Abstract
To examine the role of retinoids in hematopoietic cell growth in vivo, we studied female SENCAR mice made vitamin A deficient by dietary restriction. Deficient mice exhibited a dramatic increase in myeloid cells in bone marrow, spleen, and peripheral blood. The abnormal expansion of myeloid cells was detected from an early stage of vitamin A deficiency and contrasted with essentially normal profiles of T and B lymphocytes. This abnormality was reversed on addition of retinoic acid to the vitamin A–deficient diet, indicating that the myeloid cell expansion is a direct result of retinoic acid deficiency. TUNEL analysis indicated that spontaneous apoptosis, a normal process in the life cycle of myeloid cells, was impaired in vitamin A–deficient mice, which may play a role in the increased myeloid cell population. Quantitative reverse transcriptase-polymerase chain reaction analysis of purified granulocytes showed that expression of not only RAR, but RXRs, 2 nuclear receptors that mediate biologic activities of retinoids, was significantly reduced in cells of deficient mice. This work shows that retinoids critically control the homeostasis of myeloid cell population in vivo and suggests that deficiency in this signaling pathway may contribute to various myeloproliferative disorders.
Retinoids are derivatives of vitamin A that control cell growth, differentiation, and apoptosis in many types of cells. They are also essential for embryonic development of many vertebrate species. In addition, retinoids confer resistance to various pathogens1,2 and elicit preventive as well as therapeutic activities against certain tumors.3-5
Two types of nuclear hormone receptors, RAR and RXR, are responsible for mediating biologic activities of retinoids.6-8 Both RAR and RXR bind to retinoids and their analogues through the ligand binding domain. These receptors form RXR/RAR heterodimers, bind to the retinoic acid (RA)-responsive element through their DNA binding domain, and activate transcription of many retinoid responsive genes. Both RAR and RXR have 3 subtypes, α, β, and γ. The α and β subtypes of RAR and RXR are broadly expressed in many cells and tissues in an overlapping manner, whereas RARγ and RXRγ appear to be expressed in a tissue-restricted manner.9 Because of the potential protective role for retinoids in various diseases, their in vivo functions have been a subject of considerable interest, particularly in mammalian species. Receptor-null mice generated by gene disruption have provided valuable animal models with which to study the role of the receptors and retinoids in vivo.10-12 However, due to the redundancy of both receptors, only limited aspects of retinoid action have been elucidated by this approach. Another, less exploited approach has been to study vitamin A–deficient rodents.13-17 By strictly regulating the dietary components, mice and other rodents can be rendered selectively deficient in vitamin A for an extended period of time. Studies of vitamin A–deficient (retinoid-ligand knockout) animals may have an advantage over receptor-null mice when studying retinoid action in adults, because this can be more difficult to assess in the latter model.18,19 Studies with vitamin A–deficient animals showed that retinoids profoundly affect physiology and gene expression in various tissues including germ cells, bone, and skin.13,15,16 In addition, a number of studies have demonstrated that vitamin A–deficient rodents fail to elicit full immune responses to viruses, bacteria, and other antigens.2,14,20 Results of these studies complement clinical observations linking vitamin A deficiency with increased susceptibility to infections in humans.1
This study was designed to investigate the effects of vitamin A deficiency on mouse hematopoietic cell growth. This work was prompted by previous observations made with tissue culture cells of hematopoietic origin, which showed that retinoids have a pivotal role in their development.21-24 We found a dramatic increase in granulocytes in virtually all deficient mice, which occurred at a relatively early stage of deficiency and persisted for the duration of dietary restriction. Supporting a direct role of retinoids in regulating myelopoiesis, on dietary supplementation with a physiologic dose of RA, the number of granulocytes fell to a nearly normal level. Our results indicate that this increase was due to impaired apoptosis of granulocytes. Further, we show that expression of RXRα and β is down-regulated in vitamin A–deficient mice, which may be linked to defective apoptosis.
Materials and methods
Vitamin A–deficient (RA−) and sufficient (RA+) mice
Vitamin A–deficient female mice of the SENCAR strain (NCI, Frederick, MD) were produced as described previously.13 16Briefly, pregnant mice were fed with lab chow diet (no. 8604, Harlan Teklad, Madison, WI). At birth, dams were divided into 2 groups. One group was fed the vitamin A–deficient (RA−) diet and the other was fed the same diet supplemented with all-trans RA (3 μg/g of the diet) (RA+). At weaning, the female pups were culled and maintained on their respective diet. To study the reversibility of the effect of retinoid deficiency, mice from the RA−group were switched to the RA+ diet beginning at 15 weeks of age and maintained on the RA+ diet for 2 weeks. At least 3 mice from each group were examined for the period of 10 weeks, starting at 8 weeks of age. All mice were given sterilized distilled water and maintained at 71 ± 3°F with a 12-hour light-dark cycle in a specific pathogen-free environment. Sera from some of the 14- to 18-week-old mice in both groups were tested for a standard set of bacterial and viral pathogens, including Car bacillus, lymphocytic choriomeningitis virus, murine cytomegalovirus, mycoplasma, polyoma, Reo 3, and Sendai virus, and were negative throughout experiments.
Measurement of liver retinol and retinylpalmitate levels
Liver tissues were homogenized and extracted in chloroform/methanol (2:1). The organic extracts were dried under nitrogen and the dry residues were extracted in ethanol, filtered, and analyzed for retinoid contents.13 (15-3H)-retinol was added to the liver samples to study recovery during high-performance liquid chromatography (HPLC) analysis. Analysis of retinol was performed on a Partisil 10 ODS-2 column (Whatman, Clifton, NJ) and detected with a UV detector (Gilson Medical Electronics, Middleton, WI) and a radiomatic radioactivity flow detector.25 Retinylpalmitate was eluted at 22′ using a C-130 guard column (Upchurch Scientific, Oak Harbor, WA) in series of a Waters C-18 (5 μm) “resolve” column with the detector set at 325 nm.26
Cytokine gene expression by RNAse protection assay
RNase protection analysis was performed using the multiprobe mouse cytokine template, mCK-2 (PharMingen, San Diego, CA), as detailed in our previous paper.27 Serum interleukin (IL)-1β (IL-1β) levels were measured by enzyme-linked immunosorbent assay using a kit according to the manufacturer's instructions (R& D Systems, Minneapolis, MN).
Flow cytometry analysis and histology
Cells (5 × 105) suspended in RPMI 1640 were first treated with anti-FcRγ antibody, washed, and then incubated with fluorescein isothiocyanate (FITC)-conjugated monoclonal antimouse CD4, anti-IgM, or anti–Mac-1 (CD11b) antibodies in combination with phycoerythrin (PE)-conjugated anti-CD8, anti-B220 (CD45), or anti–Gr-1(Ly-6G) antibodies, respectively (all antibodies from PharMingen). Stained cells were analyzed on a FACScan interfaced with the Cellquest software (Becton Dickinson, San Jose, CA). For reverse transcriptase–polymerase chain reaction (RT-PCR) analysis, granulocytes were purified from RA+ or RA−spleens by FACS sorting using FITC-labeled anti–Gr-1 antibody. Spleens of 6 mice were pooled prior to sorting. Sorting was performed on Becton Dickinson FACS Vantage Cell Sorter equipped with a Turbo-Sort option. The RA− sorted cells were 95% pure and RA+sorted cells were 90% pure.
For histologic studies, paraffin sections (5 μm) were prepared from spleens fixed in 10% formaldehyde and stained with hematoxylin-eosin. Blood smear and cytospin preparations of peripheral blood and bone marrow cells were stained with Wright-Giemsa solution.
Colony-forming assay
Colony-forming assays were performed using the methylcellulose-based media (Stem Cell Technology, Vancouver, Canada). Bone marrow cells (15 × 103) were cultured for 10 days in 1 mL of the media (MethoCult GF M 3534) designed to support the formation of colonies derived from the myeloid lineage for 10 days. The media contained 50 ng/mL of recombinant mouse stem cell factor, 10 ng/mL of recombinant murine IL-3 (rmIL-3) and 10 ng/mL of recombinant human IL-6, among other components. Colony-forming assays were also performed using a serum-free medium, MethoCult M3236, from the same vender.
TdT-mediated dUTP biotin nick end labeling (TUNEL) assay
Spleen cells (2 × 107) were incubated in 5 mL of RPMI 1640 supplemented with 10% fetal bovine serum (FBS) for 24 hours. Cells stained with PE-conjugated monoclonal antibodies to CD3, B220, or Gr-1 antibodies were fixed with 1% paraformaldehyde in PBS for 15 minutes, washed in PBS, and incubated in 1 nmol/L biotin-conjugated dUTP and 5 U terminal transferase (both from Boehringer Mannheim, IN) in 50 μL reaction mixture.28 29Cells were washed in sodium-citrate buffer (0.1% TritonX-100 and 5% dry milk in 4 × standard sodium citrate), incubated with 2.5 μL of avidin-FITC (Boehringer Mannheim) for 30 minutes at room temperature and then washed in Hanks balanced salt solution containing 0.1% TritonX-100 and 0.5% BSA. Stained cells were analyzed on FACScan interfaced with Cellquest.
Quantitative RT-PCR
Total RNA was prepared from Gr-1+ cells sorted from 5 spleens or from bone marrow of individual mice in RNAsol (Tel-Test, Friendswood, TX). First strand complementary DNA (cDNA) was synthesized with Superscript II (Life Technology, Rockville, MD) according to the manufacturer's instruction. PCR reaction was performed at 94°C for 1 minute, at 55°C for 1 minute, at 72°C for 1.5 minutes for 35 cycles in the presence of 250 μmol/L dNTPs, 2.5 mmol/L MgCl2, and 500 nmol/L of 5′ and 3′ primers.30,31 The following pairs of PCR primers were used. RARα 5′-: CCGCATCTACAAGCCTTGC 3′-: TCCAGGGAGACTCGTTGTTC, RARγ RARγ1 5′-:AGCCTGGCCCAGTATGTAGG, RARγ2, 5′-:ATCCCTTACCCCCCATGC, 3′-: CTTCACAGGAGCTGACCCCAT (RARγ common),12 RXRα 5′-;GGTGAACTCTTCGTCCCTCAACT 3′-: GTGAAGGAGGCCATATTTC, RARβ 5′-:TAGGACCCGCGCGCTCCGGAG, 3′-ATTGAGCAGTATGCCGGTGCT, RXRβ 5′-: AGCCCAGACAGCTCCTCCCCAAAT 3′-: GGACCACCTGGAGGGGGTGGA. Bax-1 5′-:GAGACACCTGAGCTGACCTT, 3′-GCACCAGTTTGCTAGCAAAG, BclX-L5′: GTGGCCTTTTTCTCCTTGG,3′-TTGAAGCGCTCCTGGCCTTT, MPO 5′:TGTCAGTGAGAGGAGTTGAC, 3′:ACCTCAGCTCAGGAAGTATC. Primer sets for other genes were synthesized as described previously.32 33PCR products were resolved on 1% agarose gel electrophoresis and stained with ethidium bromide. Bands visualized in the UV transilluminator were quantified in the Eagle-Eye system (Stratagene, CA).
Results
Establishment of vitamin A deficiency in female SENCAR mice (RA− mice)
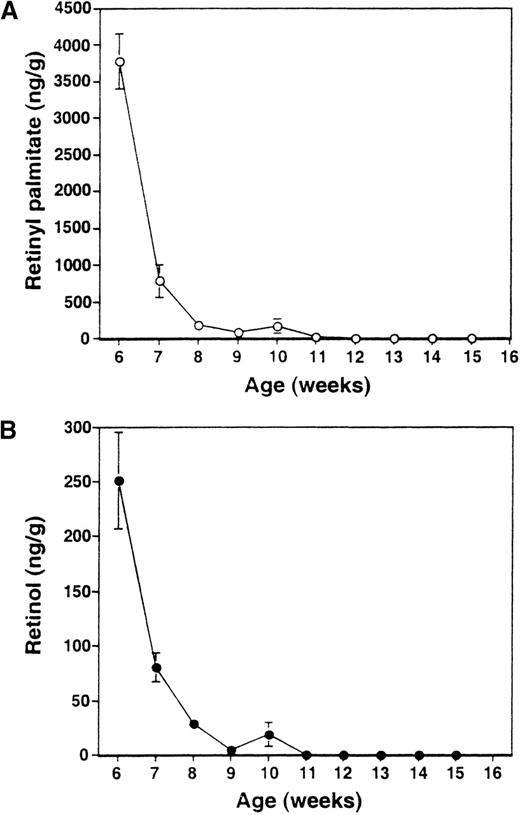
The dietary scheme used in this work has been previously shown to render mice completely depleted of retinoids.13,16 To ascertain that RA− mice studied in this work were likewise depleted of retinoids, we measured, by HPLC analysis, levels of retinylpalmitate and retinol in liver, where much of the retinoids are reposited.34 As seen in Figure1, the mean levels of retinylpalmitate and retinol both fell precipitously within 5 weeks after the initiation of dietary restriction. No retinol could be detected 2 weeks later, when mice were 10 weeks of age. Similarly, retinylpalmitate levels reached near zero levels starting at 8 weeks of feeding the deficient diet (11 weeks of age) and remained essentially undetectable thereafter. These results confirm that complete retinoid deficiency in mice can be attained by feeding a diet deficient in vitamin A, which provided a foundation for this work. The RA− mice did not show external signs of infection and their sera were negative for a panel of 13 pathogens routinely tested in an animal facility. To look for other signs of infection, expression of a series of cytokine genes known to be stimulated in response to infections35-37 was examined by RNase protection assay. Spleen samples from both RA−mice and control RA+ mice did not express detectable levels of IL-12p40, IL-1α, IL-1β, IL-6, and interferon-γ transcripts (not shown). In addition, serum levels of IL-1β in RA−mice were as low as control RA+ mice (17 and 18 pg/mL, respectively).
HPLC analysis of liver retinoids in RA−mice.
Levels of retinylpalmitate (A) and retinol (B) were measured each week starting 3 weeks after the initiation of RA− diet (6 weeks of age). The value represents the average of 4 mice ± SD.
HPLC analysis of liver retinoids in RA−mice.
Levels of retinylpalmitate (A) and retinol (B) were measured each week starting 3 weeks after the initiation of RA− diet (6 weeks of age). The value represents the average of 4 mice ± SD.
Systemic expansion of myeloid cells in vitamin A–deficient mice
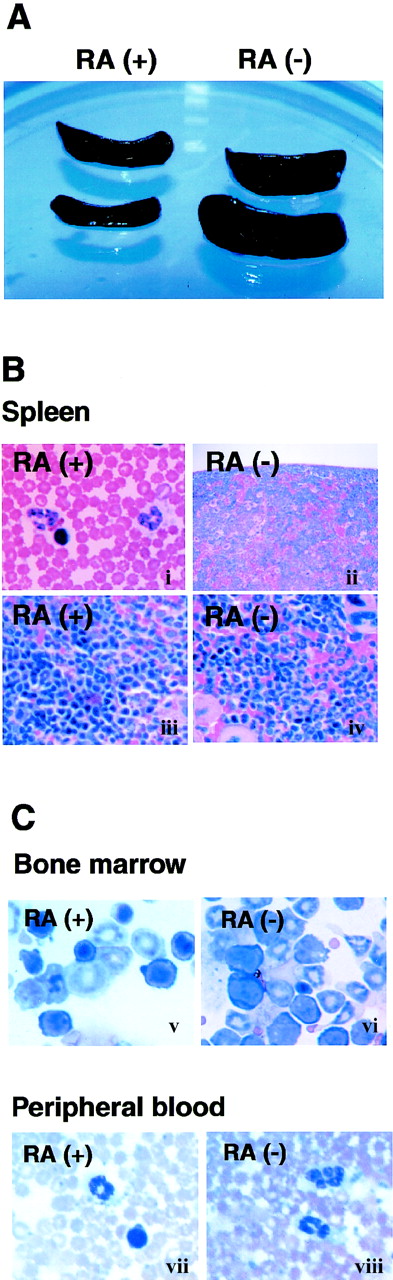
Lymphoid tissues from RA− mice were compared with those of age- and sex- matched RA+ mice. Almost all mice in the RA− group exhibited marked splenomegaly by 14 weeks of age, whereas spleens of RA+ mice were of normal size (Figure 2A). The splenomegaly coincided with a marked increase in total cell number; spleens from RA− mice contained 50% to 70% more cells than those of RA+ mice. Although less conspicuous, popliteal and mesenteric lymph nodes of RA− mice were also enlarged; however, thymi appeared unaltered (not shown). Histologic examination of RA− spleens revealed extensive infiltration of granulocytes obscuring the border between the white and red pulp (Figure 2B). The cellular composition of bone marrow was also drastically altered in RA− mice; cells of the myeloid lineage, both immature cells and mature granulocytes, were greatly increased in RA− bone marrow (Figure 2C). Peripheral blood of RA− mice also contained more granulocytes than in control mice: 2900 to 3000 cells in 1 μL of RA−blood and 375 to 415 cells in 1 μL in RA+ blood (Figure3B). Granulocytes in peripheral blood often exhibited unusual hypersegmentation (Figure 2C).
Expansion of granulocyte population in RA−mice.
(A) Spleens from 17-week-old mice fed with the RA+ or RA− diet. (B) Histology of spleens from RA+and RA− mice (17 weeks old). The border between the white pulp and red pulp is discernible in the RA+ spleen (i, iii), but not in the RA− spleen (ii, iv) due to overpopulation of granulocytes. (Original magnification: i and ii, × 100; iii and iv, × 400). (C) Cytology of bone marrow cells (i, ii) and peripheral blood (iii, iv) (original magnification × 630). Myeloid cells at various stages of maturation were increased in the RA− bone marrow, relative to the RA+ samples. Panels iii and iv illustrate hypersegmented nuclei in granulocytes of RA− peripheral blood, not found in RA+ cells.
Expansion of granulocyte population in RA−mice.
(A) Spleens from 17-week-old mice fed with the RA+ or RA− diet. (B) Histology of spleens from RA+and RA− mice (17 weeks old). The border between the white pulp and red pulp is discernible in the RA+ spleen (i, iii), but not in the RA− spleen (ii, iv) due to overpopulation of granulocytes. (Original magnification: i and ii, × 100; iii and iv, × 400). (C) Cytology of bone marrow cells (i, ii) and peripheral blood (iii, iv) (original magnification × 630). Myeloid cells at various stages of maturation were increased in the RA− bone marrow, relative to the RA+ samples. Panels iii and iv illustrate hypersegmented nuclei in granulocytes of RA− peripheral blood, not found in RA+ cells.
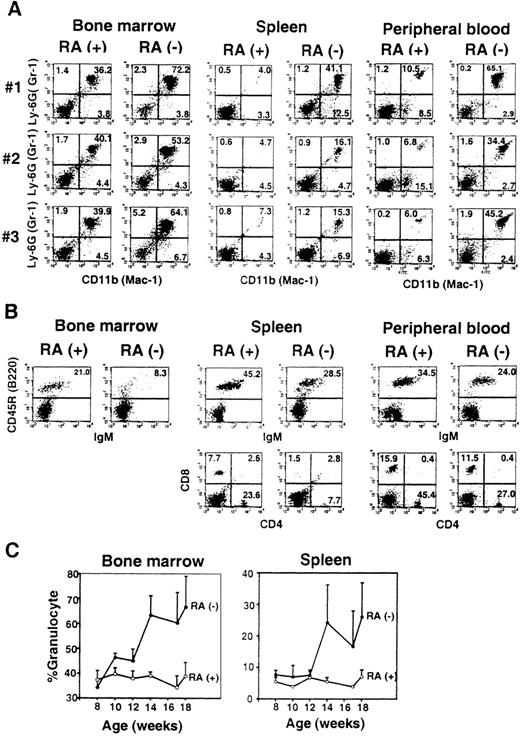
FACS analysis.
(A) Bone marrow, spleen, and peripheral blood cells from 3 individual mice (no. 1-3) at 14 weeks of age were analyzed for Gr-1 (Ly-6G) and Mac-1 (CD11b) staining. (B) Cells from 1 mouse (no. 1) from the RA+ and RA− group were tested for markers for B cells (B220/IgM) and T cells (CD4/CD8). (C) Time course analysis. Percent Mac-1+/Gr-1+ cells in bone marrow and spleen were determined by FACS analysis every 2 weeks. The values represent the average of 3 to 4 mice ± SD.
FACS analysis.
(A) Bone marrow, spleen, and peripheral blood cells from 3 individual mice (no. 1-3) at 14 weeks of age were analyzed for Gr-1 (Ly-6G) and Mac-1 (CD11b) staining. (B) Cells from 1 mouse (no. 1) from the RA+ and RA− group were tested for markers for B cells (B220/IgM) and T cells (CD4/CD8). (C) Time course analysis. Percent Mac-1+/Gr-1+ cells in bone marrow and spleen were determined by FACS analysis every 2 weeks. The values represent the average of 3 to 4 mice ± SD.
Flow cytometry analysis was performed to detect Mac-1+(CD11b)+ and Gr-1 (Ly-6G)+cells in bone marrow, spleen, and peripheral blood in 3 individual RA− and RA+ mice (Figure 3A). There were consistent and substantial increases in Mac-1+/Gr-1+ cells for all samples in RA− mice. Mac-1+/Gr-1+ cells in bone marrow consisted of both granulocytes and myeloid precursors, whereas in spleen this population consisted almost entirely of granulocytes in both RA+ and RA− mice. Mac-1+/Gr-1+ cells in RA− spleens were of the mature type, because they were devoid of myeloperoxidase transcripts, a marker for immature myeloid cells (not shown).38 Mac-1+/Gr-1− cells were also increased in spleens of some mice (eg, mouse no. 1), but not in others (eg, mouse no. 2). These changes reflected the marked increase in the total number of Mac-1+/GR-1+cells; their number in RA+ and RA− femurs were 4 to 6 × 106 and 6.5 to 8 × 106, respectively, and those in RA+and RA− spleens were 4 to 6 × 106 and 2 to 5 × 107, respectively.
In contrast to the marked expansion of granulocytes, total numbers of B and T lymphocytes in RA− mice were comparable to those of RA+ mice, although their frequencies were decreased in RA− mice, due to the increase in granulocyte numbers (Figure 3B).
Figure 3C shows the kinetics of changes in Mac-1+/Gr-1+cell population over 10 weeks of retinoid deficiency, starting at 8 weeks of age. In bone marrow, an increase was evident as early as 10 weeks, which coincided with the time when liver retinol and retinylpalmitate were completely depleted (Figure 1). Levels of Mac-1+/Gr-1+ cells rose significantly after 14 weeks and remained high thereafter. In spleen, an increase in granulocytes was detected at 14 weeks, significantly later than that in bone marrow.
Reversal of granulocyte increase after dietary RA repletion
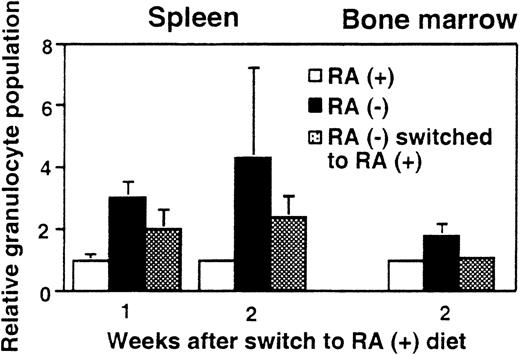
To test whether the observed granulocyte increase is reversible, 15-week-old mice maintained on the deficient diet were switched to the diet containing RA (3 μg/g diet). Figure4 shows the percentage of Mac-1+/Gr-1+ cells in spleen and bone marrow after RA supplementation. As expected, mice fed continuously with the RA− diet had more than twice the frequency of Mac-1+/Gr-1+cells as those fed continuously with the RA+ diet. However, when RA− mice were supplemented with RA for 2 weeks, the frequency of Mac-1+/Gr-1+ cells in bone marrow fell to an essentially normal level (Figure 4), although 1 week of RA supplementation did not have an effect (not shown). In spleen, RA-supplemented mice showed a significant reduction in granulocytes at 1 week and further reduction in 2 weeks, although at the end of 2 weeks, their frequency was still slightly higher in RA-supplemented mice than in control RA+ mice. Thus, vitamin A–deficient mice can respond to RA and recover from abnormal expansion of myeloid cells. The rapid reversibility by RA indicates that granulocyte expansion is a direct result of vitamin A deficiency, rather than that of an irreversible, secondary change.
Reversal of granulocyte expansion following dietary RA repletion.
Mice aged 15 weeks maintained on the RA− diet were switched to the RA+ diet for 1 or 2 weeks and the percent Mac-1+/Gr-1+ cells in spleen and bone marrow was determined. RA− mice continued on the deficient diet, whereas RA+ mice continued the diet with RA. In each graph, percent granuloctyes from RA+ mice are taken as 1. The values represent the average of 3 mice.
Reversal of granulocyte expansion following dietary RA repletion.
Mice aged 15 weeks maintained on the RA− diet were switched to the RA+ diet for 1 or 2 weeks and the percent Mac-1+/Gr-1+ cells in spleen and bone marrow was determined. RA− mice continued on the deficient diet, whereas RA+ mice continued the diet with RA. In each graph, percent granuloctyes from RA+ mice are taken as 1. The values represent the average of 3 mice.
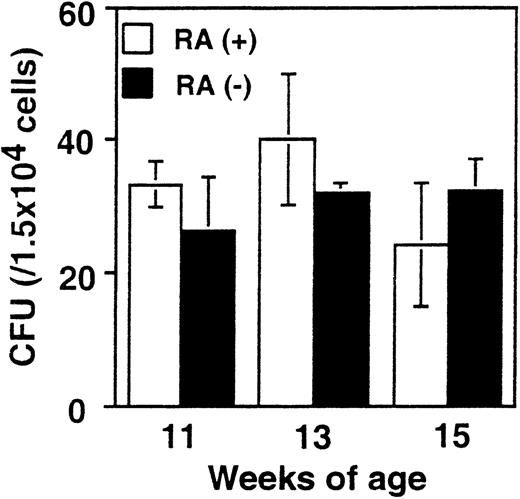
Comparable colony formation by RA+ and RA− bone marrow cells
It was important to assess whether the increase in myeloid cells in RA− mice is attributed to alterations in the frequency of myeloid cell progenitors. To this end, in vitro colony-forming assays were performed with bone marrow cells from RA+ and RA− mice using media designed to support the growth of myeloid cell progenitors (see “Materials and methods”). Results obtained from mice at 11, 13, and 15 weeks are shown in Figure5. The number of colonies formed by RA+ and RA− bone marrow was similar in all cases (20-40/104 cells). Differential counting of the number of colony-forming units–granulocyte, –macrophage, and –granulocyte/macrophage also yielded comparable results with RA+ and RA− cells. To exclude the possibility that retinoids that might be present in the FBS in the media could have obscured a difference in the 2 groups, similar assays were performed with serum-free medium. Again, no significant difference was observed between the 2 groups, ranging from 10/104 to 13/104 cells. These results indicate that the frequency of progenitor cells capable of generating myeloid cell colonies was not affected in RA− mice.
Colony formation efficiency in RA+ and RA− bone marrow.
Bone marrow cells from RA+ or RA− mice aged 11, 13, and 15 weeks were cultured in the methylcellulose-based colony formation media for 10 days. The values represent the average colony-forming units obtained from 3 mice ± SD.
Colony formation efficiency in RA+ and RA− bone marrow.
Bone marrow cells from RA+ or RA− mice aged 11, 13, and 15 weeks were cultured in the methylcellulose-based colony formation media for 10 days. The values represent the average colony-forming units obtained from 3 mice ± SD.
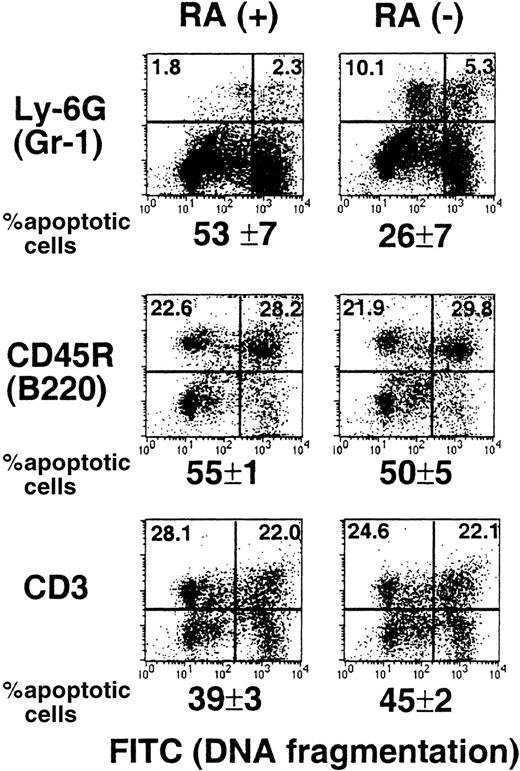
Reduced spontaneous apoptosis of RA−granulocytes
Apoptosis is a normal process in the life cycle of myeloid cells.39,40 Retinoids may have a role in this process because they have been shown to promote apoptosis in various leukemic cells of myeloid origin.22,23 It is possible that myeloid cells in RA− mice are defective in apopotosis, causing an abnormal increase in their number. To examine this possibility, TUNEL assay was used to detect spontaneous apoptosis of spleen cells from RA+ and RA− mice. Briefly, cells were cultured overnight, marked for Gr-1, B220, or CD3, and then subjected to TUNEL assay.29 Results obtained from 15-week-old mice are shown in Figure 6. Neither B (B220+) nor T cells (CD3+) from the 2 groups showed a significant difference in the extent of apoptosis. In contrast, the frequency of apoptotic cells in Gr-1+ cells from RA− mice was significantly lower than that from RA+ mice (26% versus 53%). As might have been expected, the frequency of apoptotic cells in total spleen cells were roughly comparable between the RA+ and RA− groups (36%). Similar TUNEL assays were performed with 17- and 18-week-old mice, and the percent apoptotic cells in the Gr-1+ cell population was again lower in RA− mice than that in RA+ mice; the frequency of apoptotic cells in the RA− group ranged from 32% to 41%, whereas that in the RA+ group ranged from 50% to 57%. These results indicate that vitamin A deficiency leads to a reduction in spontaneous apoptosis of the granulocyte population, although it does not completely eliminate apoptosis.
Impaired spontaneous apoptosis in RA−granulocytes.
Spleen cells from a representative RA+ or RA−mouse (15 weeks old) were cultured overnight and stained with PE-labeled antibodies to Gr-1, B220, and CD3, and then subjected to TUNEL assay. The X axis (FITC) represents DNA fragmentation and the Y axis cell surface markers. The number below the FACS profile represents percent apoptotic cells of 3 mice ± SD.
Impaired spontaneous apoptosis in RA−granulocytes.
Spleen cells from a representative RA+ or RA−mouse (15 weeks old) were cultured overnight and stained with PE-labeled antibodies to Gr-1, B220, and CD3, and then subjected to TUNEL assay. The X axis (FITC) represents DNA fragmentation and the Y axis cell surface markers. The number below the FACS profile represents percent apoptotic cells of 3 mice ± SD.
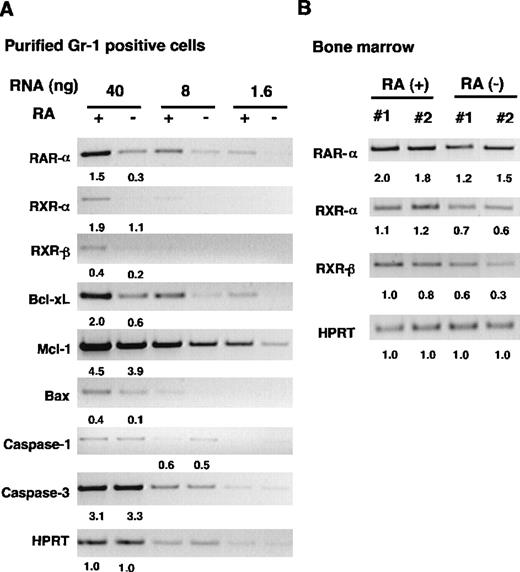
Down-regulation of RAR and RXR expression in RA− granulocytes
To gain insight into the molecular mechanism by which vitamin A deficiency causes granulocyte expansion, we examined expression of 2 nuclear receptors, RAR and RXR, major mediators of retinoid action.6-8 Semiquantitative PCR analysis was used to detect α and β subtypes of the receptors. We also tested RARγ because this receptor is expressed in bone marrow Gr-1+cells.12 To avoid a potential complication derived from cellular heterogeneity, PCR analysis was performed with FACS-purified granulocytes obtained from spleens. Three serially diluted RNA samples were tested to ensure a linear range of reaction. Neither RARβ nor RARγ was detected in any samples tested (not shown). By contrast, expression of RARα, RXRα, and RXRβ was clearly detectable in the samples from both groups (Figure 7A). Importantly, expression of the 3 receptors was significantly lower in RA− cells than in RA+ cells. Levels of hypoxanthine-guanine phosphoribosyltransferase (HPRT) RNA, run as a control, were comparable in the 2 samples. To verify down-regulation of RAR and RXR in RA− cells, we tested bone marrow RNA from 2 individual mice in each group. As seen in Figure7B, levels of RARα, RXRα, and RXRβ were again lower in RA− cells than in RA+ cells. Expression of RARβ was undetectable in bone marrow RNA, similar to the results seen with purified granulocytes. Thus, expression of RAR and RXR is down-regulated in myeloid cells in vitamin A–deficient mice, indicating that expression of these receptors is regulated by the retinoid status of the cells.
RT-PCR analysis of gene expression in RA+and RA− granuloctyes.
(A) Analysis of FACS-purified granulocytes. Serially diluted RNAs from RA+ and RA− granulocytes were subjected to RT-PCR analysis for indicated genes using specific primers (see “Materials and methods”). (B) RT-PCR analysis of bone marrow samples from 16-week-old mice. The numbers indicate relative levels of transcripts.
RT-PCR analysis of gene expression in RA+and RA− granuloctyes.
(A) Analysis of FACS-purified granulocytes. Serially diluted RNAs from RA+ and RA− granulocytes were subjected to RT-PCR analysis for indicated genes using specific primers (see “Materials and methods”). (B) RT-PCR analysis of bone marrow samples from 16-week-old mice. The numbers indicate relative levels of transcripts.
Impaired apoptosis in RA− granulocytes noted above prompted us to examine expression of genes involved in apoptosis. Caspase-1 and caspase-3 were tested because they are thought to act at the effector phase and induce cell death by cleaving various substrates.33,41 We also tested expression of genes belonging to the Bcl-2 family,42 because some members of the family are shown to protect cells from apoptosis and prolong survival of myeloid leukemia cells.43 As seen in Figure 7A, levels of transcripts for caspase-1 and caspase-3 were similar in RA+ and RA− granulocytes, whereas those for Bcl-2 family members with anti-apoptotic property, Bcl-xL and Mcl-1,40,42 were lower in RA− cells than in RA+ cells. Expression of Bax, a pro-apoptotic gene, was also reduced in RA− cells. Although Bcl-2 is expressed in human myeloid cell lines,44 the transcripts were undetectable in these samples (not shown). Levels of Bcl-xL and Mcl-1RNAs were also reduced in RA− bone marrow samples (not shown), suggesting that these genes are down-regulated, not only in granulocytes, but in other cells in bone marrow. Taken together, these results indicate that mechanisms underlying impaired apoptosis in RA− granulocytes are complex and may not be dependent on changes in the expression of caspases or Bcl-2 family members.
Discussion
We have shown that vitamin A deficiency leads to a striking expansion of myeloid cells in mice. The increase in Mac-1+/ Gr-1+ cells occurred at an early stage of deficiency and persisted throughout the remaining period. The increase was first noted in bone marrow and subsequently spread to peripheral tissues, indicating that the abnormality originates in bone marrow. The onset of the abnormality was noticeable within 1 to 2 weeks of retinoid depletion in liver and established 5 to 7 weeks after the initiation of the RA− diet. The incidence of granulocyte expansion was remarkably high, seen in practically 100% of RA− mice by 14 weeks of age, which was in contrast to the total absence of abnormality in the RA+ group. In addition to Mac-1+/ Gr-1+ cells, Mac-1+/ Gr-1−cells were also increased both in bone marrow and spleen, albeit to a modest level, and at a lower incidence (∼ 65%). Another remarkable aspect of granulocyte expansion is its ready reversibility on dietary switch from the RA− to the RA+ diet (Figure 4). In bone marrow, myeloid counts returned to a normal level in 2 weeks after switch. The almost 100% incidence of abnormalities in RA− mice and rapid reversibility on addition of RA indicate that the expansion of granulocytes is a direct consequence of vitamin A deficiency, rather than due to secondary changes such as alterations in the cytokine network or of opportunistic infection. In support of this idea, RA− mice showed no increase in expression of proinflammatory cytokine genes, and their sera were negative for a panel of routine pathogens. Although we believe that this is the first comprehensive report on the systemic granulocyte expansion caused by vitamin A deficiency in mice, our results are in line with previous observations that vitamin A–deficient rats have higher granulocyte counts in peripheral blood.45
Despite the absence of marked alterations in the number of T and B lymphocytes in RA− mice, extensive literature exists documenting that vitamin A–deficient rodents are defective in both T-cell–mediated and antibody-dependent immune responses.14,20 Consequently, vitamin A–deficient rats are highly susceptible to various infectious pathogens,2indicating that the retinoid status governs broad aspects of immune responses. In light of these results, it might be of interest to study the functionality of RA− granulocytes.
Results obtained with this animal model are relevant to many studies performed with human myeloid cells in culture, showing that retinoids affect myeloid cell growth, differentiation, and apoptosis.21-24 The ability of RA to induce differentiation has been demonstrated for the cells of acute promyelocytic leukemia, in which RA can confer clinical remission.4,5 RARs, rather than RXRs, appear to play a dominant role in inducing differentiation in these cells. Although these reports may suggest that granulocytes in RA− mice are deficient in differentiation, we found that they were morphologically mature, exhibit high Gr-1 staining46 (Figures 2 and 4) and were negative for the expression of myeloperoxidase, as well as RARγ, both expressed in immature granulocytes.12 38 Although a clear explanation is not available, it is possible that malignant myeloid cells are more responsive to RA and differentiate more readily than normal myeloid cells in vivo. Alternatively, retinoids may be more efficient in inducing differentiation in human than mouse cells.
Our data indicate that the frequency of myeloid progenitor cells is not affected in RA− bone marrow, because the efficiency of colony formation in vitro was unaffected (Figure 5). However, granulocytes in RA− mice were defective in apoptosis, as evidenced by reduced spontaneous cell death. Contrary to results with granulocytes, apoptosis in T- and B-cell populations was not significantly changed in RA− mice (Figure 6). Thus, the expansion of granulocytes seen in RA− mice may be partly attributed to the impaired apoptosis. Apoptosis is believed to be a normal part of the myeloid cell life cycle.39,40 Our data indicate that retinoids play a critical role in regulating this process in vivo, consistent with reports on cultured cells.22,23How vitamin A deficiency leads to impaired apoptosis in myeloid cells is currently not fully known. The Bcl family does not appear to play a major role in this process, because expression of anti-apoptotic genes such as Bcl-xL and Mcl-1 was not increased in RA− cells. Similarly, it is not likely that this defect involves expression of effector caspases-1 and -3 because their RNA levels are comparable in the 2 groups. Rather, RA-mediated apoptosis may involve retinoid receptors themselves, because retinoid-induced apoptosis in HL-60 cells is shown to be dependent on activation of RXR, but not RAR.22 23 Thus, decreased RXR expression in RA− granulocytes may diminish the ability of these cells to undergo spontaneous apoptosis.
Down-regulation of RXRs observed in RA− granulocytes indicates that expression of RXRs is affected by retinoid levels in mice. This is interesting, because RXRs are thought to be expressed constitutively and, to our knowledge, their regulation by retinoids has not been described before. At present we do not know the mechanism by which vitamin A deficiency results in down-regulation of RXRs. Because RXRs are not immediately induced by retinoids, and the presence of RAR is not reported for an RXR gene,47 this down-regulation may be due to an indirect mechanism. Irrespective of the mechanism, a direct consequence of RXR down-regulation may be the reduced apoptosis in myeloid cells. In addition, because RXR is a heterodimer partner for a number of other nuclear receptors,6-8 the reduced RXR expression may have a negative impact on the ability of cells to respond to other ligands and hormones. In light of the results that RARα is also strongly down-regulated in RA− cells, the overall retinoid signaling pathway seems substantially attenuated in RA− cells.
The increase in granulocytes found in vitamin A–deficient mice is reminiscent of that found in ICSBP −/− mice, in which granulocytes are markedly increased,48 and which is also attributed, at least partly, to a defect in spontaneous apoptosis.29 In light of the association between oncogenesis of myeloid cells and apoptosis,39,43,49 and the proposed role for ICSBP in myelogenous leukemia,48 the retinoid status may have an important role in the pathogenesis of various myeproliferative disorders.
Acknowledgments
We thank David Stephany and Ruth Swofford in the NIAID Flow Cytometry Unit for FACS analysis and granulocyte sorting, and Susan Kirkhoff for preparation of mice.
Reprints:Keiko Ozato, Laboratory of Molecular Growth Regulation, Bldg 6, Rm 2A01, National Institute of Child Health and Human Development, National Institutes of Health, Bethesda, MD 20892-2753; e-mail: ozatok@nih.gov.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal