Abstract
The nuclear factor-kappa B (NF-κB) gene transactivator serves in the formation of immune, inflammatory, and stress responses. In quiescent cells, NF-κB principally resides within the cytoplasm in association with inhibitory κ (IκB) proteins. The status of IκB and NF-κB proteins was evaluated for promyelocytic leukemia HL-60 cells treated at different intensities of photodynamic therapy (PDT). The action of the potent photosensitizer, benzoporphyrin derivative monoacid ring A (verteporfin), and visible light irradiation were assessed. At a verteporfin concentration that produced the death of a high proportion of cells after light irradiation, evidence of caspase-3 and caspase-9 processing and of poly(ADP-ribose) polymerase cleavage was present within whole cell lysates. The general caspase inhibitor Z-Val-Ala-Asp-fluoromethylketone (ZVAD.fmk) effectively blocked these apoptosis-related changes. Recent studies indicate that IκB proteins may be caspase substrates during apoptosis. However, the level of IκBβ was unchanged for HL-60 cells undergoing PDT-induced apoptosis. IκB levels decreased during PDT-induced apoptosis, though ZVAD.fmk did not affect this change. At a less intensive level of photosensitization, cellular IκB levels were transiently depressed after PDT. At these times, p50 and RelA NF-κB species were increased within nuclear extracts, as revealed by electrophoretic mobility supershift assays. HL-60 cells transiently transfected with a κB-luciferase reporter construct exhibited elevated luciferase activity after PDT or treatment with tumor necrosis factor-, a well-characterized NF-κB activator. Productive NF-κB activation and associated gene transcription may influence the phenotype and behavior of cells exposed to less intensive PDT regimens. However, IκB is not subject to caspase-mediated degradation as a component of PDT-induced apoptosis. (Blood. 2000;95:256-262)
Nuclear factor-kappa B (NF-κ B) participates in the transcriptional activation of genes encoding a number of immunoregulatory and proinflammatory proteins.1-4 It is widely recognized that NF-κB activation can result from cellular oxidative stress.5 The effect of H2O2, ultraviolet light, and ionizing radiation on the NF-κB system may be inhibited by antioxidants, suggesting that the formation of oxidative stress is a unifying feature for agents that activate NF-κB.3,5 NF-κB is regulated by members of a group of inhibitory (IκB) proteins that sequester NF-κB within the cytoplasm.6 Phosphorylation of serine residues 32 and 36 within the N-terminal region of IκBα targets the protein for ubiquitination and destruction by the 26S proteasome.7-9 The lysine residues at positions 20 and 21 of IκBα are required for the ubiquitination process.9These phosphorylation and ubiquitination events rapidly liberate NF-κB for nuclear translocation.7-9 The transcriptionally active forms of NF-κB consist of dimers, most commonly the p50 and p65 (RelA) species, that bind specific DNA sequences with high affinity.1-5 In addition to its well-documented action in promoting the transcription of immunomodulatory and proinflammatory genes, NF-κB contributes to the maintenance of cell viability by promoting the transcription of antiapoptotic genes.10-13
Photodynamic therapy (PDT), widely studied for its anticancer activity, uses light-absorbing compounds and visible light irradiation to elicit antitumor effects.14,15 Through the generation of reactive oxygen intermediates, PDT affects various aspects of cell biology, particularly singlet oxygen.16 Tumor cells treated with a cytotoxic combination of photosensitizer and light rapidly undergo apoptosis in vitro.17-19 Separately, neither the photosensitizer nor the light is cytotoxic. The potent photosensitizer benzoporphyrin derivative (verteporfin) has been used for oncologic, ocular, and immune indications.20-22 Human leukemic cell lines exhibit greater verteporfin uptake and higher susceptibility to photodynamic killing than normal blood mononuclear cells.20,23,24 The influence of PDT on cell signaling pathways has not been extensively studied. Combined verteporfin and light treatment altered the tyrosine phosphorylation status of various proteins within murine P815 mastocytoma cells25 and Pam212 keratinocytes,26 including the activation of the stress-activated protein kinase and p38 high-osmolarity glycerol protein kinase signaling pathways.26 NF-κB activation has been described for PDT-treated cells, though its significance is unclear. The photosensitizer Photofrin produced NF-κB translocation in light-irradiated murine L1210 lymphoma cells under conditions that permitted approximately 20% cell survival.27 In contrast, HeLa cells treated with Photofrin and light exhibited activation of the AP-1 transcription factor but not NF-κB.28 Oxidative damage produced by the DNA-intercalating photosensitizer proflavine triggered a transient decrease in IκBα levels and the nuclear translocation of NF-κB p50 and RelA species in human immunodeficiency virus-infected lymphoid ACH-2 cells.29 Treatment of human colon carcinoma HCT-116 cells with the photosensitizer pyropheophorbide-a methyl ester (PPME) and red light lead to IκBα processing and productive NF-κB activation.30It is likely that NF-κB has an integral role in defining cellular responses to the oxidative stress conditions produced by PDT.31
The induction of apoptosis by many chemotherapeutic agents is associated with mitochondrial events. With the release of cytochrome-c from mitochondria, as instigated by various cellular signals and in the presence of deoxyadenosine triphosphate, the apoptotic protease activating factor-1, or apaf-1, adaptor molecule binds procaspase-9 to promote the activation of caspase-9.32,33 Caspase-9 acts in the processing and activation of other caspases, including caspase-3,32,33 the best-characterized protease of the apoptosis pathway.34-36 Caspase activation may ensue from the removal of the regulatory prodomain through self-catalysis or the action of other caspases37 or by the serine protease granzyme B used by cytotoxic T cells,38 leading to the assembly of the active protease.37 Activated caspase-3 cleaves substrates after aspartic acid-X-X-aspartic acid (where X is any amino acid) sequences that typically precede glycine, serine, or alanine residues.39 Numerous caspase-3 substrates have been identified. These include poly(ADP-ribose) polymerase (PARP),36 sterol regulatory element binding proteins,40 U1-associated 70-kDa protein,41 the catalytic subunit of DNA-dependent protein kinase (DNA-PKCS),41 and DNA fragmentation factor (DFF).42 It has been suggested that activated caspases may remove the N-terminal region from IκB proteins and thereby incapacitate the NF-κB signaling pathway during apoptosis.42-46 Importantly, serine phosphorylation of IκBα prevented caspase-mediated of IκBα in vitro, suggesting the existence of a close biochemical relationship between the NF-κB signaling pathway and the cell death-associated protease cascade.45
At lethal levels of verteporfin and visible light, HL-60 cells rapidly exhibit evidence of caspase-3 activation, PARP, DNA-PKCS, DFF cleavage that lead to DNA fragmentation.19,47Comparable events were described for HeLa cells rendered apoptotic with verteporfin and light.48-50 The potent cytotoxic action of this photosensitizer may be related to its capacity to elicit a remarkably swift translocation of cytochrome-c to the cytosolic fraction on light irradiation.49,50 As described above, the release of mitochondrial cytochrome-c may be a pivotal event in triggering the caspase cascade with certain apoptosis-inducing agents.32,33,51,52 PDT with verteporfin triggers cellular events consistent with many of the changes described for cells rendered apoptotic by different chemotherapeutic agents, albeit with more rapid kinetics.49-51
The current study was performed to determine whether NF-κB was involved in the photodynamic response of HL-60 cells to verteporfin and whether the state of IκB proteins was altered during PDT-induced apoptosis. With a relatively low-intensity photodynamic regimen, cellular IκBα levels were transiently reduced and productive NF-κB activation ensued. For cells treated with an amount of verteporfin that rapidly induced apoptosis on light irradiation, no changes in IκBβ levels were discernible. In these cells, the level of IκBα was reduced, but this was not a direct consequence of caspase activity.
Materials and methods
Cell culture
Human HL-60 cells (American Type Culture Collection, Rockville, MD) were maintained in RPMI 1640 medium supplemented with 10% heat-inactivated fetal bovine serum, 4 mmol/L L-glutamine, 1 mmol/L sodium pyruvate, 1 mmol/L HEPES, 100 U/mL penicillin, and 100 μg/mL streptomycin, all from Gibco BRL (Burlington, Canada).
Photosensitization
Lipid-formulated verteporfin was obtained from QLT PhotoTherapeutics (Vancouver, Canada). All cell work with the photosensitizer was performed under low light. Cells were incubated for 60 minutes at 37°C with or without verteporfin in culture medium in clear 12 × 75-mm polystyrene tubes.19 For caspase inhibition studies, Z-Val-Ala-Asp-fluoromethylketone (ZVAD.fmk) (Enzyme Systems Products, Dublin, CA) was added for the final 30 minutes of the incubation period. After exposure to red fluorescent light (wavelength = 620-700 nm) at 5.6 mW/cm2 (total delivered dose = 2 J/cm2), the cells were returned to the incubator.
Measurement of DNA fragmentation
Propidium iodide (PI) dye staining and flow cytometric analysis were used to assess the status of cellular DNA.19 53 At 3 hours after photoirradiation, 1 × 106 cells were washed twice with ice-cold phosphate-buffered saline (PBS) then permeabilized and fixed in 80% ethanol at 4°C for 1 hour. Cells were rewashed and then treated with PBS containing PI (Sigma, St. Louis, MO) at 50 μg/mL and DNAse-free RNAse (Sigma) at 5 U/mL. The percentage of cells containing hypodiploid DNA levels was calculated from PI fluorescence analysis using an XL flow cytometer (Coulter Electronics, Hialeah, FL).
Measurement of cell viability
To assess their survival after PDT, 2 × 105cells were transferred to quadruplicate wells of 96-well microtiter plates in 0.2 mL culture medium. Plates were returned to the incubator, and cell viability was assessed by the MTT (3-[4-,5-dimethylthiazol-2-yl]-2,4-diphenyl tetrazolium bromide; Sigma) colorimetric assay54 24 hours later. Color development was terminated after 1 hour at 37°C in the presence of the MTT reagent. Color intensity was measured with a microtiter plate reader (Dynatech, Hamilton, VA) at a wavelength of 590 nm. Absorbance values for wells containing medium alone were subtracted from the result obtained with the test wells.
Electrophoretic mobility shift assay
Nuclear extracts were isolated by a rapid micropreparation technique adapted from a large-scale procedure55 that uses lysis with NP40 detergent followed by high salt extraction.56Radiolabeled, double-stranded oligonucleotide probes (100 ng) (Eurogentech, Liege, Belgium) were generated by end filling with the Klenow fragment of Escherichia coli DNA polymerase I (Boehringer Mannheim, Mannheim, Germany) with 3 μCi each of α-32P]-α-dATP and 32P]-α-dCTP (3000 Ci/nmol; NEN Life Science Products, Brussels, Belgium) in the presence of unlabeled dTTP and dGTP. Labeled probes were purified by spin chromatography on Sephadex G-25 (Amersham Pharmacia Biotech, Buckinghamshire, UK) columns. Specific activity of the probe was >108 dpm/μg.
Wild type NF-κB probe was: 5′-GGTTACAAGGGACTTTCCGCTG TGTTCCCTGAAAGGCGACGGTT-5′
Mutated NF-κB probe was: 5′-GGTTAACAACTCACTTTCCGCTGTTGTTGAGTGAAAGGCGACGGTT-5′
Binding reactions were performed with 7.5 μg nuclear protein in 20 μL of 20 mmol/L HEPES–KOH (pH 7.9) containing 75 mmol/L NaCl, 1 mmol/L EDTA, 5% (vol/vol) glycerol, 0.5 mmol/L MgCl2, 2 μg acetylated bovine serum albumin, 4 μg poly(dI-dC)-poly(dI-dC) (Amersham Pharmacia Biotech), 1 mmol/L dithiothreitol, and 0.2 ng32P-labeled oligonucleotide. DNA-protein complexes were resolved by a 3-hour electrophoresis in native 6% polyacrylamide gels at 200 V using 0.25 mol/L Na borate, 0.5 mmol/L EDTA, and 0.25 mol/L Tris, pH 8.0. Gels were vacuum-dried and exposed to Fuji x-ray film at −80°C for 16 to 25 hours. Supershift experiments were carried out as described.29 Polyclonal antibodies against RelA, c-Rel, p50, and RelB were purchased from Santa Cruz (SanverTECH, Boechout, Belgium).
κB-luciferase reporter assay
The pNF-κB-luciferase (Luc) reporter construct contains 5 κB sites of the human immunodeficiency virus type-1 terminal repeat cloned upstream of the luciferase gene (Stratagene GmbH, Heidelberg, Germany). HL-60 cells (7 × 106) were transiently transfected with 30 μg of the κB-Luc reporter plasmid in 0.3 mL RPMI 1640 medium containing 10% FCS and 1.25% dimethyl sulfoxide using a Gene Pulser (Bio-Rad Laboratories, Munich, Germany) at 960 μm FD and 290 V at room temperature.57 58 Immediately after the pulse, the cells were transferred to 5 mL of medium containing 20% fetal calf serum and 1.25% dimethyl sulfoxide. After 24 hours the cells were treated with recombinant human tumor necrosis factor-α (TNF-α; Roche Molecular Biochemicals, Mannheim, Germany) at 200 U/mL. Separate cell aliquots were incubated with verteporfin for 1 hour and then light-irradiated. After an additional 24 hours, cells were washed with PBS and lysed in the buffer provided with the Luciferase Reporter Gene Assay kit (Roche Molecular Biochemicals). Luciferase activity was normalized for protein concentration as determined by the Bio-Rad protein assay. Results are given as a ratio of the reporter activity for κB-Luc-transfected cells maintained in the culture medium.
Preparation of cellular protein extracts
To obtain lysates, cells were initially washed twice with ice-cold PBS. Cell pellets were treated with 1 mL lysis buffer (1% Nonidet P-40, 137 mmol/L NaCl, 10% glycerol, 1 mmol/L phenylmethylsulfonyl fluoride, aprotinin [0.15 U/mL], 1 mmol/L sodium orthovanadate, 20 mmol/L Tris, pH 8.0) for 20 minutes on ice.19 Lysates were centrifuged for 10 minutes at 15 800g at 4°C. Protein concentrations were determined with the BCA Protein Assay (Pierce, Rockford, IL).
Immunoblot analysis
Detergent-soluble proteins (30 μg) were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis in 12% gels, under reducing conditions. Proteins were transferred to nitrocellulose membranes at 100 V for 1 hour. Membranes were blocked for 30 minutes at room temperature with PBS containing 0.05% Tween 20 (PBS-T) and 5% skim milk powder. Membranes were incubated with mouse monoclonal antibodies against caspase-3 (Santa Cruz Biotechnology, Santa Cruz, CA) or the N-terminal region (p25 fragment) of PARP (BIOMOL, Plymouth Meeting, PA). A goat polyclonal antibody against the C-terminal region (p85 fragment) of PARP (Santa Cruz Biotechnology) was used in some experiments. Rabbit polyclonal antibodies against caspase-9, IκBα (residues 27–38) and IκBβ (residues 21–40) were from PharMingen (San Diego, CA), New England BioLabs (Mississauga, Ontario), and Santa Cruz Biotechnology, respectively. Mouse monoclonal antibody (clone MAD3 10B) against an epitope between amino acids 21–48 within the N-terminal region of IκBα59 was obtained from Dr R. T. Hay (University of St. Andrews, Fife, UK). Antibodies were used at 1 μg/mL in PBS-T with 5% skim milk powder for 45 minutes at room temperature. After extensive washing with PBS-T, membranes were probed with antirabbit, antigoat, or antimouse IgG horseradish peroxidase-conjugates at 1:5000 in PBS-T containing 5% skim milk powder for 30 minutes. Membranes were extensively washed with PBS-T. Proteins were detected using the enhanced chemiluminescence detection system (Amersham Pharmacia Biotech), and labeled bands were visualized by autoradiography.
Results
Impact of photodynamic therapy on DNA fragmentation and cell viability
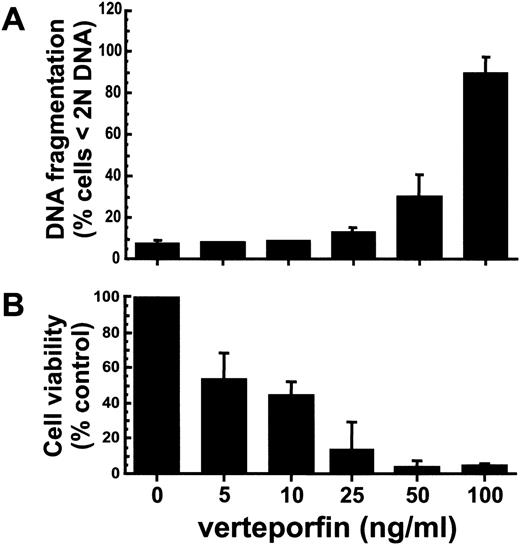
A hypodiploid level of DNA was present in more than 90% of HL-60 cells treated with verteporfin at 100 ng/mL and light-irradiated 3 hours earlier (Fig. 1A). At this time, approximately 25% of irradiated cells treated with verteporfin at 50 ng/mL exhibited DNA fragmentation. At lower concentrations of the photosensitizer, few (∼5%) cells contained a hypodiploid amount of DNA. For cells treated with verteporfin at ≥25 ng/mL, there was an 85% or greater loss in viability as determined by MTT assays performed 24 hours after irradiation (Fig. 1B). Light alone or verteporfin in the absence of light did not induce DNA fragmentation or affect cell viability (data not shown).
HL-60 cells treated with verteporfin and red light exhibit a drug dose-dependent increase in DNA fragmentation levels and a loss in viability.
Cells were incubated with different concentrations of verteporfin for 60 minutes at 37° C, exposed to red fluorescent light (2 J/cm2), and returned to the incubator. (A) The percentage of cells exhibiting DNA fragmentation at 3 hours after treatment was determined by PI staining and flow cytometric analysis (3 independent experiments). (B) Cell survival was assessed by the MTT colorimetric assay 24 hours after PDT. Results are given as a percentage of the absorbance value obtained with cells treated with light alone. MTT values for cells treated with light alone (100% viability) corresponded to a mean absorbance of 0.712 ± 0.215 above background values (3 independent experiments). Means with standard deviations are given.
HL-60 cells treated with verteporfin and red light exhibit a drug dose-dependent increase in DNA fragmentation levels and a loss in viability.
Cells were incubated with different concentrations of verteporfin for 60 minutes at 37° C, exposed to red fluorescent light (2 J/cm2), and returned to the incubator. (A) The percentage of cells exhibiting DNA fragmentation at 3 hours after treatment was determined by PI staining and flow cytometric analysis (3 independent experiments). (B) Cell survival was assessed by the MTT colorimetric assay 24 hours after PDT. Results are given as a percentage of the absorbance value obtained with cells treated with light alone. MTT values for cells treated with light alone (100% viability) corresponded to a mean absorbance of 0.712 ± 0.215 above background values (3 independent experiments). Means with standard deviations are given.
State of caspases, PARP, and IκB proteins during photodynamic therapy-mediated apoptosis
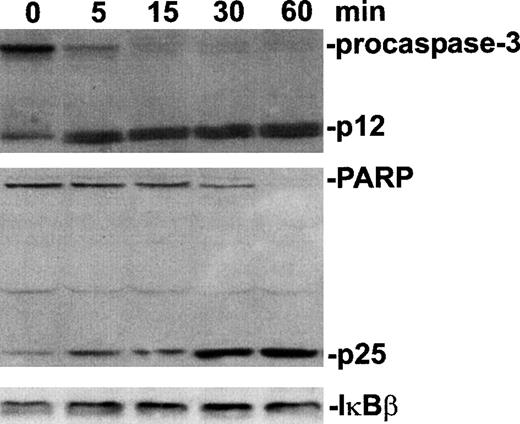
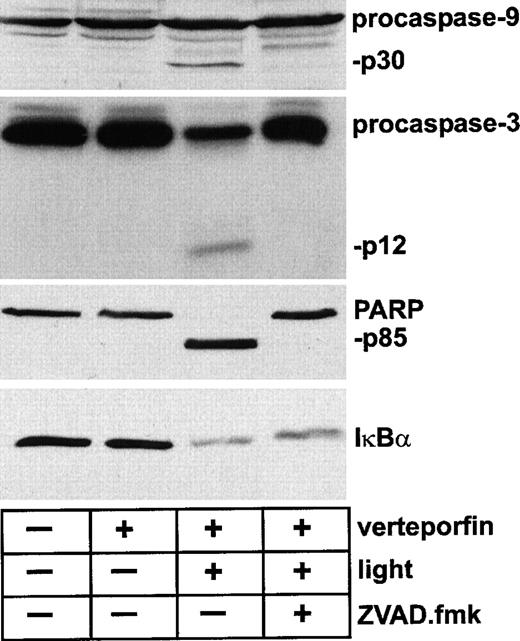
A large proportion of procaspase-3 was processed within 5 minutes after light irradiation with an amount of verteporfin that was lethal for >90% of the cells (Fig. 2). Cleavage and an accumulation of the p25 fragment of PARP, a known caspase-3 substrate, were evident by 5 minutes after PDT. A complete loss of the native form of PARP was achieved by 60 minutes after irradiation. In contrast, the photodynamic treatment of these cells with the same concentration of verteporfin had no detectable effect on cellular IκBβ levels by 1 hour. Furthermore, lysates prepared up to 3 hours after PDT did not contain detectable IκBβ degradation products or reveal a change in IκBβ band density as determined by immunoblot analysis (data not shown). The status of IκBα during PDT-apoptosis was also assessed. With immunoblot analysis, caspase-3 and caspase-9 activation was indicated by the presence of specific cleavage products within 60 minutes of PDT (Fig. 3). As expected, complete processing of PARP to its p85 fragment was also evident at this time. Although ZVAD.fmk effectively blocked the PDT-induced processing of procaspase-3, procaspase-9, and PARP, the level of IκBα within lysates of PDT-treated cells was markedly reduced regardless of whether the cells had been exposed to ZVAD.fmk. In the absence of light irradiation, verteporfin did not affect the status of any of these proteins as assessed by immunoblot analysis.
Procaspase-3 and PARP, but not IκBβ, processed during PDT-induced apoptosis.
HL-60 cells were treated with verteporfin (100 ng/mL) and red light (2 J/cm2). Cell lysates were prepared at increasing times and subjected to sodium dodecyl sulfate–polyacrylamide gel electrophoresis and Western immunoblot analysis. Bands were detected using a chemiluminescence detection system and visualized by autoradiography. Lanes designated 0 minute contain lysates of samples prepared immediately after light irradiation.
Procaspase-3 and PARP, but not IκBβ, processed during PDT-induced apoptosis.
HL-60 cells were treated with verteporfin (100 ng/mL) and red light (2 J/cm2). Cell lysates were prepared at increasing times and subjected to sodium dodecyl sulfate–polyacrylamide gel electrophoresis and Western immunoblot analysis. Bands were detected using a chemiluminescence detection system and visualized by autoradiography. Lanes designated 0 minute contain lysates of samples prepared immediately after light irradiation.
Change in the status of PARP, but not IκBα, attributable to caspase activity in HL-60 cells undergoing PDT-induced apoptosis.
Cells were incubated with verteporfin (100 ng/mL) for 60 minutes at 37°C and then were kept in the dark or were irradiated with red light (2 J/cm2). The general caspase inhibitor ZVAD.fmk (100 μmol/L) was added 30 minutes before light irradiation. Cell lysates were prepared 60 minutes after irradiation and were subjected to Western immunoblot analysis with antibodies against caspase-3, caspase-9, PARP, and IκBα. The rabbit anti-IκBα polyclonal antibody was raised against residues 27-38 of the molecule. Molecular sizes of caspase-3, caspase-9, and PARP fragments are indicated.
Change in the status of PARP, but not IκBα, attributable to caspase activity in HL-60 cells undergoing PDT-induced apoptosis.
Cells were incubated with verteporfin (100 ng/mL) for 60 minutes at 37°C and then were kept in the dark or were irradiated with red light (2 J/cm2). The general caspase inhibitor ZVAD.fmk (100 μmol/L) was added 30 minutes before light irradiation. Cell lysates were prepared 60 minutes after irradiation and were subjected to Western immunoblot analysis with antibodies against caspase-3, caspase-9, PARP, and IκBα. The rabbit anti-IκBα polyclonal antibody was raised against residues 27-38 of the molecule. Molecular sizes of caspase-3, caspase-9, and PARP fragments are indicated.
Gel mobility shift assays
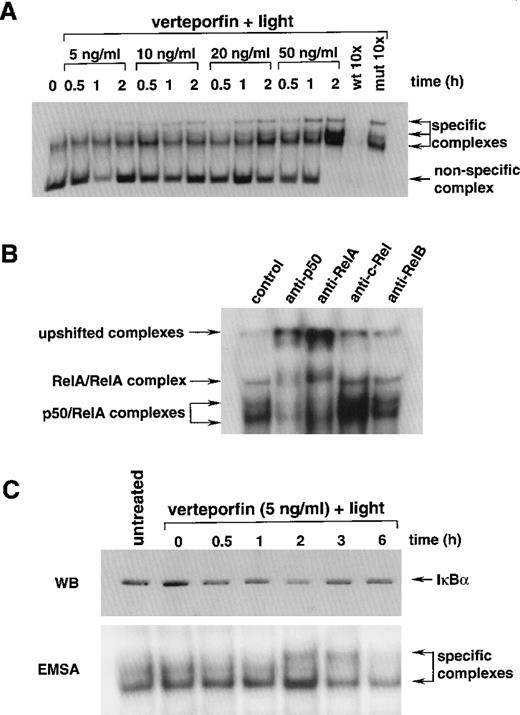
Nuclear extracts prepared from light-irradiated cells incubated with increasing amounts of verteporfin were analyzed by electrophoretic mobility shift assay with a 32P-labeled κB probe. All nuclear extracts displayed binding activity for the κB probe, as evidenced by the presence of at least 1 specific band within the gels (Fig. 4A). Constitutive NF-κB activity for HL-60 cells and other monocyte lineage cells has been described.60 Nuclear protein extracts prepared from cells treated with verteporfin or light alone also yielded a single band after incubation with the 32P-labeled NF-κB probe (data not shown). However, more than 1 specific band was evident for nuclear extracts of cells treated with PDT. The highest electrophoretic band densities were associated with nuclear extracts prepared from cells treated with verteporfin at 20 and 50 ng/mL. Lower photosensitizer concentrations had a lower, yet still detectable, impact in this regard. For nuclear extracts from PDT-treated cells, 3 specific bands were present. Competition studies using an excess of unlabeled wild-type probe, but not a construct with a mutated κB nucleotide-binding motif, abrogated detection of these complexes. The 2 lower bands within these complexes corresponded to those of p50/RelA heterodimer because higher molecular weight complexes were formed when antibodies against these structures were added to the reaction mixture (Fig. 4B). Differential phosphorylation levels for RelA may account for the presence of 2 RelA-κB complexes. The third band, the slowest-migrating complex, likely consisted of RelA homodimers because the anti-p65 antibody affected its electrophoretic mobility. Anti-c-Rel and RelB antibodies did not alter the apparent size of the κB complexes. Nuclear extracts prepared from light-irradiated cells treated with verteporfin (100 ng/mL) contained low protein levels, preventing reliable assessment of κB binding activity for these samples. Immunoblot analysis revealed a reduction in IκBα staining intensity for cytoplasmic extracts prepared from cells treated with verteporfin and irradiated 0.5 to 2 hours before (Fig. 4C). By 3 hours after irradiation, IκBα band density returned to the level of that for untreated control extract. Nuclear extracts contained maximal κB binding activity at 2 hours after PDT. This activity was lower at 3 hours and at background levels by 6 hours after irradiation. Corresponding IκBα immunoblot and κB electrophoretic mobility shift assay results were obtained with whole cell and nuclear extracts, respectively, prepared from cells treated with verteporfin at 10 or 25 ng/mL and light-irradiated up to 6 hours earlier (data not shown).
Effect of verteporfin photosensitization on κB binding activity in HL-60 cells.
(A) Nuclear extracts were prepared at increasing times after the light irradiation of cells incubated with different amounts of verteporfin. Equal amounts of protein were incubated with a 32P-labeled κB probe and separated in 6% acrylamide gels. Nonspecific and specific DNA-protein complexes are indicated. For competitor studies, nuclear extracts prepared from cells treated with verteporfin (50 ng/mL) and light 2 hours earlier were incubated with the32P-labeled κB probe in the presence of a 10 mol/L excess of unlabeled wild-type or mutated κB preparations. (B) Protein-κB complexes were characterized by gel supershift analysis. Nuclear extracts prepared from cells treated with verteporfin (50 ng/mL) and light 2 hours before were mixed with the 32P-labeled κB probe in the presence of antisera against p50, RelA, c-Rel, or RelB. Samples were separated in 4% polyacrylamide gels under nonreducing conditions. Arrows indicate the positions of κB complexes. (C) The status of IκBα was monitored after photosensitization of HL-60 cells with verteporfin and light. Cytoplasmic extracts were prepared at various times after light irradiation and were analyzed by Western immunoblot (WB) analysis using mouse monoclonal antibody MAD3 10B developed against amino acids 21-48 within the N-terminal region of IκBα. The corresponding nuclear extracts were analyzed by electrophoretic mobility shift assay as performed in part A.
Effect of verteporfin photosensitization on κB binding activity in HL-60 cells.
(A) Nuclear extracts were prepared at increasing times after the light irradiation of cells incubated with different amounts of verteporfin. Equal amounts of protein were incubated with a 32P-labeled κB probe and separated in 6% acrylamide gels. Nonspecific and specific DNA-protein complexes are indicated. For competitor studies, nuclear extracts prepared from cells treated with verteporfin (50 ng/mL) and light 2 hours earlier were incubated with the32P-labeled κB probe in the presence of a 10 mol/L excess of unlabeled wild-type or mutated κB preparations. (B) Protein-κB complexes were characterized by gel supershift analysis. Nuclear extracts prepared from cells treated with verteporfin (50 ng/mL) and light 2 hours before were mixed with the 32P-labeled κB probe in the presence of antisera against p50, RelA, c-Rel, or RelB. Samples were separated in 4% polyacrylamide gels under nonreducing conditions. Arrows indicate the positions of κB complexes. (C) The status of IκBα was monitored after photosensitization of HL-60 cells with verteporfin and light. Cytoplasmic extracts were prepared at various times after light irradiation and were analyzed by Western immunoblot (WB) analysis using mouse monoclonal antibody MAD3 10B developed against amino acids 21-48 within the N-terminal region of IκBα. The corresponding nuclear extracts were analyzed by electrophoretic mobility shift assay as performed in part A.
Nuclear reporter assay
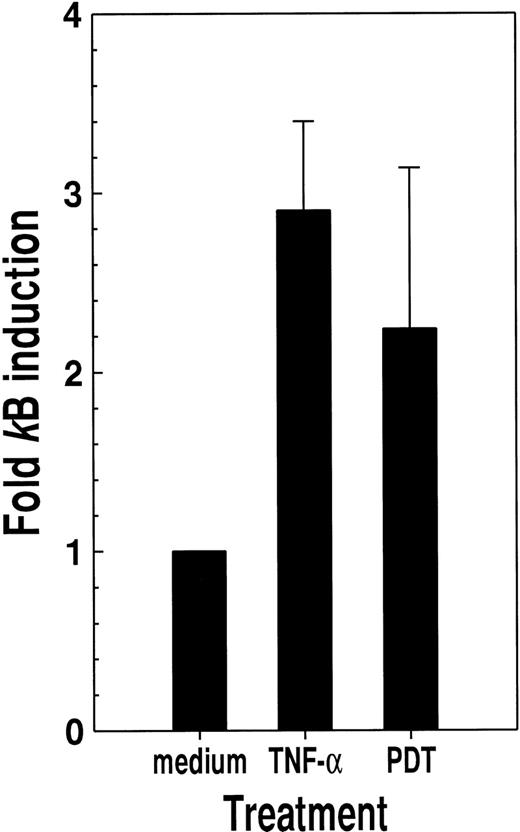
For HL-60 cells transfected with a κB-Luc reporter plasmid construct, PDT with verteporfin and light produced a more than 2-fold increase in κB-specific luciferase activity (Fig.5) This effect was of a magnitude similar to that elicited by TNF-α, a well-characterized activator of NF-κB.10-12
Photosensitization with verteporfin and red light causes productive κB activation.
HL-60 cells transfected with a κB-luciferase reporter plasmid construct were treated with either tumor necrosis factor-α (200 U/mL) or verteporfin (5 ng/mL) followed by red light (2 J/cm2) irradiation. Luciferase activity was determined 24 hours later. Results are given as the ratio of luciferase activity for cells maintained in medium alone. Means with standard deviations for the results of 3 independent experiments are shown.
Photosensitization with verteporfin and red light causes productive κB activation.
HL-60 cells transfected with a κB-luciferase reporter plasmid construct were treated with either tumor necrosis factor-α (200 U/mL) or verteporfin (5 ng/mL) followed by red light (2 J/cm2) irradiation. Luciferase activity was determined 24 hours later. Results are given as the ratio of luciferase activity for cells maintained in medium alone. Means with standard deviations for the results of 3 independent experiments are shown.
Discussion
IκBα degradation is a primary event related to the activation of NF-κB-regulated genes.61 A reduction in cellular IκBα levels provides an indication that the NF-κB pathway has been mobilized. A partial and reversible decrease in IκBα levels was observed for HL-60 cells exposed to sublethal levels of photosensitizer and light. NF-κB stimulates IκBα gene transcription as a component of the mechanism to regulate NF-κB activity,62and this may account for the observed rebound in IκBα levels after photosensitization. An unexplored possibility is that PDT-induced NF-κB nuclear translocation could ensue in the absence of IκBα degradation. Such an action was described for pervanadate, a protein tyrosine phosphatase inhibitor that liberates IκBα from NF-κB by promoting tyrosine phosphorylation at residue 42 of IκBα.63 Further, the level of IκBα degradation did not fully account for the extent of NF-κB DNA binding activity induced by TNF-α and interleukin-1β.64 This suggests that in addition to the well-characterized IκBα degradation pathway, other signaling routes may participate in NF-κB activation.64 After PDT treatment, HL-60 cells transfected with a Luc reporter gene exhibited increased luciferase activity, indicating that photosensitization likely leads to productive NF-κB-mediated gene transcription. It is unclear how PDT leads to NF-κB activation, though the photoxidative stress produced by the treatment presumably contributes directly to the effect.31A novel biochemical pathway contributing to NF-κB activation with PDT was recently delineated.30 The photodynamic treatment of human HCT-116 carcinoma cells with PPME, a photosensitizer that localizes within the cell membrane and lysosomes, produced 2 distinct waves of NF-κB activation first by promoting the internalization of surface interleukin-1 receptors and then by ceramide generation.30 The localization of a photosensitizer within cells may influence the mode, kinetics and magnitude of the NF-κB response.
NF-κB activation may promote the transcription of genes encoding proteins that counterbalance pro-apoptotic biochemical changes triggered by various agents.10-13 Cells transfected with IκBα constructs, containing amino acid substitutions for serines 32 and 36, exhibit deficient NF-κB responses and heightened sensitivity to apoptosis induced by TNF-α and chemotherapeutic agents.11 The NF-κB regulated inhibitor of apoptosis gene family-encoded proteins may act to counteract the apoptotic process by directly inhibiting caspase activity.12 Human HCT-116 colon carcinoma cells overexpressing IκBα mutated at serines 32 and 36 exhibit a greater susceptibility to PDT-induced apoptosis with the photosensitizer PPME and red light irradiation than wild-type cells (manuscript in preparation). This finding is supportive of the concept that NF-κB has a role in influencing cellular resistance to PDT-induced apoptosis. Elucidation of the genes transcribed after PDT-mediated NF-κB activation will aid in understanding the ramifications of this form of treatment.
HL-60 cells treated with a cytotoxic combination of verteporfin and light rapidly exhibited apoptotic changes reflected by caspase-3 and caspase-9 activation and PARP cleavage, changes that were blocked by the general caspase inhibitor ZVAD.fmk. These effects are similar to those described for HeLa cells undergoing PDT-induced apoptosis using verteporfin as the photosensitizer.48-50 Caspase-3 and caspase-9 activation and PARP cleavage are hallmarks of cells undergoing apoptosis.32,33,36,37,39,41,51 IκBα cleavage by a factor with enzymatic activity, consistent with that of caspase-3, was described for human fibroblasts rendered apoptotic by ionizing radiation.46 Activated caspase-3, but not caspase-1 or caspase-2, cleaved recombinant chicken and human IκBα in a cell-free system, and proteolysis of IκBα was blocked by a caspase-3-specific inhibitor peptide.45 A putative caspase-3 cleavage site exists between amino acids 35 and 36 within the N-terminal region of IκBα.45 The level of IκBα was substantially reduced during PDT-induced apoptosis. However, this diminishment in IκBα levels was unaffected by ZVAD.fmk, a peptide that effectively blocked the cleavage of PARP, a well-defined caspase-3 substrate.36,37 IκBβ also contains a putative caspase cleavage site in its N-terminal region sensitive to caspase-3-mediated cleavage in vitro.27 However, the level of IκBβ levels was unchanged for HL-60 cells rendered apoptotic with PDT under conditions that led to complete PARP cleavage. The presence of a caspase recognition consensus sequence within a protein or the capacity of a caspase to cleave the protein in a cell-free system does not consistently signify that it will be a caspase substrate during apoptosis.65 Although we did not observe evidence of IκBα cleavage in HeLa cells undergoing PDT-induced apoptosis (data not shown), other cell types may exhibit caspase-mediated cleavage of IκB proteins during PDT-induced apoptosis or after the application of other pro-apoptotic stimuli.
Serine phosphorylation of IκBα blocked caspase-mediated of IκBα in vitro.45 In contrast, coordinate phosphorylation of serine residues of the PITSLRE p110 protein kinase predisposed its processing by caspases during Fas-mediated apoptosis.66 The phosphorylation state of certain proteins may influence their susceptibility to caspase-mediated cleavage during apoptosis.45 66 Photosensitization with verteporfin may rapidly induce serine phosphorylation of IκBα and trigger the cell death pathway in temporal proximity. The absence of caspase-mediated cleavage of IκBα during PDT-induced apoptosis may be explained if the treatment causes widespread phosphorylation of IκBα molecules within these cells.
It is important to understand the impact of PDT on NF-κB-mediated gene expression because this technique is used for the treatment of human oncologic, dermatologic, and ophthalmologic conditions.21 NF-κB activation in cells given a nonlethal PDT dose may have various consequences for these and neighboring cells by promoting the expression of factors whose encoding genes are regulated by this transcription regulator. Of importance, NF-κB activation may contribute to the modification of immune responses, a widely described effect of this form of phototherapy.21,22 67
Reprints:David W. C. Hunt, QLT PhotoTherapeutics, Inc, 520 West 6th Avenue, Vancouver, BC, Canada V5Z 4H5; e-mail:dhunt@qlt-pdt.com.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal