Abstract
Several activating mutations have recently been described in the common β subunit for the human interleukin(IL)-3, IL-5, and granulocyte-macrophage colony-stimulating factor (GM-CSF) receptors (hβc). Two of these, FIΔ and I374N, result, respectively, in a 37–amino acid duplication and an isoleucine-to-asparagine substitution in the extracellular domain. A third, V449E, leads to valine-to–glutamic acid substitution in the transmembrane domain. Previous studies have shown that when expressed in murine hemopoietic cells in vitro, the extracellular mutants can confer factor independence on only the granulocyte-macrophage lineage while the transmembrane mutant can do so to all cell types of the myeloid and erythroid compartments. To further study the signaling properties of the constitutively active hβc mutants, we have used novel murine hemopoietic cell lines, which we describe in this report. These lines, FDB1 and FDB2, proliferate in murine IL-3 and undergo granulocyte-macrophage differentiation in response to murine GM-CSF. We find that while the transmembrane mutant, V449E, confers factor-independent proliferation on these cell lines, the extracellular hβc mutants promote differentiation. Hence, in addition to their ability to confer factor independence on distinct cell types, transmembrane and extracellular activated hβc mutants deliver distinct signals to the same cell type. Thus, the FDB cell lines, in combination with activated hβc mutants, constitute a powerful new system to distinguish between signals that determine hemopoietic proliferation or differentiation. (Blood. 2000;95:120-127)
Human interleukin (IL)-3, IL-5, and granulocyte-macrophage colony-stimulating factor (GM-CSF) are cytokines that affect the survival, proliferation, differentiation, and functional activation of cells within the hemopoietic system. Their many functions are mediated by membrane-bound receptors that are composed of 2 subunits. These are (1) specific α subunits that bind each growth factor with low affinity but that cannot signal and (2) a common β subunit (hβc) that cannot detectably bind growth factor but that complexes with the α subunits to form a high-affinity receptor and is thought to be responsible for the majority of signaling through the IL-3/IL-5/GM-CSF receptors.1-3
Recently, several constitutively active mutant forms of hβc have been identified via their ability to confer growth factor independence on the otherwise GM-CSF–dependent murine myelomonocytic cell line, FDC-P1. Two of these factors, FIδ and I374N, contain mutations in the extracellular domain of hβc. FIδ contains a 37–amino acid duplication within the membrane-proximal region, while I374N has an isoleucine-to-asparagine substitution in the same region. A third mutant, V449E, contains an amino acid substitution in the transmembrane domain of the receptor, converting a valine residue to glutamic acid.4
Several lines of evidence suggest that the transmembrane and extracellular hβc mutants signal via different mechanisms. First, V449E, but not FIδ or I374N, could confer factor independence on the murine IL-3–dependant pro-B cell line Ba/F3.4 Second, when expressed in primary murine hemopoietic cells, FIδ and I374N could confer factor independence on the granulocyte-macrophage lineages only, while V449E could do so to all myeloid and erythroid cell types.5 These results have recently been explained in part by the finding that the extracellular mutant I374N associates with the murine GM-CSF receptor α subunit (mGMRα) and requires its expression for function in murine cell lines.6 Third, the extracellular hβc mutants are defective in a number of aspects of signaling, including receptor phosphorylation7 and phosphorylation of Shc (T. Blake and TJG, unpublished data September 1997).
Differentiation-competent hemopoietic cell lines have provided defined systems in which to study the cellular differentiation induced by signaling through the receptors for a number of cytokines, including granulocyte colony-stimulating factor (G-CSF), Thrombopoietin, Erythropoietin (Epo), and GM-CSF.8-11 Here we describe the isolation and characterization of 2 cell lines, FDB1 and FDB2, which proliferate continuously in murine IL-3 but undergo growth arrest and granulocyte-macrophage differentiation in response to murine GM-CSF. To study the effects of constitutively active hβc mutants on hemopoietic proliferation and differentiation, we have expressed both transmembrane and extracellular hβc mutants in the FDB cell lines. We show that the extracellular hβc mutants, like murine GM-CSF, promote differentiation of these lines while the V449E, like IL-3, confers factor-independent proliferation. Hence, transmembrane and extracellular hβc mutants, in addition to their previously documented ability to confer factor independence on distinct hemopoietic lineages, deliver distinct signals to the same cell type.
Methods
Cytokines
Recombinant murine GM-CSF (mGM-CSF) was obtained and used as a crude yeast supernatant, kindly supplied by Dr Tracy Wilson (Walter and Eliza Hall Institute, Melbourne, Australia). Recombinant murine IL-3 (mIL-3) was produced from a baculovirus vector kindly supplied by Dr Andrew Hapel (John Curtin School of Medical Research, Canberra, Australia). Recombinant human Epo (hEpo) was purchased from Janssen Cilag (Lane Cove, NSW, Australia).
Cell lines
BOSC-23 ecotropic retrovirus packaging cells12 were maintained in Dulbecco's modified Eagle's medium supplemented with 10% fetal calf serum. The FDB1 and FDB2 cell lines were maintained in Iscove's modified Dulbecco's medium supplemented with 15% fetal calf serum and 1000 U/mL mIL-3. Infected pools were maintained in the above medium plus 1 μg/mL Puromycin (Calbiochem, San Diego, CA).
Construction of retroviral expression plasmids
The hβc cDNA1 used here was that described by Barry et al.13 The FIδb14 V449E, and I374N4 mutants have been described previously. The pRufPuro retroviral expression plasmid was described previously by Jenkins et al.4 To construct pRufPuro vectors containing FLAG-tagged (F) wild-type and mutant hβc cDNAs, an approximately 650 bp AccI restriction fragment incorporating the FLAG epitope tag (sequence DYKDDDDK) was excised from the N-terminus of pRufNeo-Fhβc15 and inserted into the corresponding region of pRufNeo-V449E and pRufNeo-I374N.16FLAG-tagged hβc subunits were recovered from the resulting constructs as well as pRufNeo-FFIδ15 by digestion withBamHI and EcoRI. These were inserted into the corresponding sites of pRufPuro.
Infection of FDB2 cells with RufPuro constructs
FDB2 cells were infected by co-cultivation with transiently transfected BOSC-23 cells essentially as described by Jenkins et al.16 Briefly, 1.5 × 106 BOSC-23 cells were seeded into 60-mm dishes containing 4 mL medium and incubated overnight. The next day, the medium was replaced with fresh medium containing 25 μM chloroquine. We used 20 μg retroviral DNA to transfect cells in each dish by the calcium phosphate procedure as described by Pear et al.12 The medium was replaced with fresh medium without chloroquine 7 hours after transfection, and the cells were incubated overnight. The next day, the medium was removed, and 2 × 105 FDB2 cells were added in 4 mL Iscove's modified Dulbecco's medium containing 15% fetal calf serum, 1000 U/mL mIL-3, and 4 μg/mL polybrene. After 48 hours, the FDB2 cells were harvested and selected in Iscove's modified Dulbecco's medium containing 15% fetal calf serum, 1000 U/mL mIL-3, and 1 μg/mL puromycin.
Southern analysis of genomic DNA
10 μg genomic DNA was digested with BamHI or KpnI, separated by agarose gel electrophoresis, transferred to nylon membranes, and hybridized with a random probe prepared with use of the Megaprime DNA labeling kit (Amersham, Buckinghamshire, UK) and directed against the 1096 bp MC1-Neo cassette derived from pRufNeo by digestion with BglII and ClaI.
Flow cytometry
The rat anti-mouse Mac-1, Gr-1, and F4/80 monoclonal antibodies were used as described by Gonda et al.17 The murine anti-FLAG monoclonal antibody M2 was purchased from Kodak (New Haven, CT). Cells were stained with the above antibodies followed by fluorescein isothiocyanate–conjugated anti-isotype monoclonal antibodies (Silenus, Hawthorn, Victoria, Australia) and analyzed by flow cytometry with the use of an Epics-Profile II analyzer (Coulter Electronics, Hialeah, FL). Professor Angel Lopez (Hanson Centre for Cancer Research, Adelaide, South Australia) generously provided the murine anti-hβc monoclonal antibody 4F3.18 Cells stained with this antibody were subsequently incubated with a biotin-conjugated anti-mouse immunoglobulin antibody (Vector Lab Inc, Burlingame, CA) followed by phycoerythrin-conjugated streptavidin (Caltag Laboratories, San Francisco, CA), and analyzed as above.
Differentiation assays
FDB1 cells, FDB2 cells, and stably infected FDB2 pools were washed 3 times in Dulbecco's modified Eagle's medium and cultured in 500 U/mL mIL-3, in 500 U/mL mGM-CSF, or in the absence of growth factors. At the indicated times, samples were cytocentrifuged and Wright-Giemsa stained, and the proportions of differentiated cells were determined microscopically.
Viability assays
To assess cell viability, the percentage of cells excluding trypan blue was determined microscopically from at least 200 cells scored.
Results
Isolation and characterization of pluripotent hemopoietic cell lines
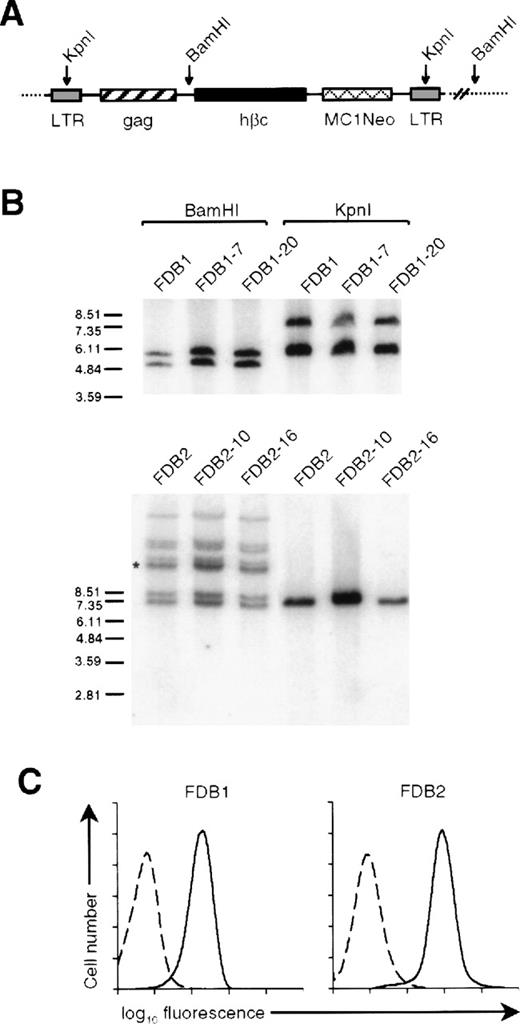
During experiments involving the retroviral expression of constitutively active hβc mutants in primary hemopoietic cells,5 murine fetal liver cells were infected with the RufNeo retroviral vector containing the wild-type hβc cDNA and placed in a long-term culture in a cocktail of mIL-3, mGM-CSF, and hEpo. From 2 such cultures, transformed cell lines arose, which we have termed FDB1 and FDB2. Both cell lines were resistant to G418, implying that they contained RufNeo-hβc proviruses. To confirm this, genomic DNA was prepared from these cell lines, and Southern analysis was performed with the use of a probe to the MC1Neo cassette contained within the RufNeo provirus (Figure 1A). To assess the number of proviruses present, the genomic DNAs were digested withBamHI, which cuts the RufNeo provirus once, generating a single band for each proviral integration (Figure 1A). As shown in Figure 1B, the FDB1 cell line contains 2 RufNeo-hβc proviruses whereas FDB2 contains 8. The proviral integration pattern of each cell line was identical to those of clones derived from these cell lines, implying that both cell lines are clonal. To confirm the size of the integrated RufNeo-hβc proviruses, the genomic DNAs were digested withKpnI, which cuts within the retroviral long terminal repeats. With the use of this approach, it was determined that 1 of the 2 RufNeo-hβc proviruses present in the FDB1 genome contains an approximately 1.2 kb deletion, while all proviruses present in the FDB2 genome are of the expected size (Figure 1B).
Retroviral integration and hβc expression on the FDB1 and FDB2 cell lines.
(A) Schematic representation of an integrated RufNeo-hβc provirus showing the position of BamHI and KpnI restriction sites. Long terminal repeat sequences (LTR) and the neomycin resistance gene under control of the MC1 promoter (MC1Neo) are indicated. (B) Southern analysis of BamHI- and KpnI-digested genomic DNA (as indicated) derived from the FDB1 and FDB2 cell lines, and from clones of each of these lines, with the use of a Neo probe. The sizes of molecular weight markers (EcoRI-digested SPP1 phage DNA) are shown in kilobase. (Note that the band marked with the asterisk is in fact a doublet.) (C) Surface expression of hβc on FDB cell lines. FDB1 and FDB2 cells were stained with an anti-hβc monoclonal antibody 4F3 (solid lines) or an irrelevant isotype control antibody (dashed lines).
Retroviral integration and hβc expression on the FDB1 and FDB2 cell lines.
(A) Schematic representation of an integrated RufNeo-hβc provirus showing the position of BamHI and KpnI restriction sites. Long terminal repeat sequences (LTR) and the neomycin resistance gene under control of the MC1 promoter (MC1Neo) are indicated. (B) Southern analysis of BamHI- and KpnI-digested genomic DNA (as indicated) derived from the FDB1 and FDB2 cell lines, and from clones of each of these lines, with the use of a Neo probe. The sizes of molecular weight markers (EcoRI-digested SPP1 phage DNA) are shown in kilobase. (Note that the band marked with the asterisk is in fact a doublet.) (C) Surface expression of hβc on FDB cell lines. FDB1 and FDB2 cells were stained with an anti-hβc monoclonal antibody 4F3 (solid lines) or an irrelevant isotype control antibody (dashed lines).
To assess hβc protein expression, cells of each line were stained with a monoclonal antibody directed against hβc and analyzed by flow cytometry. As shown in Figure 1C, both FDB1 and FDB2 cell lines express high levels of hβc protein.
Growth factor requirements of FDB1 and FDB2 cell lines
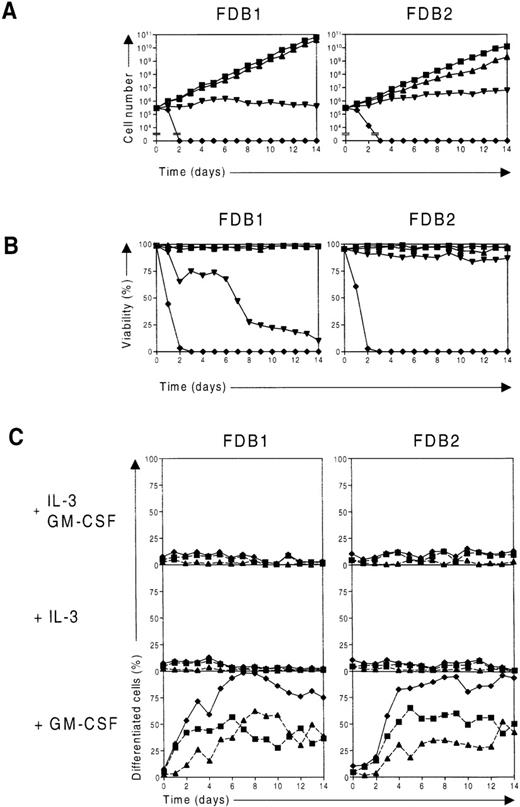
As stated above, the FDB1 and FDB2 cell lines were obtained following culture in a cocktail of mIL-3, mGM-CSF, and hEpo. To determine the growth factor requirements of the these lines, each was washed extensively and cultured in either IL-3, GM-CSF, or Epo, and cell proliferation and differentiation were monitored over time. When deprived of growth factors, cells of each line rapidly died (Figures2A and 2B), as did those placed in Epo alone (data not shown). However, when cells of either cell line were placed in IL-3, they continued to proliferate and remained morphologically similar to blast cells with a small amount of spontaneous differentiation giving rise to neutrophils, monocytes, and megakaryocyte-like cells (Figures 2A, 2C, and3). In contrast, when cultured in GM-CSF, both FDB1 and FDB2 cells rapidly differentiated along the neutrophil and monocyte lineages (Figures 2C and 3). A small amount (≤ 2%) of apparent megakaryocytic differentiation was also observed in both IL-3 and GM-CSF, particularly in the case of the FDB2 cell line. Differentiation correlated with a decline in the proliferation of both cell lines, which in the case of FDB1 cells was complete (Figure 2A), and with a loss of cell viability, particularly in the case of FDB1 (Figure 2B). When either cell line was cultured in the presence of both IL-3 and GM-CSF, the IL-3 response was dominant, the only apparent difference from culture in IL-3 alone being a slightly higher rate of proliferation of FDB2 cells (Figure 2A).
Differentiation of FDB1 and FDB2 cell lines in response to murine GM-CSF.
(A) Time course of proliferation of FDB cell lines. FDB1 and FDB2 cells were washed and placed in culture in the presence of IL-3 plus GM-CSF (▪), IL-3 (▴), or GM-CSF (▾) or in the absence of growth factors (⧫), and the number of viable cells was determined daily with the use of a hemocytometer. (B) Time course of viability of FDB cell lines. In the same experiment as in A, the percentage of viable FDB1 and FDB2 cells cultured in the presence of IL-3 plus GM-CSF (▪), IL-3 (▴), or GM-CSF (▾) or in the absence of growth factors (⧫) were determined from at least 200 cells scored. (C) Time course of differentiation of FDB cell lines. In the same experiment as in A, samples of FDB1 and FDB2 cells cultured in the presence of the indicated growth factors were cytocentrifuged and stained daily, and the proportion of differentiated granulocytes (▪, dashed lines), the proportion of macrophages (▴, dashed lines), and the total proportion of differentiated cells (⧫, solid lines) were determined from at least 200 cells scored microscopically.
Differentiation of FDB1 and FDB2 cell lines in response to murine GM-CSF.
(A) Time course of proliferation of FDB cell lines. FDB1 and FDB2 cells were washed and placed in culture in the presence of IL-3 plus GM-CSF (▪), IL-3 (▴), or GM-CSF (▾) or in the absence of growth factors (⧫), and the number of viable cells was determined daily with the use of a hemocytometer. (B) Time course of viability of FDB cell lines. In the same experiment as in A, the percentage of viable FDB1 and FDB2 cells cultured in the presence of IL-3 plus GM-CSF (▪), IL-3 (▴), or GM-CSF (▾) or in the absence of growth factors (⧫) were determined from at least 200 cells scored. (C) Time course of differentiation of FDB cell lines. In the same experiment as in A, samples of FDB1 and FDB2 cells cultured in the presence of the indicated growth factors were cytocentrifuged and stained daily, and the proportion of differentiated granulocytes (▪, dashed lines), the proportion of macrophages (▴, dashed lines), and the total proportion of differentiated cells (⧫, solid lines) were determined from at least 200 cells scored microscopically.
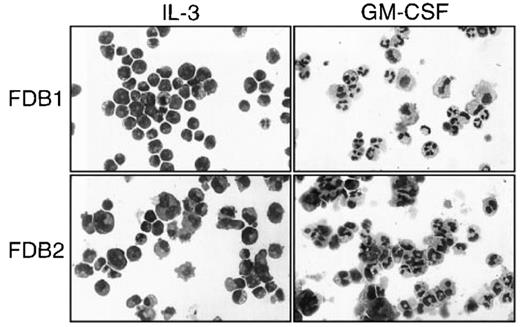
Morphology of the FDB1 and FDB2 cell lines.
FDB1 and FDB2 cells cultured in the presence of the indicated growth factors for 5 days were cytocentrifuged and Wright-Giemsa stained. Photographs are at 270 × magnification.
Morphology of the FDB1 and FDB2 cell lines.
FDB1 and FDB2 cells cultured in the presence of the indicated growth factors for 5 days were cytocentrifuged and Wright-Giemsa stained. Photographs are at 270 × magnification.
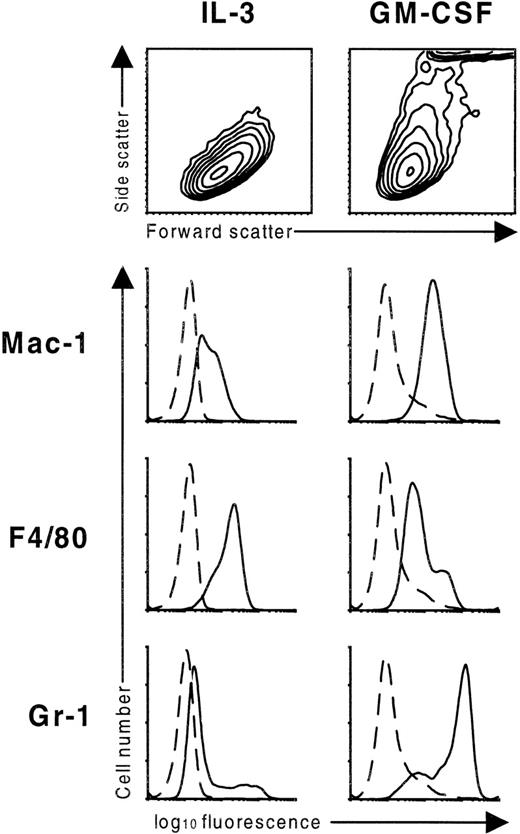
GM-CSF–induced differentiation of FDB1 cells was accompanied by a loss of size and an increase in granularity, as measured by forward scatter and side scatter of the cells, respectively (Figure4). Both are consistent with the observed granulocytic differentiation of these cells. Moreover, there was strong induction of the granulocyte-macrophage markers Mac-1 and Gr-1 (Figure4). The monocytic marker, F4/80, was expressed at high levels on cells cultured in IL-3, indicating that this line has monocytic characteristics in its undifferentiated state, and was slightly reduced when cells were switched to GM-CSF. All 3 of these markers were induced on FDB2 cells upon culture in GM-CSF (see Fhβc panels in Figure 5).
Scatter profile and surface antigen expression of the FDB1 cell line.
FDB1 cells cultured in the presence of the indicated growth factors for 7 days were stained with rat monoclonal antibodies specific for the indicated surface antigens (solid lines) or an irrelevant isotype control (dashed lines). Scatter profiles are shown as contour maps.
Scatter profile and surface antigen expression of the FDB1 cell line.
FDB1 cells cultured in the presence of the indicated growth factors for 7 days were stained with rat monoclonal antibodies specific for the indicated surface antigens (solid lines) or an irrelevant isotype control (dashed lines). Scatter profiles are shown as contour maps.
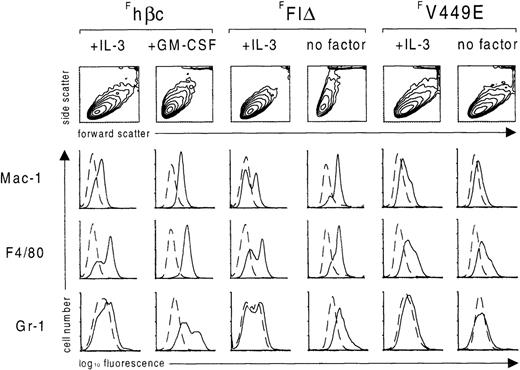
Flow cytometric analysis of FDB2 cells expressing FLAG-tagged hβc subunits.
After culture for 7 days in the presence of the indicated growth factors or in the absence of growth factors (no factor), FDB2 cells expressing the indicated FLAG-tagged hβc proteins were stained with rat monoclonal antibodies specific for the indicated surface antigens (solid lines) or an irrelevant isotype control (dashed lines). Scatter profiles are shown as contour maps.
Flow cytometric analysis of FDB2 cells expressing FLAG-tagged hβc subunits.
After culture for 7 days in the presence of the indicated growth factors or in the absence of growth factors (no factor), FDB2 cells expressing the indicated FLAG-tagged hβc proteins were stained with rat monoclonal antibodies specific for the indicated surface antigens (solid lines) or an irrelevant isotype control (dashed lines). Scatter profiles are shown as contour maps.
To confirm that the IL-3/GM-CSF differentiation switch exhibited by the FDB1 and FDB2 cell lines was not peculiar to the cytokine concentrations used, FDB2 cells were washed extensively and cultured in varying concentrations of IL-3 and GM-CSF for 6 days. As shown in Table1, very low levels of IL-3 promoted differentiation of the FDB2 cell line; however, concentrations as low as 2 U/mL were sufficient to maintain the line in a predominantly undifferentiated state. In contrast, concentrations of GM-CSF from 0.03 U/mL to 500 U/mL promoted differentiation of this cell line along the granulocyte-macrophage lineages. Thus these data indicate that FDB1 and FDB2 are cytokine-dependent cell lines that exhibit a switch between proliferation and differentiation in response to IL-3 and GM-CSF, respectively.
Effect of growth factor concentration on FDB2 cells.*
| Growth Factor (U/mL) . | Blast/Pro . | Myel/Meta . | Band/Neut . | Mono . | MK . |
|---|---|---|---|---|---|
| IL-3 | |||||
| 500 | 66 | 22 | 6 | 4 | 2 |
| 50 | 63 | 23 | 9 | 4 | 1 |
| 10 | 54 | 36 | 5 | 4 | 2 |
| 2 | 39 | 47 | 8 | 6 | 2 |
| 0.4 | 0 | 28 | 43 | 29 | 0 |
| 0.08 | 0 | 30 | 51 | 19 | 0 |
| 0.016 | Nonviable† | ||||
| GM-CSF | |||||
| 500 | 4 | 17 | 68 | 12 | 0 |
| 100 | 0 | 4 | 67 | 30 | 0 |
| 20 | 0 | 0 | 63 | 37 | 0 |
| 4 | 0 | 10 | 46 | 45 | 0 |
| 0.8 | 1 | 19 | 46 | 35 | 0 |
| 0.16 | 2 | 18 | 40 | 41 | 0 |
| 0.032 | 3 | 35 | 38 | 24 | 0 |
| Growth Factor (U/mL) . | Blast/Pro . | Myel/Meta . | Band/Neut . | Mono . | MK . |
|---|---|---|---|---|---|
| IL-3 | |||||
| 500 | 66 | 22 | 6 | 4 | 2 |
| 50 | 63 | 23 | 9 | 4 | 1 |
| 10 | 54 | 36 | 5 | 4 | 2 |
| 2 | 39 | 47 | 8 | 6 | 2 |
| 0.4 | 0 | 28 | 43 | 29 | 0 |
| 0.08 | 0 | 30 | 51 | 19 | 0 |
| 0.016 | Nonviable† | ||||
| GM-CSF | |||||
| 500 | 4 | 17 | 68 | 12 | 0 |
| 100 | 0 | 4 | 67 | 30 | 0 |
| 20 | 0 | 0 | 63 | 37 | 0 |
| 4 | 0 | 10 | 46 | 45 | 0 |
| 0.8 | 1 | 19 | 46 | 35 | 0 |
| 0.16 | 2 | 18 | 40 | 41 | 0 |
| 0.032 | 3 | 35 | 38 | 24 | 0 |
Numbers are percentages of blasts and promyelocytes (blast/pro), myelocytes and metamyelocytes (myel/meta), bands and mature neutrophils (band/neut), monocytes and macrophages (mono), and megakaryocyte-like cells (MK) of 200 cells scored at day 6 of culture.
No significant viability after 6 days' culture.
Function of constitutive hβc mutants in the FDB2 cell line
To determine the effects of constitutively active hβc mutants on the growth and differentiation of the FDB cell lines, 2 extracellular mutants, FIδ and I374N, and the transmembrane mutant, V449E, were expressed in this cell line by infection with the corresponding RufPuro retroviral vectors. As shown in Table 2, when the resultant puromycin-resistant pools were washed and cultured in the absence of growth factors for 5 days, the extracellular hβc mutants induced granulocyte-macrophage differentiation of the FDB cell lines, similar to that induced by GM-CSF. In contrast, the transmembrane mutant V449E maintained the cells in an undifferentiated state, similar to cells cultured in IL-3.
Extracellular but not transmembrane activated hβc mutants induce differentiation of FDB cells.
| Cell Line . | RufPuro Construct . | Growth Factor . | Blast/Pro . | Myel/Meta . | Band/Neut . | Mono . | MK . |
|---|---|---|---|---|---|---|---|
| FDB1 | — | IL-3 | 90 | 7 | 1 | 1 | 1 |
| — | GM-CSF | 0 | 3 | 59 | 37 | 1 | |
| FIΔ | — | 1 | 5 | 51 | 44 | 0 | |
| V449E | — | 82 | 8 | 7 | 2 | 1 | |
| I374N | — | 0 | 7 | 58 | 34 | 1 | |
| FDB2 | — | IL-3 | 72 | 20 | 7 | 1 | 0 |
| — | GM-CSF | 3 | 8 | 65 | 24 | 0 | |
| FIΔ | — | 5 | 6 | 77 | 12 | 0 | |
| V449E | — | 82 | 12 | 5 | 1 | 1 | |
| I374N | — | 8 | 8 | 76 | 8 | 0 |
| Cell Line . | RufPuro Construct . | Growth Factor . | Blast/Pro . | Myel/Meta . | Band/Neut . | Mono . | MK . |
|---|---|---|---|---|---|---|---|
| FDB1 | — | IL-3 | 90 | 7 | 1 | 1 | 1 |
| — | GM-CSF | 0 | 3 | 59 | 37 | 1 | |
| FIΔ | — | 1 | 5 | 51 | 44 | 0 | |
| V449E | — | 82 | 8 | 7 | 2 | 1 | |
| I374N | — | 0 | 7 | 58 | 34 | 1 | |
| FDB2 | — | IL-3 | 72 | 20 | 7 | 1 | 0 |
| — | GM-CSF | 3 | 8 | 65 | 24 | 0 | |
| FIΔ | — | 5 | 6 | 77 | 12 | 0 | |
| V449E | — | 82 | 12 | 5 | 1 | 1 | |
| I374N | — | 8 | 8 | 76 | 8 | 0 |
Numbers are the percentage of blasts and promyelocytes (blast/pro), myelocytes and metamyelocytes (myel/meta), bands and mature neutrophils (band/neut), monocytes and macrophages (mono), and megakaryocyte-like cells (MK) of 200 cells scored at day 5 of culture.
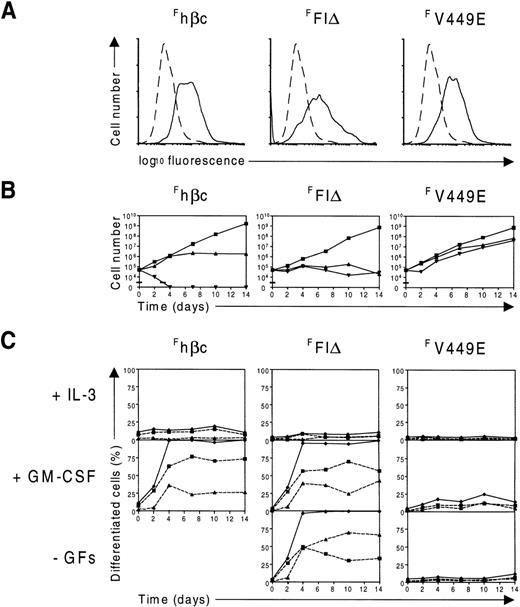
To confirm that the disparate effects of transmembrane and extracellular hβc mutants on the FDB cell lines did not reflect different expression levels, we sought to determine the level of expression of each of the hβc mutants on these cells. As these lines also expressed wild-type hβc (Figure 1C), the FLAG epitope tag was added to wild-type hβc and each of the FIδ and V449E mutants to facilitate detection by flow cytometry. As shown in Figure 6A, FLAG-tagged (F)hβc, FFIδ, and FV449E were all expressed on FDB2 cells at comparable levels. To confirm the effects of these proteins on FDB2 cell growth and differentiation, the cells were washed extensively and cultured in the presence of IL-3 or GM-CSF or in the absence of growth factors. Not surprisingly, FDB2 cells containing Fhβc proliferated in IL-3 with a low level of spontaneous differentiation, differentiated along the granulocyte-macrophage lineages in response to GM-CSF, and rapidly died when deprived of growth factors (Figure 6B, C), similarly to uninfected FDB2 cells (see above). When deprived of growth factors, FDB2 cells expressing FFIδ ceased proliferation and differentiated along the granulocyte-macrophage lineages, while those expressingFV449E continued to proliferate (Figure 6B, C, Figure7). Similar results were obtained when theFFIδ and FV449E proteins were expressed in the FDB1 cell line (Figure 7 and data not shown).
Expression and function of FLAG-tagged hβc proteins in FDB2 cells.
(A) Flow cytometric analysis of FDB2 cells expressing FLAG-tagged hβc subunits. FDB2 cells expressing the indicated FLAG-tagged hβc subunits were stained with the anti-FLAG monoclonal antibody M2 (solid lines). As controls, uninfected FDB2 cells were stained identically (dashed lines). (B) Time course of proliferation of FDB2 cells expressing FLAG-tagged hβc proteins. FDB2 cells were washed and cultured in IL-3 (▪) or GM-CSF (▴) or in the absence of growth factors (▾), and the number of viable cells was determined periodically with the use of a hemocytometer. (C) Time course of differentiation of FDB2 cells expressing FLAG-tagged hβc proteins. In the same experiment as in B, samples of FDB1 and FDB2 cells cultured in the presence of the indicated growth factors or in the absence of growth factors (-GFs) were cytocentrifuged periodically, and the proportion of differentiated granulocytes (▪, dashed lines), the proportion of macrophages (▴, dashed lines), and the total proportion of differentiated cells (♦, solid lines) were determined from at least 200 cells scored microscopically. As shown in B, no viable cells were present in Fhβc -GFs beyond the second day of culture.
Expression and function of FLAG-tagged hβc proteins in FDB2 cells.
(A) Flow cytometric analysis of FDB2 cells expressing FLAG-tagged hβc subunits. FDB2 cells expressing the indicated FLAG-tagged hβc subunits were stained with the anti-FLAG monoclonal antibody M2 (solid lines). As controls, uninfected FDB2 cells were stained identically (dashed lines). (B) Time course of proliferation of FDB2 cells expressing FLAG-tagged hβc proteins. FDB2 cells were washed and cultured in IL-3 (▪) or GM-CSF (▴) or in the absence of growth factors (▾), and the number of viable cells was determined periodically with the use of a hemocytometer. (C) Time course of differentiation of FDB2 cells expressing FLAG-tagged hβc proteins. In the same experiment as in B, samples of FDB1 and FDB2 cells cultured in the presence of the indicated growth factors or in the absence of growth factors (-GFs) were cytocentrifuged periodically, and the proportion of differentiated granulocytes (▪, dashed lines), the proportion of macrophages (▴, dashed lines), and the total proportion of differentiated cells (♦, solid lines) were determined from at least 200 cells scored microscopically. As shown in B, no viable cells were present in Fhβc -GFs beyond the second day of culture.
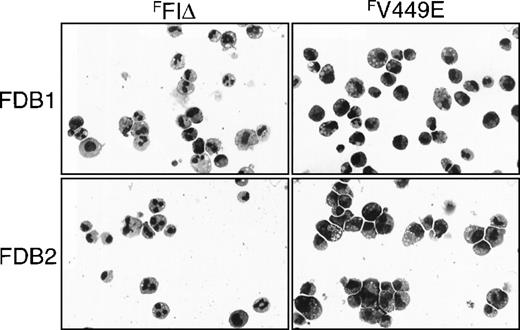
Morphology of factor-independent FDB1 and FDB2 cells expressing FLAG-tagged hβc mutants.
FDB1 and FDB2 cells expressing the indicated FLAG-tagged hβc proteins and cultured in the absence of growth factors for 5 days were cytocentrifuged and Wright-Giemsa stained. Photographs are at 270 × magnification.
Morphology of factor-independent FDB1 and FDB2 cells expressing FLAG-tagged hβc mutants.
FDB1 and FDB2 cells expressing the indicated FLAG-tagged hβc proteins and cultured in the absence of growth factors for 5 days were cytocentrifuged and Wright-Giemsa stained. Photographs are at 270 × magnification.
Finally, to confirm the identities of the cell types observed, we investigated the expression of several myeloid surface antigens on FDB2 cells induced to differentiate by GM-CSF or the FFIδ protein using flow cytometry. As shown in Figure 5, when FDB2-Fhβc cells were induced to differentiate with GM-CSF for 7 days, there was a decrease in cell size and an increase in granularity as measured by forward scatter and side scatter, respectively. There was induction of the granulocyte-macrophage markers Mac-1 and Gr-1 and of the monocytic marker F4/80. These changes are consistent with the morphological observation that these cells had undergone granulocyte-macrophage maturation (above). Similar changes in scatter profile and expression of myeloid cell surface markers were observed in FDB2-FFIδ cells deprived of growth factors, which is also consistent with the above observations that these cells undergo granulocyte-macrophage maturation upon cytokine deprivation. In contrast, FDB2-FV449E cells deprived of growth factors did not exhibit altered scatter profile or cell surface antigen expression, which is consistent with the above observations that these cells are morphologically similar to cells grown in IL-3.
Discussion
Properties of the FDB1 and FDB2 cell lines
We have isolated 2 novel murine myeloid cell lines that proliferate in murine IL-3 but cease growth and differentiate in response to murine GM-CSF. Other cell lines displaying an IL-3/GM-CSF differentiation switch have been previously reported. When the FDCP-mix cell line is infected with a retroviral vector expressing mGM-CSF, multipotent variants of this line can be selected in IL-3 that proliferate in IL-3 and differentiate upon IL-3 withdrawal owing to autocrine GM-CSF stimulation.9 However, uninfected FDCP-mix cells respond poorly to exogenous GM-CSF unless only low levels of IL-3 are present and the horse serum used for passage of this line is replaced by fetal calf serum.19 Also, a variant of the FDC-P1 cell line, WT19, which is dependent on IL-3 for growth, differentiates toward the monocyte lineage in response to GM-CSF.20,21 However, this differentiation is partial and reversible and does not lead to growth arrest. Furthermore, an IL-3–dependent cell line derived from a leukemic mouse containing a retrovirus encoding IL-11 differentiates in GM-CSF.22However, as this cell line also produces autocrine IL-11, the role of each growth factor in the growth and differentiation of this cell line remains uncertain. Hence, FDB1 and FDB2 are the first reported cell lines having the properties of growth in IL-3 alone and having complete and terminal differentiation in response to exogenous GM-CSF. As such, they have potential utility in determining the molecular characteristics of this unique differentiation switch (see also below).
Implications for βc signaling
In this study we have expressed constitutively active hβc mutants in the multipotential myeloid cell lines FDB1 and FDB2, and shown that while extracellular mutants deliver a differentiation signal to these lines, a transmembrane mutant, V449E, confers factor-independent proliferation. There are at least 2 possible explanations for the different effects of transmembrane and extracellular hβc mutants in these cell lines. First, it is possible that V449E delivers a quantitatively stronger signal than the extracellular hβc mutants. Since very low concentrations of mIL-3 (≤ 0.4 U/mL) lead to differentiation of FDB2 cells (Table 1), it is possible that, in like manner, extracellular hβc mutants deliver a weaker form of the same signals generated by V449E, resulting in differentiation. However, there are no large differences in the proliferation rates or activation of downstream signal transduction pathways in FDC-P1 cells bearing the FIδ and V449E mutants4 7 or in the levels of expression of FFIδ and FV449E in FDB2 cells in this study (Figure 6A).
Rather, the disparate cellular outcomes elicited by transmembrane and extracellular hβc mutants in the FDB cell lines are more likely to be due to qualitative differences in signal transduction by these 2 classes of hβc mutants. This would result from different modes of activation, for which there is recent evidence. It has been shown that I374N, an extracellular point-mutant, forms a heterodimeric complex with mGMRα in murine cell lines and requires expression of mGMRα for function, while V449E does not.4,6 A similar requirement of mGMRα for function in murine cell lines has been observed for the FIδ mutant (R. D'Andrea, oral communication, August 1997). In contrast, the V449E mutant is analogous to the V664E transmembrane mutant of the c-Neu receptor tyrosine kinase, which leads to ligand-independent aggregation and activation of this receptor.23 24 By analogy, it is probable that this mutant functions by inducing hβc homodimerization in the absence of ligand leading to constitutive activity.
The signaling properties of the transmembrane and extracellular hβc mutants have been studied previously.7Although JAK2, STAT, and MAP kinase proteins were activated by both classes, several defects were found in the signaling of I374N; among these are receptor phosphorylation7 and phosphorylation of Shc (T. Blake, unpublished data, September 1997). Taken together with the results of this study, this implies that these properties are dispensable for hβc-induced differentiation.
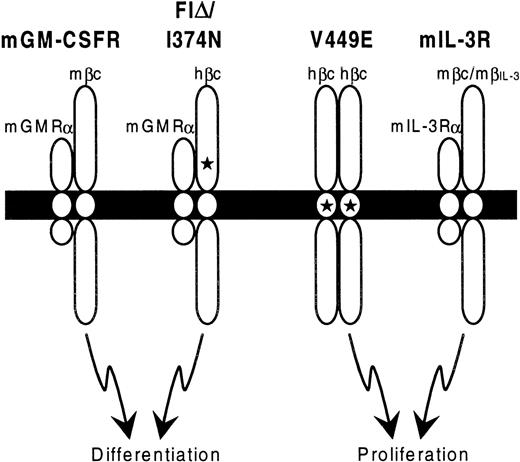
Taking these findings together with those presented here, a model is proposed in Figure 8 that involves 2 modes of signaling through hβc. Heterodimerization with mGMRα leads to a signal that is predominantly differentiative. However, since FIδ and I374N are able to mediate factor-independent proliferation of the FDC-P1 cell line, some aspects of mitogenic signaling must also be entailed in this mode of signaling. As the cytoplasmic region of mGMRα is required for function of FIδ and I374N in murine cell lines,6 this subunit is likely to play a key role in signaling, either by inducing differentiation pathways directly or by suppressing pathways leading to proliferative signaling. As mGMRα is also a component of the mGM-CSFR, it is possible that its presence is a key determinant of the different cellular responses of FDB cell lines to mIL-3 and mGM-CSF. In contrast, hβc homodimerization caused by V449E (presumably in the absence of heterodimerization with an α subunit) leads to a proliferation signal in these lines.
Model for signaling by constitutively active hβc mutants.
Schematic diagram showing the proposed stoichiometry of constitutively active hβc mutant receptor complexes. The cell membrane is represented by a solid bar, with extracellular regions at the top. Stars indicate the positions of activating mutations in hβc. Note that the wild-type mGM-CSFR and mIL-3R may in fact be tetrameric,25 but for simplicity only heterodimers are shown.
Model for signaling by constitutively active hβc mutants.
Schematic diagram showing the proposed stoichiometry of constitutively active hβc mutant receptor complexes. The cell membrane is represented by a solid bar, with extracellular regions at the top. Stars indicate the positions of activating mutations in hβc. Note that the wild-type mGM-CSFR and mIL-3R may in fact be tetrameric,25 but for simplicity only heterodimers are shown.
As wild-type hβc is expressed in the FDB cell lines, it is formally possible that it contributes to either of the above signaling complexes involving constitutively active hβc mutants. However, as the extracellular hβc mutants exhibit similar differentiative activities in primary murine hemopoietic cells5 and in the FDB cell lines, the presence of hβc appears not to be required for their function.
The ability of transmembrane and extracellular mutants to deliver qualitatively different signals to hemopoietic precursors must be borne in mind when considering the function of these mutants in vivo. Indeed, recent experiments indicate that transmembrane and extracellular hβc mutants induce distinct hemopoietic disorders in bone marrow–reconstituted mice, with extracellular mutants causing chronic myeloproliferative disorders while transmembrane mutants lead to acute leukemia.26 This implies that the differences in signaling between transmembrane and extracellular hβc mutants reported here may also apply in vivo.
The ectopically expressed constitutively active hβc mutants employed in this study functionally mimic the endogenous IL-3/GM-CSF differentiation switch in the FDB cell lines. Thus, this system should prove useful in studying differentiation and proliferation by, for example, introducing constitutive hβc mutants with second-site mutations in regions of hβc that affect various downstream signaling pathways.
Note added in proof: Since the manuscript was accepted for publication, Evans et al.27 reported that the (human) IL-3 and GM-CSF receptors can switch FDCP-mix cells between proliferation and differentiation. Moreover they have shown, as we speculated, that the differential response is a function of the GM-CSF receptor α subunit.
Supported in part by grants (to TJG) from the National Health and Medical Research Council of Australia. MPM is a recipient of an Australian Postgraduate Award from the University of Adelaide. TJG is a Senior Research Fellow of the National Health and Medical Research Council of Australia.
Reprints:Thomas J. Gonda PhD, the Hanson Centre for Cancer Research, Institute of Medical and Veterinary Science, Frome Road, Adelaide, SA 5000, Australia
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal