Abstract
Human γδ T lymphocytes respond to viral, bacterial, protozoal, and tumoral antigens, but their precise function remains unknown. In adults the major circulating γδ T-cell subset expresses the Vγ9Vδ2 T-cell receptor and responds to protease-resistant phosphorylated derivatives found in many pathogens. In this study we show that activation of Vδ2+ cells with the nonpeptidic antigen isopentenyl pyrophosphate (IPP) rapidly induces (within 4-12 hours) the C-C chemokines MIP-1, MIP-1β, and lymphotactin but not MCP-1. The most robust response was obtained for MIP-1β. IPP induction of MIP-1 and MIP-1β was not affected by costimulation with interleukin-4 (IL-4), IL-10, TGF-β, or interferon-γ (INF-γ). However, IL-12 significantly enhanced IPP-induced expression and release of MIP-1 that was down-regulated by TGF-β whereas the induction of MIP-1β by IPP+IL-12 was refractory to cotreatment with TGFβ indicating that these chemokines are differentially regulated by these cytokines. Vδ2+ T cells also expressed a wide range of C-C chemokine receptors including CCR1, CCR5, and CCR8, all of which were down-regulated following activation. We conclude that Vδ2+ cells can be rapidly induced by components of bacterial cell walls to express high levels of proinflammatory chemokines, supporting an important role for these cells in the early stages of the inflammatory responses to many common pathogens. (Blood. 2000, 95:39-47)
T cells that express the γδ T-cell receptor (TCR) form a minor component of the peripheral circulating T-cell pool.1,2 In human peripheral blood, 2 main populations of γδ T cells have been identified based on the TCR composition. The predominant subset expresses the Vδ2 chain associated with Vγ9 and represents 70% of the circulating γδ T cells in adults, while a minor subset (approximately 30%) expresses a Vδ1-J δ1 chain linked to a chain different from Vγ9. At birth the Vδ1 population predominates, whereas Vδ2 T lymphocytes are almost completely absent.3 It has been proposed that the shift from Vδ1 predominance in the blood of newborns to Vδ2-expressing cells in the blood of adults may be due to a selective response to environmental stimuli such as commonly encountered bacteria.4 5
γδ T cells that express the Vδ2/Vγ9 rearrangement of the TCR are known to respond, in a major histocompatibility complex (MHC) independent manner,6,7 to antigens that differ from conventional peptidic antigens recognized by α β T lymphocytes.The nature of these compounds, which are characterized by a low molecular weight (100-160 Da) and the presence of phosphate groups, have recently been summarized .8
Studies that have addressed whether the diversity of the TCR CDR3 region contributes to the fine specificity of the Vδ2/Vγ9 T cells, allowing them to discriminate between stimulatory metabolites, have shown that different Vδ2/Vγ9 T-cell clones present the same pattern of cross-reactivity toward these compounds. However, although these cells are broadly cross-reactive, they are also highly specific for ligand structure, since the number and position of phosphate groups are important for T-cell activation.9 The expansion of these cells during the first years of life is thought to reflect a selective response of this γδ T-cell subset to these nonpeptidic antigens associated with common pathogens. Little is known about the function or the ligands recognized by Vδ1 T cells, although their expansion has been observed in several pathological conditions, especially in patients with human immunodeficiency virus (HIV).10 11
Because the Vδ2/Vγ9 T cells are prevalent in human peripheral blood and lymphoid organs and react to phosphorylated protease-resistant bacterial antigens, it has been suggested that they could perform a sentinel function by responding to either products released by bacteria or to ligands released by autologous damaged cells (infected or necrotic).8 Previously we have shown that Vδ2 Vγ9+ T cells from peripheral blood of healthy donors can be induced to release proinflammatory cytokines, particularly TNF- α and INF-γ when challenged in vitro with either phosphoantigens or with PMA + ionomycin .12 13 These cells have also been shown to possess potent cytotoxic activity and to kill via a perforin-dependent process.
In this study we address the role of these phosphoantigens in inducing chemokine production in Vδ2Vγ9+ cells. Interest in studying chemokines has relevance not only to the potential role of γδ T cells in inflammatory reactions but also to certain infections, such as HIV type 1 (HIV-1), because chemokine receptors have been shown to function as obligate coreceptors for HIV-1.14 15
Chemokines are small cytokines that are classified into different subfamilies depending upon the positioning of 4 N-terminal conserved cysteine residues involved in disulfide bond formation.16The presence or absence of an intervening amino acid in the first 2 cysteine residues defines the 2 main chemokine families. Chemokines with a C-X-C structure ( α-chemokines) are potent chemoattractants for neutrophils, while chemokines with a C-C structure (β-chemokines) preferentially attract monocytes but not neutrophils. In addition, certain C-C chemokines, such as MIP-1α, MIP-1β, and RANTES, induce the migration of activated T lymphocytes.17-20 More recently 2 other families of chemokines have been identified: lymphotactin,21 which lacks 2 of the 4 cysteine residues and is a powerful attractant for T lymphocytes, and fractalkine, which contains 3 amino acids between the first 2 cysteines.22
Chemokines are known to regulate leukocyte movement in development, homeostasis, and inflammation by binding to specific G-protein–coupled cell-surface receptors on target cells.23 24 Triggering of chemokine receptors leads to the generation of biochemical signaling events, such as release of intracellular calcium and activation of protein kinase C, which regulates specific directional migration. Some chemokine receptors are restricted to particular cell types, while others are widely expressed or may be constitutively expressed on some cells and inducible in others.
Activation of αβ TCR+ T cells has also been shown to lead to the production of chemokines. Mitogenic stimuli generally leads to a low-level transient expression of chemokines, such as MIP-1α and MIP-1β, and ligation of CD3, particularly when there is costimulation with CD28, which leads to a more sustained stimulation and release.25
In this study we have examined the ability of phosphoantigens to activate chemokine expression in Vδ2+ T cells. We chose to focus our efforts on expression of the C-C chemokines and their receptors because of the potential contribution of these cells and their response to bacterial cell products, such as phosphoantigens. In the transition from the innate to the acquired immune response, the ability to induce the chemoattraction of specific lymphocyte subsets could play an important role in the development of antigen-specific responses. Our data show that phosphoantigen-activation of Vδ2+ T cells induces synthesis and releases the β-chemokines MIP-1α and MIP-1β (but not MCP-1) by phosphoantigens. They express a wide range of C-C chemokine receptors. The data also show that chemokine induction by isopentenyl pyrophosphate (IPP) can be further enhanced by the addition of interleukin 12 (IL-12) and, for MIP-1α but not MIP-1β, can be down-regulated by TGFβ. The immunomodulatory cytokines IL-4, IL-10, and interferon (INF) γ are without effect.
Materials and methods
Cell preparation and stimulation
Peripheral blood mononuclear cells (PBMCs) were isolated from heparinized blood obtained from healthy donors by gradient centrifugation (Ficoll-Hypaque; Pharmacia Biotech, Uppsala, Sweden). PBMCs were cultured at 106 cells/mL in medium composed of RPMI 1640 supplemented with 10% (vol/vol) heat-inactivated fetal bovine serum, 2 mmol/L L-glutamine, 20 mmol/L HEPES, and 10 units/mL penicillin and streptomycin (Life Technologies, Grand Island, NY). Long-term cultures (20-30 days) were maintained with 50 units/mL human recombinant IL-2 (SIGMA, St.Louis, MO). Phosphoantigen-specific stimulation of Vδ2+ T cells was performed using the synthetic compound isopentenyl pyrophosphate (IPP; SIGMA) at 30 μg/mL final concentration. Vδ2+ T cells were expanded with IPP for approximately 4 weeks to obtain Vδ2+ T cell lines. FACS analysis was performed to determine the percentage of Vδ2+ and Vδ1+cells in the cultures by using the B6 monoclonal antibody (mAb) (IgG1; PharMingen, San Diego, CA) coupled with PE and the TS8.2 mAb (IgG1; Endogen, Woburn, MA) coupled to FITC. Lipopolysaccharide (SIGMA) was also used in some cultures at 10 ng/mL. We used the cytokines INFγ, TGFβ, IL-4, and IL-10 (R & D Systems, Minneapolis, MN).
Vδ2+ T cell lines and clones
PBMCs were stained with PE-conjugated mAb to anti-Vδ2 (see above) by incubation for 30 minutes at 4°C. After 2 washes in phosphate-buffered saline, positive cells were sorted at 1 or 10 cells/well into 96 well plates (Costar, Cambridge, MA) using a cell sorter (MoFlo High Speed Cell Sorter; Cytomation, Fort Collins, CO).
Cells were cultured in RPMI 1640 (Life Technologies) supplemented with 10% human serum (BioWhittaker, Walkersville, MD), 5% heat-inactivated FCS (HyClone, Logan, UT), 200 mmol/L L-glutamine, 100 μg/mL MEM nonessential amino acids, 0.5 mg/mL 2-ME (Life Technologies), 10 μg/mL penn-strept, 1 mg/mL MEM sodium pyruvate (Life Technologies), 0.5 μg/mL PHA (Murex, Dartford, England) and 100 units/mL human recombinant IL-2 (Boehringer Mannheim, Mannheim, Germany).
Cells were expanded with IL-2 and restimulated every 2 weeks with PHA and irradiated feeder cells (3000 rad) according to standard procedures. Cells were activated with plate-bound antibodies to CD3, the γδ TCR, and CD28, as described previously.13
Detection of chemokine production by sandwich ELISA
For detection of chemokines, freshly isolated PBMCs were plated in 96 well plates at 1 × 105 cells/well in the presence of the following stimuli: 50 units/mL IL-2, 30 μg/mL IPP + IL-2, or 10 ng/mL LPS + IL-2. Cells were stimulated every 7 days. On days 1, 7, and 14, supernatants were harvested at time 0 (immediately after IPP or LPS was added); 30 minutes; and hours 1, 2, 6, 20, 24, or 48. For Vδ2+ T cell lines and clones, cells were cultured in 96 well plates, and supernatants were harvested 48 hours after stimulation.
To quantify the amount of chemokines secreted in the medium, sandwich enzyme-linked immunosorbent assay (ELISA) was performed using matched antibody pairs, 13 as previously described, and a standard curve was established using human recombinant chemokines (R&D System).
Modulation of chemokine production by cytokines
Vδ2+ T cell lines were obtained by stimulating freshly isolated human PBMCs, derived from 3 healthy donors, with 30 μg/mL of IPP and culturing the cells for 1 month in the presence of 50 units/mL of human recombinant IL-2 (SIGMA). Enriched Vδ2+ T cells were cultured in 96 well plates at 2 × 105cells/well and divided into 2 groups: 1 was stimulated with IPP for a second time (IPP 2 × ), and the other was incubated in medium alone and used as a control group. IL-2 (10 units/mL) was given to both groups during the experiment. Cells were then cultured for 48 hours in the absence or presence of the following human recombinant cytokines (given alone or in combination): IL-4 (2.5 ng/mL), IL-10 (5 ng/mL), TGF-β (10 ng/mL), IL-12 (2 units/mL), or INF-γ (100 units/mL). Supernatants were harvested, and chemokine ELISA was performed as described above.
Ribonuclease protection assay
Chemokine and chemokine receptor mRNA expression was determined using multiprobe ribonuclease protection assay26 27 (RPA) (Riboquant; PharMingen, San Diego, CA). Cells were harvested and washed twice in phosphate-buffered solution. Total RNA was extracted according to standard procedures (RNAzol B; TEL-TEST, Frienswood, TX) according to standard procedures. Twenty μg of RNA was hybridized overnight at 43°C to specific probe sets containing 32P-UTP labeled transcripts using the RPA kit (RPA II kit; Ambion, Austin, TX) per manufacturers instructions. Single-stranded RNA was digested with RNase A/T1 mixture (Ambion), and the hybrids were analyzed on denaturing urea/polyacrylamide denaturing gels. Bands were detected by autoradiography and were quantified by phosphoimaging with a scanner and software package (Storm 860, ImageQuaNT 3.01; Molecular Dynamics, San Francisco, CA). Results were calculated as a ratio of the volume of the band of interest to the sum of the bands for the housekeeping genes. The housekeeping genes were large ribosomal protein subunit L32 and glyceraldehyde 3-phosphate dehydrogenase (GAPDH). To compare data for different chemokines, gel imaging data were also corrected for the number of 32P-dUTP incorporation sites for each of the chemokines examined. Differences between data sets were analyzed by ANOVA, using P < 0.05 as the significant measurement.
Results
MIP-1β, MIP-1, and RANTES induction by IPP stimulation
We and others have shown that IPP exclusively stimulates proliferation of the Vδ2Vγ9 T-cell subset (Figure 1) and also induces cytokine production in the same γδ T-cell population.7,9,12 28 To determine whether IPP selectively stimulated chemokines within the Vδ2+ population, freshly isolated PBMCs from 2 healthy donors were cultured with either IL-2, IL-2 + IPP, or IL-2 + LPS. LPS was used as a positive control since it is known to induce the expression of both MIP-1α and MCP-1 in PBMCs.
FACS analysis of PBMC after stimulation with IPP.
PBMC isolated by density centrifugation (Ficoll-Hypaque) from a healthy donor were stained to detect the percentage of Vδ2+ and Vδ1+ T cells before (B) and after culture for 21 days in vitro with either IL-2 alone (C) or IL-2+IPP (D).Cells were gated for the expression of the gene product Vδ2 or Vδ1. The gates are indicated by boxes, and the numbers indicate the percentage of positive cells within the total T-cell population. Panel A shows reactivity for the isotype control antibodies. Data shown are representative of 3 separate donors.
FACS analysis of PBMC after stimulation with IPP.
PBMC isolated by density centrifugation (Ficoll-Hypaque) from a healthy donor were stained to detect the percentage of Vδ2+ and Vδ1+ T cells before (B) and after culture for 21 days in vitro with either IL-2 alone (C) or IL-2+IPP (D).Cells were gated for the expression of the gene product Vδ2 or Vδ1. The gates are indicated by boxes, and the numbers indicate the percentage of positive cells within the total T-cell population. Panel A shows reactivity for the isotype control antibodies. Data shown are representative of 3 separate donors.
Cells were stimulated 3 times at weekly intervals to expand γδ T cells. At the time of each stimulation, supernatants were harvested, and ELISA determined the release of chemokines MIP-1α, MIP-1β, RANTES, and MCP-1 into the medium. FACS analysis was performed to determine the percentage of cells present in the culture that expressed the Vδ2 gene product. Culture with IPP led to a significant increase in the number of Vδ2+ T cells. By 15 days in culture, approximately 50% of the total lymphocyte population was positive for Vδ2 antigen (data not shown). No change was noted in the percent representation of Vδ2+ cells in cultures incubated in IL-2 or LPS. None of the culture conditions altered the representation of Vδ1 cells in these cultures, which was always <10% (data not shown). In these 2 donors the initial percentage of Vδ2 cells was 0.8% for LP3 and 15% for XC.
Following the first stimulation (Figure 2), only treatment with LPS led to the induction and release of MIP-1α MIP-1β, and to a lesser extent, MCP-1. RANTES was produced at low levels in all culture conditions. After the second stimulation on day 7, exposure to IPP led to the release of MIP-1α, MIP-1β, and RANTES in both donors, which was equivalent to levels induced by LPS. In contrast, levels of MCP-1 remained low to nondetectable in both donors following stimulation with IPP. Stimulation with LPS in these same donors led to significant release of MCP-1 (data not shown). Following the third stimulation on day 14, the amount of MIP-1α MIP-1β, and RANTES increased further in IPP-stimulated cells, and in 1 donor exceeded that induced by LPS (Figure 2). MCP-1 was found at high levels (12-13 ng/mL) in supernatants from LPS-stimulated cells, but it was almost undetectable following the third IPP stimulation.
Chemokine expression as determined by ELISA in PBMC cultured in vitro in IL-2 or IL-2+IPP.
PBMCs were cultured in medium IL-2 (open bars), IL-2+IPP (hatched bars), or LPS (closed bars) either once (day 1) or 3 times (day 14). Supernatants were harvested at the times shown. The presence of MIP-1α, MIP-1β, RANTES, and MCP-1 in these supernatants was determined by ELISA. Data shown are for cultures that were challenged either once (day 1) or that had been stimulated with these agents at weekly intervals (day 14).
Chemokine expression as determined by ELISA in PBMC cultured in vitro in IL-2 or IL-2+IPP.
PBMCs were cultured in medium IL-2 (open bars), IL-2+IPP (hatched bars), or LPS (closed bars) either once (day 1) or 3 times (day 14). Supernatants were harvested at the times shown. The presence of MIP-1α, MIP-1β, RANTES, and MCP-1 in these supernatants was determined by ELISA. Data shown are for cultures that were challenged either once (day 1) or that had been stimulated with these agents at weekly intervals (day 14).
These data indicate that IPP selectively stimulates MIP-1α, MIP-1β, and RANTES but not MCP-1 production in the Vδ2 subpopulation of T cells. It also suggests that IPP does not lead to the release of chemokines from other subsets of mononuclear cells. This suggests that IPP selectively stimulates MIP-1αb MIP-1β, and RANTES production but does not stimulate MCP-1 production in the Vδ2 subpopulation of T cells. It also suggests that IPP does not lead to the release of chemokines from other subsets of mononuclear cells.
To investigate the potential role of antigen presenting cells (APC) in this response, we performed 2 additional experiments. In the first, we sorted Vδ2+ cells from the total peripheral blood population and activated them with either IPP or PHA in the presence or absence of APC. Activation with IPP for 24 hours led to the release of MIP-1β and IFNγ, which did not require the presence of APC; although an enhanced response was noted when APC were present (Figure3). In contrast, no response to PHA was noted in the absence of APC. APC alone did not release MIP-1β or IFNγ in response to either IPP or PHA.
Chemokine and cytokine expression in freshly sorted Vδ2+ cells in response to IPP and PHA.
Vδ2+ cells were FACS sorted from the total PBMCs, seeded at 40 000 cells per well, activated for 24 hours with IPP (30 μg/mL) or PHA (0.5 μg/mL) in the presence or absence of autologous irradiated APC (10 000 cells/well), and supernatants collected. Autologous irradiated APC were also cultured alone or with either IPP or PHA. The levels of MIP-1β and IFNγ in the supernatants were determined by ELISA. Data shown are from 1 representative donor of 2 tested.
Chemokine and cytokine expression in freshly sorted Vδ2+ cells in response to IPP and PHA.
Vδ2+ cells were FACS sorted from the total PBMCs, seeded at 40 000 cells per well, activated for 24 hours with IPP (30 μg/mL) or PHA (0.5 μg/mL) in the presence or absence of autologous irradiated APC (10 000 cells/well), and supernatants collected. Autologous irradiated APC were also cultured alone or with either IPP or PHA. The levels of MIP-1β and IFNγ in the supernatants were determined by ELISA. Data shown are from 1 representative donor of 2 tested.
In the second experiment, Vδ2+ clones were prepared and activated with IPP in the presence or absence of APC. In these cells the presence of APC had little to no effect on the release of MIP-1β or IFNγ. For example, in 4 different clones the levels of MIP-1β in the cell supernatant at 6 hours following IPP stimulation without/with APC were 2310/3200, 2330/2270, 1800/2700, and 3650/3550 pg/mL. At 24 hours, the levels without/with APC were 10160/8060, 2140/2590, 4410/4370, and 6490/3700 pg/mL. APC alone did not secrete MIP-1β following stimulation with IPP. From these data we conclude that the induction of chemokines in Vδ2+ T cells in response to IPP does not require the presence of APC.
Analysis of chemokine production by γδ T cell lines
To further investigate the possibility that Vδ2+ T cells can be induced to express certain members of the C-C chemokine family following activation, we prepared highly enriched lines of Vδ2+ T cells from healthy donors by FACS sorting. We then stimulated the cells through the TCR using plate-bound mAbs to CD3 and CD28 or the γδ TCR. Cells were activated overnight, and the amount of MIP-1α, RANTES, and MCP-1 in the supernatants were measured by ELISA.
The results showed that compared to unstimulated controls, Vδ2+ T cell lines produced significant amounts of MIP-1α and RANTES (Figure 4). In contrast, MCP-1 was expressed at very low levels, and this did not change following stimulation through the TCR (data not shown). These data support the conclusion that activated Vδ2+ T cells are a potent source of chemokines such as MIP-1α but not MCP-1.
Chemokine expression as determined by ELISA for Vδ2+ T cell lines.
Vδ2+ T cell lines were established by FACS sorting at 10 cells per well and expanded in vitro using PHA and IL-2. Cells were then activated by plate-bound anti-CD3 or anti-γδ TCR and anti-CD28 for 24 hours. Control cells were maintained in IL-2 alone. Supernatants were harvested, and chemokine expression was determined by ELISA. Data for 8 different Vδ2+ T cell lines are shown. All of the lines produced MIP-1α and RANTES when stimulated with anti-CD3 + anti-CD28. However, MCP-1 was not detected in the same supernatants (data not shown).
Chemokine expression as determined by ELISA for Vδ2+ T cell lines.
Vδ2+ T cell lines were established by FACS sorting at 10 cells per well and expanded in vitro using PHA and IL-2. Cells were then activated by plate-bound anti-CD3 or anti-γδ TCR and anti-CD28 for 24 hours. Control cells were maintained in IL-2 alone. Supernatants were harvested, and chemokine expression was determined by ELISA. Data for 8 different Vδ2+ T cell lines are shown. All of the lines produced MIP-1α and RANTES when stimulated with anti-CD3 + anti-CD28. However, MCP-1 was not detected in the same supernatants (data not shown).
Chemokine and chemokine receptor expression by RPA
It has been suggested that the expression of MIP-1α and its receptors (CCR5 and CCR1) by activated T cells is characteristic of a response that has been biased toward a Th1 cytokine profile,29 whereas expression of MCP-1 and its receptor (CCR2) may be more characteristic of a Th2 response. To investigate whether incubation with IPP led to the selective expression of chemokines, we assessed the expression of additional members of the C-C and CXC chemokine families by γδ T cell lines using multiprobe RPA. Vδ2+ T cells were expanded for 4 weeks in the presence of IL-2 and IPP (IPP × 1), at which time γδ T cells represented approximately 90% of the lymphocyte population. The T cells were then activated again with IPP (IPP × 2); supernatants were collected; and mRNA was extracted at 4, 12, 24, and 48 hours post-challenge. Cells cultured with IL-2 alone were examined as the control population. The results showed that following exposure to IPP, there was a rapid up-regulation of the mRNA expression for lymphotactin, MIP-1α, MIP-1β, and RANTES, whereas the expression of a protected band for IP-10 was variable from 1 donor to another. Protected bands for MCP-1, IL-8, and I-309 were not visible (Figure5A).
Chemokine mRNA expression induced by IPP in IPP-expanded Vδ2+ T cell lines as determined by RPA.
Vδ2+ T cell lines were established by culturing PBMCs from a healthy donor for 4 weeks in vitro, following a single stimulation with IPP (IPP 1 × ). A population of the same PBMCs was also maintained in IL-2 alone (IL-2). The cells that had been stimulated 4 weeks previously were then stimulated again with IPP (IPP 2 × ), and RNA was extracted at 4, 12, 24, and 48 hours post-stimulation. (A) The result of the RPA analysis for chemokine mRNA expression using a multiprobe RPA system (hCK5). The undigested probe set (U) and the digested probe set (D) are shown in the first 2 lanes respectively, and the control RNA (ctr) provided with the kit is shown in the extreme right-hand lane. (B) Quantitative analysis of the bands by phosphoimaging are shown and are expressed as a ratio of the gene of interest to the sum of the housekeeping genes L32 and GAPDH. Data shown are representative of 3 different healthy donors.
Chemokine mRNA expression induced by IPP in IPP-expanded Vδ2+ T cell lines as determined by RPA.
Vδ2+ T cell lines were established by culturing PBMCs from a healthy donor for 4 weeks in vitro, following a single stimulation with IPP (IPP 1 × ). A population of the same PBMCs was also maintained in IL-2 alone (IL-2). The cells that had been stimulated 4 weeks previously were then stimulated again with IPP (IPP 2 × ), and RNA was extracted at 4, 12, 24, and 48 hours post-stimulation. (A) The result of the RPA analysis for chemokine mRNA expression using a multiprobe RPA system (hCK5). The undigested probe set (U) and the digested probe set (D) are shown in the first 2 lanes respectively, and the control RNA (ctr) provided with the kit is shown in the extreme right-hand lane. (B) Quantitative analysis of the bands by phosphoimaging are shown and are expressed as a ratio of the gene of interest to the sum of the housekeeping genes L32 and GAPDH. Data shown are representative of 3 different healthy donors.
The gels were then subjected to phosphoimaging. Differences in expression of the mRNA over time were determined as a ratio of the protected band for each chemokine to the sum of the protected bands for L32 and GAPDH. Gel imaging data were also corrected for the number of32P-dUTP incorporation sites for each of the chemokines examined. The results showed that peak expression of mRNA for these chemokines was observed at 4 hour post-challenge (Figure 5B), and that values had returned to near baseline levels by 48 hour post-challenge. The most robust response was noted for MIP-1β. These data show, therefore, that exposure to IPP leads to the induction of these C-C chemokines in Vδ2+ cells. However, no differences were noted in the overall pattern of chemokine mRNA expression from that observed in the same population of cells that had been incubated for the same length of time in high-dose (50 units/mL) IL-2, indicating that the effect of IPP was quantitative rather than qualitative.
ELISA data for MIP-1α, MIP-1β, and RANTES in these culture supernatants are shown in Figure 6. Consistent with the mRNA data, levels of MIP-1β were higher than for the other chemokines in all 3 donors. In addition, MIP-1β levels were elevated earlier (at 12 hours) than MIP-1α. No release of MIP-1α or MIP-1β into the medium was found in the cells cultured with IL-2 or stimulated with IPP 4 weeks earlier (IPP × 1). In contrast, RANTES was expressed at low levels in all of these cultures, and this increased only slightly following exposure to IPP.
Chemokine expression induced by IPP in IPP-expanded Vδ2+ T cell lines as determined by ELISA.
The supernatants from the cultures shown in Figure 4 were analyzed for expression of MIP-1α, MIP-1β, and RANTES by ELISA. Pooled data for all 3 donors are shown (mean ± SD).
Chemokine expression induced by IPP in IPP-expanded Vδ2+ T cell lines as determined by ELISA.
The supernatants from the cultures shown in Figure 4 were analyzed for expression of MIP-1α, MIP-1β, and RANTES by ELISA. Pooled data for all 3 donors are shown (mean ± SD).
The effect of immunomodulatory cytokines on the induction of chemokine expression in Vδ2+ cells
Studies in the mouse have shown that the induction of the chemokines MIP-1α and MIP-1β by LPS can be differentially regulated by the immunomodulatory cytokines INFγ, IL-10, IL-4, and TGFβ.30 To determine whether these cytokines modulated IPP-induced chemokine production, Vδ2-enriched T cell lines were established from 3 healthy donors by activating with IPP (IPP × 1) and culturing for an additional 4 weeks. At the end of this culture period, Vδ2+ cells represented 93%, 90%, and 70%, respectively, of the total T-cell population, as assessed by FACS analysis (data not shown). The cells were then stimulated again with IPP (IPP × 2) either alone (IL-2 at 50 U/ml) or in the presence of cytokines IL-4, IL-10, TGFβ, or INFγ. After 48 hours the supernatants were harvested, and MIP-1α and MIP-1β release was determined by ELISA. Cells that had been activated 4 weeks previously and maintained in IL-2–containing medium showed no chemokine release (Figure 7). However, following activation again with IPP (IPP × 2), high-level release of both MIP-1α and MIP-1β was observed for all 3 donors. Co-culture with IL-4, IL-10, TGFβ, or INFγ did not substantially affect chemokine release induced by IPP. In an additional experiment, we also tested whether pretreatment with INFγ (100 U/ml) for 2h modulated chemokine release induced by IPP. No effect was observed (data not shown). These data show that the activatory effects of IPP for MIP-1α and MIP-1β expression are not altered by regulatory cytokines.
Cytokine regulation of IPP-induced chemokine expression as determined by ELISA.
Vδ2+–enriched cultures were established by stimulating with IPP and culturing in IL-2 for 4 weeks. Cells were then activated again with IPP (IPP × 2) in the presence of IL-2 alone and IL-2 +IL-4, +IL-10, +TGFβ, and +INFγ. Culture supernatants were harvested 48 hours later, and MIP-1α and MIP-1β were released into the medium as determined by ELISA. Data shown represent the mean ± SD of 3 healthy donors.
Cytokine regulation of IPP-induced chemokine expression as determined by ELISA.
Vδ2+–enriched cultures were established by stimulating with IPP and culturing in IL-2 for 4 weeks. Cells were then activated again with IPP (IPP × 2) in the presence of IL-2 alone and IL-2 +IL-4, +IL-10, +TGFβ, and +INFγ. Culture supernatants were harvested 48 hours later, and MIP-1α and MIP-1β were released into the medium as determined by ELISA. Data shown represent the mean ± SD of 3 healthy donors.
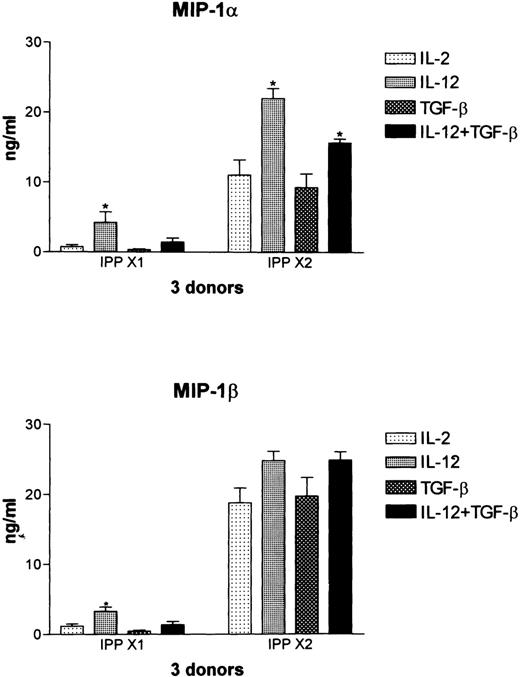
In previous studies we have shown that Vδ2+ T cells prominently express both IL-12rβ1 and IL-12Rβ2 and can be shown to up-regulate cell surface expression of the C-type lectin NKR-P1A in response to IL-12.31 Therefore, we tested whether culture with IL-12 affected the production of MIP-1α and MIP-1β in these cultures and if this could be modulated by co-culture with TGFβ. In resting cultures maintained with low-dose IL-2 (10 U/ml), culture with IL-12 led to low-level release of both MIP-1α and MIP-1β in all 3 donors tested. This was down-regulated by co-culture with TGFβ (Figure 8). In cultures activated again with IPP (IPP × 2), co-culture with IL-12 led to a significant increase (P < .01) in the levels of MIP-1α, whereas the release of MIP-1β was increased to a lesser extent. In agreement with the data shown in Figure 7, co-culture with TGFβ had no effect on IPP-induced MIP-1α or MIP-1β production, demonstrating that differences in the levels of IL-2 in the medium (10 units/mL for the data shown in Figure 8 and 50 units/mL for the data shown in Figure 7) did not influence this result.
Regulation by IL-12 of IPP-induced chemokine expression as determined by ELISA.
Vδ2-enriched cultures were established as described in Figure 6 and cultured with IL-12 and TGFβ, either alone or in combination, in control cultures maintained in IL-2 (IPP × 1) or in cultures that had been stimulated again with IPP (IPP × 2). Supernatants were harvested at 48 hours, and MIP-1α and MIP-1β expression was determined by ELISA. Data shown represent the mean ± SD of 3 healthy donors. The values for IPP × 2, IPP × 2 + IL-12, and IPP × 2 + IL-12 + TGFβ were significantly different from each, with a P > .01.
Regulation by IL-12 of IPP-induced chemokine expression as determined by ELISA.
Vδ2-enriched cultures were established as described in Figure 6 and cultured with IL-12 and TGFβ, either alone or in combination, in control cultures maintained in IL-2 (IPP × 1) or in cultures that had been stimulated again with IPP (IPP × 2). Supernatants were harvested at 48 hours, and MIP-1α and MIP-1β expression was determined by ELISA. Data shown represent the mean ± SD of 3 healthy donors. The values for IPP × 2, IPP × 2 + IL-12, and IPP × 2 + IL-12 + TGFβ were significantly different from each, with a P > .01.
Interestingly, however, the presence of both IL-12 and TGFβ in the culture medium differentially affected IPP-induced release of MIP-1α and MIP-1β. TGFβ significantly (P < .01) down-modulated the IL-12-induced augmentation of IPP-induced MIP-1α, but co-culture with TGFβ had no effect on the IL-12–induced augmentation of IPP-induced MIP-1β (Figure 8). These data support the conclusion that these 2 β chemokines, although highly homologous, are under different regulatory controls, as has been previously documented in mouse macrophages activated with LPS.30 Co-culture with IL-4, IL-10, and INFγ had no significant effect on the levels of either MIP-1α or MIP-1β in cultures stimulated with IPP+IL-12 (data not shown).
The effect of IPP on chemokine receptor expression
To determine whether activation with IPP led to altered expression of chemokine receptors, the RNA samples shown in Figure 4 were studied by RPA for the expression of C-C chemokine receptors (Figure9A). Quantitation of these data by phosphoimaging of the gels is shown in Figure 9B. The data showed that resting Vδ2+ cells, previously expanded by challenge with IPP and analyzed after 4 weeks, expressed predominantly CCR5 and also CCR1, CCR4, CCR8, and CCR2a+b (Figure 9A, black bars). Re-exposure to IPP (IPP × 2) led to rapid down-regulation of the mRNA signal for all of these chemokine receptors; gradual recovery occurred over time. In the donor shown in Figure 9A, no signal for CCR3 was detected, whereas it was possible to detect it in other donors (data not shown), which may reflect polymorphism within this receptor.32
Chemokine mRNA receptor analysis as determined by RPA.
(A)The samples shown in Figure 4 were also analyzed by the RPA multiprobe system for chemokine receptor expression. (B) Quantitative analysis of the data determined by phosphoimaging of the gels are shown and are expressed as a ratio of the band of interest to the sum of the housekeeping genes L32 and GAPDH. Following the second activation with IPP expression, all of the chemokine receptors were rapidly down-regulated but gradually recovered over time. Data shown are representative of 3 donors.
Chemokine mRNA receptor analysis as determined by RPA.
(A)The samples shown in Figure 4 were also analyzed by the RPA multiprobe system for chemokine receptor expression. (B) Quantitative analysis of the data determined by phosphoimaging of the gels are shown and are expressed as a ratio of the band of interest to the sum of the housekeeping genes L32 and GAPDH. Following the second activation with IPP expression, all of the chemokine receptors were rapidly down-regulated but gradually recovered over time. Data shown are representative of 3 donors.
Discussion
Peripheral circulating human γδ T cells have been shown to respond to viral, bacterial, protozoal, and tumoral antigens, but the specific nature of the antigens involved remains to be defined.4-8,33 34 Because these cells share many features in common with both natural killer cells as well as B and T lymphocytes, it has been suggested that they might form a bridge between the innate and acquired immune response by functioning as a source of cytokines involved in activation of specific arms of the immune response.
Two observations are consistent with such a notion. The γδ T-cell subset expresses the Vδ2 Vγ9+CD3+CD4−CD8−phenotype and represents the major γδ T-cell subset found in the circulation of most normal adults. As such, the γδ T-cell subset specifically responds to nonprotein compounds, such as prenyl pyrophosphate derivatives, by rapidly producing high levels of the cytokines INFγ and TNFα.8,12,28 These nonprotein ligands are components of the cell wall of many common pathogens and, as such, would fit well into the hypothesis of Janeway and colleagues35 36 that components of the innate immune response are specialized to recognize and respond rapidly to conserved molecular patterns found in microorganisms.
In this study we have investigated whether Vδ2+ T cells from human peripheral blood can also be induced to secrete C-C chemokines in response to prenyl pyrophosphate derivatives such as IPP, a synthetic phosphoantigen. The results show that these cells are specifically activated by IPP to release large quantities of the β-chemokines MIP-1α and MIP-1β. Studies at both the mRNA and protein levels indicated that the most robust response was obtained for MIP-1β. We also detected differences in the kinetics of release, with MIP-1β being induced and released rapidly and MIP-1α induction occurring more gradually.
Although MIP-1α and MIP-1β share significant sequence homology and may use the same receptor,37 they are known to have distinct and sometimes opposing properties.38 In vitro, human MIP-1α and MIP-1β recruit different populations of T cells, with MIP-1α attracting mainly CD4+ T cells and MIP-1β inducing chemotaxis of predominantly CD8+ T cells.18,19 Consistent with this are the observations that MIP-1 peptides display differential agonist activity for different chemokine receptors. MIP-1α activates CCR1, CCR5, and perhaps CCR4, whereas MIP-1β more selectively interacts with CCR5. Furthermore, MIP-1α has been shown to activate macrophages, eosinophils, and basophils, whereas MIP-1β lacks this activity.39
The production of MIP-1α and MIP-1β, as well as INFγ and TNFα, in response to IPP stimulation would add further support to the conclusion that IPP activates a Th1-type response in these Vδ2+ T cells.12,28 IL-12 is a potent proinflammatory cytokine and acts on activated T cells and NK cells to stimulate cytokine production and cytotoxicity.40 We have previously shown that Vδ2+ T cells express both IL-12rβ1 and IL-12rβ2, and they respond to IL-12 by up-regulation of the activation marker NKR-P1A.31 In Vδ2+ T cells stimulated with IPP, IL-12 has been shown to increase the number of cells expressing INFγ.28 We now show that IL-12 also induces the expression of MIP-1α and MIP-1β in these cells and augments the induction of these chemokines by IPP. IL-12 is induced in macrophages following phagocytosis of different intracellular organisms, including mycobacteria, which are a potent source of these phosphorylated ligands.6 7 As a result, chemokine expression by Vδ2+ T cells could significantly contribute to the proinflammatory microenvironment at sites of infection.
In T cells the regulatory cytokine TGFβ has been shown to down-regulate IL-12 responsiveness by inhibiting the early signaling events essential to IL-12–induced gene expression.41 In Vδ2+ T cells, we found that TGFβ strikingly reduced the effect of IL-12 on MIP-1α production while sparing the IPP+IL-12–induced expression of MIP-1β. This differential effect of regulatory cytokines on MIP-1α and MIP-1β expression has been previously noted in mouse macrophages activated with LPS.30The authors of that report speculate that this may reflect a response to different roles that these chemokines play in the immune response. MIP-1α, in addition to its potent chemotactic activity, is a potent activator of the immune response, which could become detrimental over time, once repair mechanisms have become activated. In contrast, the production of MIP-1β, which lacks the cellular activating functions characteristic of MIP-1α, could be maintained and still contribute to the wound-healing process at later stages of the response. However, in contrast to the report where the cytokines IL-4, IL-10, TGFβ, and INFγ all down-regulated LPS induction of MIP-1α in macrophages,30 we failed to find a regulatory effect of these cytokines on IPP-induced expression in γδ T cells. At the present time, the IPP-activated signaling pathway, which is involved in proinflammatory gene expression, has yet to be defined, and thus potential regulatory pathways involved in this response must await further study.
In addition to these chemokines, the RPA data showed that in some donors, Vδ2+ cells could be activated by IPP to express mRNA for LTN and IP-10, but they could not express MCP-1. This profile of chemokine expression in human γδ T cells is remarkably similar to that found in intraepithelial γδ T (DETC) cells in the mouse.42 In this study, LTN was the most abundantly expressed chemokine. LTN is a major chemotactic factor for CD8+ T cells in the mouse, and in human peripheral blood cells, LTN has been shown to be chemotactic for T lymphocytes and NK cells.21 NK cells are also a significant source of this chemokine, again suggesting close similarities between Vδ2+ and NK cells. The fact that we did not find expression of MCP-1 in Vδ2+ cells may mark a distinct difference in chemokine expression between the major γδ T-cell subsets in PBMCs. We have found that some populations of Vδ1 cells express both mRNA and protein for MCP-1 following activation through the TCR (unpublished observations).
With RPA we have also analyzed the levels of mRNA for chemokine receptors in Vγ9Vδ2 cells prior to and at varying times following restimulation with IPP. In resting γδ T cells we observed protected bands for all of the β-chemokine receptors analyzed, including CCR1, CCR2, CCR3, CCR4, CCR5, and CCR8 (TER 1). The presence of these receptors in these cells in culture would be consistent with the known effect of prolonged culture with IL-2 on chemokine receptor expression in αβ TCR+ T cells. However, in contrast to the chemokine data, all the transcripts for these receptors were strongly down-regulated following activation with IPP. The kinetics of this response, as well as the fact that CCR2 was also down-regulated even though MCP-1 was not detected in the supernatant, suggest that this occurred by a ligand-independent mechanism.
It is of interest to note that a similar ligand-independent mechanism of down-regulation of C-C chemokine receptors has been noted in monocytes in response to other bacterial superantigens including staphylococcal enterotoxins A and B43 and lipopolysaccharide.44 This mechanism involved activation of protein kinase signaling and secreted serine proteinases. Future studies will address the mechanisms involved in this response in γδ T cells. However, following long-term stimulation with IPP (4 to 6 weeks), these cells expressed high levels of mRNA for CCR5, consistent with a bias toward a Th1-type response in cells stimulated with IPP. In addition, these cells expressed CCR8. This receptor is known to be highly specific for the β-chemokine I-309.45 It has been demonstrated that I-309 is produced mainly by activated T lymphocytes and is an inflammatory mediator that specifically stimulates human monocytes.46 We did not find that γδ T cells expressed I-309, suggesting that this chemokine could function to specifically attract and activate γδ T cells to sites of antigen activation.
Recently it has also been shown that NK cells isolated from the peripheral blood of either healthy donors or HIV-infected patients produce the chemokines MIP-1α, MIP-1β, and RANTES after stimulation with IL-12 and IL-15. Supernatants from these cultures partially inhibit HIV-1 replication in vitro compared with supernatants from unstimulated controls.47 Vγ9Vδ2 cells may also play an important role in HIV immunity, since supernatants from Vγ9Vδ2 cells isolated from healthy donors and stimulated with phosphoantigens (TubAg or IPP) inhibit in vitro replication and infectivity of both the monocytotropic and T-tropic strains of HIV.48
Taken together, our findings indicate that Vδ2+ T cells respond to components of the bacterial cell wall by rapidly releasing high levels of C-C chemokines involved in T-cell recruitment and activation. Cells, such as the NK cells, could play an important role as a bridge between the innate and acquired immune response to bacterial challenge. This would add further support to the hypothesis that certain subpopulations of γδ T cells may be specialized to form part of the early response to infectious agents through the rapid secretion of proinflammatory cytokines and chemokines.
Supported by grants from the Progretto Sclerosi Multipla, Istituto Superiore Sanita, Rome, Italy; by MURST, Finalizzato 1998, Ministero della Sanita, Italy, Telethon-Italy (project 1033); by the United States Public Health Service (grant NS31919); and by the National Multiple Sclerosis Society, USA (RG #3037A3).
Reprints: Barbara Cipriani, the Department of Pathology, Albert Einstein College of Medicine, 1300 Morris Park Avenue, Bronx, NY 10461.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.











This feature is available to Subscribers Only
Sign In or Create an Account Close Modal