Abstract
Angiogenesis plays a key role in solid tumor growth. The purpose of this work was to study angiogenesis in acute myeloid leukemia (AML). We stained bone marrow samples from 20 adult patients with untreated AML and 20 normal controls using endothelial cell markers (ULEX-E and von Willebrand factor [vWF]). The number of vessels per millimeter length of bone marrow core biopsy specimen was scored by light microscopy. Using ULEX-E staining, AML marrows had (average ± SEM) 8.3 ± 3.6 vessels/mm (range, 3.7-19.3), whereas normal marrows had 4.3 ± 1.8 vessels/mm (range, 1.6-7.9). A similar difference was noted using vWF staining (8.6 ± 3.0 vessels/mm vs 4.9 ± 2.2 vessels/mm in AML vs normal bone marrows, respectively). The differences between the numbers of vessels/mm in AML and normal marrows were highly significant (P < .0001 for both ULEX-E and vWF staining). When analyzed by FAB category, there was no difference in the average number of vessels/mm among the different subgroups of AML. Using reverse transcriptase polymerase chain reaction, we observed that the HL-60 and U937 human AML cell lines and 4 of 4 freshly isolated AML cells from untreated patients expressed mRNA for vascular endothelial growth factor (VEGF). Both cell lines as well as all fresh AML isolates tested expressed VEGF protein. Basic fibroblast growth factor was expressed only in HL-60 cells and in only 3 of 4 fresh AML samples. These observations suggest that angiogenesis may play a role in the pathogenesis of AML. Inhibition of angiogenesis could constitute a novel strategy for the treatment of AML. (Blood. 2000;95:309-313).
Angiogenesis is a highly regulated process under the tight control of inducers and inhibitors.1-3 It involves degradation of the parent venule basement membrane, endothelial cell proliferation and migration, development of sprouts, and generation of new basement membrane.1-3 Angiogenesis plays a critical role in solid tumor development and metastasis.1,2Recently, a role for angiogenesis in the pathophysiology of hematologic malignancies has been suggested.4-6 Vascular endothelial growth factor (VEGF) and basic fibroblastic growth factor (bFGF) are 2 of the best characterized angiogenic factors.1,2 They are produced by a number of neoplastic and non-neoplastic cell types.1-3 Acute myeloid leukemia (AML) cells express VEGF.5 Furthermore, elevated levels of bFGF were detected in urine from patients with acute lymphoblastic leukemia (ALL) and lymphoma.4,6 In children with ALL, elevated levels of bFGF in the urine were associated with increased density of bone marrow vessels.4
The purpose of the current study was to determine the extent of angiogenesis in AML. Our results show increased vessel density in bone marrow specimens from patients with AML, thus suggesting a possible role for angiogenesis in the pathophysiology of this disease.
Materials and methods
Histologic analysis
Twenty archival, paraffin-embedded bone marrow core biopsy specimens from adult patients with AML were evaluated. The diagnosis of AML was made according to standard French-American–British (FAB) criteria. In addition, 20 bone marrow core biopsy specimens from adult patients without evidence of malignancy were used as controls. The control patients had undergone bone marrow biopsy for a number of different reasons, including tumor staging and evaluation of cytopenias. All of the specimens had been fixed in formalin or B-5 and embedded in paraffin. The hematoxylin and eosin (H&E)-stained sections from each corresponding biopsy were reviewed and the histologic diagnosis was confirmed. Bone marrow cellularity and blast counts also were reviewed.
Bone marrow sections were stained immunohistochemically for the endothelial cell marker von Willebrand factor (vWF) (Dako, Carpinteria, CA; 1:1600) or ULEX-E (Vector, Burlingame, CA; 1:500). Immunohistochemistry was performed with a Ventana 320 automated stainer (Ventana, Tucson, AZ) using an indirect avidin-biotin-peroxidase detection method. The tissues were cut into 3-μm sections, placed on silanated slides, and incubated at 56°C for 30 minutes. The sections were dewaxed and dehydrated through serially diluted ethanol solutions to distilled water. All sections underwent antigen retrieval in 10 mmol/L citrate buffer using a microwave pressure cooker for 15 and 30 minutes for vWF and ULEX-E, respectively. Vessels were identified based on the combination of endothelial cell staining for vWF and ULEX-E and morphology. The total number of vessels was counted over the whole length of the biopsy core specimen. Vessel scoring was expressed as the number of vessels per millimeter length of the biopsy core.
Leukemia cells and nucleic acid isolation
HL-60 and U937 cells were from American Type Culture Collection (Rockville, MD). Cells were maintained in suspension culture at a density of 150 000 cells/mL in RPMI-1640 with 10% fetal bovine serum at 37°C in a 5% CO2 humidified atmosphere. Fresh leukemia samples were obtained from the peripheral blood of untreated patients newly diagnosed with AML according to the FAB criteria. All patients had circulating leukemic blasts. The protocol was approved by the University of Utah and the Salt Lake City VA Institutional Review Boards. After subjects gave informed consent, 10 mL of heparinized blood was obtained. The mononuclear fraction was isolated on a Ficoll-Hypaque density gradient. The percentage of leukemia cells was determined using Wright staining of a cytospin from the mononuclear cell fraction. All samples had more than 95% leukemic blasts. Fresh leukemic cells thus obtained were used for RNA isolation. For both leukemic cell lines and freshly isolated AML cells, total cellular RNA was isolated using the QIAGEN RNeasy Mini Kit (QIAGEN, Santa Clara, CA) using the manufacturer's protocol.
Reverse transcriptase polymerase chain reaction (RT-PCR)
RT-PCR was done using the GeneAmp RNA PCR kit from Perkin-Elmer (Foster City, CA). In brief, cDNA was reverse transcribed from total cellular RNA using oligo dT priming following the manufacturer's protocol. PCR amplification was done under the following conditions: 95°C for 3 minutes for initial melting; 95°C for 1 minute, 55°C for 1 minute, and 72°C for 1 minute for a total of 35 cycles; and 72°C for 7 minutes for final extension. PCR primers were as follows: upstream actin, 5′-CGCTGCGCTGGTCGTCGACA-3′; downstream actin, 5′-GTCACGCACGATTTCCCGCT-3′; upstream VEGF, 5′-TCGGGCCTCCGAAACCATGA-3′; downstream VEGF, 5′-CCTGGTGAGAGATCTGGTTC-3′; upstream bFGF, 5′-GGTCCTGTTTTGGATCCA-3′; and downstream bFGF, 5′-AGAGAGAGGAGTTGTGTC-3′.
Immunohistochemical stains for VEGF expression
Cytospin slides from HL-60, U937, and representative fresh AML cell isolates were stained immunohistochemically using an anti-human VEGF antibody (1:100) from Santa Cruz Biotechnology Inc. (Santa Cruz, CA). Immunohistochemistry was performed with a Ventana 320 automated stainer (Ventana) using an indirect avidin-biotin-peroxidase detection method. The cytospin preparations were fixed in cold 100% acetone for 10 minutes. All preparations were pretreated with Immuno+Master antigen enhancer (American Histology Reagent Company, Inc., Lodi, CA) before staining.
Statistical analysis
Results are expressed as the averages of vessel scores from normal or leukemic marrows with standard error of the mean (SEM). SEM was calculated as the standard deviation divided by the square root of the number of samples. Statistical analysis was done using the Student's t test. Differences were considered statistically significant at P < .05.
Results
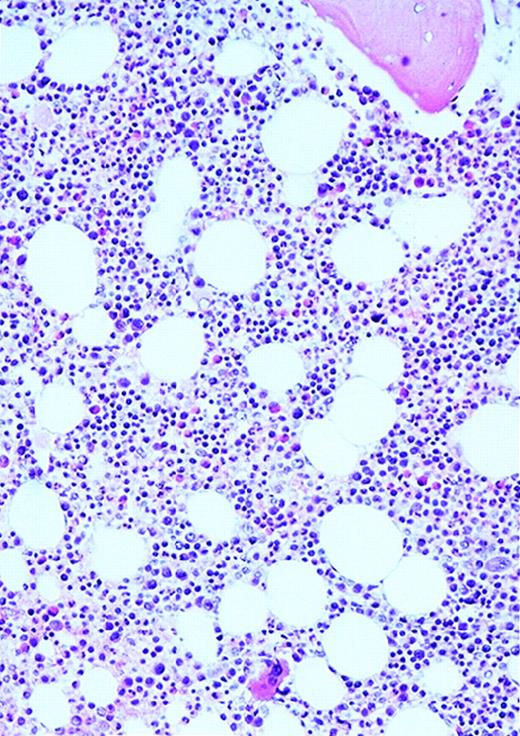
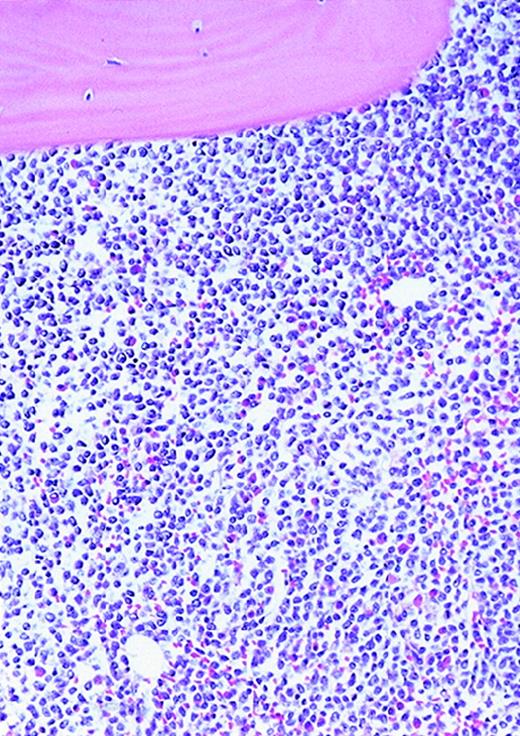
The patients with AML consisted of 8 women and 12 men; the control patients consisted of 12 women and 8 men. The mean age of the AML patients was 59 years (range, 24-87 years). In the control group, the mean age was 49 years (range, 27-71 years). The FAB distribution of the AML cases was as follows: 2 M0, 6 M1, 6 M2, 4 M4, and 2 M7. The cellularity (average ± SEM) was 78% ± 26% (range, 25% to 100%) and 49% ± 8% (range, 35% to 65%) for AML and normal marrows, respectively (P < .0001). Using ULEX-E staining, AML marrows had (average ± SEM) 8.3 ± 3.6 vessels/mm (range, 3.7-19.3) while normal marrows had 4.3 ± 1.8 vessels/mm (range, 1.6-7.9). Using vWF staining of the same specimens, AML marrows had 8.6 ± 3.0 vessels/mm (range, 3.7-15.8) and normal marrows had 4.9 ± 2.2 vessels/mm (range, 1.5-10.1). The differences between the numbers of vessels/mm in AML and normal marrows were highly significant (P < .0001 for both ULEX-E and vWF staining). ULEX-E and vWF exhibited similar staining patterns. Figures1 and 2 show examples of vascularity in normal and AML marrows.
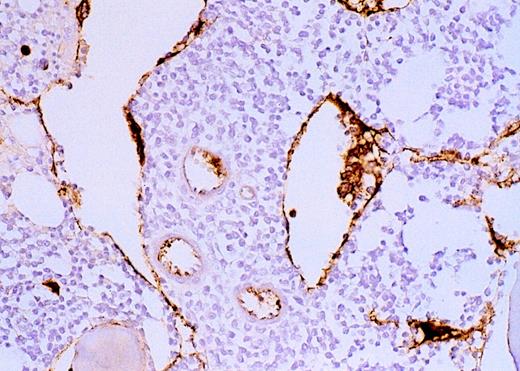
Vascularity of normal and AML marrow specimens.
Normal or AML bone marrow samples were stained with H&E or for vWF expression (see Methods) for vessel scoring. Legends (all ×200): A = normal bone marrow with H&E stain; B = normal bone marrow with vWF stain; C = AML bone marrow with H&E stain; D = AML bone marrow with vWF stain. The normal bone marrow shows strong staining for vWF in megakaryocytes but no evidence of increased vessels. The AML marrow has significantly more vessels than the normal marrow. These sections are representative of the whole series.
Vascularity of normal and AML marrow specimens.
Normal or AML bone marrow samples were stained with H&E or for vWF expression (see Methods) for vessel scoring. Legends (all ×200): A = normal bone marrow with H&E stain; B = normal bone marrow with vWF stain; C = AML bone marrow with H&E stain; D = AML bone marrow with vWF stain. The normal bone marrow shows strong staining for vWF in megakaryocytes but no evidence of increased vessels. The AML marrow has significantly more vessels than the normal marrow. These sections are representative of the whole series.
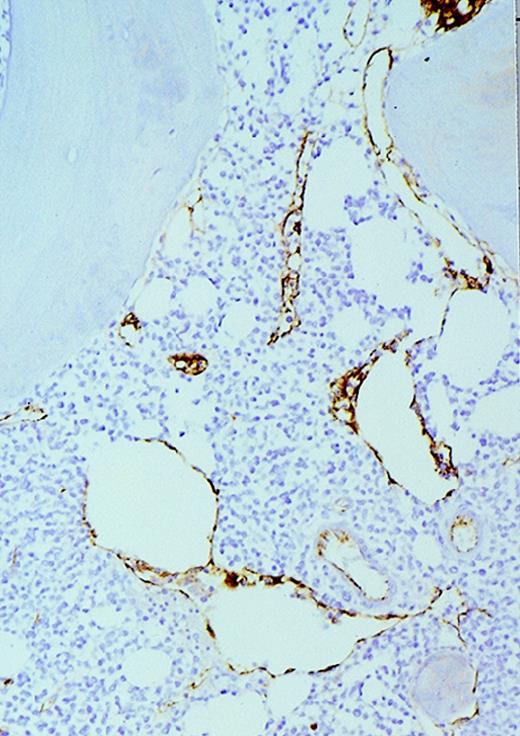
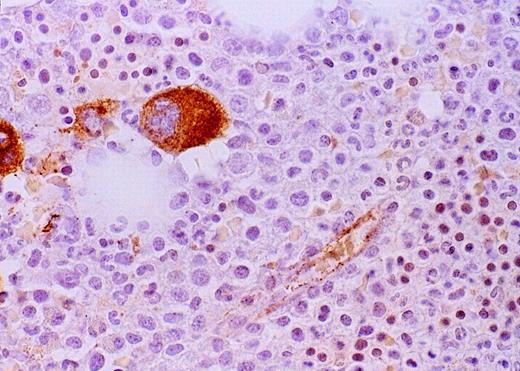
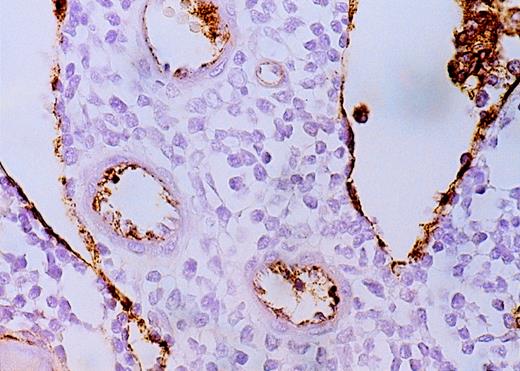
High-power view of vascularity of normal and AML marrow specimens.
Normal or AML bone marrow samples were stained for vWF expression (see Methods). A = 600× view of normal bone marrow showing positive staining in a megakaryocyte as well as 1 vessel. B = 400× view of representative AML marrow showing numerous vessels. Note that some of the vessels are large with irregular and bizarre shapes. C = 600× view of representative AML marrow showing details of vascular endothelial cell staining.
High-power view of vascularity of normal and AML marrow specimens.
Normal or AML bone marrow samples were stained for vWF expression (see Methods). A = 600× view of normal bone marrow showing positive staining in a megakaryocyte as well as 1 vessel. B = 400× view of representative AML marrow showing numerous vessels. Note that some of the vessels are large with irregular and bizarre shapes. C = 600× view of representative AML marrow showing details of vascular endothelial cell staining.
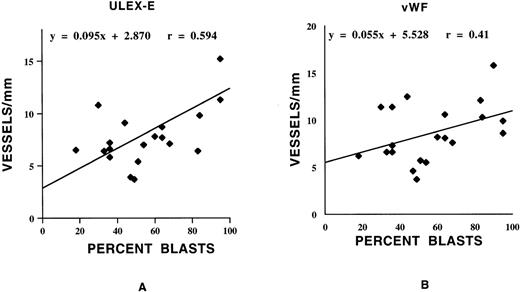
There was no significant difference in vessel scores among samples from different FAB subcategories (not shown). There was also no correlation between bone marrow cellularity and vessel score in either normal or AML bone marrows (Figure 3). In AML samples, there was a positive correlation between vessel score and percentage of marrow myeloblasts (Figure4).
Vessel score and bone marrow cellularity.
Normal (A) and AML (B) marrows were scored for vessel number (see Methods) using vWF staining. Scores were correlated with cellularity. There was no clear correlation between the number of vessels/mm and marrow cellularity in either normal or AML marrows. Similar results were obtained with ULEX-E staining.
Vessel score and bone marrow cellularity.
Normal (A) and AML (B) marrows were scored for vessel number (see Methods) using vWF staining. Scores were correlated with cellularity. There was no clear correlation between the number of vessels/mm and marrow cellularity in either normal or AML marrows. Similar results were obtained with ULEX-E staining.
Correlation between vessel score and percentage of blasts in AML bone marrow specimens.
AML marrows were scored for vessel number using ULEX-E (A) or vWF (B) staining (see Methods). Scores were correlated with percentage of blasts. Using either stain, there was a positive correlation between vessel scores and percentage of marrow blasts.
Correlation between vessel score and percentage of blasts in AML bone marrow specimens.
AML marrows were scored for vessel number using ULEX-E (A) or vWF (B) staining (see Methods). Scores were correlated with percentage of blasts. Using either stain, there was a positive correlation between vessel scores and percentage of marrow blasts.
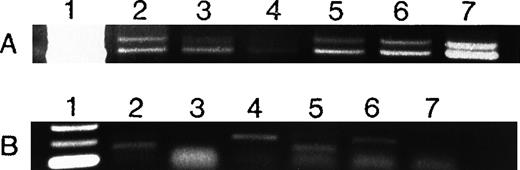
To determine whether AML cells are a source of angiogenic factors and therefore could stimulate angiogenesis in vivo, we used RT-PCR to study the mRNA expression of VEGF and bFGF in 2 human AML cell lines (HL-60 and U937) as well as in AML cells freshly isolated from untreated patients. VEGF was expressed by HL-60 and U937 cells as well as all fresh AML samples (Figure 5A). The extent of VEGF mRNA expression varied among the different patient isolates tested (Figure 5A). In contrast, only HL-60 cells and 3 of 4 fresh AML cells expressed bFGF (Figure 5B). Immunohistochemical stains of cytospins from HL-60, U937, and fresh AML samples showed that they expressed VEGF protein (Figure 6).
Expression of VEGF (A) and bFGF (B) RNA in AML cells.
VEGF and bFGF expression was assayed by RT-PCR (see Methods) in AML cells freshly isolated from untreated patients and in HL-60 and U937 cells. Lane 1 = molecular weight (MW) marker; lanes 2 to 5 = AML patients; lane 6 = HL-60 cells; lane 7 = U937 cells. All samples expressed VEGF, whereas only 3 of 4 fresh samples and HL-60 cells expressed bFGF. Patient 4 had weaker VEGF expression than the others. Actin controls showed equal amounts of RNA (not shown). Negative controls without RNA and without reverse transcriptase were negative (not shown).
Expression of VEGF (A) and bFGF (B) RNA in AML cells.
VEGF and bFGF expression was assayed by RT-PCR (see Methods) in AML cells freshly isolated from untreated patients and in HL-60 and U937 cells. Lane 1 = molecular weight (MW) marker; lanes 2 to 5 = AML patients; lane 6 = HL-60 cells; lane 7 = U937 cells. All samples expressed VEGF, whereas only 3 of 4 fresh samples and HL-60 cells expressed bFGF. Patient 4 had weaker VEGF expression than the others. Actin controls showed equal amounts of RNA (not shown). Negative controls without RNA and without reverse transcriptase were negative (not shown).
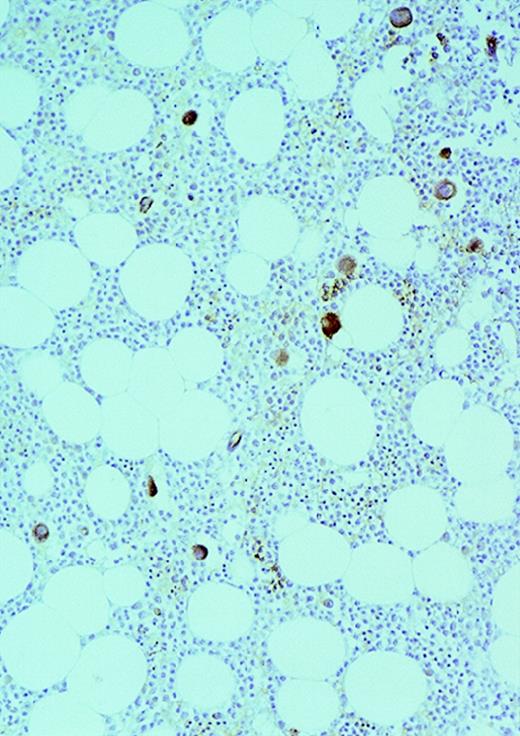
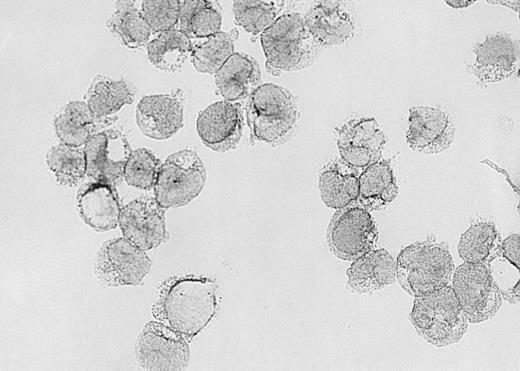
Expression of VEGF protein in AML cells.
Cytospin of AML cells freshly isolated from an untreated patient was stained immunohistochemically for VEGF expression (see Methods). AML cells expressed VEGF protein in the cytoplasm, which is typical of this growth factor. Similar results were obtained with HL-60, U937, and 5 other fresh AML samples.
Expression of VEGF protein in AML cells.
Cytospin of AML cells freshly isolated from an untreated patient was stained immunohistochemically for VEGF expression (see Methods). AML cells expressed VEGF protein in the cytoplasm, which is typical of this growth factor. Similar results were obtained with HL-60, U937, and 5 other fresh AML samples.
Discussion
We demonstrate in this study evidence of increased angiogenesis in AML. Although bone marrow specimens from AML patients were more cellular than those of normal controls, there was no clear correlation between cellularity and vessel scores in either group. Consequently, it is unlikely that increased cellularity would be the only cause of increased angiogenesis in AML. A more likely explanation would be the secretion of angiogenic factors by leukemia cells, which in turn stimulate the development of new vessel beds. Indeed, we demonstrated that AML cells from all samples studied expressed VEGF mRNA, and the majority of them also expressed bFGF. We also demonstrated that AML cells expressed VEGF protein. Furthermore, there was a positive correlation between the percentage of marrow blasts and vessel score. Both VEGF and bFGF are among the most potent mitogens for endothelial cells and stimulators of angiogenesis.1,2 They also work synergistically to stimulate angiogenesis.7
Most of the early work on angiogenesis was done in solid tumors. More recently, a mounting body of evidence has been accumulating suggesting a role for angiogenesis in the pathophysiology of hematopoietic malignancies. Children with ALL have increased angiogenesis in their marrow and increased urinary levels of bFGF.4Interestingly, urinary levels of bFGF did not decrease significantly when the patients were in remission 31 days after cytotoxic chemotherapy.4 Although the authors did not report long-term follow-up measurements, this suggests that the source of bFGF in ALL may not be the leukemia cells. Fiedler et al5 have shown that AML cells express VEGF as well as the VEGF receptors (VEGFR-1 and VEGFR-2). These findings raise the possibility that VEGF may play the role of an autocrine growth factor for AML cells. Along these lines, VEGF was shown to protect AML cells from chemotherapy-induced apoptosis by upregulating MCL1 (a member of the BCL2 family).8 Bellamy et al9 studied a panel of hematopoietic tumor cell lines and found that they all expressed VEGF, whereas only 50% of them expressed bFGF.
Perhaps the most compelling evidence showing a role for angiogenesis in hematopoietic malignancies has been generated from patients with multiple myeloma. In multiple myeloma, there is a correlation between the extent of bone marrow angiogenesis and plasma cell proliferative index.10 In the latter study, there was also a correlation between angiogenesis and disease progression. Furthermore, patients with active multiple myeloma have increased angiogenesis, whereas patients with inactive disease or monoclonal gammopathy of undetermined significance do not.11 The same group of investigators also has demonstrated that human lymphoblastoid cell lines produce angiogenic factors, induce an angiogenic phenotype in endothelial cells, and secrete various matrix metalloproteinases.12
The question arises as to why there would be increased angiogenesis in the bone marrow of AML patients. A strictly “mechanical” explanation for the supply of nutrients and oxygen to the leukemic cells and the removal of metabolites from these cells is not completely satisfying because AML cells invading the marrow do not form a well-circumscribed “mass” like solid tumors. Furthermore, our results do not support this explanation because we did not observe a correlation between marrow cellularity and vessel score. A more plausible explanation would be a positive synergistic relationship between AML and endothelial cells. We and others have shown that AML cells make angiogenic factors.5,9 As part of their study of VEGF expression in AML cells, Fiedler et al5 showed that VEGF stimulates granulocyte macrophage colony-stimulating factor (GM-CSF) production by human umbilical cord endothelial cells. Likewise, Bellamy et al9 have shown that VEGF stimulates the production of M-CSF, G-CSF, interleukin-6, and stem cell factor (SCF) in endothelial cells. All of this work supports the hypothesis of a positive synergistic relationship between AML and endothelial cells through the paracrine production by each of mutually mitogenic growth factors.
In conclusion, by demonstrating increased angiogenesis in the bone marrow of AML patients, we lend support to previous studies suggesting that angiogenesis may play a role in the pathophysiology of hematopoietic malignancies. Furthermore, our work shows that angiogenesis may be clinically relevant in the pathophysiology of AML and thus raises the possibility of using angiogenesis inhibitors as a novel therapeutic strategy for this disease.
Acknowledgments
We would like to acknowledge Sheryl Tripp and Lai Yi Wang for excellent technical support.
P.J.S is a Translational Research Awardee from the Leukemia Society of America. This work was also supported by the VA Research Service (GMR) and ARUP Laboratories (J.W.H).
Reprints:Paul J. Shami, University of Utah and Salt Lake City VA Medical Centers, Box 151M, 500 Foothill Blvd, Salt Lake City, UT 84148; e-mail: p.shami@m.cc.utah.edu.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal