Abstract
Chronic neutropenia, often associated with rheumatoid arthritis, is a characteristic finding in large granular lymphocyte (LGL) leukemia. The mechanism of neutropenia is not known. Normal neutrophil survival is regulated by the Fas–Fas ligand apoptotic system. We hypothesized that neutropenia in LGL leukemia is mediated by dysregulated expression of Fas ligand. Levels of Fas ligand in serum samples from patients with LGL leukemia were measured with a Fas ligand enzyme-linked immunosorbent assay. The effects of serum from patients with LGL leukemia on apoptosis of normal neutrophils were determined by flow cytometry and morphologic assessment. High levels of circulating Fas ligand were detected in 39 of 44 serum samples from patients with LGL leukemia. In contrast, Fas ligand was undetectable in 10 samples from healthy donors. Serum from the patients triggered apoptosis of normal neutrophils that depended partly on the Fas pathway. Resolution of neutropenia was associated with disappearance or marked reduction in Fas ligand levels in 10 of 11 treated patients. These data suggest that high levels of Fas ligand are a pathogenetic mechanism in human disease.
Clonal diseases of large granular lymphocytes (LGLs) result from proliferation of either CD3-negative (CD−) or CD3-positive (CD3+) lymphocytes and have been designated natural killer (NK) and T-LGL leukemia, respectively.1 LGLs comprise 10% to 15% of peripheral blood mononuclear cells.2 CD3− LGLs are NK cells that mediate non-major histocompatibility complex (MHC)–restricted cytotoxicity and do not express the CD3–T-cell receptor (TCR) complex or rearrange TCR genes. CD3+ LGLs are T cells that express the CD3–TCR complex and rearrange TCR genes. These cells mediate non-MHC–restricted cytotoxicity in vitro and are thought to represent in vivo activated cytotoxic T cells.3 Patients with LGL leukemia have marked rates of morbidity and mortality from bacterial infections acquired during severe neutropenia. In some patients, severe anemia is a prominent feature.4
Leukemic LGLs constitutively express high levels of Fas ligand.5-7 Fas ligand, a member of the tumor necrosis factor (TNF) family, induces apoptosis by binding to its receptor, Fas, which is also known as APO-1 or CD95.8,9 Fas ligand is synthesized as a type II membrane protein and then cleaved from the membrane by a metalloproteinase.10 The physiologic role of shedding of TNF family members has not been well characterized. It is not known whether high levels of circulating Fas ligand can cause human disease. Because Fas is expressed ubiquitously in a variety of normal cells, it is possible that systemic expression of Fas ligand is pathologic.8 Indeed, treatment of mice with anti-Fas antibody, which mimics the actions of Fas ligand, causes hepatic necrosis.11
The mechanism causing neutropenia in LGL leukemia has not been defined. It is known that neutrophils undergo apoptosis through Fas triggering.12 Furthermore, growth of hematopoietic colonies in vitro can be negatively regulated by activation of the Fas pathway.13 Therefore, in this study, we examined the possibility that circulating Fas ligand is involved in mediating neutropenia in LGL leukemia. Our results suggest that high levels of Fas ligand contribute directly to a manifestation of human disease.
Patients, materials, and methods
Patients
All patients met clinical criteria of T-LGL leukemia, with increased LGL counts ranging from 0.6 to 27 × 109/L (normal, 0.223 ± 0.099 × 109/L) and evidence of clonal TCR gene rearrangement. Two patients had an aggressive form of LGL leukemia (unpublished data). Serum samples were obtained from the patients at diagnosis, before initiation of treatment. Additional samples were obtained from some patients while they were receiving treatment.
Detection of Fas ligand
Quantitative assessment of the amount of Fas ligand circulating in serum from patients was done with a Fas ligand enzyme-linked immunosorbent assay (ELISA) (MBL, Nagoya, Japan). Serum samples from 10 healthy donors were used as controls. The sensitivity of this assay for detecting Fas ligand is 0.1 mmol/L.
Immunoblotting
Detection of soluble Fas ligand in serum samples from 9 patients with LGL leukemia and 5 healthy donors was done with Western blot analysis. The samples (20 μL) were boiled for 5 minutes in Laemmli sample-loading buffer for sodium dodecyl sulfate (SDS)–polyacrylamide gel electrophoresis and separated on a 10% SDS-polyacrylamide gel. The proteins were then transferred to Immobilon membranes and allowed to react with the anti-Fas ligand antibody (clone C20; Santa Cruz Biotechnology, Santa Cruz, CA) for Western blot analysis. The proteins were detected by an enhanced chemiluminescence detection system (Amersham, Piscataway, NJ), according to the manufacturer's recommendations.
Preparation of polymorphonuclear neutrophils (PMN)
Leukocyte buffy coats from healthy volunteers were obtained from the Southwest Florida Blood Bank (Tampa, FL) and purified as described previously.14 After Ficoll-Hypaque separation at 400g for 30 minutes at room temperature, the layer PMN on the surface of erythrocyte cell pellet was collected, and contaminating erythrocytes were lysed by hypotonic shock with sterile distilled water for 30 seconds. The PMN were then washed twice in phosphate-buffered saline (PBS). Purity of the PMN was more than 95% on morphologic assessment.14 All procedures were performed with endotoxin-free media and supplies to avoid nonspecific activation of PMN.
Apoptosis assays of PMN
Apoptosis was determined by both flow cytometry15 and a Diff-Quik Stain as described previously.14 Briefly, cultured PMN were washed once with PBS, and 1 mL of hypotonic propidium iodide (PI) solution (50 g/mL PI in 0.1% sodium citrate solution plus 0.1% Triton X-100) was added. Cells were kept overnight at 4°C and then analyzed in their staining solution on a flow cytometer (Becton Dickinson, San Jose, CA). The morphologic features of apoptotic PMN were also studied with use of staining with a Diff-Quik Stain Set. At least 500 cells/slide were counted for assessment of the percentage of cells showing apoptotic morphologic features.
To examine whether Fas ligand secreted by leukemic LGLs is functional and responsible for induction of neutropenia, PMN from healthy donors were incubated in RPMI 1640 media containing 10% serum from healthy donors or patients with LGL leukemia. One million PMN were also incubated in the presence or absence of 100 ng/mL of apoptosis-inducing anti-Fas antibody CH11 (Kamiya Biomedical, Tukwilla, WA) for 1 day at 37°C as a positive control. To block apoptosis induced by Fas ligand, some PMN were treated with 500 ng/mL of a blocking antibody (ZB4; Kamiya Biomedical) for 1 hour before the apoptosis experiments. As a control for specificity of the anti-Fas blocking antibody, PMN were also incubated for 1 hour before the apoptosis experiments with an irrelevant, IgG1-isotype control monoclonal antibody. Kinetic studies were also done to examine whether PMN from patients with LGL leukemia were more susceptible than PMN from healthy donors to apoptosis induced by anti-Fas monoclonal antibody.
Results
Constitutive expression of high levels of Fas ligand in serum from patients with LGL leukemia
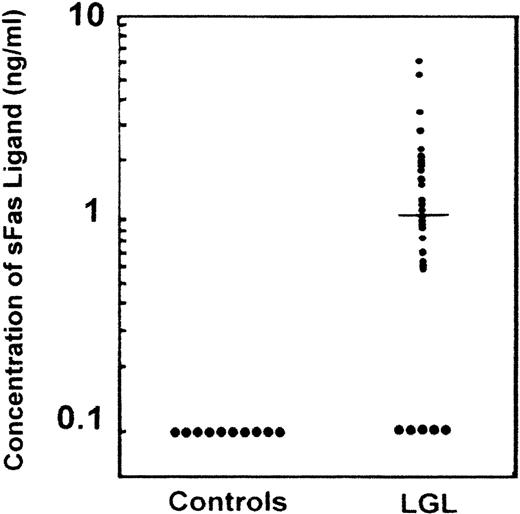
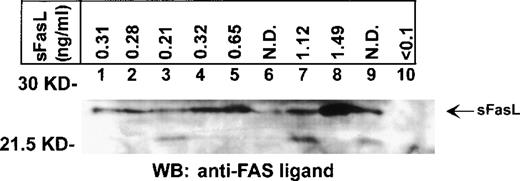
We previously detected constitutive expression of Fas ligand gene transcripts and protein in leukemic LGLs.6 7 In this study we detected circulating Fas ligand by ELISA assessment in 39 of 44 serum samples from patients with LGL leukemia (Figure1). In contrast, Fas ligand was undetectable in all 10 samples from healthy donors. Elevated levels of Fas ligand were found in 31 of 34 serum samples from patients with LGL leukemia and neutropenia. Therefore, there was a significant association between detectable levels of Fas ligand and neutropenia (P < .001). All but 1 of the 31 patients with elevated Fas ligand levels had severe neutropenia, with neutrophil counts below 0.5 × 109/L. The remaining patient had moderate neutropenia (1.5 × 109/L) but a clinically aggressive form of LGL leukemia with massive hepatosplenomegaly. Two of the 3 patients with undetectable levels of Fas ligand had only moderate neutropenia (1.2 × 109/L and 1.7 × 109/L, respectively). Elevated levels of Fas ligand were also found in 8 of 10 patients with transfusion-dependent anemia. In 1 patient with undetectable Fas ligand, the mechanism of anemia was thought to be autoimmune mediated. The mean level of circulating Fas ligand in the patients with LGL leukemia was 1.00 mmol/L (median, 0.62 mmol/L; Figure 1). Detection of Fas ligand in serum samples from patients (n = 9) and its absence in samples from healthy donors (n = 5) was confirmed by immunoblotting (Figure 2).
Detection of circulating Fas ligand in serum from patients with large granular lymphocyte (LGL) leukemia.
Serum samples from healthy donors (n = 10) and from patients with LGL leukemia (n = 44) were tested for the presence of Fas ligand by using a Fas ligand enzyme-linked immunosorbent assay (ELISA). The threshold for detecting Fas ligand was 0.1 mmol/L.
Detection of circulating Fas ligand in serum from patients with large granular lymphocyte (LGL) leukemia.
Serum samples from healthy donors (n = 10) and from patients with LGL leukemia (n = 44) were tested for the presence of Fas ligand by using a Fas ligand enzyme-linked immunosorbent assay (ELISA). The threshold for detecting Fas ligand was 0.1 mmol/L.
Detection of Fas ligand in serum from patients by Western blot analysis.
Serum samples from 9 patients with LGL leukemia (lanes 1-9) and 1 healthy donor (lane 10) were fractionated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis and subjected to Western immunoblotting with an anti-Fas ligand antibody that recognizes the soluble form of Fas ligand. Arrow indicates the position of the 26-kd soluble Fas ligand protein. The levels of soluble Fas ligand in the serum determined by ELISA are shown in the upper panel. Each blot is representative of 3 experiments.
Detection of Fas ligand in serum from patients by Western blot analysis.
Serum samples from 9 patients with LGL leukemia (lanes 1-9) and 1 healthy donor (lane 10) were fractionated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis and subjected to Western immunoblotting with an anti-Fas ligand antibody that recognizes the soluble form of Fas ligand. Arrow indicates the position of the 26-kd soluble Fas ligand protein. The levels of soluble Fas ligand in the serum determined by ELISA are shown in the upper panel. Each blot is representative of 3 experiments.
Mediation of Fas-dependent apoptosis of normal PMN by serum from patients with LGL leukemia
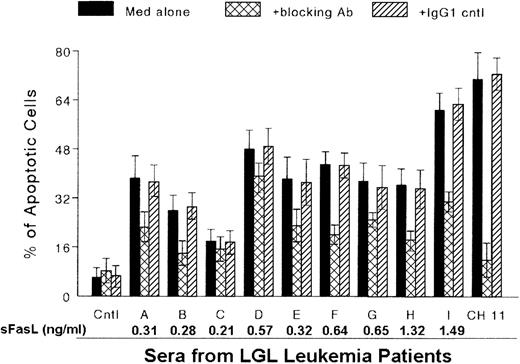
We investigated whether serum from patients with LGL leukemia and severe neutropenia (neutrophil count < 0.5 × 109/L) would induce apoptosis of normal PMN through a Fas-dependent mechanism. We first examined PI-stained PMN from healthy donors to detect the rate of spontaneous apoptosis of PMN. We found that 90% to 95% of PMN were still viable after culturing for 24 hours in complete medium with or without 5% to 10% normal human serum. In contrast, a 1:10 dilution of serum from patients with LGL caused apoptosis of normal PMN, in proportions ranging from 18% ± 3.8% to 61% ± 5.6% (Figure3). Prior treatment of normal PMN with a neutralizing antibody to Fas inhibited apoptosis produced by each LGL leukemia serum sample, with a range of inhibition of 15% to 53%. These results show that the apoptosis triggered by serum from patients with LGL leukemia occurred partly through the Fas pathway.
Induction of apoptosis of normal polymorphonuclear neutrophils (PMN) with serum from patients with LGL leukemia.
PMN were purified from healthy donors and incubated for 1 day in RPMI medium with 10% normal human serum in the absence (Cntl) and presence of anti-Fas monoclonal antibody (MAb) CH11 (100 ng/mL) or with serum from patients with LGL leukemia (A-I). Apoptotic cells were examined with use of both a Diff-Quik Stain Set and flow cytometry. Apoptosis induced by serum from patients with LGL leukemia was inhibited with a blocking anti-Fas MAb (ZB4). In contrast, no inhibition of apoptosis induced by serum from patients was observed with the addition of an irrelevant, IgG1-isotype control MAb. Data represent results of 3 separate experiments.
Induction of apoptosis of normal polymorphonuclear neutrophils (PMN) with serum from patients with LGL leukemia.
PMN were purified from healthy donors and incubated for 1 day in RPMI medium with 10% normal human serum in the absence (Cntl) and presence of anti-Fas monoclonal antibody (MAb) CH11 (100 ng/mL) or with serum from patients with LGL leukemia (A-I). Apoptotic cells were examined with use of both a Diff-Quik Stain Set and flow cytometry. Apoptosis induced by serum from patients with LGL leukemia was inhibited with a blocking anti-Fas MAb (ZB4). In contrast, no inhibition of apoptosis induced by serum from patients was observed with the addition of an irrelevant, IgG1-isotype control MAb. Data represent results of 3 separate experiments.
Clinical responses to treatment
Levels of Fas ligand were measured in patients responding to treatment for neutropenia or anemia (Table1). Indications for treatment included severe neutropenia (neutrophil count < 0.5 × 109/L) or transfusion-dependent anemia. The response to oral low-dose methotrexate for treatment of neutropenia in patients 1 to 6 was described previously.16 Levels of Fas ligand were markedly reduced or not detectable in 12 of 13 patients who had a response to treatment. Three patients with a partial response had resolution of neutropenia or anemia with therapy; however, increased numbers of LGLs persisted in the peripheral blood. Fas ligand levels appeared to be reduced to similar levels in patients with a partial response and those with a complete response (Table 1). Similarly, among patients who had a complete clinical response, Fas ligand levels were reduced to comparable levels in patients with molecular remissions and those in whom the abnormal clone could still be detected by Southern blot analysis. The spontaneous resolution of neutropenia observed in Patient 12 was also associated with undetectable levels of circulating Fas ligand. Treatment was stopped in patients 5, 13, and 14 after resolution of neutropenia or anemia, but all 3 subsequently required additional treatment because of recurrent cytopenias. Fas ligand levels in patient 13 fell to undetectable levels at remission but rose to 0.61 mmol/L at relapse, a level similar to that observed at initial presentation.
Decrease in levels of Fas ligand with treatment (Rx) in 14 patients with large granulocyte lymphocyte leukemia
| Patient . | Indication for Rx . | Rx . | Clinical Response . | Molecular Response . | Fas Ligand Before Rx (mmol/L) . | Fas Ligand During Rx (mmol/L) . |
|---|---|---|---|---|---|---|
| 1 | Neutropenia | MTX | CR | Yes | 0.56 | Undetectable |
| 2 | Neutropenia | MTX | CR | No | 0.28 | Undetectable |
| 3 | Neutropenia | MTX | CR | Yes | 0.21 | Undetectable |
| 4 | Neutropenia | MTX | CR | No | 0.57 | 0.12 |
| 5 | Neutropenia | MTX | CR | Yes | 0.32 | Undetectable |
| 6 | Neutropenia | MTX | PR | — | 0.64 | 0.27 |
| 7 | Neutropenia | MTX | PR | — | 0.54 | Undetectable |
| 8 | Neutropenia | MTX | CR | ND | 2.18 | 0.81 |
| 9 | Neutropenia | MTX | CR | No | 1.38 | Undetectable |
| 10 | Neutropenia | 2CdA | CR | Yes | 0.39 | 0.11 |
| 11 | Neutropenia | MTX | CR | Yes | 0.31 | 0.29 |
| 12 | Neutropenia | None | CR | No | 1.24 | Undetectable |
| 13 | Anemia | MTX | CR | ND | 0.81 | Undetectable |
| 14 | Anemia | Cy | PR | — | 0.47 | 0.13 |
| Patient . | Indication for Rx . | Rx . | Clinical Response . | Molecular Response . | Fas Ligand Before Rx (mmol/L) . | Fas Ligand During Rx (mmol/L) . |
|---|---|---|---|---|---|---|
| 1 | Neutropenia | MTX | CR | Yes | 0.56 | Undetectable |
| 2 | Neutropenia | MTX | CR | No | 0.28 | Undetectable |
| 3 | Neutropenia | MTX | CR | Yes | 0.21 | Undetectable |
| 4 | Neutropenia | MTX | CR | No | 0.57 | 0.12 |
| 5 | Neutropenia | MTX | CR | Yes | 0.32 | Undetectable |
| 6 | Neutropenia | MTX | PR | — | 0.64 | 0.27 |
| 7 | Neutropenia | MTX | PR | — | 0.54 | Undetectable |
| 8 | Neutropenia | MTX | CR | ND | 2.18 | 0.81 |
| 9 | Neutropenia | MTX | CR | No | 1.38 | Undetectable |
| 10 | Neutropenia | 2CdA | CR | Yes | 0.39 | 0.11 |
| 11 | Neutropenia | MTX | CR | Yes | 0.31 | 0.29 |
| 12 | Neutropenia | None | CR | No | 1.24 | Undetectable |
| 13 | Anemia | MTX | CR | ND | 0.81 | Undetectable |
| 14 | Anemia | Cy | PR | — | 0.47 | 0.13 |
MTX indicates oral low-dose methotrexate; CR, complete response; PR, partial response; ND, evaluation not done; 2CdA, 2-chlorodeoxyadenosine; and Cy, oral low-dose cyclophosphamide. A dash indicates not applicable, as patient did not achieve CR.
Discussion
Our results strongly suggest that elevated levels of circulating Fas ligand mediate neutropenia occurring in LGL leukemia. Leukemic LGLs appear to be clonally expanded, antigen-driven cytotoxic T lymphocytes (CTL).17,18 It was previously suggested that the Fas system may be involved in CTL-mediated diseases involving tissues that express abundant amounts of Fas, such as the liver and lung.8Indeed, Fas ligand has been implicated in the pathogenesis of liver diseases, including viral hepatitis.19 Moreover, dysregulated expression of Fas ligand appears to explain the pathogenesis of lung and liver injury in patients with aggressive forms of LGL leukemia.20 In this study, we hypothesized that secretion of Fas ligand by leukemic LGLs is involved in the pathogenesis of neutropenia, since normal neutrophils express Fas and are susceptible to Fas-mediated apoptosis.12 We found elevated levels of Fas ligand in 39 of 44 serum samples from patients with LGL leukemia. These results confirm and extend findings of an earlier study in which circulating Fas ligand was detected in a few patients with the T-cell form of LGL leukemia.5 Moreover, we demonstrated that serum from patients with LGL leukemia mediated Fas-dependent apoptosis of neutrophils from healthy donors. Neutrophils from the patients also were more sensitive to Fas-dependent apoptosis than those from the healthy donors (not shown).
It is possible that mechanisms other than Fas-dependent apoptosis contribute to the pathogenesis of neutropenia occurring in LGL leukemia. Supporting this idea was the observation that the apoptosis of neutrophils from healthy donors produced by serum from patients with LGL leukemia was not completely inhibited by neutralizing antibody to Fas. Moreover, Fas ligand was not detected in 3 of 34 serum samples from neutropenic patients with LGL leukemia, including 1 patient who had severe neutropenia. In addition, no direct correlation was observed between the level of detectable Fas ligand and the degree of neutropenia. More work is needed to define additional mechanisms that may lead to neutropenia in patients with LGL leukemia.
Findings regarding the pathologic role of Fas ligand in LGL leukemia are similar to observations made in Fas-ligand transgenic mice. In this animal model, tissue injury is due to the effects of Fas ligand on normal cells expressing Fas.21 Moreover, both leukemic LGLs and a novel population of activated T cells from Fas ligand transgenic mice are resistant to Fas-mediated death, even though they express high levels of Fas.7,21 Fas resistance is the underlying pathogenetic mechanism in animal models of lymphoproliferation and autoimmune disease.22,23 The autoimmune phenotype that is characteristic of LGL leukemia resembles that in these lpr/lprmice and includes such autoantibodies as rheumatoid factor, polyclonal hypergammaglobulinemia, and circulating immune complexes. However, Fas resistance in LGL leukemia is not due to function-ablating mutations in the Fas gene,6,7 as was observed in mice and also in children with autoimmune lymphoproliferative syndrome.22,24 25
In addition to serologic abnormalities, patients with LGL leukemia often have autoimmune diseases, particularly rheumatoid arthritis. Therefore, it is interesting that elevated levels of Fas ligand were also observed in serum from patients with rheumatoid arthritis.26 Thirteen of the patients in this study had rheumatoid arthritis and could be considered to have Felty syndrome. Similar clinical features, a common immunogenetic background of HLA-DR4 inheritance, and a possible pathogenetic role for Fas ligand indicate that LGL leukemia with rheumatoid arthritis and Felty syndrome are part of the spectrum of a single disease process.27 28
Clinical response to low-dose oral methotrexate, in a regimen similar to that used to treat rheumatoid arthritis, was associated with reduced levels of circulating Fas ligand. Moreover, increased serum levels of Fas ligand were again observed at relapse in 1 patient. These data further support the hypothesis that Fas ligand mediates cytopenias in LGL leukemia. How methotrexate exerts its beneficial effect in LGL leukemia is not known. Methotrexate may act by modulating Fas resistance and causing apoptosis of leukemic LGLs.29Alternatively, methotrexate may affect regulation of Fas ligand gene expression. A nuclear factor–activated T-cell (NFAT) response element in the promoter region of the Fas-ligand gene is critical for up-regulation of expression of the gene in antigen-activated T cells.30 Treatment with cyclosporine has led to resolution of neutropenia despite persistence of leukemic LGLs.31 It is likely that the therapeutic benefit of cyclosporine resulted from a reduction in Fas ligand secretion due to inhibition of NFAT activation. It is notable that leukemic LGLs express a multidrug-resistance phenotype (P-glycoprotein positive, lung resistance gene positive32) and that aggressive cases of LGL leukemia are often resistant to intensive chemotherapy.33 Taken together, these observations suggest that novel strategies targeting Fas ligand may be more efficacious than treatments aimed at killing leukemic cells. Therefore, an understanding of the regulation of expression of the Fas ligand gene in leukemic LGLs will be important in determining optimal therapy for patients with LGL leukemia.
Supported by the Veterans Administration, the American Cancer Society's Institutional Research grant 93-032, and grant CA78724 from the National Cancer Institute. The Flow Cytometry Core Laboratory and the Biostatistics Core at the H. Lee Moffitt Cancer Center and Research Institute were used in this work.
Reprints: Thomas P. Loughran Jr, Suite 3157, H. Lee Moffitt Cancer Center and Research Institute, 12902 Magnolia Dr, Tampa, FL 33612, e-mail: loughrat@moffitt.usf.edu.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal