Abstract
Castleman's disease, an atypical lymphoproliferative disorder, can be classified into 2 types: hyaline-vascular and plasma cell types according to the histologic features of the affected lymph nodes. The plasma cell type is frequently associated with systemic manifestations and is often refractory to systemic therapy including corticosteroids and chemotherapy, particularly in multicentric form. Dysregulated overproduction of interleukin-6 (IL-6) from affected lymph nodes is thought to be responsible for the systemic manifestations of this disease. Therefore, interference with IL-6 signal transduction may constitute a new therapeutic strategy for this disease. We used humanized anti-IL-6 receptor antibody (rhPM-1) to treat 7 patients with multicentric plasma cell or mixed type Castleman's disease. All patients had systemic manifestations including secondary amyloidosis in 3. With the approval of our institution's ethics committee and the consent of the patients, they were treated with 50 to 100 mg rhPM-1 either once or twice weekly. Immediately after administration of rhPM-1, fever and fatigue disappeared, and anemia as well as serum levels of C-reactive protein (CRP), fibrinogen, and albumin started to improve. After 3 months of treatment, hypergammaglobulinemia and lymphadenopathy were remarkably alleviated, as were renal function abnormalities in patients with amyloidosis. Treatment was well tolerated with only transient leukopenia. Histopathologic examination revealed reduced follicular hyperplasia and vascularity after rhPM-1 treatment. The pathophysiologic significance of IL-6 in Castleman's disease was thus confirmed, and blockade of the IL-6 signal by rhPM-1 is thought to have potential as a new therapy based on the pathophysiologic mechanism of multicentric Castleman's disease. (Blood. 2000;95:56-61)
Castleman's disease is a lymphoproliferative disorder with benign hyperplastic lymph nodes characterized histologically by follicular hyperplasia and capillary proliferation with endothelial hyperplasia.1 Castleman's disease has been classified, according to the histopathologic findings, as either hyalinevascular or plasma cell type.2,3 Patients with plasma cell type or a mixed hyaline-vascular and plasma cell type frequently have systemic manifestations such as fever, anemia, hypergammaglobulinemia, hypoalbuminemia, and an increase in acute phase proteins.2-4 In cases of localized Castleman's disease, these clinical abnormalities may resolve after excision of the affected lymph nodes.3-5 On the other hand, the multicentric form of Castleman's disease is often refractory to treatment even with corticosteroids or chemotherapy, and consequently the prognosis for such patients is poor. Infections are a common cause of death in multicentric Castleman's disease, as well as renal failure and other malignancies including malignant lymphoma and Kaposi's sarcoma.6 Recently, Kaposi's sarcoma-associated herpesvirus (also called human herpesvirus type 8, KSHV/HHV-8) was reported to be an etiologic agent of Castleman's disease, especially in patients infected with human immunodeficiency virus (HIV).7-9
Interleukin-6 (IL-6) is a pleiotropic cytokine with a wide range of biologic activities, such as support of hematopoiesis, regulation of immune responses, and generation of acute phase reactions.10 We previously demonstrated the generation of large quantities of IL-6 from the germinal centers of hyperplastic lymph nodes of patients with plasma cell type Castleman's disease.5 We also showed a correlation between serum IL-6 concentration and clinical features, suggesting that dysregulated IL-6 production from affected lymph nodes may be responsible for the systemic manifestations of this disease. On the basis of these findings, Beck et al11 showed that the in vivo administration of murine anti-IL-6 monoclonal antibody (mAb) to a patient with Castleman's disease seemed to be therapeutically effective, thus confirming the in vivo function of IL-6 in this disease. However, such a therapeutic effect was transient, because of either the emergence of neutralizing human antibodies against murine anti-IL-6 mAb or the inability to attain serum concentrations of anti-IL-6 mAb sufficient to neutralize increasing IL-6 levels.12 The therapeutic value of murine anti-IL-6 mAb for human patients thus remains limited. We therefore attempted to block IL-6 signal transduction by using mAb against the IL-6 receptor (IL-6R). Furthermore, to be effective as a therapeutic agent administered to patients in repeated doses, murine anti-IL-6R mAb, PM-1, was engineered to be a human antibody by grafting the complimentarity-determining regions from the murine anti-IL-6R mAb into human IgG, thereby creating a functioning antigen-binding site in a reshaped human antibody, rhPM-1.13 We used this humanized anti-IL-6R mAb to treat 7 patients with the multicentric form of plasma cell or mixed type Castleman's disease.
Patients and methods
Patients
Seven patients in Osaka University Hospital were recruited, each of whom had lymphadenopathy at multiple sites, mild splenomegaly, and constitutional symptoms such as fever and fatigue. Laboratory findings included anemia, polyclonal hypergammaglobulinemia, hypoalbuminemia, and increased acute phase proteins. All patients had plasma cell or mixed type Castleman's disease, based on histologic examination of the biopsy specimens. The clinical characteristics of the patients are shown in Table 1. All patients had active disease at the start of anti-IL-6R mAb treatment, and 4 of them had previously been treated with prednisolone or immunosuppressive therapy for more than 3 months. Three patients had amyloidosis secondary to Castleman's disease, and 5 had lymphocytic interstitial pneumonia, based on histologic examination of the lung biopsy specimens. None of the patients had autoimmune disorders, such as rheumatoid arthritis or Sjögren syndrome, or malignancies. Both HIV and KSHV/HHV-8 were undetectable by serologic assays and polymerase chain reaction (PCR). All patients gave their informed consent for the anti-IL-6R mAb therapy under the auspices of an approval approved by the ethics committee of Osaka University.
Clinical profiles of 7 patients with multicentric form of Castleman's disease who received humanized anti-IL-6R Ab therapy
| Patient . | Age/ Sex . | Disease Duration* (years) . | Histologic Diagnosis† . | Complications† . | Total Amount of Anti-IL-6R Ab (mg) . | Number of Administrations . | Duration of Anti-IL-6R Ab Therapy (mo) . | Concominant Therapy† . | Serum IL-6 Concentration Before Therapy (pg/mL)‡ . |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 33/M | 3 | Mixed type | LIP | 4561 | 67 | 11 | Pred 10 mg | 40.1 |
| 2 | 43/F | 1 | Plasma cell type | LIP | 4060 | 46 | 10 | (−) | 93.0 |
| 3 | 59/F | 1 | Plasma cell type | Secondary amyloidosis | 1661 | 35 | 4 | (−) | 44.1 |
| 4 | 23/M | 1 | Mixed type | Secondary amyloidosis LIP | 1860 | 25 | 4 | (−) | 20.6 |
| 5 | 51/M | 3 | Plasma cell type | Secondary amyloidosis | 1200 | 26 | 41-153 | Pred 10 mg | 6.5 |
| l-PAM 2 mg | |||||||||
| 6 | 54/F | 1 | Plasma cell type | LIP | 660 | 15 | 2 | Pred 40 mg | 8.4 |
| CYC 50 mg | |||||||||
| 7 | 49/F | 1 | Plasma cell type | LIP | 410 | 10 | 1 | Pred 20 mg | 24.5 |
| Patient . | Age/ Sex . | Disease Duration* (years) . | Histologic Diagnosis† . | Complications† . | Total Amount of Anti-IL-6R Ab (mg) . | Number of Administrations . | Duration of Anti-IL-6R Ab Therapy (mo) . | Concominant Therapy† . | Serum IL-6 Concentration Before Therapy (pg/mL)‡ . |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 33/M | 3 | Mixed type | LIP | 4561 | 67 | 11 | Pred 10 mg | 40.1 |
| 2 | 43/F | 1 | Plasma cell type | LIP | 4060 | 46 | 10 | (−) | 93.0 |
| 3 | 59/F | 1 | Plasma cell type | Secondary amyloidosis | 1661 | 35 | 4 | (−) | 44.1 |
| 4 | 23/M | 1 | Mixed type | Secondary amyloidosis LIP | 1860 | 25 | 4 | (−) | 20.6 |
| 5 | 51/M | 3 | Plasma cell type | Secondary amyloidosis | 1200 | 26 | 41-153 | Pred 10 mg | 6.5 |
| l-PAM 2 mg | |||||||||
| 6 | 54/F | 1 | Plasma cell type | LIP | 660 | 15 | 2 | Pred 40 mg | 8.4 |
| CYC 50 mg | |||||||||
| 7 | 49/F | 1 | Plasma cell type | LIP | 410 | 10 | 1 | Pred 20 mg | 24.5 |
Disease duration is defined as the period since diagnosis.
Pred indicates prednisolone; CYC, cyclophosphamide;l-PAM, melphalan; mixed type, mixed plasma cell and hyaline vascular type; LIP, lymphocytic interstitial pneumonia.
Normal range of serum IL-6 is < 1 pg/ml.
The patient received anti-IL-6R antibody therapy for a total of 4 months after a 2-month interval.
PCR/Southern hybridization and serologic analysis of KSHV/HHV-8
The PCR/Southern hybridization analysis of KSHV/HHV-8 was performed as follows. Genomic DNA was extracted from peripheral blood mononuclear cells or frozen specimens of lymph nodes or both. The DNA extracted from peripheral blood mononuclear cells of a Japanese patient with acquired immunodeficiency syndrome-Kaposi's sarcoma (AIDS-KS) with KSHV/HHV-8 infection was also used as a positive control for KSHV/HHV-8. Each 50-μL reaction mixture for PCR contained 0.4 to 0.6 μg total DNA, 2.5 U Ex Taq DNA polymerase (Takara, Kyoto, Japan), dNTP (0.25 mM each), 25 mM TAPS buffer (pH 9.3), 50 mM KCl, 2 mM MgCl2, 1 mM 2-mercaptoethanol, and the primers (10 pmol each) the sequences of which were as follows: outer forward primer: 5′-atggcactcgacaagagtatagtgg-3′; outer reverse primer: 5′-cgttagcgtggggaataccaacagg-3′ (from nucleotide 633 to nucleotide 1552 in KSU 18 551)14; inner forward primer: 5′-ctatccaagtgcacactcgctgtcc-3′; inner reverse primer: 5′-ggaaccaaggctgataggatacaaagg-3′ (from nucleotide 828 to nucleotide 1288 in KSU 18 551). The PCR procedure consisted of 1 cycle at 94°C for 2 minutes, then 40 cycles at 94°C for 1 minute, at 58°C for 1 minute, and at 72°C for 2 minutes, and 1 cycle at 72°C extension for 5 minutes with a DNA thermal cycler, Gene Amp PCR system 2400® (Perkin Elmer, Norwalk, CT). First, the DNA samples were amplified with the pair of outer primers and then with the pair of inner primers. The PCR products (10 μL) were then electrophoresed in a 2.0% agarose gel containing ethidium bromide and visualized with UV light. The PCR products were transferred to a hybond N+ (Amersham Inc., Arlington Heights, IL), hybridized with a DIG-labeled KSHV/HHV-8 specific probe: 5′-tgttggtgtaccacatctactccaaaatat-3′, and colorimetrically detected using a DIG DNA Labeling and Detection Kit (Boehringer Mannheim, Germany) in accordance with the manufacturer's instructions.
Immunofluorescence assay was performed as previously described.15 Briefly, BCBL-1 cells (106/mL), the body cavity-based lymphoma cell line that is latently infected with KSHV/HHV-8 but not Epstein-Barr virus, were treated with 20 ng/mL phorbol ester (TPA: Sigma, St. Louis, MO) for 48 hours to induce the viral lytic antigen. Uninduced and TPA-induced BCBL-1 cells were then washed in phosphate-buffered saline (PBS), spotted on slides (2 × 104 cells/well), air dried in a laminar flow hood, and fixed in acetone at −20°C for 10 minutes. All sera from the patients with Castleman's disease and the Japanese patient with AIDS-KS were stored at −20°C and heat inactivated at 56°C for 30 minutes before use. Fixed cells were incubated at 37°C for 30 minutes with human sera serially diluted 1:2 beginning at 10-fold dilution. After washing rigorously in PBS, the slides were incubated with fluorescein isothiocyanate-conjugated goat anti-human IgG (Dako, Copenhagen, Denmark) at 37°C for 30 minutes. The slides were counterstained with a 1:20 000 dilution of Evan's blue (Sigma) at room temperature for 5 minutes, washed, mounted with 50% (vol/vol) glycerol in PBS, and examined under a fluorescence microscope.
Administration of humanized anti-IL-6R mAb, rhPM-1
Humanized anti-IL-6R mAb, rhPM-1, (IgG1 class) was produced in Chinese hamster ovary cells and purified on protein A.13rhPM-1 retains its specificity for natural and recombinant human IL-6R with the same affinity as possessed by the original murine anti-IL-6R mAb, PM-1, and inhibits IL-6 function both in vitro and in vivo.13 16 rhPM-1 was dissolved in PBS and stored at −20°C until administration. The appropriate amount of rhPM-1 was then diluted to a volume of 50 mL in saline and administered intravenously over a period of 1 hour only after a skin test using 100 μL of the antibody was negative. Increasing doses (1, 10, 50, and 100 mg/patient) of rhPM-1 were administered intravenously twice weekly to establish the maximal tolerated dose in every patient. The dose of 100 mg/patient was chosen as the maximal dose because higher doses exceeded permitted levels of contaminating DNA after purification, in compliance with the guideline for the manufacture and testing of mAb products for human use suggested by the Ministry of Health and Welfare, Japan, although no dose-limiting toxicity was observed. Fifty to 100 mg rhPM-1 was then administered either once or twice weekly for 5 to 42 weeks as maintenance therapy until completion of the study and cessation of rhPM-1 production. Total amounts of rhPM-1 administered, the number of administrations, and the duration of rhPM-1 therapy for each patient are summarized in Table 1.
Serum IL-6 and soluble IL-6R (sIL-6R) levels
Serum IL-6 levels were determined with a chemiluminescent enzyme immunoassay (CLEIA).17 The principle of this assay is based on 2-site immunometric reverse sandwich reaction with the aid of a Lumipulse 1200 (Fujirebio Inc., Tokyo, Japan). Briefly, the mixture of the sample and mouse anti-IL-6 mAb conjugated with alkaline phosphatase was incubated at 37°C for 10 minutes, and then added to particles covalently linked to a murine anti-IL-6 mAb that recognizes a different epitope than that recognized by the original mAb. After a 10-minute incubation at 37°C, the particles were separated magnetically and washed in buffer. Subsequently, the substrate solution, 3-(2′-spiroadamantane)-4-methoxy-4-(3″-phosphoryloxy) phenyl-1, 2-dioxetane disodium salt was added at 37°C, and the chemiluminescent signal was photoncounted after 5 minutes. The detection limit for serum IL-6 was 0.1 pg/mL.
sIL-6R was measured by means of a dissociation-enhanced fluoroimmunoassay (DELFIA® Pharmacia, Uppsala, Sweden), as previously described.18 Briefly, 200 μL of sample was added to microstrips coated with 200 μL anti-human IL-6R mAb (MT18) (1 μg/mL), and incubated at 4°C, followed by a reaction with 200 μL europium-labeled PM-1 mAb (100 ng/mL) at room temperature for 15 minutes. Fluorescence in each well was measured with a 1230 ARCUS fluorometer (Pharmacia).
Serum rhPM-1 and anti-rhPM-1 antibody levels
Serum rhPM-1 levels were assessed by enzyme-linked immunosorbent assay (ELISA). Briefly, 100 μL recombinant human sIL-6R (1 μg/mL) was added to the wells of an immunoplate precoated with MT18 and incubated at room temperature for 2 hours. After washing, 100 μL serum was added and incubated for additional 2 hours. After washing, bound rhPM-1 was measured using alkaline phosphatase-conjugated goat anti-human IgG. The calorimetric reaction was measured with a microplate reader.
Serum anti-rhPM-1 antibody levels were also determined by ELISA. Serum was added to the wells coated with 100 μL rhPM-1 (5 μg/mL) and incubated for 2 hours. After washing, biotin-conjugated rhPM-1 was added and developed with alkaline phosphatase-conjugated to streptavidin.
Results
Efficacy of humanized anti-IL-6R mAb, rhPM-1
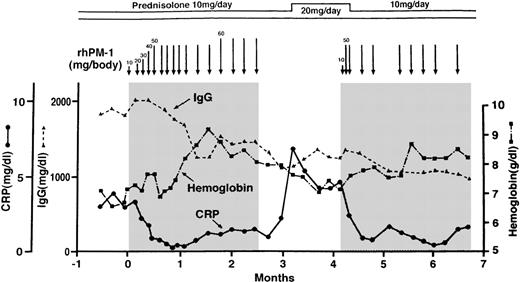
Data representative of patients with Castleman's disease treated with rhPM-1 are shown in Figure 1. The patient was a 51-year-old man who suffered from amyloidosis secondary to Castleman's disease (patient 5 in Table 1). His disease was refractory to steroid therapy and chemotherapy consisting of melphalan, vincristine, and doxorubicin hydrochloride. Immediately after the administration of 20 mg rhPM-1, fever and malaise disappeared, whereas CRP and fibrinogen started to decrease within 24 hours, but this treatment was insufficient to obtain a satisfactory therapeutic effect. Treatment with repeated doses of 50 mg rhPM-1 twice a week completely normalized serum CRP levels within 4 weeks; 8 weeks of therapy was required to increase hemoglobin and albumin levels. After 2 months of treatment, hypergammaglobulinemia and lymphadenopathy also improved. Extension of the interval between administrations caused a slight increase in CRP and a decrease in hemoglobin levels, but constitutional symptoms did not reappear. Two weeks after termination of the therapy, fever, malaise, and anemia recurred, and treatment with increased doses of prednisolone had little effect on his symptoms. Readministration of rhPM-1, however, was as effective as the first treatment course, evidenced by improvements in CRP and hemoglobin levels. The data confirmed that the improvement was due to rhPM-1 therapy but not influenced by concomitant therapy. A similar therapeutic effect was obtained by treatment with 100 mg rhPM-1 once a week, without any significant adverse events. Thus, a total dose of 100 mg rhPM-1 per week was determined to be an effective therapy.
Representative changes in CRP, hemoglobin, and IgG concentrations in a patient with multicentric Castleman's disease during treatment with anit-IL-6R (rhPM-1).
Arrows and shadings indicate the administration of rhPM-1. Note that treatment with rhPM-1 improved the clinical abnormalities, but that these recurred 2 weeks after the cessation of the rhPM-1 regimen. Readministration of rhPM-1 was as effective as the first treatment.
Representative changes in CRP, hemoglobin, and IgG concentrations in a patient with multicentric Castleman's disease during treatment with anit-IL-6R (rhPM-1).
Arrows and shadings indicate the administration of rhPM-1. Note that treatment with rhPM-1 improved the clinical abnormalities, but that these recurred 2 weeks after the cessation of the rhPM-1 regimen. Readministration of rhPM-1 was as effective as the first treatment.
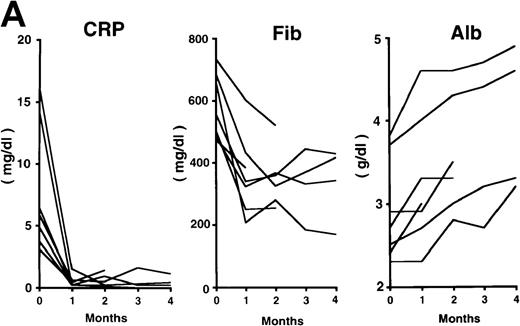
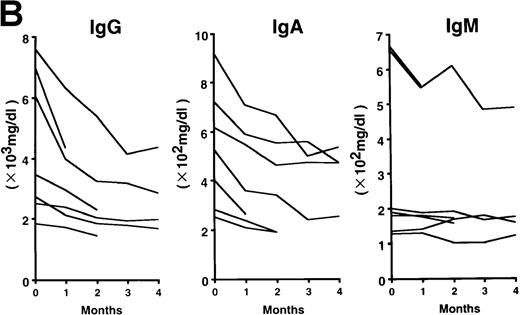
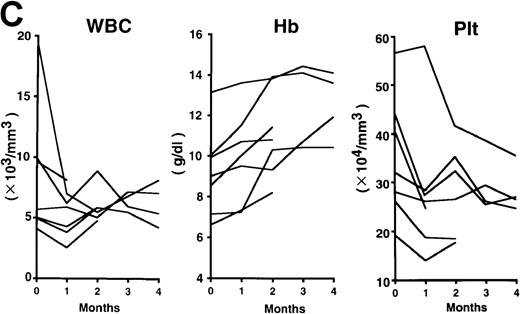
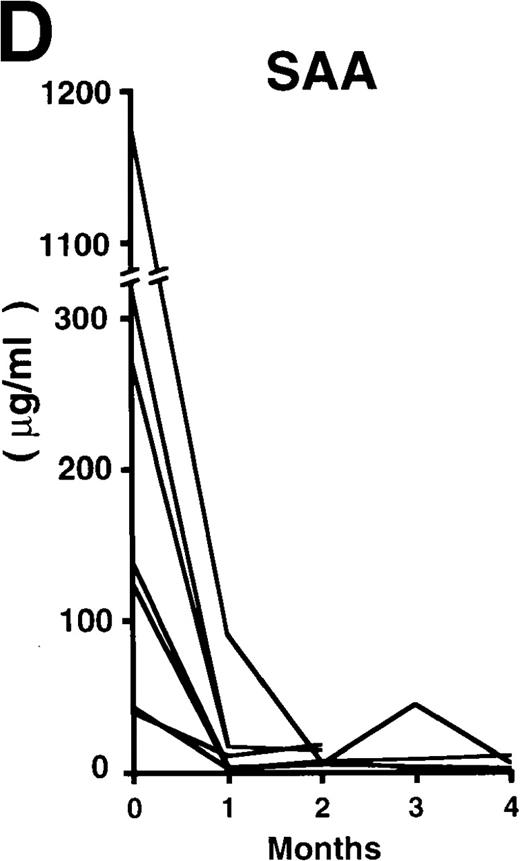
The patterns of response to rhPM-1 therapy are shown in Figure2. Improvements in thrombocytosis, serum levels of CRP, fibrinogen, and serum amyloid A (SAA) protein were observed within 1 month in most cases, whereas hemoglobin, albumin, and immunoglobulin levels more gradually improved over a 3- to 4-month period. Two of the 7 patients showed increased levels of IgE (patient 2, 5300 IU/mL; patient 7, 650 IU/mL) before treatment, which were also reduced by rhPM-1 therapy (1100 IU/mL and 310 IU/mL, respectively). Autoantibodies (antinuclear antibody, anti-DNA antibody) observed in patients 2 and 6 disappeared after rhPM-1 therapy. Platelet counts and fibrinogen and immunoglobulin levels decreased only to the low-normal range. Similar therapeutic effects were observed in patients both with and without concomitant therapy. As a result of decrease in disease activity, it was possible to discontinue concomitant therapy in some patients. Importantly, these therapeutic effects did not diminish even after continuous 11-month treatment (patient 1). Therapy with rhPM-1 remarkably improved lymphadenopathy in all patients. Only in patient 4 were a few residual lymph nodes palpable, although their sizes were significantly reduced by the treatment. Computed tomography scanning confirmed the disappearance of pathologically significant visceral lymph nodes (> 1 cm in diameter) in all patients. During the treatment of patients 3, 4, and 5, who had secondary amyloidosis, constitutional symptoms such as appetite loss, constipation, and diarrhea (in all 3 patients) gradually improved. Bradyphasia and bradypragia, which might not be directly caused by the deposition of amyloid in the brain, also improved in patient 3. Furthermore, improvements in serum creatinine and daily urine protein levels were also observed after 4-month therapy in patient 3: before treatment, the serum creatinine level was 1.8 mg/dL, urine protein 3.1 g/d, serum β2m 7.1 mg/L, and urine β2 microglobulin 55.5 mg/d; after treatment, the corresponding values were 1.1 mg/dL, 0.9 g/d, 5.3 mg/L, and 7.4 mg/d. No major side effects of rhPM-1 therapy were observed, except for a transient and mild decrease in granulocyte counts on the day following mAb administration in 2 patients, which spontaneously recovered within 2 days. No decrease in T-cell function was observed, assessed either by a skin test with purified protein derivative of tuberculin or by a mixed lymphocyte culture test with allogeneic T cells.
Clinical response patterns in 7 patients treated with rhPM-1.
CRP indicates C-reactive protein; Fib, fibrinogen; Alb, albumin; IgG, immunoglobulin G; IgA, immunoglobulin A; IgM, immunoglobulin M; WBC, white blood cells; Hb, hemoglobin; Plt, platelets; and SAA, serum amyloid A.
Clinical response patterns in 7 patients treated with rhPM-1.
CRP indicates C-reactive protein; Fib, fibrinogen; Alb, albumin; IgG, immunoglobulin G; IgA, immunoglobulin A; IgM, immunoglobulin M; WBC, white blood cells; Hb, hemoglobin; Plt, platelets; and SAA, serum amyloid A.
Histologic examinations of affected lymph nodes
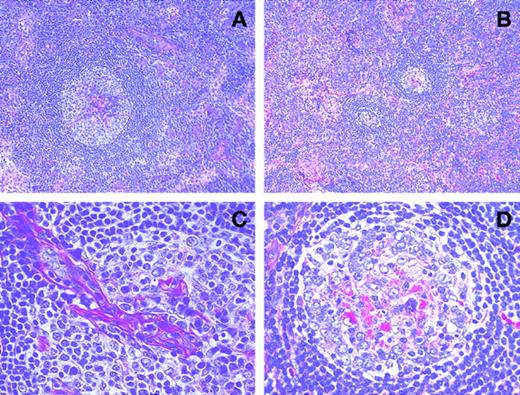
Therapy with rhPM-1 remarkably improved lymphadenopathy in all patients, and a few small residual lymph nodes remained palpable only in patient 4. To examine the histologic changes in the affected lymph nodes resulting from rhPM-1 therapy, a cervical lymph node that decreased in size on therapy was resected after 3 months of therapy in patient 4 and compared to pretreatment histopathologic appearance. Lymph nodes before rhPM-1 treatment showed features of a mixed hyaline-vascular and plasma cell type: (1) follicular hyperplasia with a large germinal center penetrated by branching hyaline blood vessels, (2) concentric appearance of mantle zone lymphocytes, and (3) proliferation of hyaline capillaries and numerous plasma cells in the interfollicular areas (Figure 3A and C). However, after treatment there was (1) a reduction in the size of lymph nodes, (2) a reduction in both the number and the size of follicles, resulting in a relative increase in the interfollicular areas (Figure3B), and (3) a reduction in the vascularity of germinal centers with residual eosinophilic deposits (Figure 3D). However, the number of plasma cells and the extent of vascularity in the interfollicular areas did not show significant changes (Figure 3B).
Serial histopathologic changes with rhPM-1 therapy.
Hyperplastic lymph follicle with hyalinous capillary vessels penetrates into the germinal center before rhPM-1 treatment (A and C). Note reduction in both the size of lymph follicles and in the vascularity of germinal centers with residual eosinophilic deposits after rhPM-1 therapy (B and D). (Hematoxylin-eosin, A and B, original magnification ×100; C and D, original magnification ×400).
Serial histopathologic changes with rhPM-1 therapy.
Hyperplastic lymph follicle with hyalinous capillary vessels penetrates into the germinal center before rhPM-1 treatment (A and C). Note reduction in both the size of lymph follicles and in the vascularity of germinal centers with residual eosinophilic deposits after rhPM-1 therapy (B and D). (Hematoxylin-eosin, A and B, original magnification ×100; C and D, original magnification ×400).
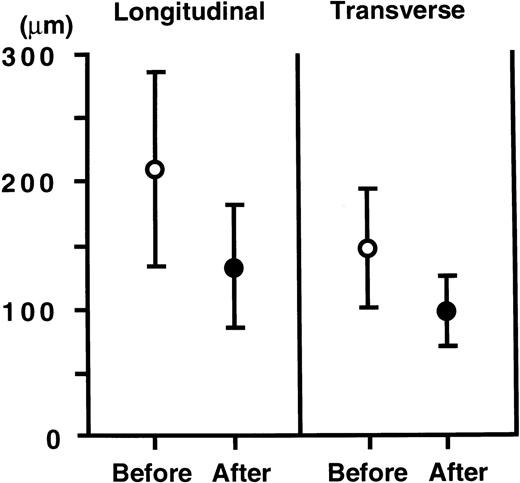
Quantification of these changes was attempted by measuring both the longitudinal and transverse diameters of the germinal centers. As shown in Figure 4, both the longitudinal (210 ± 76 μm, mean ± SD, n = 109) and transverse diameters (150 ± 45 mm, n = 109) of the germinal centers before therapy were markedly reduced (133 ± 48 mm, and 98 ± 27 mm, n = 15, respectively) after therapy (P < 0.005 andP < 0.001, respectively, assessed by Mann-Whitney's Utest).
Changes in longitudinal and transverse diameters of the germinal centers.
Open circles indicate mean diameters of germinal centers before treatment (n = 109) and closed circles indicate those after treatment (n = 15). The points and vertical bars indicate mean and SDs, respectively. Both the longitudinal and transverse diameters significantly decreased (P < 0.005, P < 0.001, respectively).
Changes in longitudinal and transverse diameters of the germinal centers.
Open circles indicate mean diameters of germinal centers before treatment (n = 109) and closed circles indicate those after treatment (n = 15). The points and vertical bars indicate mean and SDs, respectively. Both the longitudinal and transverse diameters significantly decreased (P < 0.005, P < 0.001, respectively).
Serum IL-6 and sIL-6R levels
Serum IL-6 levels did not change essentially, although they showed fluctuations unrelated temporally to the rhPM-1 treatment, whereas sIL-6R levels increased in the first month after the administration of rhPM-1, with peak serum levels of up to 700 ng/mL. Repeated administration of rhPM-1 uniformly and rapidly reduced the sIL-6R levels to normal in all patients. The maximum concentrations of serum sIL-6R did not increase with rhPM-1 maintenance therapy.
Serum rhPM-1 and anti-rhPM-1 antibody levels
The trough level of serum rhPM-1 was 10 μg/mL during maintenance therapy using 50 mg rhPM-1 twice a week and decreased to 5 μg/mL using treatment at 100 mg rhPM-1 once a week. Because a slight increase in CRP was observed with the latter regimen, the former schedule was considered to be more effective. There were no differences in responses in terms of constitutional symptoms observed between the 2 regimens. Ten μg/mL rhPM-1 had previously been proven to inhibit the IL-6-induced proliferation of myeloma/plasmacytoma cells in vitro.16
No serum anti-rhPM-1 antibodies were detected during the course of therapy of these 7 patients.
Discussion
The clinical course of multicentric Castleman's disease is unpredictable, but the prognosis is generally poor.4,6 The accompanying constitutive inflammatory state may result in complications such as pneumonia, amyloidosis, and malignancies, and treatment should therefore be introduced as early as possible. Although spontaneous remissions are observed in some patients,4 6 in other patients the disease is refractory to conventional therapies such as corticosteroids, cytotoxic agents, or radiation. Thus, a new therapeutic strategy for multicentric Castleman's disease is needed.
The present study showed that the humanized anti-IL-6R mAb, rhPM-1, can achieve marked responses in the refractory form of this disease without significant adverse reactions or development of neutralizing antibodies. Furthermore, rhPM-1 therapy also improved the symptoms and biochemical abnormalities of secondary amyloidosis, which is often otherwise untreatable at present, although successful therapy to reduce the supply of amyloid fibril protein precursors is followed by substantial regression of amyloid.19 Although re-biopsy of the kidney was not performed after rhPM-1 therapy, reduced serum creatinine and urine protein levels suggest that rhPM-1 therapy may alleviate renal amyloidosis. In addition, rhPM-1 therapy improved the hydronephrosis in patient 3 who, before therapy, required nephrostomy due to ureterostenosis resulting from amyloidosis of the ureter. These findings suggest that visceral amyloid deposition in organs may also be reversible, if SAA production is inhibited by blocking IL-6 signaling. rhPM-1 may also reduce the likelihood of malignant lymphoma and Kaposi's sarcoma because IL-6 is a potent growth factor for these malignancies.20 21
The fact that the blockade of the IL-6 signal by humanized anti-IL-6R mAb improved the systemic manifestations of Castleman's disease confirms a central role for IL-6 in the pathogenesis of this disease. In particular, overproduction of IL-6 causes inflammatory symptoms, such as low-grade fever and generalized malaise, as well as laboratory findings, such as leukocytosis, anemia, thrombocytosis, hypergammaglobulinemia, hypoalbuminemia, and an increase in CRP, fibrinogen, and SAA levels. The observation that elevation in serum IgE levels also resolved provides further evidence that IL-6 plays some role in regulating IgE production in vivo and supports the view that IL-6 induces differentiation of naive T cells into Th2 cells in vitro.22 Autoantibodies frequently observed in this disease also disappeared during rhPM-1 treatment, suggesting that IL-6 may also mediate autoimmune phenomena.
Histologic changes observed in the lymph nodes after treatment imply an important pathogenic mechanism of this disease. That is, IL-6 may be involved in the follicular hyperplasia of affected lymph nodes because blockade of the IL-6 signal reduced both their number and size. Because IL-6 is mainly produced by activated B cells in the germinal center of lymph follicles,5,11 an autocrine growth mechanism may be mediated by IL-6 in follicular B cells of patients with this disease. The disappearance of hyaline capillaries in the follicles after treatment with rhPM-1 indicates that IL-6 may also play a role in angiogenesis, although it remains to be determined whether or not IL-6 acts directly on endothelial cells. IL-6 may promote angiogenesis through the induction of other factors such as vascular endothelial growth factor,23 because patients with a typical hyaline-vascular variant of Castleman's disease do not always show an increase in serum IL-6 levels.
We have shown the efficacy of humanized antibodies for anti-cytokine therapy because no anti-rhPM-1 antibodies were detectable, even in the patient who received multiple injections (67 doses) for 11 months. Klein et al12 reported that anti-IL-6 mAb therapy induced a continuous increase in IL-6 production in vivo, and consequently was unable to administer sufficient anti-IL-6 mAb to neutralize IL-6 activity. A similar phenomenon was observed during anti-IL-6R mAb therapy as serum sIL-6R increased after administration of rhPM-1. However, this tendency did not continue, and the highest concentration observed was about 700 ng/mL, with trough levels of the serum rhPM-1 concentration of 5 to 10 μg/mL. Moreover, maximum sIL-6R levels gradually decreased with repeated administrations of rhPM-1 over 2 months. This may be the reason the efficacy of humanized anti-IL-6R mAb therapy did not diminish over time. On the other hand, serum IL-6 levels did not change essentially although they showed some fluctuations. This is probably because rhPM-1 reacts with IL-6R and interferes with IL-6-binding to IL-6R, but does not react with IL-6 itself. It may also be the reason a relatively prompt relapse occurred (after 2 weeks) when the treatment was stopped. Two weeks is consistent with the period when rhPM-1 was detectable in blood after termination of the therapy. However, serum IL-6 levels may decrease if we continue the treatment further, because lymphadenopathy improved during the treatment and a reduction in both the number and size of the follicles, which are the main sources of IL-6 in this disease,5 11 was observed in a lymph node.
Although rhPM-1 therapy improved the symptoms associated with Castleman's disease and reduced lymphadenopathy, recurrence of the disease was observed after termination of this therapy. Our data indicate that overproduction of IL-6 causes most of the abnormalities of Castleman's disease, but the cause of the constitutive overproduction of IL-6 is at present unknown. This may be due to a lack of NF-IL6, resulting from genetic abnormalities,24 although familial aggregation has not been reported. Because Castleman's disease is frequently associated with HIV infection6 and HIV infection also causes overproduction of IL-6,25infection with a retrovirus may also play a role in pathogenesis. In this regard, KSHV/HHV-8 was recently reported to be frequently positive in multicentric Castleman's disease, especially in HIV-infected patients.7-9 Because a homologue to human IL-6, viral IL-6 (vIL-6), has been identified in the KSHV/HHV-8 genome,26 27which retains biologic activities similar to human IL-6, KSHV/HHV-8 is suspected of being involved in the pathogenesis of Castleman's disease. Although serologic and PCR assays in our patients were negative for HIV and KSHV/HHV-8, infection with another organism that can promote IL-6 production might be involved in this disease.
Further studies will be required to identify the etiology of the deregulated production of human IL-6 in Castleman's disease. It is also necessary to examine the effect of rhPM-1 therapy on IL-6 gene, steroid-responsive gene, and multidrug-resistant gene expression in germinal center cells because incorporation of immunotherapy may cause the emergence of drug resistance as a consequence of perturbations in intracellular signaling and the population kinetics of tumor cell adaptation and selection. Nevertheless, the pathophysiologic significance of human IL-6 is confirmed by this study because the clinical manifestations of Castleman's disease are alleviated by blockade of human IL-6R. The present study, therefore, demonstrates a novel and promising therapy, based on blockade of IL-6 signaling with a humanized anti-IL-6R antibody, which may also be effective for other diseases characterized by overproduction of IL-6.
Acknowledgments
We wish to thank Dr R. Inagi for performing the KSHV/HHV-8 assay, Ms C. Aoki and Ms M. Ohno for their outstanding technical assistance, and Ms T. Nakao for preparing the manuscript.
Supported by grants from the Ministry of Education, Science, Sports and Culture of Japan, and from the Osaka Foundation for Promotion of Clinical Immunology.
Reprints:Norihiro Nishimoto, Department of Medical Science I, School of Health and Sport Sciences, Osaka University, 2-1 Yamada-oka, Suita-city, Osaka 565-0871 Japan; e-mail:norihiro@imed3.med.osaka-u.ac.jp.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal