Abstract
Our recent finding that resistance to lymphoma cell metastasis in intercellular adhesion molecule-1-(ICAM-1)–deficient mice was manifested after homing suggested that the mechanism could involve the capacity of ICAM-1 to induce, via leukocyte function-associated antigen-1 (LFA-1) signaling, the expression of new genes necessary for migration and survival of lymphoma cells after homing. This hypothesis would imply that lymphoma cells, on repeated metastatic cycles, would acquire such a highly aggressive phenotype that they no longer require contact with ICAM-1 at later stages of metastasis. We addressed this question by generating highly aggressive lymphoma variants to determine if increased tumorigenicity would allow lymphoma cells to grow into tumors in ICAM-1–deficient mice. We found that on repeated in vivo passages, a selective pressure favored the lymphoma cells that constitutively express high levels of matrix metalloproteainse-9 (MMP-9), a gene associated with a poor clinical outcome in non-Hodgkins's lymphoma. We further found that although the parent lymphoma cells could not grow tumors in ICAM-1–deficient mice, the aggressive lymphoma variants could. This indicates that, at late stages of the disease, tumor cells with a high metastatic efficiency, encoded by the repertoire of selected genes, no longer require some of the signals normally delivered by cell adhesion molecules. In light of these findings, the possibility of inhibiting dissemination of lymphoma cells at the late stage of the disease by acting against cell adhesion molecules must be reconsidered. (Blood. 2000;95:314-319)
Cell adhesion molecules play a variety of roles during distinct stages of metastasis. In the initial stage, down-regulation of cell membrane integrins that interact with extracellular matrix proteins favors the spread of tumor cells by allowing them to detach from the primary tumor and enter the circulation.1 In later stages of metastasis, however, expression of adhesion molecules favoring intercellular contacts is necessary for the efficient spreading of metastatic cells.2-5 Although the latter was first thought to provide specific homing signals for the invasion of target organs, the observation that metastatic and nonmetastatic cells home to target organs with the same efficiency and kinetics supports the view that adhesion molecules rather control processes after homing.6-8 The most compelling evidence supporting this view is that mice deficient in intercellular adhesion molecule (ICAM-1) are resistant to dissemination of lymphoma cells to peripheral organs, yet lymphoma cells migrate with the same efficiency to target organs in both normal and ICAM-1–deficient mice.5
Although the resistance mechanism of ICAM-1–deficient mice to lymphoma metastasis is still unclear, 2 possible scenarios deserve particular attention. First, it is conceivable that resistance simply consists in the absence of ICAM-1 induced “tumor welcoming signals” in stromal cells. Indeed, ICAM-1 is a known transducer of intracellular signals via their cytoplasmic domains.9-11 Moreover, the essential role of peritumoral cells in cancer metastasis is now well accepted, most notably following the results of Masson et al,12 who recently showed that fibroblasts isolated from stromelysin-3–deficient mice failed to support the growth of human breast cancer cells in nude mice. The second scenario explaining the resistance of ICAM-1–deficient mice could be that contact of leukocyte function-associated antigen (LFA-1)–bearing lymphoma cells to ICAM-1 normally up-regulates the expression of specific genes in the tumor cells, providing them with the ability to grow into tumors in the target organs. Integrins generally activate, via their cytoplasmic domains, a number of physiologic processes that provide normal and transformed cells with the ability to disseminate.13 Thus, the truncation of the cytoplasmic domain of the α-4 chain down-regulates the dissemination of lymphoma cells in vivo.14 Our group has also shown that contact of lymphoma cells with endothelial cells via ICAM-1 up-regulates the expression of matrix metalloproteinase-9 (MMP-9) in lymphoma cells.15Interestingly, the more aggressive human lymphomas display a constitutive expression of high levels of MMP-9.16 It is therefore possible that intercellular contact at late stages of metastasis induce the expression of new aggressive properties in tumor cells that are essential for their growth in the target organ. If so, constitutive expression of such a repertoire of tumor-promoting gene(s) would allow aggressive lymphoma cells to bypass the traditionally required exchange of intercellular signals during the establishment of a tumor in its target organs. We addressed this question in the present work and injected highly aggressive lymphoma cells in ICAM-1–deficient mice to determine whether their aggressive phenotype would allow these lymphoma cells to grow into tumors. To obtain such highly aggressive lymphoma cells, we carried out serial in vivo passages of 164T2 lymphoma cells, a cell line capable of forming tumors following intravenous injection in normal, but not in ICAM-1–deficient mice.5 We found that as we increased aggressiveness of the lymphoma cells, we did not observe any change in their homing characteristics, but an increased production of MMP-9. When we tested their ability to overcome the resistance of ICAM-1–deficient mice, we found that although the parent cell line 164T2 could not induce lymphoid tumors in ICAM-1–deficient mice, the aggressive daughter cells could.
Materials and methods
Mice
The ICAM-1 mutant mice (Icam1tm1Bay) backcrossed on a C57BL/6 background were obtained from Jackson Laboratories (Bar Harbor, MA). Mice of this strain carry a deletional mutation in the fifth exon of the icam-1 gene.17 They were age- and sex-matched with their wild-type controls. All mice were bred in our animal facility and maintained under specific pathogen-free conditions.
Antibodies
Monoclonal antibodies (mAbs) were purified from hybridoma culture supernatants by chromatography on protein G-Sepharose (Pharmacia Fine Chemicals, Piscataway, NJ). The following mAbs were used: M17/4.2 (anti-mouse LFA-1),18 PS/2 (anti-mouse 4 chain),19 145-2C11 (anti-mouse CD3ε chain),20and YN1/1.7.4 (anti-mouse ICAM-1).21 Fluoresceination and biotinylation of antibodies were carried out using standard protocols. The fluorescein isothyocyanate (FITC)-conjugated anti-mouse CD31, CD44, VCAM-1, VLA-5, and ICAM-2 were obtained from Pharmingen (San Diego, CA). The streptavidin-phycoerythrin (PE) conjugate was obtained from GIBCO/BRL (Mississauga, Ontario) The PE-conjugated anti-mouse CD4 was obtained from Boehringer Mannheim (Laval, QC). The FITC-conjugated anti-CD8 was obtained from Becton Dickinson (Mountain View, CA).
Culture of cell lines
The mouse thymic lymphoma lines 164T2 and 374 were developed in vitro from a radiation-induced thymic lymphoma in C57BL/Ka mice, as previously described.5 All cell lines were maintained in culture using RPMI 1640 supplemented with 10% fetal calf serum (FCS), 2 mM glutamine, and 10 mM HEPES buffer (N-2-hydroxyethyl-piperazine-N-2-ethane sulfonic acid).
Lymphoid tumor growth in spleen, kidneys, liver, and lymph nodes
To induce lymphoma, mice at least 6 to 8 weeks old were injected via the tail vein with 106 lymphoma cells resuspended in 200 μL phosphate-buffered saline (PBS). When clinical signs of lymphoma became evident (dyspnea, runting, and splenomegaly), the animals were killed, and spleen, lungs, ovaries, kidneys, liver, and lymph nodes harvested, weighed, and fixed in 10% formalin for histologic examination.
In vivo selection of highly metastatic lymphoma cell lines
The aggressive 164T2S11 (S11) and 164T2S19 (S19) cell lines were obtained from serial in vivo passages of the parent 164T2 line in young adult C57BL/6 males using the spleen as the organ from which lymphoma cells were harvested after each passage. Briefly, for each passage, 6- to 8-week-old mice were injected via the tail vein with 106lymphoma cells. When mice presented clinical signs of lymphoma, they were killed, and lymphoma cells were isolated from the spleen. A leukocyte suspension was obtained by removing red blood cells using the standard ammonium chloride-based protocol. Two clones were finally established after 11 and 19 passages, respectively (S11 and S19). These clones were maintained in culture without loss of aggressiveness.
To test for the ability of S11 and S19 to induce tumor dissemination in normal male and female C57BL/6 and ICAM-1–deficient mice, 1 to 5 × 105 lymphoma cells were injected into the tail vein of 6- to10-week-old mice. Mice were observed at regular intervals for clinical signs of lymphoma development, and when moribund, they were killed and tissues harvested and fixed in 10% formalin for histologic examination.
RNA isolation and analysis
Total cellular RNA was isolated from lymphoma cells using Trizol reagent (Life Technologies, Mississauga, Canada) according to the manufacturer's instructions. First-strand cDNA was prepared from 3 μg total cellular RNA in a 30-μL reaction volume, using 40 U M-MuLV Reverse Transcriptase (Boehringer Mannheim, Laval, Canada). After reverse transcription, the MMP-2, MMP-9, and tissue inhibitor of metalloproteinase (TIMP) cDNA were amplified using specific primers (Table 1). Expression of β-actin (Stratagene, La Jolla, CA) mRNA was monitored as a control using commercially available primers. TIMP-2–specific primers were carefully chosen to react with the different mRNA messages possibly resulting from alternative polyadenylation/termination site usage observed previously.22 Amplification was conducted using the polymerase chain reaction (PCR) core kit (Boehringer Mannheim). Thirty cycles of amplification were performed in a thermal cycler (model PTC-100TM, MJ Research, Watertown, MA) using the following programmed step cycle: 94°C for 1 minute, 58°C for 2 minutes, and 72°C for 3 minutes. Five to 10 μL of the reaction mixture was size-separated on a 1.5% agarose gel and specifically amplified products were detected by ethidium bromide staining and UV transillumination. Semiquantitative analysis was conducted using a computerized densitometric imager (Model GS-670; Bio-Rad, Mississauga, Canada).
Sequences of primers used for the detection of MMPs and TIMPs
| Gene* . | NT (bp) . | Sense Sequence . | Antisense Sequence . |
|---|---|---|---|
| MMP-2 | 705 | CTTGCAGGAGACAAGTTCTGG | TTAAGGTGGTGCAGGTATCTGG |
| MMP-9 | 380 | CCATGAGTCCCTGGCAG | AGTATGGATGTTATGATG |
| TIMP-1 | 655 | CTTTGCATCTCTGGCATCTGG | AAGTAGACAGTGTTCAGGC |
| TIMP-2 | 584 | TGCAGCTGCTCCCCGGTGCAC | TTATGGGTCCTCGATGTCAAG |
| TIMP-3 | 392 | AACTCCGACATCGTGATCCGGGCC | GGAGAGCATGTCGGTCCAGAGACA |
| TIMP-4 | 491 | TCGGCTCTAGTGATACGGGCC | CCTCGGTACCAGCTGCAGATG |
| Gene* . | NT (bp) . | Sense Sequence . | Antisense Sequence . |
|---|---|---|---|
| MMP-2 | 705 | CTTGCAGGAGACAAGTTCTGG | TTAAGGTGGTGCAGGTATCTGG |
| MMP-9 | 380 | CCATGAGTCCCTGGCAG | AGTATGGATGTTATGATG |
| TIMP-1 | 655 | CTTTGCATCTCTGGCATCTGG | AAGTAGACAGTGTTCAGGC |
| TIMP-2 | 584 | TGCAGCTGCTCCCCGGTGCAC | TTATGGGTCCTCGATGTCAAG |
| TIMP-3 | 392 | AACTCCGACATCGTGATCCGGGCC | GGAGAGCATGTCGGTCCAGAGACA |
| TIMP-4 | 491 | TCGGCTCTAGTGATACGGGCC | CCTCGGTACCAGCTGCAGATG |
Each pair of primers were tested for their functional integrity. For TIMP-3 and TIMP-4, the primers were tested using mRNA extracted from C6 glioma cells or freshly isolated murine spleen cells.
Zymography
Production and secretion of MMP in cell culture supernatants was measured as previously described.23 Briefly, cells were cultured in 24-well plates for 24 hours, in serum-free medium with or without PMA (10 ng/mL). Supernatants (1 mL) were then collected and centrifuged at 1200 rpm for 10 minutes to remove contaminating cells and debris. The supernatants were lyophilized and resuspended in 100 μL Dulbecco's modified Eagle's medium. Aliquots of 20 μL were subjected to electrophoresis on a 7.5% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) containing 1 mg/mL denatured collagen (Sigma, St. Louis, MO). After electrophoresis, the gel was washed to remove SDS and incubated in a renaturating buffer (50 mM Tris, 5 mM CaCl2, 0.02% NaN3, 3.1% Triton X-100) for 18 hours at 37°C. The gels were stained with Coomassie brilliant blue and destained in 30% (v/v) methanol/10% (v/v) acetic acid. The proteolytic activity was identified as a clear band on a blue background. Quantitative analysis of activity was conducted using a computerized densitometric imager.
Flow cytometric analysis
Cells were stained at 4°C and washed with PBS containing 0.5% bovine serum albumin (BSA) and 0.2% sodium azide (PBA). Prior to staining, cells were incubated with 10 μg/mL human IgG (Sigma) for 20 minutes at 4°C to block nonspecific binding. Fluorochrome- or biotin-labeled mAbs were then added at appropriate concentrations and incubated for another 20 minutes. Cells were then washed 4 times with PBA. For indirect staining with streptavidin-PE, cells were washed 3 times following the reaction with the first mAb and then incubated 20 minutes on ice with the fluorescent conjugate. Results shown are representative of at least 3 independent experiments. Flow cytometric analyses were performed on a Coulter XL-MCL™ flow cytometer (Coulter Electronics, Hialeah, FL).
In vivo migration assays
The ability of lymphoma cells to migrate to target organs was analyzed using standard indium (In) labeling of lymphoma cells, as previously described.7 Briefly, 107 cells were labeled with 10 mCi 111In in 0.5 mL RPMI for 15 minutes at room temperature. The cells were washed 4 times with RPMI containing serum and resuspended in PBS. The viability of labeled cells was over 95% as determined by trypan blue exclusion. Each mouse was injected intravenously with 106 cells (0.5 to 1 × 106 cpm). At 3, 12, and 24 hours, animals (5 mice for each time point) were killed and kidneys, spleen, liver, brain, ovaries, testis, and thymus, as well as heparinized blood samples, were collected. Calculations of the percentage of lymphoma cells per target organs were corrected to take into account the short-half-life of111In. The total radioactivity in circulating blood was estimated in 400 μL aliquots of blood and assumed a total volume of 2 mL circulating blood per mouse.
Statistical analysis
Data are presented as means ± SD. Student t test was used to test for statistical significance.
Results
Increased aggressiveness of lymphoma cells induced by serial in vivo passages
To determine whether the resistance of ICAM-1–deficient mice could be overcome by increasing the aggressiveness of the lymphoma cells, we generated highly metastatic clones by serial in vivo passages of 164T2 cells in C57BL/6 mice. After 11 such passages, a first clone, 164T2S11 (S11) was obtained and maintained in vitro without loss of aggressiveness. The S11 lymphoma cells induced lymphoid tumors in 99% of injected male or female C57BL/6 mice (Table2), a significant increase over the parent 164T2 clone (P ≤ 0.001), which induced tumors in < 50% of male C57BL/6 mice,5 and rarely (1 of 11) in females. Furthermore, in both male and female C57BL/6 mice, the lymphoid tumors induced by S11 developed much more rapidly than those induced by 164T2 cells (P ≤ 0.001). The size and organ distribution of the lymphoid tumors induced by both cell lines did not differ significantly (data not shown). Despite the fact that lymphoma cells were selected from the spleen throughout their in vivo passages, no changes in organ distribution were observed, because both S11 and 164T2 consistently induced lymphoid tumors in kidneys, spleen, ovaries, and liver. Thus, in vivo passages increased aggressiveness of 164T2 clone but did not modify target organ distribution. This observation was consistent with the results of the homing test. We injected 106111In-labeled cells intravenously to determine the distribution of the cells in the mouse. The in vivo selection process did not alter the homing pattern of the lymphoma cells. Both 164T2 and S11 have identical homing kinetics at 30 minutes, 3 hours, and 24 hours after injection. Both cell lines showed a transient homing to the lungs before colonizing kidneys, liver, and spleen. Less than 0.5% of the cells remained in circulation 24 hours after injection. Similarly, using a panel of 16 antibodies specific for cell surface molecules normally expressed by T-lymphocyte subsets, we found that increased aggressiveness did not modify the repertoire of cell adhesion molecules expressed by the parent cell line (Table3) nor their expression level (data not shown).
Frequency of lymphoma and mean survival time (in brackets) of C57BL/6 mice injected intravenously with 164T2 and 164T2S11 lymphoma cells
| Lymphoma Cell Line . | Male . | Female . |
|---|---|---|
| 164T2 | 3/8 (58 days) | 1/11 (62 days) |
| 164T2S11 | 8/8 (16 days) | 15/16 (16.5 days) |
| Lymphoma Cell Line . | Male . | Female . |
|---|---|---|
| 164T2 | 3/8 (58 days) | 1/11 (62 days) |
| 164T2S11 | 8/8 (16 days) | 15/16 (16.5 days) |
The experiment was terminated at day 90. The data are representative of at least 3 independent experiments.
Phenotype of the lymphoma cell lines
| Receptor . | 164T2 . | 164T2S11 . |
|---|---|---|
| CD3ε | + | + |
| CD4 | − | − |
| CD8 | − | − |
| CD11a/CD18 (LFA-1) | + | + |
| CD29/CD49d (VLA-4) | − | − |
| CD29/CD49e (VLA-5) | − | − |
| CD31 | + | + |
| CD44 | − | − |
| CD54 (ICAM-1) | + | + |
| CD62L (l-selectin) | + | + |
| CD90 (Thy-1.2) | + | + |
| CD102 (ICAM-2) | + | + |
| CD106 (VCAM-1) | − | − |
| gp70 | + | + |
| αβTcR | + | + |
| TL | + | + |
| Receptor . | 164T2 . | 164T2S11 . |
|---|---|---|
| CD3ε | + | + |
| CD4 | − | − |
| CD8 | − | − |
| CD11a/CD18 (LFA-1) | + | + |
| CD29/CD49d (VLA-4) | − | − |
| CD29/CD49e (VLA-5) | − | − |
| CD31 | + | + |
| CD44 | − | − |
| CD54 (ICAM-1) | + | + |
| CD62L (l-selectin) | + | + |
| CD90 (Thy-1.2) | + | + |
| CD102 (ICAM-2) | + | + |
| CD106 (VCAM-1) | − | − |
| gp70 | + | + |
| αβTcR | + | + |
| TL | + | + |
Increased aggressiveness correlates with increased MMP-9 expression in lymphoma cells
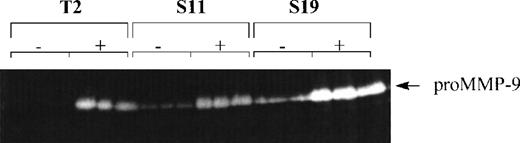
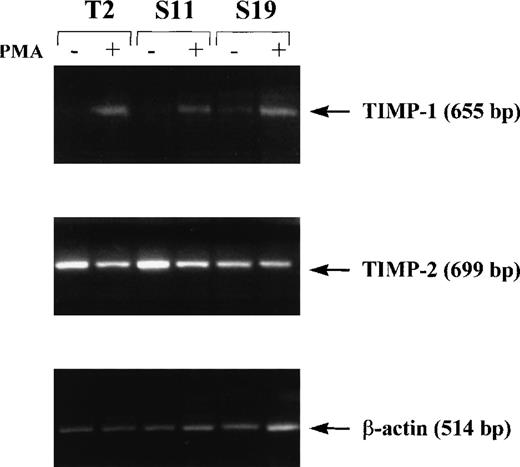
Because production of high levels of MMP-9 is a characteristic of aggressive lymphoma cells,16,24 we compared the production of MMP-9 in the parent 164T2 clone and the S11 subclone. We found that S11 cells produced more MMP-9 constitutively, whereas expression of MMP-9 in 164T2 required activation (Figure1). This increase of MMP-9 expression on passage of the lymphoma cells in vivo was even more noticeable when the 164T2 cells were passaged 19 times. Because tumor growth and metastasis of T-cell lymphoma are inhibited by high-level TIMP-1 production,25 we also measured the level of TIMP-1, TIMP-2, TIMP-3, and TIMP-4 in both cell lines. As in the case of 164T2, we found that in S11 TIMP-1 expression was only detectable on activation with PMA (Figure 2). The expression level of TIMP-1 on activation, however, significantly decreased with increased numbers of in vivo passages. In contrast to TIMP-1, the expression level of TIMP-2 was constitutive in all cell lines but showed variable expression on passages. No expression of TIMP-3 and TIMP-4 were detected (data not shown).
Production and expression of MMP-9 by lymphoma cells in vitro.
Cells were cultured in triplicate in the absence (−) or presence (+) of 50 nM PMA for 12 hours. Supernatants were collected, lyophilized, and assayed for their gelatinase content by zymography. Results are representative of at least 3 independent experiments.
Production and expression of MMP-9 by lymphoma cells in vitro.
Cells were cultured in triplicate in the absence (−) or presence (+) of 50 nM PMA for 12 hours. Supernatants were collected, lyophilized, and assayed for their gelatinase content by zymography. Results are representative of at least 3 independent experiments.
Expression of TIMP-1 and TIMP-2 in lymphoma cells.
Cells were cultured in the absence (−) or presence (+) of 50 nM PMA for 12 hours. Induction of TIMP-1 and TIMP-2 was measured by reverse transcriptase-polymerase chain reaction. Results are representative of 2 independent experiments.
Expression of TIMP-1 and TIMP-2 in lymphoma cells.
Cells were cultured in the absence (−) or presence (+) of 50 nM PMA for 12 hours. Induction of TIMP-1 and TIMP-2 was measured by reverse transcriptase-polymerase chain reaction. Results are representative of 2 independent experiments.
Ability of aggressive lymphoma cells to overcome the resistance of ICAM-1–deficient mice
We tested whether the highly aggressive phenotype of S11 allowed these cells to overcome the resistance of ICAM-1–deficient mice. For this purpose, 106 S11 and 164T2 (control) cells were injected intravenously to C57BL/6tm1bay mice. As we previously reported,5 there was no evidence of tumors in any organ of male (0 of 15) and female (0 of 4) ICAM-1–deficient mice injected with the 164T2 cells (Table4). In contrast, S11 lymphoma cells induced tumors in all male (9 of 9) and almost all female (7 of 8) ICAM-1–deficient mice. The resistance of ICAM-1–deficient mice could also be overcome by the injection of S19 lymphoma cells (data not shown). The distribution pattern of tumors induced by S11 in male ICAM-1–deficient mice was identical to that induced by 164T2 in normal male or female C57BL/6 mice (i.e., spleen, liver, lymph nodes, and kidneys). In female ICAM-1–deficient mice, however, tumors developed first in the ovaries, although histologic examination of these mice showed that lymphoma cells significantly invaded the other organs as well. The homing property of S11 was identical with that of 164T2 in both normal and ICAM-1–deficient mice, as determined by the111In migration assay (data not shown). Whether this distinct pattern of organ distribution between male and female ICAM-1–deficient mice is due to the repertoire of ICAM-1 isoforms is under investigation.
Frequency of lymphoma and mean survival time (in brackets) of ICAM-1–deficient mice injected intravenously with 164T2 and 164T2S11 lymphoma cells
| Lymphoma Cell Line . | Male . | Female . |
|---|---|---|
| 164T2 | 0/15 | 0/4 |
| 164T2S11 | 9/9 (16.8 days) | 7/8 (19 days) |
| Lymphoma Cell Line . | Male . | Female . |
|---|---|---|
| 164T2 | 0/15 | 0/4 |
| 164T2S11 | 9/9 (16.8 days) | 7/8 (19 days) |
The experiment was terminated at day 90. The data are representative of at least 4 independent experiments.
Ability of LFA-1–negative T lymphoma cells to spread in normal mice
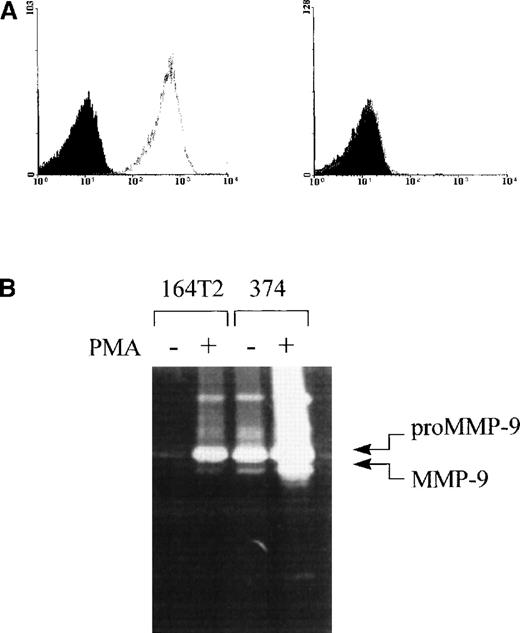
The above data suggest that the increased aggressiveness of S11 can overcome the resistance of ICAM-1–deficient mice. However, alternative splicing of the icam-1 gene and the localization of the mutation in ICAM-1–deficient mice leave a basal level of ICAM-1 expression in these mice, and because S11 expresses high levels of LFA-1, which can bind to the residual ICAM-1 isoforms, it was still conceivable that increased aggressiveness alone was not sufficient to produce lymphoma in absence of ICAM-1-mediated signals. In fact, we had no hard evidence to indicate that lymphoma dissemination could occur in absence of LFA-1/ICAM-1-mediated adhesion. To directly address this issue, we have established an independent new lymphoma cell line, 374. Just like the parent 164T2 line, this T-lymphoma cell line (as evidenced by the expression of the TcRαβ) was also isolated from a radiation-induced thymic lymphoma and maintained in culture. In contrast to other T-lymphoma cell lines, however, we found that the 374 cells lacked any detectable expression of LFA-1 on its surface (Figure3A). The 374 cells produced lymphoma systemically in normal mice when injected intravenously (Table5). Moreover, just like other aggressive lymphoma cells, the 374 cells produced high levels of MMP-9 constitutively (Figure 3B). We thus intravenously injected ICAM-1–deficient mice with 374 and found that these mice developed tumors despite the absence of LFA-1 on the surface of the tumor cells (Table 5). The organ distribution pattern induced by 374 cells was identical in both male and female ICAM-1–deficient mice and showed tumor development in kidneys, liver, spleen, lungs, and lymph nodes.
Expression of LFA-1 and MMP-9 by 374 lymphoma cell line.
(A) Expression of LFA-1 on 164T2 (left histogram) and 374 cells (right histogram). Expression of LFA-1 was determined using biotinylated anti-LFA-1 antibodies plus SA-PE (gray lines). Controls included cells incubated with SA-PE alone (black lines) and autofluorescence control, represented by the population filled in gray. The T-cell origin of 374 cells was ascertained by the expression of the CD3ε and TcRαβ-specific mAbs (data not shown). (B) Secretion of high levels of MMP-9 by 164T2 and 374 T lymphoma cells. Secretion of MMP-9 by resting or PMA-stimulated 164T2 and 374 lymphoma cells was measured by gelatin-zymography. Results are representative of at least 3 independent experiments.
Expression of LFA-1 and MMP-9 by 374 lymphoma cell line.
(A) Expression of LFA-1 on 164T2 (left histogram) and 374 cells (right histogram). Expression of LFA-1 was determined using biotinylated anti-LFA-1 antibodies plus SA-PE (gray lines). Controls included cells incubated with SA-PE alone (black lines) and autofluorescence control, represented by the population filled in gray. The T-cell origin of 374 cells was ascertained by the expression of the CD3ε and TcRαβ-specific mAbs (data not shown). (B) Secretion of high levels of MMP-9 by 164T2 and 374 T lymphoma cells. Secretion of MMP-9 by resting or PMA-stimulated 164T2 and 374 lymphoma cells was measured by gelatin-zymography. Results are representative of at least 3 independent experiments.
Frequency of lymphoma and mean time of latency (in brackets) of mice injected intravenously with 374 lymphoma cells
| Mouse Strain . | Male . | Female . |
|---|---|---|
| C57BL6 | 13/14 (54.3 ± 18.4 d) | 4/9 (54.8 ± 3.34 d) |
| C57BL6tm1Bay | 3/8 (72 ± 30.4 d) | 8/10 (54.2 ± 15.7 d) |
| Mouse Strain . | Male . | Female . |
|---|---|---|
| C57BL6 | 13/14 (54.3 ± 18.4 d) | 4/9 (54.8 ± 3.34 d) |
| C57BL6tm1Bay | 3/8 (72 ± 30.4 d) | 8/10 (54.2 ± 15.7 d) |
The experiment was terminated at day 90. The data are representative of the cumulative results obtained in 2 independent experiments.
Discussion
The most compelling evidence that intercellular contact via cell adhesion molecules plays a crucial role in the control of lymphoma metastasis is the fact that genetic ablation of the icam-1 gene in mice confers resistance to dissemination of lymphoma following intravenous injection of malignant lymphoma cells.5 In the present work, we have shown: (1) that in vivo passage failed to modify the repertoire of cell adhesion molecules at their surface, nor did it exert a selective pressure that would modify the homing pattern of the lymphoma cells; (2) that a selective pressure favored the emergence of aggressive lymphoma cells that constitutively expressed MMP-9; and (3) that increased aggressiveness overcame the resistance of ICAM-1–deficient mice to lymphoma metastasis. These results are consistent with the idea that intercellular adhesion-dependent post-homing events not only control lymphoma metastasis, but apply a selective pressure that favors the constitutive expression of tumor-specific genes that normally are induced on intercellular contact.
We have previously shown that intercellular contact via ICAM-1 is required for the up-regulation of MMP-9 in lymphoma cells.15 Furthermore, our findings that MMP-9 was overexpressed in mice bearing large thymic lymphoma was consistent with the important role of MMP-9 in lymphoma development.23 More recently, we also showed that transfection of MMP-9 cDNA in murine lymphoma cells accelerated the development of thymic lymphoma.26 It would thus be tempting to conclude that the obvious reason why S11 can overcome the resistance of ICAM-1–deficient mice was directly related to their capacity to express MMP-9 constitutively. Indeed, both S11 and S19 clones expressed higher levels of MMP-9 than the 164T2 parent clone. The other clone that can form tumors in ICAM-1–deficient mice, 374, also constitutively expressed high levels of MMP-9. However, resistance of ICAM-1–deficient mice could not be overcome solely by transfecting the MMP-9 cDNA in nonaggressive lymphoma cells lines (Aoudjit et al, unpublished observation), indicating that although MMP-9 could be an important element for lymphomagenesis, other yet unidentified genes must be expressed to confer to lymphoma cells an aggressive phenotype to overcome the resistance of ICAM-1–deficiency.
These findings led us to propose that although less aggressive lymphoma metastasis requires the expression of ICAM-1 by the host, as lymphomas undergo repeated cycles of metastasis, they stabilize a more metastatic phenotype that is likely induced by cell adhesion molecules during the post-homing events. This scenario is supported by our data showing that although MMP-9 is expressed in the parent clone only on ICAM-1–dependent activation, its expression becomes constitutive and observed levels increase as lymphoma cells acquire a more aggressive phenotype. This up-regulation was confined to MMP-9; no such increase was found for MMP-2 or any of the 4 TIMP molecules. Our results may thus explain why MMP-9 has been previously associated with aggressive lymphoma behavior in non-Hodgkin's lymphoma.16 Whether the expression of other extracellular proteases is also modified by the selective pressure on lymphoma cells is currently under investigation.
The potential of targeting cell adhesion molecules to control cancer metastasis has recently been the focus of attention.27,28The fact that ICAM-1–deficient mice show resistance to the lymphoma development supports this approach and logically justifies the rationale for developing new anticancer therapies specific for adhesion molecules to reduce the spread of tumor cells, at least in the case of lymphoma.29 However, our data demonstrating that increased aggressiveness of tumor cells can override the resistance of ICAM-1–deficient mice must somewhat restrain the original enthusiasm for antiadhesion therapy in lymphomagenesis, particularly in the late stages of lymphoma development. These data, in fact, suggest that antiadhesion therapies directed against specific adhesion molecules might be very effective in inhibiting critical post-homing events in the early stages of the disease, but might lose their effectiveness once tumor cells have acquired a more aggressive state that allows them to ignore the need for some signals delivered through interactions with the host environment. Our data with 374 cells are consistent with this possibility, establishing for the first time that lymphoma development can still occur in complete absence of LFA-1/ICAM-1 interaction, given that the lymphoma cells had time to develop and stabilize a repertoire of tumorigenic genes.
Acknowledgments
The authors thank Ms Claire Beauchemin and Ms Doris Legault for their excellent technical support.
Supported by the Medical Research Council of Canada and by the Fonds pour la Formation de Chercheur et d'Aide à la Recherche (FCAR).
Reprints:Yves St-Pierre, INRS-Institut Armand-Frappier, 531 Boul. des Prairies Laval, Québec, Canada H7V1B7; e-mail: yves_st-pierre@inrs-iaf.uquebec.ca.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal