient to induce cAMP accumulation in A2AR −/− thymocytes. Taken together, these data support the conclusion that observed effects of extAdo are mediated by A2A receptors.
Studies of messenger RNA (mRNA) expression revealed the strong expression of A2A receptors in thymocytes, but not of the other (A3 or A1) extAdo receptors (Figure 2C). The correct size of mRNA transcripts was detected in mastocytoma cells or brain tissues, which were used as positive controls for the expression of A3 and A1 receptors, respectively.
Thus, the above described data suggest that A2A receptors are responsible for extAdo-induced cAMP accumulation in thymocytes. The genetic evidence is provided from the observation that extAdo does not induce cAMP accumulation in A2AR-deficient thymocytes. Figures 1 and 2 provide the results of parallel studies of A2AR expressing (+/+) and A2AR deficient (−/−) thymocytes from wild type and homozygous A2AR littermates. It is shown in Figure 1 that, although extAdo did induce cAMP accumulation in wild type +/+ thymocytes, no cAMP response could be detected in A2AR-deficient (−/−) thymocytes (Figure 1B) in a parallel assay. Similar data were obtained when A2AR agonist CGS21680 was used with thymocytes from +/+ and −/− mice (Figure 2A and B). These data provide genetic evidence that A2A receptors are the only extAdo receptors responsible for cAMP response in thymocytes. Because no cAMP accumulation was observed in thymocytes from A2AR (−/−) mice, compared with cells from wild type mice (Figures 1 and 2), these experiments provided biochemical validation of genetic deficiency in A2AR −/− mice and demonstrated that A2ARs are completely responsible for adenosine-induced accumulation of cAMP in mouse thymocytes.
As described above, the characterization of A2AR −/− mice intended to approach the main goal of this study and to ask whether adenosine-induced lymphotoxicity and extAdo-mediated inhibition of TCR-triggered activation of thymocytes will be observed in A2AR deficient mice.
ExtAdo-induced apoptosis in thymocytes mediated by signaling through A2A receptors
The design of the experiments to test the effects of adenosine on ex vivo thymocytes after they were incubated for 16 to 22 hours in vitro was complicated by having the apoptotic effects of added adenosine superimposed over the background of the already “spontaneously” dying thymocytes. Indeed, at any given moment, the thymus contains several subpopulations of thymocytes in terms of their “location” in the apoptotic pathway as a result of the processes of thymocyte selection. It was expected that after 16 to 24 hours of in vitro culturing, those cells that have received an apoptotic selection signal through their TCR molecules will be detected as “dead” even in the absence of added adenosine or anti-TCR mAb. Only cells that have not received an apoptotic signal in vivo and are “intact” at the moment of harvesting provided an opportunity to be tested for their response in vitro to the addition of extracellular adenosine and/or of activating anti-CD3 mAb. Accordingly, results of assays are presented both conventionally (eg, Annexin V vs PI) to enable the direct enumeration of apoptotic versus necrotic versus dead cells and as a proportion/number of surviving thymocytes. Incubation of thymocytes with extracellular adenosine or selective A2AR agonist CGS21680 (Figures 3,4, and 5) causes cell death as demonstrated by using several independent assays. It is shown that some ex vivo thymocytes die “spontaneously” when incubated in vitro in the culture media, but the rate of their death is significantly increased by adding extracellular adenosine.
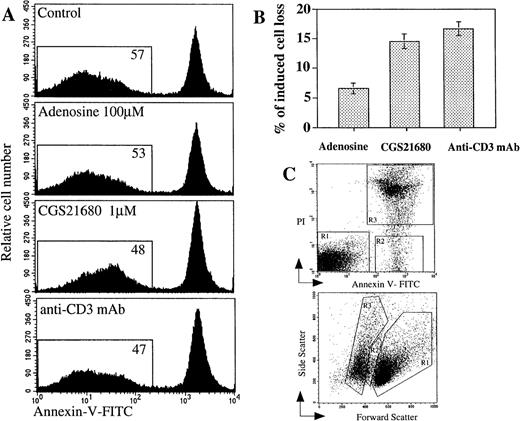
Comparison of extracellular adenosine, CGS21680 and anti-CD3 mAb-induced apoptosis and cell death in mouse thymocytes.
Ex vivo thymocytes were incubated 16 hours at 37°C in 5% CO2 incubator in the presence of adenosine or adenosine analog CGS21680 or anti-CD3 mAb, and the extent of apoptosis or cell death was measured by flow cytometry as described in “Materials and methods.” (A) Detection of apoptotic events in thymocytes using Annexin V assay; (B) comparable proportion of thymocytes is lost after extAdo-induced and TCR/CD3-induced apoptosis. Cell loss was estimated by flow cytometry by PI staining of dead cells. (C) Demonstration of typical experimental gate selection for evaluation of number and proportion of live (gate R1), apoptotic (gate R2), and dead (gate R3) after 16 hours of incubation of thymocytes in vitro with CGS21680 as panel A. The status of cells was studied by flow cytometry after their staining with propidium iodide (PI) and Annexin V (upper graph) and by their site versus forward scatter (lower graph).
Comparison of extracellular adenosine, CGS21680 and anti-CD3 mAb-induced apoptosis and cell death in mouse thymocytes.
Ex vivo thymocytes were incubated 16 hours at 37°C in 5% CO2 incubator in the presence of adenosine or adenosine analog CGS21680 or anti-CD3 mAb, and the extent of apoptosis or cell death was measured by flow cytometry as described in “Materials and methods.” (A) Detection of apoptotic events in thymocytes using Annexin V assay; (B) comparable proportion of thymocytes is lost after extAdo-induced and TCR/CD3-induced apoptosis. Cell loss was estimated by flow cytometry by PI staining of dead cells. (C) Demonstration of typical experimental gate selection for evaluation of number and proportion of live (gate R1), apoptotic (gate R2), and dead (gate R3) after 16 hours of incubation of thymocytes in vitro with CGS21680 as panel A. The status of cells was studied by flow cytometry after their staining with propidium iodide (PI) and Annexin V (upper graph) and by their site versus forward scatter (lower graph).
Demonstration of extracellular adenosine or CGS21680-induced thymocyte death by PI versus Annexin V staining of cells.
Ex vivo thymocytes were incubated 16 to 20 hours at 37°C in 5% CO2 incubator in the presence of adenosine or adenosine analog CGS21680, and the extent of apoptosis or cell death was measured by flow cytometry as described in “Materials and methods.” (A) Loss of live cells in the presence of adenosine or CGS21680. (B) Effects of different concentrations of adenosine and CGS21680 on thymocytes. CGS21680-induced apoptosis of thymocytes reached plateau at as low as 0.3 μmol/L.
Demonstration of extracellular adenosine or CGS21680-induced thymocyte death by PI versus Annexin V staining of cells.
Ex vivo thymocytes were incubated 16 to 20 hours at 37°C in 5% CO2 incubator in the presence of adenosine or adenosine analog CGS21680, and the extent of apoptosis or cell death was measured by flow cytometry as described in “Materials and methods.” (A) Loss of live cells in the presence of adenosine or CGS21680. (B) Effects of different concentrations of adenosine and CGS21680 on thymocytes. CGS21680-induced apoptosis of thymocytes reached plateau at as low as 0.3 μmol/L.
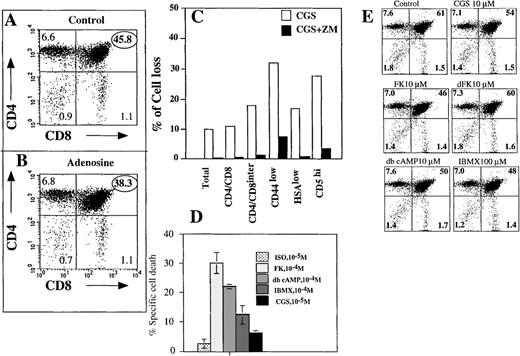
CD4+CD8+DP thymocytes are predominantly susceptible to extracellular adenosine-induced apoptosis.
Ex vivo thymocytes were incubated 16 hours at 37°C in 7% CO2 incubator in the absence (control, A) or presence of 100 μmol/L adenosine (B). Cells were triple stained with anti-CD4, anti-CD8 mAb and PI, and the extent of cell death was measured by flow cytometry as described in “Materials and methods.” (C) Adenosine/A2a receptor-mediated signaling targets CD4interCD8interCD4lowHSAlowCD5highthymocytes. Thymocytes were incubated 16 hours with A2AR selective and apoptosis-inducing agonist CGS21680 (1 μmol/L) in the presence or absence of A2AR antagonist ZM241385 (2 μmol/L) or in the media alone. Cells were then stained with Annexin V, PI, and different mAb to important surface antigens and the proportion of cells with a particular surface marker was estimated by flow cytometry among live cells, which were gated on the basis of Annexin V and PI staining. (D) Effect of cAMP and cAMP-raising agents on apoptosis of thymocytes. Cells were incubated with different agents at concentrations that are indicated and, after 16 hours of incubation, the proportion of CD4+ versus CD8+ cells among surviving cells was estimated by flow cytometry. (E) cAMP-raising agents target for apoptosis mostly CD4+CD8+ DP thymocytes.
CD4+CD8+DP thymocytes are predominantly susceptible to extracellular adenosine-induced apoptosis.
Ex vivo thymocytes were incubated 16 hours at 37°C in 7% CO2 incubator in the absence (control, A) or presence of 100 μmol/L adenosine (B). Cells were triple stained with anti-CD4, anti-CD8 mAb and PI, and the extent of cell death was measured by flow cytometry as described in “Materials and methods.” (C) Adenosine/A2a receptor-mediated signaling targets CD4interCD8interCD4lowHSAlowCD5highthymocytes. Thymocytes were incubated 16 hours with A2AR selective and apoptosis-inducing agonist CGS21680 (1 μmol/L) in the presence or absence of A2AR antagonist ZM241385 (2 μmol/L) or in the media alone. Cells were then stained with Annexin V, PI, and different mAb to important surface antigens and the proportion of cells with a particular surface marker was estimated by flow cytometry among live cells, which were gated on the basis of Annexin V and PI staining. (D) Effect of cAMP and cAMP-raising agents on apoptosis of thymocytes. Cells were incubated with different agents at concentrations that are indicated and, after 16 hours of incubation, the proportion of CD4+ versus CD8+ cells among surviving cells was estimated by flow cytometry. (E) cAMP-raising agents target for apoptosis mostly CD4+CD8+ DP thymocytes.
Three different assays for the detection of cell apoptosis were used in the described studies of the effects of extracellular nucleotides on thymocytes, including (i) detection of apoptotic events using Annexin V assay (Figure 3); (ii) detection of dead cells by both PI and Annexin V staining (Figure 4); and (iii) detection of dead cells by cell size on the basis of forward versus side-scatter measurements. Similar data were obtained in estimation of apoptotic cells in our earlier studies.10
The analysis of multiple parallel experiments in which extAdo-induced thymocyte death was compared with the thymocyte response to other apoptotic stimuli revealed that extAdo or A2AR agonist CGS21680 induced a 6% to 15% cell loss in the 16- to 20-hour assay, which is comparable to the degree of cell death induced by anti-CD3 mAb in parallel assays (Figure 3A, B). ExtAdo is found to be less potent than CGS21680 because dose-response studies indicated that much lower concentrations of CGS21680 were sufficient to accomplish similar levels of apoptosis and cell loss (Figure 4). This is most likely due to the degradation of extAdo by ADA in the absence of ADA inhibitors in this set of experiments.
In the next series of experiments (Figure 5), we attempted to identify the thymocyte subset, which was susceptible to A2AR-transduced apoptotic signals. This was performed by incubating thymocytes with or without adenosine for 18 hours, followed by the staining of surviving cells by fluoresceinated anti-CD4 or anti-CD8 mAb to discriminate between CD4−CD8−(DN), CD4+CD8−(4+SP), CD4−CD8+(8+SP), and CD4+CD8+(DP) thymocyte subsets. These experiments were expected to clarify whether susceptibility to adenosine-induced apoptosis is spread among all thymocytes subsets or is subset specific. It is shown that 45.8% of CD4+CD8+ thymocytes survived 18 hours of incubation in media alone, whereas only 38.3% did so in the presence of extAdo (Figure 5A, B). No changes in the other subsets were detected in this and other (data not shown) experiments. Thus, the extracellular adenosine induced cell death of mostly DP CD4+CD8+, but not of CD4−CD8−, CD4+CD8−, or CD4−CD8+ thymocytes. Adenosine analog CGS21680 was as efficient as the adenosine, but at lower concentrations and a similar proportion of DP thymocytes was eliminated after incubation of thymocytes in parallel assays. These experiments with extAdo and selective agonist CGS21680 provided suggestive pharmacologic evidence to implicate A2A receptors in extAdo-induced apoptosis, but the relatively high concentrations of adenosine were required to observe cytotoxic effects of extAdo on thymocytes. The estimation of 6% to 15% of thymocytes that are susceptible to adenosine-induced apoptosis and are dying after 16 to 24 hours in vitro was very consistent and was observed in many independent experiments to further identify these cells in terms of different surface markers. Thymocytes were treated with A2AR selective and apoptosis-inducing agonist CGS21680 (Figure 5C) and, after 16 hours incubation in the presence or absence of CGS21680 and A2AR antagonist ZM241385 cells, were stained with different mAbs to important surface antigens. This was followed by the evaluation of the loss of cells that express particular surface markers. It is shown that CGS21680 did induce the cell loss, whereas addition of A2Areceptor antagonist ZM241385 prevented the effect of CGS21680. The evaluation of relative cell loss among different markers allowed further description of cells that are targeted by adenosine/A2A receptor signaling as CD4interCD8interCD44lowHSAlowCD5highthymocytes, which represent a transient stage of thymocyte differentiation toward single positive T cells. Because cAMP-inducing treatment of cells with adenosine and A2A receptor agonist CGS21680 was shown to kill some thymocytes, it was important to test (i) whether different cAMP-raising agents have a different ability to kill thymocytes and (ii) whether cAMP increases can target equally well all thymocyte subsets or only DP immature thymocytes. Five different cAMP level-raising treatments were used to answer these questions. It is shown in Figure 5D, that the direct activation of adenylate cyclase by Forskolin or the addition of dbcAMP caused the strongest thymocyte death (about 30% and 22%, respectively), whereas the inhibitor of cAMP phosphodiesterase IBMX damaged about 15% of thymocytes. The triggering of A2A receptors by CGS21680 was more apoptotic than the triggering of adrenergic receptors by isoproterenol.
Figure 5E shows that mostly CD4+CD8+ DP thymocytes are susceptible to the apoptotic effects of cAMP and cAMP-raising agents. It is shown that the incubation of thymocytes with Forskolin, IBMX, and dbcAMP, but not with the inactive analog dideoxyforskolin, had decreased proportion of CD4+CD8+ DP thymocytes among the surviving cells after 16 hours in vitro incubation.
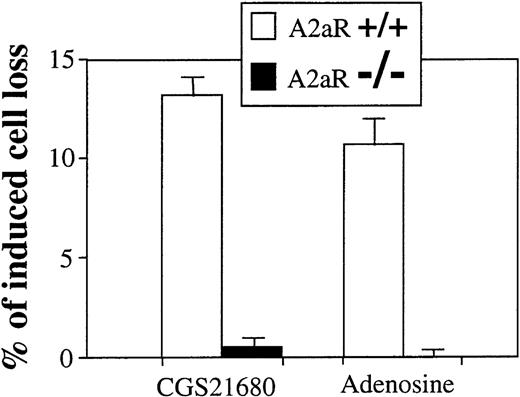
To conclusively implicate the involvement of A2ARs in the apoptotic effects of extAdo, we used thymocytes from wild type (+/+) and A2AR deficient (−/−) mice. Figure6 shows the results of a representative experiment, in which ex vivo thymocytes were tested in parallel for the ability of extAdo to trigger cAMP accumulation and apoptosis. In agreement with other experiments, CGS21680 and extAdo did induce the cell loss of thymocytes from wild type (+/+) mice, but in none of the experiments was apoptosis detected in thymocytes from A2AR deficient (−/−) mice, providing genetic evidence of the necessity of A2AR for the transmission of extAdo-initiated apoptotic signal. Because it is shown that the disruption of the A2AR gene results in the elimination of extAdo and CGS21680-induced signaling in thymocytes, these experiments conclusively prove the direct involvement of A2A receptors in adenosine-induced apoptosis of thymocytes.
A2AR deficient thymocytes are not susceptible to extracellular adenosine and CGS21680-induced apoptosis and cell death.
Ex vivo thymocytes from wild type mice (A2AR+/+) or from A2AR deficient (A2AR−/−) mice were incubated 20 hours at 37°C in 5% CO2 incubator in the presence of adenosine (100 μmol/L) or adenosine analog CGS21680 (1 μmol/L), and the extent of cell death was measured by flow cytometry as described in “Materials and methods.”
A2AR deficient thymocytes are not susceptible to extracellular adenosine and CGS21680-induced apoptosis and cell death.
Ex vivo thymocytes from wild type mice (A2AR+/+) or from A2AR deficient (A2AR−/−) mice were incubated 20 hours at 37°C in 5% CO2 incubator in the presence of adenosine (100 μmol/L) or adenosine analog CGS21680 (1 μmol/L), and the extent of cell death was measured by flow cytometry as described in “Materials and methods.”
Although only about 10% to 15% of thymocytes express apoptosis-inducing A2ARs (Figures 3 through 6), the possibility existed that many more thymocytes do express A2ARs with less efficient coupling to Gsprotein→adenylate cyclase→cAMP pathway. Accordingly, it was still possible that some thymocytes do respond to extAdo by cAMP accumulation, which is not followed by apoptosis, but does inhibit TCR-signaling in thymocytes. This A2AR-mediated mechanism was first to be considered and tested in explanation of an unusual ability of extAdo to block TCR-triggered but not the Fas- or CD45-triggered apoptosis in thymocytes.9
A2A adenosine receptors not responsible for the extAdo-mediated inhibition of TCR-induced thymocytes activation and apoptosis in conditions of inhibited ADA
We have shown recently that the up-regulation of both CD69 and CD25 (IL-2 receptor) activation markers was inhibited by extAdo in the presence of ADA inhibitor.9 The inhibition of TCR-induced thymocyte activation could be observed only in conditions of inhibited ADA, because neither adenosine alone (data not shown) nor ADA inhibitor, coformycin, alone were having an effect on thymocytes in the 16- to 24-hour activation assays presented here (Figure7) and in recently published experiments.9 However, it was important to exclude the possibility that some unknown drug interaction or synergy in the effects of adenosine and coformycin or erythro-9(2-hydroxy-3-nonyl)adenine hydrochloride (EHNA) could be the reason for the inhibition of thymocyte activation by adenosine in the presence of ADA.
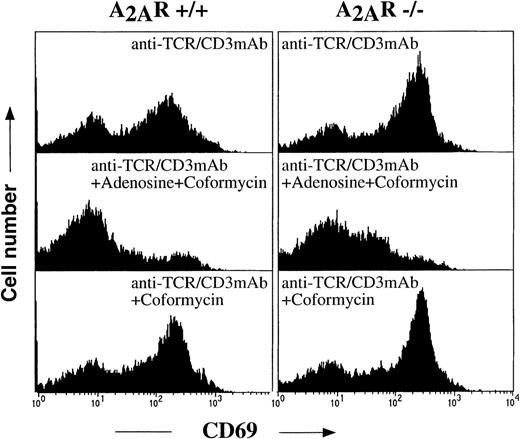
Inhibition of TCR-induced up-regulation of T-cell activation marker CD69 in both A2A receptor-expressing (+/+) and A2A receptor-deficient (−/−) thymocytes by extracellular adenosine in the presence of ADA inhibitor.
Ex vivo thymocytes from A2A receptor-expressing (+/+) and A2A receptor-deficient (−/−) mice were incubated 16 hours at 37°C in 5% CO2 incubator with immobilized anti-TCR/CD3 mAb (20 μg/mL) in the presence of extracellular adenosine (100 μmol/L) and adenosine deaminase inhibitor coformycin (cof, 10 μmol/L) as indicated on the graphs. Cells were harvested and stained with Annexin V, PI (to set gate for nonapoptotic cells), and anti-CD69-PE mAb to estimate the proportion of activated cells. The extent of up-regulation of CD69 was measured by flow cytometry as described in “Materials and methods.”
Inhibition of TCR-induced up-regulation of T-cell activation marker CD69 in both A2A receptor-expressing (+/+) and A2A receptor-deficient (−/−) thymocytes by extracellular adenosine in the presence of ADA inhibitor.
Ex vivo thymocytes from A2A receptor-expressing (+/+) and A2A receptor-deficient (−/−) mice were incubated 16 hours at 37°C in 5% CO2 incubator with immobilized anti-TCR/CD3 mAb (20 μg/mL) in the presence of extracellular adenosine (100 μmol/L) and adenosine deaminase inhibitor coformycin (cof, 10 μmol/L) as indicated on the graphs. Cells were harvested and stained with Annexin V, PI (to set gate for nonapoptotic cells), and anti-CD69-PE mAb to estimate the proportion of activated cells. The extent of up-regulation of CD69 was measured by flow cytometry as described in “Materials and methods.”
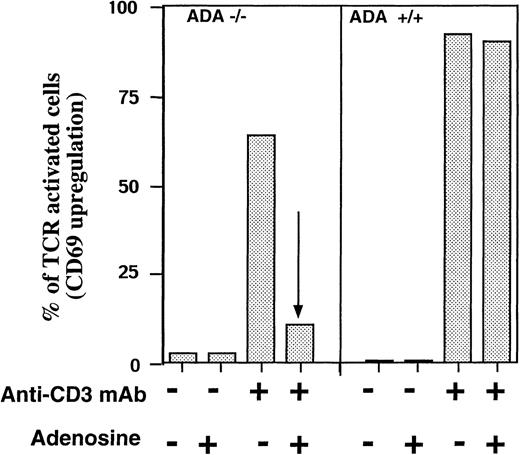
To address this issue, we took advantage of the availability of ADA-deficient mice41 and tested the effect of adenosine on TCR-induced up-regulation of CD69 activation marker in the presence or absence of exogeneously added adenosine. It is shown that anti-CD3 mAb triggered up-regulation of CD69 in more than 60% of ADA-deficient T cells and about 90% of ADA-expressing T cells. However, the addition of adenosine resulted in virtually complete inhibition of TCR-triggered activation only of ADA-deficient T cells, but not of T cells from ADA-expressing littermates (Figure 8). Thus, these experiments provided genetic evidence for the ability of adenosine to block the activation of T cells by TCR cross-linking.
Adenosine inhibits in vitro TCR-triggered activation of ADA-deficient T cells in the absence of ADA inhibitors.
Ex vivo spleen T cells from ADA −/− and ADA +/+ littermates were incubated in 96-well plates with immobilized anti-CD3 mAb (5 μg/mL mAb) or with serum-free, ADA-free media alone (0 μg/mL mAb) in the presence or absence of adenosine (100 μmol/L), and 16 hours later, the TCR-triggered up-regulation of T-cell activation markers CD69 in T cells was evaluated by flow cytometry.
Adenosine inhibits in vitro TCR-triggered activation of ADA-deficient T cells in the absence of ADA inhibitors.
Ex vivo spleen T cells from ADA −/− and ADA +/+ littermates were incubated in 96-well plates with immobilized anti-CD3 mAb (5 μg/mL mAb) or with serum-free, ADA-free media alone (0 μg/mL mAb) in the presence or absence of adenosine (100 μmol/L), and 16 hours later, the TCR-triggered up-regulation of T-cell activation markers CD69 in T cells was evaluated by flow cytometry.
It was most straightforward to test whether the elimination of A2A receptors would lead to the loss of susceptibility of thymocytes to these effects of extAdo. To this end, the ex vivo thymocytes were incubated with immobilized anti-CD3 mAb for 16 hours and then the cells were stained with both Annexin V and anti-CD69 mAb (Figure 7). Analysis of gate with Annexin V negative (live, nonapoptotic) cells reveals that the TCR-induced up-regulation of activation marker CD69 on thymocytes from wild type (A2AR+/+) mice was dramatically inhibited by extAdo. Remarkably, the same effects of extAdo were observed with thymocytes from A2AR−/− mice (Figure 7) in a parallel experiment using littermates of A2AR+/+ and A2AR−/−mice. Thus, it appears that A2ARs are not required for the inhibition of TCR-triggered activation of thymocytes by extAdo. It remains to be conclusively determined whether such TCR-antagonizing effects are mediated by a novel adenosine receptor or by non-cAMP–mediated signaling through A2B receptors or by intracellular toxic effects of exogeneously added extAdo.
Taken together, the results of the experiments presented here suggest that sustained increases in concentrations of extAdo in conditions of ADA deficiency may signal to T cells in normal extracellular environment and cause elimination of about 6% to 15% A2AR-expressing thymocytes. Moreover, the adenosine is able to block the TCR-triggered activation even in thymocytes that lack expressed A2A receptors.
Discussion
The effects of extracellular adenosine on lymphocytes that were described earlier9,10,17,33,34 and in this report, put this physiologically abundant compound among a very small and select group of molecules that have a potential to modulate normal immune response in vivo and to cause immune pathologies, including T-cell depletion and T-cell defects observed in conditions of ADA SCID.9
The attractive features of the described effects of adenosine on thymocytes include its paradoxical abilities to induce the apoptotic death of a small subset of CD4+CD8+ thymocytes (Figures 3 through 5) and to inhibit the TCR-triggered activation and TCR-induced apoptosis (Figures 7 through 9) of the majority of CD4+CD8+ DP thymocytes. The experiments with A2AR −/− thymocytes described here provide strong evidence that the apoptosis-inducing effects of extAdo are mediated by A2A receptors (Figures 3 through 5) and suggest that the TCR-antagonizing effects of extAdo are A2Areceptor independent (Figures 7 through 9).
These data provide the formal proof of earlier biochemical and pharmacologic findings that, of all 4 classes of adenosine receptors, the A2ARs are responsible for cAMP accumulation in murine17 and human32 peripheral T-lymphocytes and in murine thymocytes (Figures 1 and 2). The experiments described in Figures 3 and 4 suggest that the increases in extracellular adenosine levels in the conditions of ADA deficiency may result in different effects on murine thymocytes. These effects include both cytotoxic and cytoprotective effects of extAdo as well as the intracellular toxicity of adenosine catabolites after translocation of extAdo by nucleoside transporters. The adenosine-triggered lymphotoxicity, in turn, could be explained by both intracellular toxicity and by extracellular signaling through adenosine receptor or by both mechanisms, and it was important to evaluate the relative contribution of these 2 different mechanisms.
Strong lymphotoxicity of intracellular adenosine, especially in the conditions of deficiency of ADA, was known for several decades,2-7 but it has been difficult to estimate the contribution of extracellular signaling into the overall effects of adenosine. The attempts to interpret apoptotic effects of so-called “slow-hydrolyzable” analogs of adenosine as supporting the “signaling” mechanism of apoptosis10,13-17 were weakened by observations of these analogs being intracellularly toxic.25-28
Findings of A2AR being responsible for adenosine-induced cAMP accumulation in murine T cells17 and thymocytes (Figures 1 and 2) and the availability of A2AR-deficient mice provided a valuable opportunity to directly test the involvement of adenosine receptors in adenosine-triggered cytotoxicity. It is shown (Figure 6) that the disruption of the gene for A2AR results in the elimination of adenosine-induced apoptosis of thymocytes, thereby confirming that there are no functional A2AR receptors in these mice and that their loss was not compensated by expression of other (eg, A2B) adenosine receptors in A2AR −/− mice. These data both provide the evidence of extAdo/A2A receptor-mediated thymocyte death and validate the use of adenosine analog CGS21680 as a selective agonist of A2AR and as a selective apoptosis-inducing drug in thymocytes.
Because the apoptotic effects of exogeneous adenosine and adenosine receptor agonist CGS21680 on thymocytes are completely accounted for by signaling through A2ARs, these data demonstrate that intracellular toxicity of adenosine and adenosine catabolites was not a contributing factor in tested conditions of incubations. Interestingly, the A2AR-mediated apoptotic effects of extracellular adenosine affect only 6% to 15% of normal thymocytes. The relative small size of this subset may not, however, reflect its significance in the overall process of the positive and negative selection of thymocytes, and experiments are underway to determine the functional consequences of elimination of this subset by extAdo or adenosine analogs treatment with CGS21680.
The differentiation of thymocytes involves several stages, including transitions from CD4−CD8−(DN) to CD4+CD8+(DP) to CD4+CD8−(SP) and CD4−CD8+(SP) thymocytes that mature into peripheral CD8+ T cells or CT4+ T cells (reviewed by Robey and Fowlkes35 and Cibotti et al36). It is interesting that mostly immature CD4+CD8+(DP) thymocytes are susceptible to effects of cAMP-raising agents (Figure 5), thereby reflecting differences in intracellular signaling pathways between immature double and single positive thymocytes. This, in turn, suggests that the A2AR-mediated signaling could be involved in T-cell selection processes that use apoptotic and intracellular cAMP-pathway by selectively triggering Gs-coupled A2ARs with a subset-specific expression. These observations support the view that expression of apoptotic-signal transducing A2ARs is differentiation dependent. The exact phenomenologic and functional identity of DP cells that are susceptible to A2AR-mediated apoptosis is yet to be determined in future studies using different functional and surface markers of T cells, but data shown in Figure 5C suggest that this CD4interCD8interCD44lowCD5hisubset of DP thymocytes represents a transitional stage toward their maturation. The use of A2AR −/− mice provided genetic evidence for selectivity of a well-known A2AR agonist, CGS21680 in elimination of a numerically minor (10%-15%) individual subset of thymocytes (Figure 5C).
Especially dramatic are the effects of extAdo on thymocytes with inhibited ADA (Figures 7 through 9). The addition of extAdo to TCR-activated thymocytes prevents the TCR-induced up-regulation of cell surface markers (Figure 8) and rescues them from TCR-induced apoptosis (Figures 7 and 9). This “apoptosis rescue” assay is of special value for studies and interpretation of the effects of adenosine on thymocytes in conditions of ADA deficiency, because, in contrast to direct apoptotic effects of extAdo (Figures 3 through 6), the TCR-antagonizing effects of the adenosine require the inhibited ADA, suggesting that there is a need in sustained concentrations of added extAdo to observe protection of TCR-activated thymocytes from apoptosis.
The requirement in the low activity of ADA to observe the effects of extAdo on the majority of all TCR-activated thymocytes leads to a view that, if this mechanism does function in vivo in normal animals, it could be most likely operational only in the microenvironment with low ADA activity, which was described in the studies of distribution of ADA activities in the thymus.7This requirement is satisfied in the conditions of ADA SCID. Thus, the described experiments revealed 2 novel types of signaling effects of extracellular adenosine on thymocytes with one of them being unique for conditions of ADA deficiency.
First, in the presence of normal ADA activity, the addition of adenosine causes the elimination of about 10% of DP CD4+CD8+ thymocytes (Figures 3 through 5). This observation has the implication for both in vitro studies (eg, by enabling the selective elimination of A2AR-expressing subset in the investigation of normal thymocytes differentiation) and for the understanding of the mechanisms of ADA deficiency (Figure9). Second, in the absence of ADA activity, the TCR-triggered activation of large proportion of TCR-triggered thymocytes could be inhibited by adenosine (Figures 7 and 9), thereby interfering with the processes that are needed for the survival of thymocytes. As a result of such effects of adenosine, the thymocytes that would normally be selected for further maturation could be dying from “neglect” in an ADA-deficient environment.
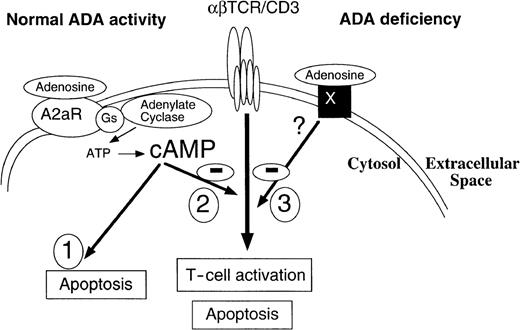
Adenosine acting through A2A receptors may directly trigger the apoptosis of murine thymocytes and inhibit TCR-triggered activation by both A2A receptor-dependent and A2A receptor-independent mechanisms.
Receptor X is postulated to stimulate yet-to-be-determined TCR inhibiting pathway only in conditions of inhibited ADA. These effects of adenosine on TCR-triggered activation are not affected by inhibition of adenosine transport.
Adenosine acting through A2A receptors may directly trigger the apoptosis of murine thymocytes and inhibit TCR-triggered activation by both A2A receptor-dependent and A2A receptor-independent mechanisms.
Receptor X is postulated to stimulate yet-to-be-determined TCR inhibiting pathway only in conditions of inhibited ADA. These effects of adenosine on TCR-triggered activation are not affected by inhibition of adenosine transport.
The molecular mechanisms of extAdo inhibition of TCR-signaling are not yet well understood. Originally, we expected that these effects could be mediated by the A2A receptors, but this was not the case, because the A2AR −/− thymocytes were as susceptible to the inhibition of TCR-triggered activation of thymocytes by extAdo as the wild type, A2AR-expressing thymocytes (Figures 7 and 8). It remains to be established whether A2ARs receptors for extAdo in thymocytes are responsible for these TCR-antagonizing effects of extAdo, but the existing data are consistent with the model (Figure 9) in which about 10% to 15% of DP thymocytes are targets for A2AR-mediated signaling, which can cause their apoptosis. The unexpected ability of adenosine to block activation, even of A2AR-deficient thymocytes and of A3 receptor-deficient thymocytes (data not shown) could be reconciled by postulating the existence of a novel adenosine receptor X. This receptor is predicted to have much lower affinity for adenosine than known adenosine receptors because much higher levels of adenosine (most likely achieved only on conditions of inhibited ADA) are required to observe its effect. These properties of the receptor may help in designing strategies of its expression cloning.
The possible clue as to what kind of signaling may be involved in TCR-antagonizing effects of extAdo is provided by studies of neuroprotective and cardioprotective37 effects of adenosine since it was shown that adenosine agonists were having cytoprotective effects when added during ischemia and epilepsy.38 39
Neuroprotective and cardioprotective effects of extracellular adenosine were explained by short-term functional antagonism between the adenosine receptor and other receptors or channels-mediated signaling.20 The alternative explanation of A2Badenosine receptor functioning through non-cAMP–based mechanism still remains to be investigated.
Independent of the exact molecular mechanism of the effects of adenosine in conditions of ADA deficiency, the ability of adenosine to directly kill 10% to 15% of thymocytes and to block TCR-induced activation in a majority of thymocytes should be taken into account in explanations of pathogenesis of ADA SCID.
Acknowledgments
We thank Dr Diana L. Marquardt (University of California, San Diego) for A1 cDNAs, Dr David Grandy (Vollum Institute for Advanced Biomedical Studies, Portland, OR) for A3 cDNA, Brenda Rae Marshall for editorial help, and Shirley Starnes for help in preparation of the manuscript.
Reprints:M. Sitkovsky, Biochemistry and Immunopharmacology Section, Laboratory of Immunology, NIAID, National Institutes of Health, Bethesda, MD 20982-1892.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal