Abstract
To date, the normal transcriptional regulation of the human β-globin gene cluster has been recapitulated most accurately in transgenic mice that carry large yeast artificial chromosome (YAC) or ligated cosmid constructs. However, these large transgenes still exhibit variegated expression levels, perhaps because they tend to rearrange upon integration, or because the cloning vectors remain attached to the globin inserts. To try to circumvent these potential problems, we investigated the transgenic properties of a 100-kb DNA fragment containing the entire human β-globin cluster propagated in a bacterial artificial chromosome (BAC). We created 9 independent mouse lines, each carrying 1 to 6 copies of the human β-globin cluster without the attached BAC vector. Five of the lines carry unrearranged copies of the cluster. Reverse-transcriptase polymerase chain reaction (RT-PCR) analysis of adult F1 mice showed that 2 lines express human β globin at levels approximately equivalent to the endogenous mouse β-major genes. One line expresses no human β globin, while the remaining 6 lines show intermediate expression levels. Complete γ→β-globin gene switching occurs, but is slightly delayed with respect to the endogenous mouse embryonic→adult switch. Since these data are similar to what has been obtained using globin YACs or ligated cosmids, we conclude that (1) globin transgenes propagated in BACs are no less likely to rearrange than their cosmid or YAC counterparts, and (2) the retention of YAC vector sequences in a transgene probably has no significant impact on globin expression when using constructs of this size.
THE HUMAN β-GLOBIN GENE cluster is composed of 5 functional genes (ε, Gγ, Aγ, δ, and β) arrayed on chromosome 11 in the order in which they are developmentally expressed. The genes are flanked upstream by a group of DNAse I hypersensitive sites collectively known as the locus control region (LCR), as well as a single downstream hypersensitive site (3′HS-1). A number of groups have used cosmid, ligated cosmid, or yeast artificial chromosome (YAC) constructs to try to recreate the native spatial architecture of the human β-globin locus in transgenic mice.1-8 These large constructs accurately recapitulate the normal cis environment required for high-level, tissue and developmental stage-specific globin gene transcription.2,7,9,10 Cosmid, ligated cosmid, and YAC β-globin cluster transgenes approximate the normal γ- to β-globin switch that occurs during human development. However, these constructs do have limitations. Ligated cosmids are restricted to approximately 70 kb in size and therefore cannot encompass the entire β-globin locus. Although larger, YAC constructs have in many cases shown a propensity to rearrange upon integration.3,11,12 Studies with YACs also have been somewhat limited by the difficulty in isolating the globin cluster insert away from the yeast vector arms, leaving open the possibility of “vector poisoning” affecting expression levels in mice.13 14
Bacterial artificial chromosomes (BACs) are a DNA vector system based on the bacterial F1 fertility factor. Propagated as single-copy plasmids in Escherichia coli, BAC vectors are capable of holding inserts of up to 300 kb.15 BACs have become an essential tool for handling large DNA fragments in the course of mapping the genomes of several organisms. More recently, BAC inserts have been used to generate transgenic mice.16-19 We have obtained a BAC clone that carries a 100-kb fragment containing the entire human β-globin cluster and flanking LCR. Using both pronuclear injection and embryonic stem (ES)-cell methodologies, we created 9 independent founder lines of transgenic mice. We sought to determine whether β-globin cluster–containing BACs would offer any advantages in providing either improved transgene integrity or high-level gene expression independent of integration site. The performance of the BAC transgene was essentially equivalent to that of YAC transgenes; the major advantage of BAC transgenesis is therefore convenience of propagation and DNA preparation. DNA rearrangement frequency appears to be primarily influenced by DNA fragment size, regardless of the vector type used. Likewise, the position effect-variegation exhibited by large transgenes appears to occur in a vector-independent manner.
MATERIALS AND METHODS
DNA constructs and DNA manipulation.
The P1-derived artificial chromosomes (PAC) clone designated 4396 was isolated from a commercial library (Genome Systems, St Louis, MO) by screening with human β-globin cluster–specific polymerase chain reaction (PCR) primers as follows. An initial screen was performed using primers specific for human ε IVS II (forward primer: 5′-AGCAGACTTCTAGTGAGCAT-3′; reverse primer: 5′-AGTTCTCAGCGGGAGTTTAA-3′; 400-bp PCR product). All clones identified as positive were then screened with 2 additional primer sets, specific for 5′HS-4 (forward primer: 5′-ATCTGCAGAGCCAGGGCCGA-3′; reverse primer: TCCTGACTTTCTGTCTAGTG-3′; 239-bp PCR product) and human β globin (forward primer: 5′-ACCTGACTCCTGAGGAGAAG-3′; reverse primer: 5′-GATCCTGAGACTTCCACACT-3′; 579-bp PCR product). Two PAC clones positive for all 3 PCR products were identified (Genome Systems control no. 4396 and 4397). PAC 4396 was arbitrarily selected for further investigation. Initial studies showed that the 180-kb PAC insert tended to randomly nick upon routine manipulation in vitro. Therefore, a HindIII partial digest was performed, and fragments of 90 to 110 kb were isolated and cloned into theHindIII site of the 7.4-kb vector pBeloBAC 11 (Genome Systems). β-globin–positive BAC clones were further investigated by short-range Southern analysis, using specific probes for HS-4, ε, Gγ, Aγ, β, and 3′HS-1. Short-range mapping, usingEcoRI-, BamHI-, and HindIII-digested DNA indicated that 1 clone, designated 4396-44, contained all the above markers: further characterization of this clone is provided in the Results section.
The BAC 4396-44 insert was isolated from its vector as follows (whenever possible, large-bore pipette tips were used to help minimize shearing): 25 μg of plasmid 4396-44 was digested with NotI at 37°C for 2 hours. The insert was then purified by pulse-field gel electrophoresis (PFGE). The digestion products were loaded into the preparative well of a 1% low-melt agarose contour-clamped homogeneous electric field (CHEF) gel made with 0.5X Tris Borate-EDTA (TBE). A 10-μL quantity of digestion products was run in lanes on either side of the preparative well to permit isolation of the insert without having to expose it to ethidium bromide. Using a Bio-Rad CHEF DR II apparatus (Hercules, CA), PFGE was performed for 16 hours at 4°C, using a field strength of 5 V/cm and switch time ramping from 5 to 15 seconds. Following PFGE, the 100-kb insert was identified by cutting the gel into thirds, removing the middle third containing the insert, and staining only the 2 flanking portions of the gel with ethidium bromide. Notches were cut in the flanking gel sections to indicate where the insert had run. The gel was reassembled, and an unstained gel slice containing the insert was excised and stored at 4°C in 50 mmol/L EDTA.
Transgenic mice.
In preparation for microinjection, a 4-mm piece of gel containing the BAC insert was equilibrated with high-salt injection buffer (100 mmol/L NaCl, 10 mmol/L Tris pH 8.0, 250 μmol/L EDTA) for 2 hours at 4°C. The gel slice was removed from the buffer, transferred to a microfuge tube, and melted at 65°C for 10 minutes. The sample was then equilibrated at 45°C for 5 minutes, and digested with 2 U of gelase (Epicentre, Madison, WI) at 45°C for 1 hour. Undigested agarose was removed by centrifuging the sample at 2,000 rpm at room temperature for 5 minutes and transferring the top 90% of the material to a new microfuge tube. DNA quality and quantity were estimated by running a small amount of the purified insert on an agarose gel. The final concentration was adjusted to 1 ng/μL using high-salt injection buffer. This material was then stored at 4°C until needed. Microinjection of fertilized mouse eggs (C3H × BL/6 F1) was performed as described.20 Ten independent founder lines were identified by Southern analysis ofEcoRI-digested tail DNA using probes specific for the human β-globin cluster. A final line (no. 3) was created by coelectroporating 250 ng of the BAC insert into 107 RW4 ES cells (strain 129/SvJ) along with 20 μg of a linearized PGK-Neo plasmid. Selection with 300 μg/mL G418 was performed for 6 days, and 110 G418-resistant ES clones were obtained. PCR and Southern analysis were performed to identify ES clones carrying a stably integrated transgene. Nine such clones were identified, of which 6 appeared to contain completely intact transgenes. One of these clones (no. 43) was then injected into e2.5 C57Bl/6 blastocysts as described.20Chimeric mice were obtained, and the transgene was bred into the germline.
Mouse genotyping.
Short-range mapping was performed by Southern blot analysis ofEcoRI-digested tail DNA using probes specific for individual regions of the human β-globin cluster. For long-range mapping, 106 mouse splenocytes were embedded in 1% agarose plugs. High-molecular-weight DNA was prepared as described,21digested with KpnI, and analyzed by PFGE and Southern blotting as described earlier. Transgene copy number was estimated by phosphorimaging a short-range Southern blot containing DNA from all transgenic lines, as well as a sample of K562 DNA, a human erythroleukemia cell line known to be triploid for the β-globin cluster. The human ε signal from each transgenic line was compared with the ε signal from the K562 DNA. DNA loads were normalized by phosphorimaging bands of approximately the same size following hybridization with a human dBpB-1 cDNA probe.22 Because this probe is 99% identical to the murine dBpB-1 cDNA, it efficiently cross-hybridizes with mouse DNA.
Expression analysis.
A reverse-transcriptase (RT)-PCR assay was used to measure human β-globin and adult mouse β-globin (β major + β minor) mRNA levels simultaneously in adult F1 transgenic mice. Peripheral blood was collected by performing retroorbital bleeds on anesthetized mice. Ten-microliter peripheral blood samples were used to prepare RNA as described.23 The RNA was reverse-transcribed to cDNA, and 1 μL cDNA was used in a 30-μL PCR reaction. A universal upstream primer spanning β-globin exons 2 and 3 was used in conjunction with species-specific downstream primers that recognize sequences in human or mouse exon 3, respectively. The primer sequences are as follows: universal upstream primer: 5′-TGAGAACTTCAGGGCTCCTG-3′; human exon 3 reverse: 5′-GCCCTTCATAATATCCCCCA-3′; mouse exon 3 reverse: 5′-ACAGGCAAGAGCAGGAAAGG-3′. The mouse β major PCR product was 166 bp, and the human β-globin product was 226 bp in length. A 1-μL cDNA sample (undiluted, or diluted 1:10 with Tris-EDTA [TE]) was amplified in the presence of 0.05 μL 32P-dATP (0.5 μCi) for 20 cycles (99°C × 30 seconds/55°C × 30 seconds/72°C × 30 seconds). The reaction products were separated by electrophoresis on 5% acrylamide gels and visualized by autoradiography. The ratios of human β-globin mRNA to mouse β major mRNA were determined by spot densitometry and/or phosphorimaging. All values were normalized for transgene copy number, as well as for the dATP content of the respective PCR products.
Analysis of β-like globin expression during development.
Timed matings were set up between male adult F1heterozygous transgenic mice and wild-type B6/C3H female mice. RNA was harvested on embryonic days 10.5 (yolk sac), 14.5 (fetal liver), and 18.5 (fetal liver), as well as from the peripheral blood of adult transgenic heterozygotes (above). RT-PCR was performed to examine relative levels of human ε, γ, and β globins, or mouse βH1 and adult β globins. The following primers were used to simultaneously amplify human γ- and β-globin cDNA sequences in a multiplex PCR reaction: human γ exon 1: (5′-AGGTGAATGTGGAAGATGCT-3′); human γ exon 2: (5′TTTGGGGTTGCCCATGATGG-3′); human β exon 1: (5′-AACTGTGTTCACTAGCAACC-3′); human β exon 2: (5′-AGCATCAGGAGTGGACAGAT –3′). The PCR conditions used were 94°C × 30 seconds/57°C × 30 seconds/72°C × 30 seconds. The size for the γ-globin product was 127 bp, and for β globin, 194 bp. The following primers were used to simultaneously amplify human ε and γ cDNA sequences: human ε exon 1, 5′-GCAATCACTAGCAAGCTCTC-3′; human ε exon 2, 5′-CAGGGGTAAACAACGAGGAG-3′; human γ exon 2, 5′-GAGATGCCATAAAGCACCTG-3′; and human γ exon 3, 5′-TCAGTGGTATCTGGAGGACA-3′. The PCR conditions used were as follows: 99°C × 30 seconds/70°C × 1 minute. The size for the ε globin product was 145 bp, and for the γ globin product, 227 bp. All primer sets flank introns, allowing authentic signals to be differentiated from any produced from a contaminating genomic template. An analogous multiplex PCR experiment was performed to simultaneously amplify mouse βH1 and β major cDNA sequences. To amplify βH1, an exon 1 forward primer (5′-CACTCGAGATCATCTCCAAGC-3′) was used with an exon 2 reverse primer (5′-TAACCCCCAAGCCCAAGGATG-3′). Adult mouse β-globin mRNA (β major + β minor) was amplified using an exon 2 forward primer (5′-CACCTTTGCCAGCCTCAGTG-3′) with an exon 3 reverse primer (5′-GGTTTAGTGGTACTTGTGAGCC-3′). The PCR conditions used were as follows: 94°C × 30 seconds/ 57°C × 30 seconds/72°C × 30 seconds. The product size for βH1-globin cDNA was 267 bp, and for β-major plus β-minor cDNA, 197 bp. Both primer sets flank introns. As in the adult expression studies, 30-μL PCR reactions were run in the presence of 0.05 μL32P-dATP. Reaction products were electrophoretically separated on 5% acrylamide gel and quantitated by spot densitometry and/or phosphorimaging.
RESULTS
Isolation and characterization of the human β-globin cluster–containing BAC.
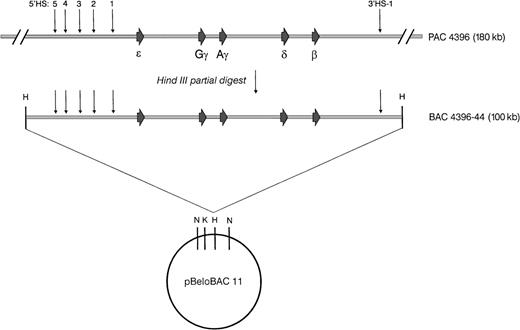
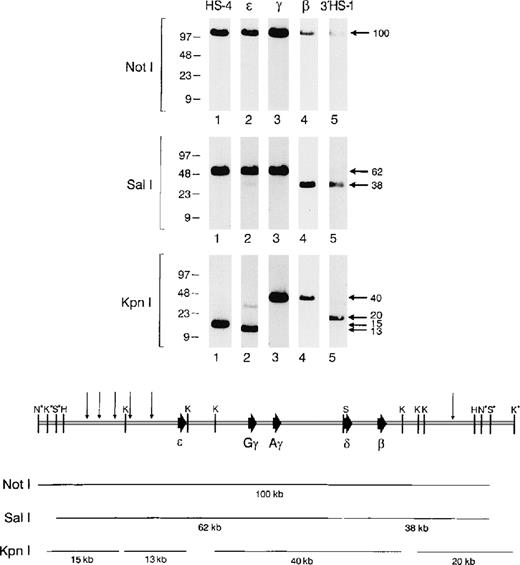
We screened a human PAC library with β-globin–specific probes and identified a 180-kb clone (designated 4396) that was determined by Southern analysis to contain the entire intact human β globin gene cluster (Fig 1). In initial experiments, the PAC insert tended to undergo random nicking upon routine manipulation in vitro. Therefore, we sought to obtain a smaller piece of DNA that still contained all potentially important functional sequences of the β-globin cluster. A HindIII partial digest was performed on PAC 4396, and fragments of 90 to 110 kb were cloned into the BAC vector pBeloBAC 11. One clone, designated 4396-44, was determined by Southern analysis to contain an approximately 100-kb insert that retained the entire human β-globin cluster (Fig 1). As shown in Fig 2, no structural rearrangements were detected in this DNA fragment. Probes for HS-4, ε, γ, β, and 3′ HS-1 all colocalize to the same approximately 100-kb NotI fragment, suggesting that the BAC insert spans the entire β-globin locus. SalI and KpnI digests likewise yield the expected fragments on long-range Southern analysis. The β-globin cluster insert, released by digestion withNotI, was used to generate all the transgenic animals described below. The NotI digest leaves 382 bp of vector sequence attached to the 5′ end of the insert, and 248 bp of vector sequence on the 3′ end.
Map of the human β-globin cluster–containing BAC. A 100-kb HindIII fragment containing the human β-globin cluster was generated by a partial digest of the 180-kb PAC 4396 and subcloned into the 7.4-kb pBeloBAC 11 vector to create BAC 4396-44. The BAC 4396-44 insert was subsequently released from the vector by aNotI digest, isolated by PFGE, and microinjected into fertilized mouse eggs or transfected into ES cells.
Map of the human β-globin cluster–containing BAC. A 100-kb HindIII fragment containing the human β-globin cluster was generated by a partial digest of the 180-kb PAC 4396 and subcloned into the 7.4-kb pBeloBAC 11 vector to create BAC 4396-44. The BAC 4396-44 insert was subsequently released from the vector by aNotI digest, isolated by PFGE, and microinjected into fertilized mouse eggs or transfected into ES cells.
Structural analysis of the human β-globin cluster–containing BAC. (A) BAC 4396-44 DNA was digested withNotI, SalI, or KpnI, and a Southern analysis was performed. The blot was hybridized sequentially with probes for HS-4 (lanes 1), ɛ (lanes 2), γ (lanes 3), β (lanes 4), and 3′HS-1 (lanes 5). The relevant lanes from each autoradiogram were cut out and reassembled as shown. (B) Restriction map of the BAC transgene. The sizes of key restriction fragments are shown below the diagram of the β-globin cluster. (N, NotI; S, SalI; K, KpnI; H; HindIII.) *Restriction sites derived from the pBeloBAC vector and not the β-globin cluster.
Structural analysis of the human β-globin cluster–containing BAC. (A) BAC 4396-44 DNA was digested withNotI, SalI, or KpnI, and a Southern analysis was performed. The blot was hybridized sequentially with probes for HS-4 (lanes 1), ɛ (lanes 2), γ (lanes 3), β (lanes 4), and 3′HS-1 (lanes 5). The relevant lanes from each autoradiogram were cut out and reassembled as shown. (B) Restriction map of the BAC transgene. The sizes of key restriction fragments are shown below the diagram of the β-globin cluster. (N, NotI; S, SalI; K, KpnI; H; HindIII.) *Restriction sites derived from the pBeloBAC vector and not the β-globin cluster.
Generation of transgenic mice.
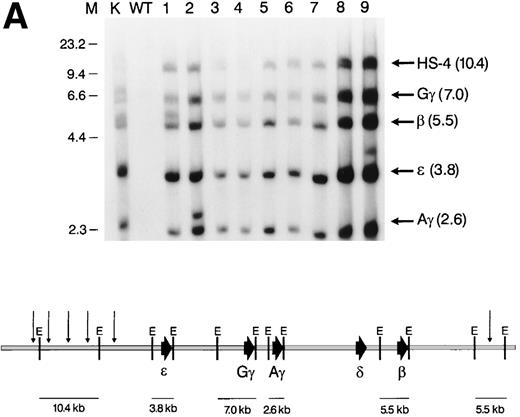
Initially, 10 of 114 potential founder mice, created by direct pronuclear injection of the globin BAC insert, were determined to be transgene-positive by Southern analysis of tail DNA. Two mice failed to transmit the transgene to the F1 generation; these animals were presumed to be germline mosaics and were not analyzed further. The other 8 transgenic lines did transmit to the germline and are characterized below. The overall number of transgenic mice generated (9% of potential founders) was the same as that observed in our laboratory when generating conventional transgenic mice. Line no. 3 was generated using ES-cell–based technology (see below). Each founder line was found to carry 1 to 6 copies of the human β-globin cluster transgene. Short- and long-range mapping studies showed that the transgene integrated without any detectable rearrangements in 5 of the 9 founder lines. As shown in Fig 3, all lines were positive by short-range Southern blot analysis for DNA fragments containing HS-4, ε, Gγ, Aγ, δ, β, and 3′-HS-1. Three lines (nos. 1, 2, and 9) contain a single abnormal DNA fragment in addition to intact copies of the transgene. As these lines contain multiple copies of the locus, the additional bands may represent junction fragments.
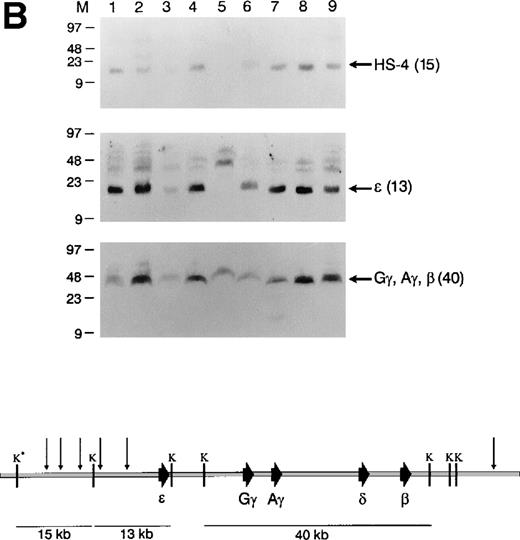
Structural analysis of the integrated β-globin BAC transgenes. (A) Top: short-range map of BAC transgenic mouse lines. K562 DNA (lane 1), wild-type mouse ES cell DNA (lane 2), and tail DNA from F1 transgenic mice (lanes 3-11) was digested withEcoRI and a Southern analysis was performed. The blot was hybridized simultaneously with radiolabeled probes for HS-4, ɛ, γ, and β; it was then stripped and rehybridized with a probe for 3′HS-1. Bottom: EcoRI restriction map of the BAC transgene. The sizes of relevant restriction fragments are shown below the diagram of the β-globin cluster. (B) Top: Splenocytes from F1 transgenic mice (lanes 1-9) were embedded in agarose plugs. High-molecular-weight DNA was prepared, digested withKpnI, and a Southern analysis was performed. The blot was hybridized sequentially with probes for HS-4, ɛ, γ, and β. Bottom: KpnI restriction map of the BAC transgene. *KpnI derived from the pBeloBAC vector; E, EcoRI; K,KpnI.
Structural analysis of the integrated β-globin BAC transgenes. (A) Top: short-range map of BAC transgenic mouse lines. K562 DNA (lane 1), wild-type mouse ES cell DNA (lane 2), and tail DNA from F1 transgenic mice (lanes 3-11) was digested withEcoRI and a Southern analysis was performed. The blot was hybridized simultaneously with radiolabeled probes for HS-4, ɛ, γ, and β; it was then stripped and rehybridized with a probe for 3′HS-1. Bottom: EcoRI restriction map of the BAC transgene. The sizes of relevant restriction fragments are shown below the diagram of the β-globin cluster. (B) Top: Splenocytes from F1 transgenic mice (lanes 1-9) were embedded in agarose plugs. High-molecular-weight DNA was prepared, digested withKpnI, and a Southern analysis was performed. The blot was hybridized sequentially with probes for HS-4, ɛ, γ, and β. Bottom: KpnI restriction map of the BAC transgene. *KpnI derived from the pBeloBAC vector; E, EcoRI; K,KpnI.
The line designated no. 3 was made using the ES-cell method: the β-globin cluster insert was isolated from the BAC vector and coelectroporated with 20 μg of a PGK-NEO plasmid into 129/SvJ ES cells. Nine G418-resistant ES-cell lines carrying the insert were obtained, of which 6 were determined to carry intact, unrearranged copies of the β-globin cluster transgene. One of these ES clones was used to create founder line no. 3.
Long-range structural analysis using the rare-cutting enzymeKpnI was performed on genomic DNA from all 9 founder lines (Fig3). Eight of the lines carry intact copies of the transgene, whereas line 5 contains a rearrangement involving the 5′ end of the globin cluster. In all lines except 5, the integrity of the 5′ end of the transgene is demonstrated by the blot hybridized with the HS-4 probe, which recognizes an approximately 15-kb DNA fragment. The endogenous human globin cluster KpnI fragment containing HS-4 is approximately 30 kb in length; a 15-kb fragment is detected for the transgene, because a KpnI site in the pBeloBAC 11 vector defines the 5′ end of the insert (Fig 1). In all lines, the γ- and β-globin genes colocalize to the same approximately 40-kb fragment. In lane 4, the appropriate HS-4–containing fragment is detected upon longer exposure of the Southern blot shown in Fig 2A, as well as in numerous other Southern analyses.
Expression of human β globin in adult transgenic mice.
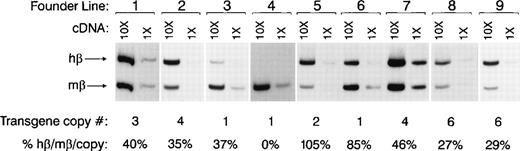
Semiquantitative RT-PCR was performed on peripheral blood RNA from adult F1 mice from each of the transgenic lines to determine the levels of human β-globin expression relative to endogenous mouse adult β globin on a per-gene-copy basis (Fig4). cDNA templates were used both undiluted or diluted 1:10 to establish the linearity of the assay. Eight of the 9 lines express detectable levels of human β-globin mRNA. The transgene copy numbers, and levels of human β globin (expressed as a percentage of mouse β major per gene copy) are shown below the autoradiograph. Two lines (no. 5 and 6) express human β globin at levels approximately equivalent to that of the endogenous β major gene (105% and 85%, respectively). Most of the lines express intermediate levels of human globin (27% to 46% of adult β globin). Line 4 does not express detectable amounts of human β globin, even though it contains the entire β-globin gene cluster with no detectable rearrangements.
Expression of human β globin by adult transgenic mice. Multiplex RT-PCR was performed on peripheral blood (PB) RNA from adult F1 transgenic mice. For each line, undiluted PB cDNA (first lane) and cDNA diluted 1:10 (second lane) were used as PCR templates to ensure that the assay was in the linear range of amplification. Both human β-globin– and adult mouse β-globin (β major + β minor)–specific primers were used in each reaction. Twenty cycles of PCR were performed in the presence of 32P-dATP, and the reaction products were separated on a 5% acrylamide gel. Levels of human β globin relative to adult mouse β globin were quantitated by phosphorimaging. Expression of human β globin as a percentage of adult mouse β-globin expression per gene copy is denoted for each founder line below each pair of lanes.
Expression of human β globin by adult transgenic mice. Multiplex RT-PCR was performed on peripheral blood (PB) RNA from adult F1 transgenic mice. For each line, undiluted PB cDNA (first lane) and cDNA diluted 1:10 (second lane) were used as PCR templates to ensure that the assay was in the linear range of amplification. Both human β-globin– and adult mouse β-globin (β major + β minor)–specific primers were used in each reaction. Twenty cycles of PCR were performed in the presence of 32P-dATP, and the reaction products were separated on a 5% acrylamide gel. Levels of human β globin relative to adult mouse β globin were quantitated by phosphorimaging. Expression of human β globin as a percentage of adult mouse β-globin expression per gene copy is denoted for each founder line below each pair of lanes.
Temporal regulation of globin gene expression.
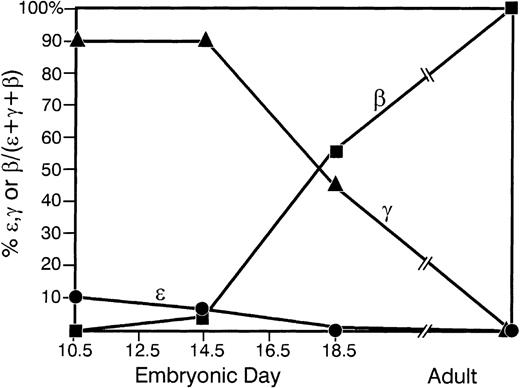
Relative levels of human ε-, γ-, and β-globin RNA were measured at different time points during development in 2 of the transgenic mouse lines. The data for line no. 1 are plotted in Fig5. Human γ-globin expression predominates on day 10.5 and day 14.5 of development. By day 18.5, β is the dominant human globin expressed, and is the only human globin detected in animals greater than 6 weeks of age. Low-level human ε-globin expression is restricted to embryonic days 10.5 and 14.5. Similar data were obtained for line no. 9. The endogenous βH1→β major switch occurred as expected between e10.5 and e14.5 (data not shown).
Developmental regulation of the globin BAC transgene. Timed matings were performed between transgenic F1 male mice from line no. 1 and WT B6/C3H female mice. RNA was harvested on e10.5 (yolk sac), e14.5 (fetal liver), e18.5 (fetal liver), or from adult mice >6 weeks old (peripheral blood). Multiplex RT-PCR was performed to determine the relative levels of human ɛ-, γ-, or β-globin mRNA at different stages of development. (•) Human ɛ-globin mRNA; (▴) human γ-globin mRNA; (▪) human β-globin mRNA. Each data point represents an average value for several experiments.
Developmental regulation of the globin BAC transgene. Timed matings were performed between transgenic F1 male mice from line no. 1 and WT B6/C3H female mice. RNA was harvested on e10.5 (yolk sac), e14.5 (fetal liver), e18.5 (fetal liver), or from adult mice >6 weeks old (peripheral blood). Multiplex RT-PCR was performed to determine the relative levels of human ɛ-, γ-, or β-globin mRNA at different stages of development. (•) Human ɛ-globin mRNA; (▴) human γ-globin mRNA; (▪) human β-globin mRNA. Each data point represents an average value for several experiments.
DISCUSSION
In this report, we have used a 100-kb vector-free DNA fragment containing the entire human β-globin cluster to generate 9 independent founder lines of transgenic mice. These lines were analyzed for transgene structural integrity and expression, with the goal of determining whether a BAC-propagated DNA fragment could provide an improved model of human β-globin gene regulation in transgenic mice. We found that the biologic behavior of the BAC transgene was essentially the same as that reported for ligated cosmids and YACs. The primary advantage of BACs, therefore, is their ease of propagation and manipulation in vitro.
In this study, we tried to further define the tendency of large transgenes to rearrange either in vitro or upon integration into the mouse genome. This phenomenon has been particularly well-documented with YACs.3,11,12 We postulated that our 100-kb DNA fragment might be more stable upon genomic integration because of its smaller size. Overall though, our experience has been similar to that of Peterson et al,11 whose rigorous YAC mapping studies support a direct relationship between the size of a construct and its propensity to rearrange upon genomic integration. As mentioned earlier, our initial 180-kb PAC clone often became degraded upon routine manipulation in vitro. Our final 100-kb BAC proved to be significantly more stable, yet we still detected transgene rearrangements in 4 of our 9 mouse lines, a frequency that is comparable to what has been described in YAC studies using transgenes of 150 and 248 kb in length.3 11
In early studies of human globin genes in mice, there was a strong suggestion that retained plasmid vector backbone sequences could dramatically repress transgene expression.13,14 In later experiments using YAC-based globin constructs, several kilobases of yeast vector sequence were routinely left attached to each end of the transgene.1-3,24,25 Only recently has it been possible to remove the vector arms, through the introduction of I-Ppo restriction sites via homologous recombination in yeast.11 26 In contrast, isolation of BAC inserts from the vector can easily be accomplished without having to perform any additional modifications of the plasmid. In our adult transgenic mice, the majority of lines expressed moderate levels of human globin relative to mouse β major on a per-copy basis. Two lines expressed human β globin at high levels; 1 line (no. 4) expressed no detectable human β globin even though we could not detect rearrangement in the transgene. These data are essentially equivalent to what has been observed previously using YAC mice, even in experiments in which yeast vector arms flanked the integrated transgenes. Thus, retained vector sequences do not appear to be a problem in experiments using large globin transgenes, and in this regard, BACs offer no particular advantage over YACs. The variation in expression we observed most likely reflects some degree of position-dependent variegation, even though the LCR is included in our transgene.
Complete γ→β-globin gene switching occurs in our mice, but with delayed kinetics. In most studies of YAC or cosmid-based transgenic mice, the γ→β switch occurs on or around e12.5.1,3,6 A slightly later switch (∼e13.5) was observed by Peterson et al in mice carrying either 155- or 248-kb YAC transgenes.11 Our mice switched later still, with β-globin expression not surpassing γ expression until approximately e18.5. The delayed switch could be related to integration site (although both lines switched at the same time), or it could be related to the precise sequences that are included in the transgene. Further experiments will be required to determine the cause of this phenomenon. The expression level and developmental regulation of human ε by our mice was similar to that reported previously for mice carrying β-globin cluster YACS11 or ligated cosmids.7
Only a small number of previously published reports describe the creation of transgenic mice using BACs. Yang et al16isolated a 131-kb BAC containing the murine RU49 gene, which encodes a brain-specific zinc-finger protein. Using a novel homologous recombination procedure, the RU49 insert was marked with a lacZ cassette and then used to generate 3 lines of transgenic mice. One line was analyzed extensively, and the BAC transgene was found to confer proper tissue-specific expression of RU49. Similarly, Nielsen et al17 used transgenic BAC mice to investigate the regulation of the human apolipoprotein B gene. Single high-expressing lines generated from 207- and 145-kb BAC inserts were analyzed, leading to the discovery of a regulatory domain required for the expression of apolipoprotein B in intestine. Two groups have reported the use of BAC transgenes for in vivo complementation studies to identify genes of interest in mice. Antoch et al18 used a 140-kb circular BAC transgene to correct the abnormal circadian rhythms in mice carrying a mutation of the Clock gene. Three of 4 founder lines analyzed contained transgenes with no obvious rearrangements; all 4 lines expressed sufficient levels of Clock mRNA to rescue the phenotype. Probst et al19 used a 140-kb BAC-derived transgene to correct the auditory and vestibular defects inshaker-2 mice. This led to the identification of Myo15, a myosin gene found to be mutated in these mice. While clearly demonstrating BACs to be a convenient vector system for propagating large transgenes, the aforementioned studies did not examine whether BAC-derived transgenes provided any inherent biologic advantages over YACs or cosmids for studying human gene regulation in the mouse.
Like the investigators in the above studies, we found that using a high-salt injection buffer (100 mmol/L NaCl, 10 mmol/L Tris pH 8.0, 250 μmol/L EDTA) considerably stabilized the high–molecular-weight transgene DNA prior to microinjection. This buffer has been used successfully in the past for YAC injections.27 Second, we found that the concentration of DNA used for microinjection greatly affected our ability to generate transgenic founder animals. We achieved our best results with a DNA concentration of 1 ng/μL. This was similar to what was observed by Yang et al,16 who suggested that higher concentrations of DNA (6 ng/μL) were probably toxic to injected mouse oocytes.
In conclusion, BAC transgenes share some of the same technical limitations common to both ligated cosmids and YACs. First, the propensity for large transgenes to rearrange appears to be an inherent property of their size. Second, the variation in human β-globin expression observed using these large transgenes is apparently not caused by the attached cloning vectors. Nevertheless, several features do make BACs an attractive alternative to other vector systems. BAC vectors can hold considerably larger inserts than ligated cosmids. Unlike YACs, BACs are conveniently propagated in E coli much like conventional plasmids, allowing for the preparation of milligram quantities of DNA. BAC libraries are technically easier to generate than YAC libraries. Finally, mutations may readily be introduced into BAC plasmids, either by homologous recombination in E coli,16 or by recA-assisted restriction endonuclease cleavage (RARE).28,29 So while BACs offer no inherent biologic advantages over previous vector systems for investigating the regulation of the β-globin gene cluster, their technical convenience makes them an attractive alternative for future experiments using large transgenes. However, as in all transgenic mouse studies, the expression patterns of multiple different insertion sites must be evaluated to define the consequences of a mutation within a BAC transgene. For this reason, targeted knock-in or knock-out studies of the endogenous mouse β-globin cluster may ultimately provide a more fruitful approach for understanding how the β-globin genes are regulated.30-34
ACKNOWLEDGMENT
We thank Tim Corbin for performing our microinjection work, and Pam Goda and Kelly Schrimpf for excellent mouse care. Nancy Reidelberger provided expert assistance with the preparation of the manuscript.
Supported by Grants No. DK38682 (T.J.L.) and DK09584 (R.M.K.) from the National Institutes of Health.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Timothy J. Ley, MD, Washington University School of Medicine, Division of Bone Marrow Transplantation and Stem Cell Biology, 660 S Euclid Ave, Campus Box 8007, St Louis, MO 63110; e-mail: timley@im.wustl.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal